Abstract
Neoadjuvant chemotherapy (NAC) is an optimal option in early breast cancer, but in ER-positive/HER2-negative (luminal) is still controversial, although a survival benefit has recently been observed when a histological response by Symmans’ method type 0 or I is achieved. The 21-gene Oncotype DX Breast Recurrence Score® assay (Oncotype DX®) is a validated test to assess the survival benefit of adjuvant chemotherapy in these patients but its role in the neoadjuvant setting is less established. We analyzed the results of the Oncotype DX® test in a cohort of 122 consecutive patients selected to receive NAC based on classical clinicopathological parameters and the correlation between the Oncotype DX® results and the pathological response assessed by Symmans’ method. Median age was 56.5 (range 31–84) years. Initial tumor size was T1 (<20 mm) in 46 patients (37.7%), 57 (46.7%) had a T2 tumor (20–50 mm), and 19 (15.6%) had a tumor size more than 50 mm. 59 (48.4%) had axillary node involvement. The median expression estrogen and progesteron receptors by immunohistochemistry was 280 and 120 respectively and median Ki67 index was 28%. The Recurrence Score (RS) results were <11 in 21 patients (17.2%) patients, RS 11 to 25 in 58 (47.5%), and RS > 25 in 43 (35.2%). Considering the Oncotype DX test results, neoadjuvant chemotherapy was administered to 60 patients (49%), 11 (9%) received adjuvant chemotherapy and 51 (42%) no chemotherapy. Testing with the assay has therefore led to 42% fewer chemotherapy treatments. Among 60 patients receiving NAC, pathologic response was achieved for 5 patients (8.3%) with RCB-0 and 15 RCB-1 (25%). We did not find any pathological response RCB-0 and RCB-I in the 20 patients who received NAC and had a Recurrence Score result <21 for the premenopausal group, or a RS result <25 for the postmenopausal group. For patients with highest Recurrence Score results (RS > 21 or 25 according to menopausal status) it was 12% (5/40) RCB-0 and 40% (16/40) RCB-I.
Conclusions
The Oncotype DX test could be a useful tool to select patients candidates for neoadjuvant chemotherapy in luminal breast cancer. Neoadjuvant chemotherapy could be avoided in 42% of patients. We found a correlation between Recurrence Score results and pathological response with 14% of RCB-0 and a total of 47% of significant pathological response type RCB-0 and RCB-I in patients with highest Recurrence Score results. Interestingly, patients with a Recurrence Score result inferior to 32 did not get any histological response type 0 and only 5% RCB-I.
Highlights
-
•
All comments are reviewed in the main manuscript text.
-
•
We have introduced changes in tables and Figures according Editor′s comments.
-
•
It is an academic study and we don′t received any founding and we appreciate all patients who participated.
1. Introduction
Neoadjuvant chemotherapy (NAC) is considered an optimal choice in early breast cancer, especially in HER2-positive and triple negative phenotypes due to its higher pathological complete response (pCR) and its significant benefit in survival, but remains controversial in HR-positive and an HER2-negative (luminal) disease, as the pCR rate is much lower and therefore the impact on survival is not sufficiently established [1].
Although the relevance of pCR is lower in luminal breast cancer, there are some studies showing a difference in prognosis depending on the size of residual tumor after NAC, so the magnitude of the response to NAC, excluding patients with pCR, has also a very important prognostic value [2]. The residual cancer burden (RCB), a standardized measure of pathological response, has recently been shown to accurately correlate pathological response and prognosis in early breast cancer patients treated with NAC [3].
Classical clinicopathological indicators of patients’ prognosis include tumor size, histopathological subtype and grade, presence of lymph node metastases, and lymphovascular invasion. Nevertheless, the predictive power of these features for selection of the optimal therapeutic approach is quite limited [4]. The low expression of other biomarkers such as Ki67 index and high expression of estrogen and progesterone receptors have been recognized as associated with poor response to NAC [5]. The association between the Ki67 index and pCR has been proven in many studies but it is difficult to establish precise cut-off values for Ki67 due to the variations in testing techniques that exist between different laboratories. However, few authors were able to demonstrate Ki67 levels as an independent predictor of pCR in multivariable models [6].
Despite the selection of luminal patients with high levels of Ki67 proliferation index, pCR remains very low [7] with rates ranging from 9% to 17%, so it is necessary to considerer other factors when selecting patients who will benefit from NAC.
The 21-gene Oncotype DX Breast Recurrence Score® assay was developed by Genomic Health, Inc. (now Exact Sciences) for patients with HR-positive early-stage breast cancer. The Recurrence Score result has been shown to predict the likelihood of benefitting from the addition of chemotherapy to hormonal therapy for luminal tumors in the adjuvant setting [8,9].
Recently, there have been multiple small studies incorporating the Oncotype DX® test in the neoadjuvant setting. These studies have shown that pCR or clinical complete response (cCR) to chemotherapy almost never occurs in patients with a low Recurrence Score result by Oncotype DX [[10], [11], [12], [13]]. Using other genomic signatures in the NAC setting, very few or no pathological responses were observed among patients with a low risk of recurrence (ROR) based on the PAM50 [14].
In this study, we examine if the Oncotype DX genomic test could be effective to select luminal patients to receive neoadjuvant chemotherapy and its accuracy in predicting histological response in an unique prospective series of consecutive patients in our public hospital.
2. Methods
2.1. Patient eligibility
This prospective single-center study enrolled female patients with HR+ (defined as > 10% tumor staining by immunohistochemistry [IHC]), HER2-negative (according to ASCO/CAP guidelines [15]) invasive breast cancers, with a tumour size ≥ 2 cm. Patients had at least 18 years of age; an ECOG performance status of 0 or 1; and ipsilateral axillary lymph nodes evaluated by imaging (MRI or ultrasound) within 6 weeks prior to registration. If indicated for abnormal lymph nodes, fine needle aspirate (FNA) or core needle biopsy was performed. All patients were evaluated by a multidisciplinary team that recommended neoadjuvant chemotherapy.
2.2. Study design
The primary objective of this prospectively designed study, was to assess the distribution of Recurrence Score results in pretreatment biopsies from early breast cancer patients who were candidates for neoadjuvant chemotherapy primarily because of tumor size and/or biological criteria such as high Ki67 index, always evaluated in an multidisciplinary team. The secondary objective was to evaluate the distribution of the Recurrence Score results in patients with and without pCR according to National Surgical Adjuvant Breast Project (NSABP) criteria, or histological response based in Symmans’ criteria [3].
Tissue blocks from the biopsies were sent to the Genomic Health laboratory (Clinical Laboratory Improvement Amendments certified) for Oncotype DX testing according to standard procedures [9]. Treatment was assigned based on the Recurrence Score (RS) result by study protocol: patients with RS < 11 were to undergo initial surgery; patients with RS > 25 in postmenopausal patients or RS > 20 in premenopausal patients were to receive NAC; patients with midrange RS 11 to 25 were assigned mainly to initial surgery.
2.3. Statistical analysis
Descriptive analyses included frequencies and percentages for categorical variables and means, medians, and ranges for continuous variables. The Recurrence Score results were analyzed as a continuous variable and in Recurrence Score results groups, considering the cut-offs of 11 and 20 for women 50 years of age or younger and of 11 and 25 for more than 50 years according to the results to the TAILORx trial [16]. Fisher’s exact test and Student’s t-test were performed to compare distribution of Recurrence Score results according to pCR and breast preservation. Univariate and multivariate logistic regression models were performed to investigate the associated factors with pCR. We studied concordance between IHC analysis and RT-PCR for ER, PR, and HER2 status. Statistical analysis was performed with SAS software 9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
3. Results
Between January 2016 and September 2019, 122 consecutive patients were included with locally or advanced breast cancer, considered candidates to receive neoadjuvant chemotherapy (NAC) to achieve a survival benefit, based on clinical variables such as initial tumor size or lymph node involvement. Patients were evaluated by a multidisciplinary team and an Oncotype DX test was performed to select treatment based on the Recurrence Score result according with the outcome of the prospective TAILORx trial that demonstrated an absence of chemotherapy benefit overall in patients with a Recurrence Score 11 to 25 [16]. The mean age was 56.5 (range 31–84) years. The distribution of Recurrence Score results was RS < 11 in 21 (17.2%) patients, RS 11–25 in 58 (47.5%), and RS > 25 in 43 (35.2%). Initial tumor size was T1 (<20 mm) in 46 (37.7%), 57 (46.7%) had a T2 tumor (20–50 mm), and 19 (15.6%) had a tumor size more than 50 mm. In addition, 59 (48.4%) had axillary node involvement assessed clinically as N1 by ultrasounds and confirmed histologically. The expression of classical biological variables was: median estrogen and progesterone receptors by immunohistochemistry 280 and 120 respectively and the median Ki67 index was 28%. General characteristics of the cohort are shown in Table 1.
Table 1.
General characteristics of the cohort.
| Characteristics |
Oncotype DX Recurrence Score category |
||||
|---|---|---|---|---|---|
| Correlation to RS |
|||||
| Total | RS < 11 | RS 11-25 | RS > 25 | ||
| 122 | 21 (17,2%) | 58 (57,2%) | 43 (35,2%) | ||
| Premenopausal | 45 | 8 (18%) | 14 (31%) | 23(51%) | P = 0.01 |
| Mean age (years) | 56,8 | 57 | 59,4 | 53 | p = 0.04 |
| Mean tumour size (mm) | 32,5 | 28,1 | 32,9 | 34,1 | P = 0.572 |
| Node + (%) | 48,4 | 38,1 | 44,8 | 58,1 | P = 0.244 |
| ER (IHQ) | 264 | 281 | 274 | 242 | P = 0.001 |
| PR (IHQ) | 121 | 219 | 116 | 81 | P = 0.000 |
| Ki67 (%) | (5-85)28,8 | (5-50) 19,8 | (8-65) 17,6 | (8-85) 34,9 | P = 0.000 |
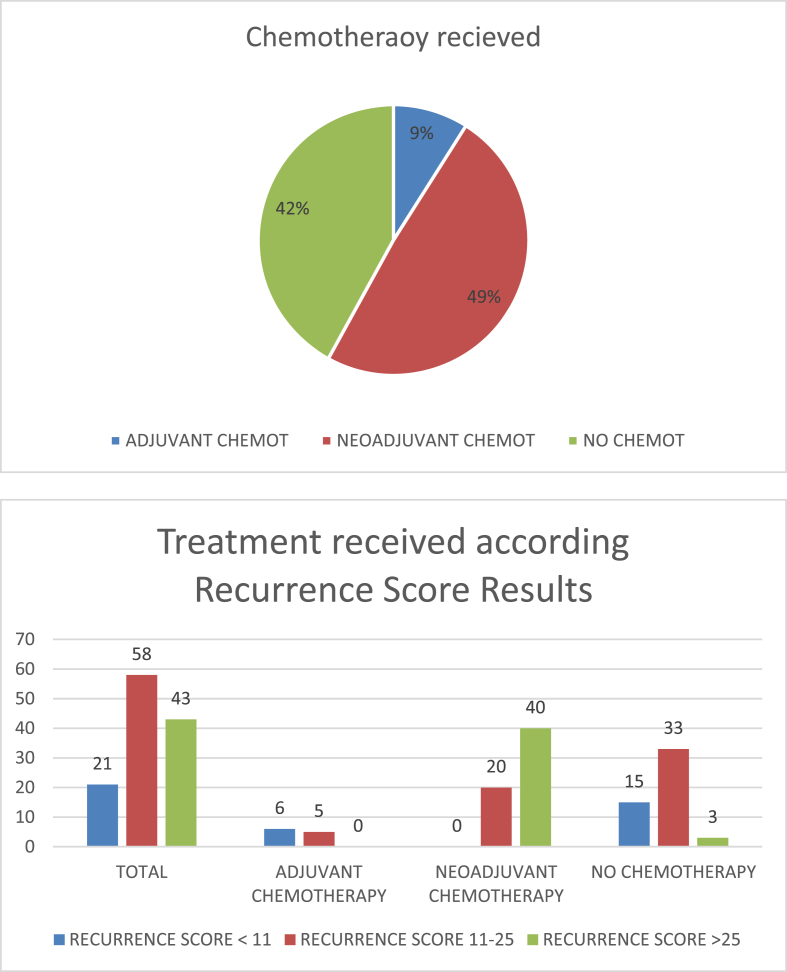
After receiving results of the Oncotype DX test, 60 patients (49%) received NAC, 11 (9%) adjuvant chemotherapy (after surgery for axillary involvement) and 51 (42%) did not receive any chemotherapy. Therefore, the Oncotype DX test has avoided a total of 42% of chemotherapy treatment in a cohort of patients with initial indication of chemotherapy by multidisciplinary team according to clinical and pathological criteria. None of the 21 patients with RS < 11 received NAC, but 6 patients ultimately received adjuvant chemotherapy because had pathologically node positive disease. In the group of RS 11–25, 20 of 58 patients received NAC and only 5 received adjuvant chemotherapy. Finally, 40 of 43 patients with Recurrence Score result >25 received NAC and the remaining three patients did not receive any type of chemotherapy treatment. The final treatment received based on the Oncotype DX test results is shown in Fig. 1.
Fig. 1.
Final treatment received based on the Oncotype DX test results.
Pathologic complete response after NAC was achieved by 5 (8.3%) patients and according to histological response to Symmans’ method, the total response type 0–1 was achieved in 20 (33,3%) patients. Among the 39 patients with initial axillary involvement that received NAC, 8 patients (20%) achieved a complete axillary response. Considering the 20 patients who received NAC in the low RS group (RS < 20 for the premenopausal patients and RS < 25 group for the postmenopausal) the complete pathological response was 0% and the pathological response type 0-I found according to Symmans’ method was <1% (1/20), while in the high risk group (RS > 20 or 25 according to menopausal status) it was 12% (5/40) and 47% (19/40) respectively. The results of the Oncotype DX test, consequent treatment recommendations and histological response to NAC are detailed in Table 2.
Table 2.
Recurrene Score Results, treatment received and histological response to NAC.
| MULTIDISCIPLINARY TEAM TREATMENT RECOMMENDATION AFTER ONCOTYPE DX TESTING: TOTAL 122 | ||||||
|---|---|---|---|---|---|---|
| PREMENOPAUSAL PATIENTS: n = 45 |
POSTMENOPAUSAL PATIENTS: n = 77 |
|||||
| Recurrence Score <11 n = 8 | Recurrence Score 11–20 n = 14 | Recurrence Score >20 n = 23 | Recurrence Score <11 n = 13 | Recurrence Score 11–25 n = 44 | Recurrence Score >25 n = 20 | |
| No CT | 6 | 9 | 2 | 9 | 24 | 1 |
| Adjuvant CT | 2 | 0 | 0 | 4 | 5 | 0 |
| Neoadjuvant CT | 0 | 5 | 21 | 0 | 15 | 19 |
| RCB TYPE 0-I | N/A | 0/5 0% |
9/21 42.9% |
N/A | 1/15 6.7% |
10/19 52.6% |
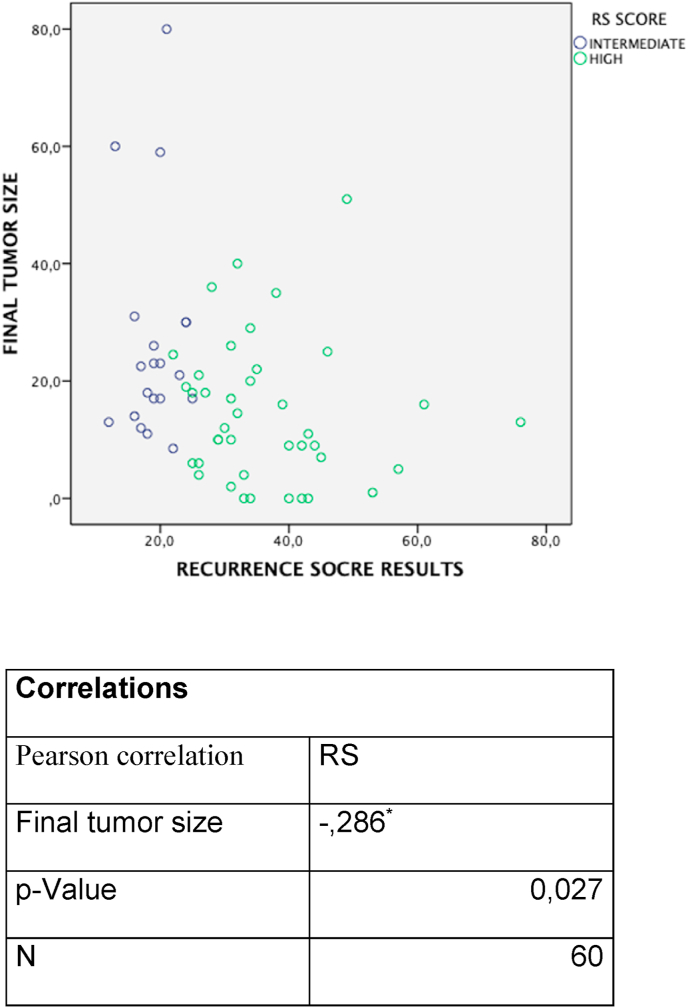
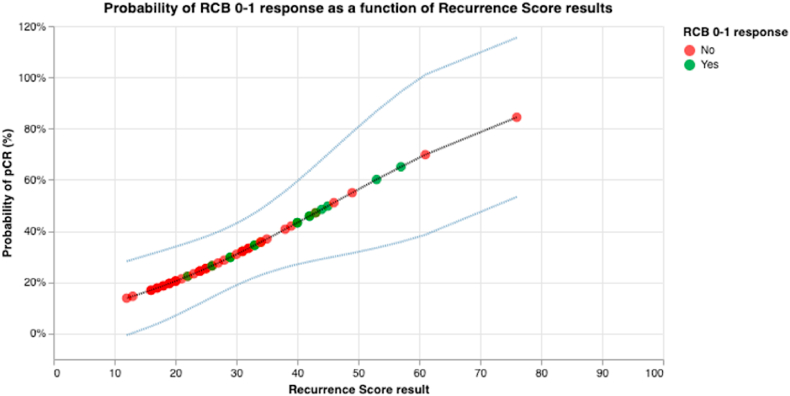
We have also assessed the correlation between Recurrence Score results and pathological response by Symmans’ method by logistic regression with the Recurrence Score results by quantitative expression and other variables such initial tumor size, hormonal receptors expression and Ki67 index. The Recurrence Score result was the only variable associated with pathological response (OR 0.94; 95% CI 0.901–0.99). Multivariable logistic regression analysis confirmed the significant relationship between pathological response and high Recurrence Score (OR 0.946; 95% CI 0.901–0.993) while controlling for other clinical variables. The distribution of Recurrence Score results in patients with respect to final tumor size after NAC status and their Pearson method correlation is represented Fig. 2. Using the probit regression method based on iteratively weighted least squares, specifically to assess the correlation of Recurrence Score to RCB 0–1 response, the global likelihood ratio test comparing the (full) model with Recurrence Score results to the (null) model excluding Recurrence Score was performed, resulting in a P value of 0.005. Predictive value of the probability of RCB 0–1 response as a function of Recurrence Score derived from the probit model fit and normal two-sided 95% CIs are provided in Fig. 3, where the probability of RCB 0–1 response increased with Recurrence Score result (see Table 3).
Fig. 2.
Distribution of Recurrence Score results in patients with respect to final tumor size after NAC.
Fig. 3.
Probablity of pathologic response RCB type 0–1 as a function of Recurrence Score results. The green circles represent patients who had a RCB type 0–1 response, whereas the red ones represent patients who did not have a RCB type 0–1 response. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Univariate and multivariate analysis correlation with pathological response.
| Variable | RCB 0–1 Univariate | RCB 0–1 Multivariate |
|---|---|---|
| Tumor size (mm) | OR 1.016 (CI 95% 0.991–1.041) | Non Significative |
| Node (N+) | OR 0.812 (CI 95% 0.336–1.964) | Non Significative |
| Age | OR 1.014 (CI 95% 0.978–1.052) | Non Significative |
| Estrogen R | OR 1.008 (CI 95% 1.000–1.017) | Non Significative |
| Progesterone R | OR 1.004 (CI 95% 1.000–1.009) | Non Significative |
| Ki67 | OR 0.97 (CI 95% 0.94–0.99) | Non Significative |
| RS | OR 0.94 (CI 95% 0.90–0.975) | OR 0.946 (CI 95% 0.901–0.993) |
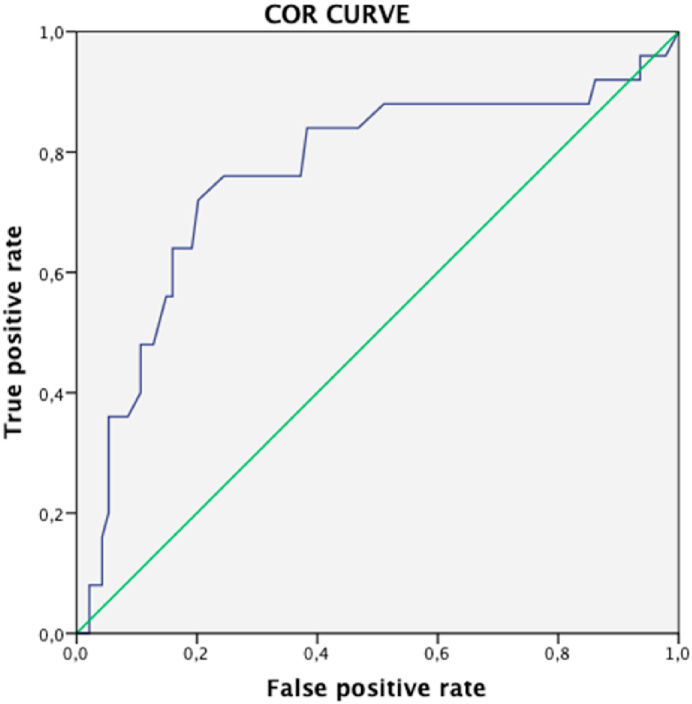
A ROC curve was designed is order to stablish a correlation within the RS and pathological response. We found a statistically significant correlation with an area under de curve of 0.763 (CI 95% 0.643 - 0.882). ROC curve is shown in Fig. 4.
Fig. 4.
Curve COR with the correlation to RS score with pathological response (RCB type 0–1).
4. Discussion
To date, the most common use of the Oncotype DX test was to guide adjuvant chemotherapy treatment decisions [16,17]. Retrospective and prospective studies have supported its clinical utility, and its use has been standardized and included in all international guidelines [18,19]. In the larger series, Surveillance, Epidemiology, and End Results (SEER), with more than 105,000 patients, the proportion of patients with Recurrence Score >25 is lower than what we observed, around 15% for N0 and 13% for >1500 N1 patients, probably because the patients tested were of better prognosis overall. In fact, in this series, most tumors had tumoral grade I or II, half had tumor size less than 2 cm and only a 16% of initial nodal involvement [20]. These observations contrast with our outcomes in a selected population with higher clinical risk, we found a significant greater proportion of patients with Recurrence Score >25, around 35%.It is very interesting that despite the high clinical risk of this population considered candidates for treatment with NAC, 65% of patients had la Recurrence Score <25 suggesting no benefit of chemotherapy treatment. Overall we avoided chemotherapy in a total of 42% of these patients. Fig. 4
In the WSG-plan B study that included high clinical risk patients [21] with a proportion of 41% of node-positive patients and only 5% of tumoral grade I, the proportion of Recurrence Score > 25 was 21% and interestingly similar in node-positive and negative-patients. We included node-positive patients (48%) in the study reported hereby because in our institution we use the Oncotype DX test to guide adjuvant chemotherapy decisions in patients with 1–3 node involvement based on the collective evidence for this test in this population with an excellent survival in patients with low Recurrence Score results without chemotherapy treatment [22]. We observed that, ultimately, 9% (11 of 122) of patients received adjuvant chemotherapy because more than 3 positive nodes were identified after surgical removal of the tumour. Importantly, all cases that had no initial axillary lymph node involvement, did not have axillary involvement after surgical resection following NAC. Half of the patients had an initial axillary node involvement, 32% in the patients who did not received NAC and 63% in the NAC group. The axillary node response were validated with the RCB symmand’s method The identification of axillary involvement prior to administering NAC can be a suboptimal especially when it comes to identifying the number of nodes affected. Generally, patients with more than 3 positive nodes would receive chemotherapy treatment due to their higher risk of relapse [23] and the test has not been validated for this patient population. Therefore, the presence of an axillary involvement can be a limitation for the selection of NAC guided by the Oncotype DX test, however, a 9% proportion of adjuvant chemotherapy, may be acceptable, especially considering the significant reduction in overall chemotherapy use for 42% of the patients.
The correlation between residual tumor size after NAC and survival has been demonstrated in multiple studies. Although in the triple negative and HER2 phenotypes this effect seems more significant, it has also been demonstrated in the luminal subtype [1]. Results from a large meta-analysis of 5100 breast cancer patients, funded by the Department of Defense, the National Institutes of Health (NIH), the Cancer Prevention Research Institute of Texas, and the Breast Cancer Research Foundation, presented recently in SABCS 2020 demonstrated that RCB after NAC is an accurate long-term predictor of recurrence and survival across all breast cancer subtypes. In the luminal subtype this correlation was the same for RCB-0 and RCB-I, so we could use this parameter as predictor to survival [24].
We found 9% of pCR in the overall series that is similar to most studies [7], but in patients with Recurrence Score > 25 this percentage increases to 14% and in patients with a Recurrence Score > 32 the pCR was 22%. It is remarkable that there was no patient with a Recurrence Score lower than 32 that achieved a pCR. We also found a significant correlation between residual size and Recurrence Score results (pearson 0,714 P:0,027). The grouped response type RCB-0 and –RCB-I is also used as a predictor of survival and thus the percentage of patients with significant histological response can be increased. In recent studies this type of response was achieved around 20% [24], in our study we found a 20% in the global series but in patients with high RS this response was increased to 40%.
Pathological response rates have been associated with higher expression of the proliferation gene group from the 21 gene assay in earlier studies [25]. Yardley et al. [26] showed an interesting correlation between achievement of pCR and Recurrence Score results with no pCR achieved in the Recurrence Score groups RS 0–30 and 26% in the Recurrence Score >30. More recently Pease et al. [27] found in an important retrospective study with 890 patients that a high Recurrence Score result (RS > 30) was associated with an increased pathologic complete response rate, with 10% of such patients achieving pCR, compared to 14% of patients with RS > 25 in our series, similarly Kantor et al. [28] found a 7,8% pCR rate in the RS > 25 group in a similar retrosprective study of the national cancer database in the US. It is important to note the concordance of low pathological response found in both studies in patients with lower Recurrence Score results (RS 0–25), suggesting these patients will not benefit from NAC despite having clinical criteria of NAC. Pivot el al [29] showed a similar effect in patients selected for NAC primarily based on large tumor size, in that patients with low RS results, with minimal if any expected long-term clinical benefit to NAC, were unlikely to achieve pCR.
The rate of pathological response in this study for patients with higher Recurrence Score results treated with NAC is consistent with observations from studies that assigned NAC to patients with higher scores. Bear et al. [30] found a 14% pCR rate in the group of patients with a RS > 25, while patients with lower RS did not receive chemotherapy treatment. We also consider important the quantification of the response according to the residual cancer burden, which impacts survival benefit. So far, few studies have assessed this type of response, although it is important for the selection of patients for NAC. In our study there is a very interesting correlation between the final size and the Recurrence Score result that shows the importance of the Oncotype DX test in the histological response. This correlation was not observed with any of the other variables assessed.
5. Conclusion
Selecting NAC based on results from the Oncotype DX test allows application of this treatment with more accuracy, and avoids chemotherapy in nearly half of patients previously selected to NAC based on clinical common parameters. We found only 35% of patients had a high Recurrence Score (>25) that would suggest a clear long-term benefit of chemotherapy treatment.
The pathological response was strongly correlated with the Recurrence Score results in the quantitative model with 14% of RCB-0 in patients with highest Recurrence Score results and conversely, patients with a RS below 32 did not obtain any RCB-0. By grouping the pathological response in RCB-0 and RCB-1 to enrich the survival benefits, we found a total of 47% response (19 of 40) in patients with high Recurrence Score results (RS > 21, premenopausal; RS > 25, postmenopausal) in contrast to only 5% (1 of 20) in the groups of lower Recurrence Score results.
This prospective study confirms the findings of several earlier studies of RS performed on archived tumors from patients treated with neoadjuvant chemotherapy. Overall, the findings suggest that a sizable proportion of patients with locally advanced ER + HER2-breast cancer do not benefit from NAC, and the Oncotype DX test substantially reduces over-treatment of such patients.
| Correlations | |
|---|---|
| Pearson correlation | RS |
| Final tumor size | -,286∗ |
| p-Value | 0,027 |
| N | 60 |
Contributor Information
Serafin Morales Murillo, Email: serafinmorales01@gmail.com, smorales.lleida.ics@gencat.cat.
Ariadna Gasol Cudos, Email: ariadna.gc.86@gmail.com.
Joel Veas Rodriguez, Email: Joelveas@gmail.com.
Carles Canosa Morales, Email: carleskan73@hotmail.com.
Jordi Melé Olivé, Email: jordimeleolive@gmail.com.
Felip Vilardell Villellas, Email: fvilardell.lleida.ics@gencat.cat.
Douglas Rene Sanchez Guzman, Email: Douglas.pathology@gmail.com.
Edelmiro Iglesias Martínez, Email: eiglesias@comll.cat.
Antonieta Salud Salvia, Email: asaluds@hotmail.com.
References
- 1.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet Lond Engl. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 2.Sheri A., Smith I.E., Johnston S.R., A’Hern R., Nerurkar A., Jones R.L. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol Off J Eur Soc Med Oncol. 2015;26(1):75–80. doi: 10.1093/annonc/mdu508. [DOI] [PubMed] [Google Scholar]
- 3.Symmans W.F., Wei C., Gould R., Yu X., Zhang Y., Liu M. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(10):1049–1060. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuksa L., Micuda S., Grim J., Ryska A., Hornychova H. Predictive biomarkers in breast cancer: their value in neoadjuvant chemotherapy. Canc Invest. 2012;30(9):663–678. doi: 10.3109/07357907.2012.725441. [DOI] [PubMed] [Google Scholar]
- 5.Keam B., Im S.-A., Kim H.-J., Oh D.-Y., Kim J.H., Lee S.-H. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Canc. 2007;7:203. doi: 10.1186/1471-2407-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasching P.A., Heusinger K., Haeberle L., Niklos M., Hein A., Bayer C.M. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Canc. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkert C., Loibl S., Müller B.M., Eidtmann H., Schmitt W.D., Eiermann W. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(11):2786–2793. doi: 10.1093/annonc/mdt350. [DOI] [PubMed] [Google Scholar]
- 8.Paik S., Shak S., Tang G., Kim C., Baker J., Cronin M. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 9.Paik S., Tang G., Shak S., Kim C., Baker J., Kim W. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 10.Pivot X., Mansi L., Chaigneau L., Montcuquet P., Thiery-Vuillemin A., Bazan F. In the era of genomics, should tumor size Be reconsidered as a criterion for neoadjuvant chemotherapy? 2015;7 doi: 10.1634/theoncologist.2014-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soran A., Bhargava R., Johnson R., Ahrendt G., Bonaventura M., Diego E. The impact of Oncotype DX® recurrence score of paraffin-embedded core biopsy tissues in predicting response to neoadjuvant chemotherapy in women with breast cancer. Breast Dis. 2016;36(2–3):65–71. doi: 10.3233/BD-150199. [DOI] [PubMed] [Google Scholar]
- 12.Petkov V.I., Miller D.P., Howlader N., Gliner N., Howe W., Schussler N. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer. 2016;2:16017. doi: 10.1038/npjbcancer.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thekkekara R.J., Bharadwaj S., Yadav U., Baranwal A., Peace D., Rogowski W. Predicting response to neoadjuvant chemotherapy in nonmetastatic hormone receptor-positive breast cancer using 21-gene Breast Recurrence Score test. J Clin Oncol. 2019;37(15_suppl) [Google Scholar]
- 14.Parker J.S., Mullins M., Cheang M.C.U., Leung S., Voduc D., Vickery T. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff A.C., Hammond M.E.H., Hicks D.G., Dowsett M., McShane L.M., Allison K.H. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 16.Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris L.N., Ismaila N., McShane L.M., Andre F., Collyar D.E., Gonzalez-Angulo A.M. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(10):1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andre F., Ismaila N., Henry N.L., Somerfield M.R., Bast R.C., Barlow W. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(22):1956–1964. doi: 10.1200/JCO.19.00945. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L., Hsieh M.-C., Petkov V., Yu Q., Chiu Y.-W., Wu X.-C. Trend and survival benefit of Oncotype DX use among female hormone receptor-positive breast cancer patients in 17 SEER registries, 2004-2015. Breast Canc Res Treat. 2020;180(2):491–501. doi: 10.1007/s10549-020-05557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitz U., Gluz O., Christgen M., Kates R.E., Clemens M., Malter W. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Canc Res Treat. 2017;165(3):573–583. doi: 10.1007/s10549-017-4358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamounas E.P., Russell C.A., Lau A., Turner M.P., Albain K.S. Clinical relevance of the 21-gene Recurrence Score® assay in treatment decisions for patients with node-positive breast cancer in the genomic era. NPJ Breast Cancer. 2018;4:27. doi: 10.1038/s41523-018-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sestak I., Dowsett M., Zabaglo L., Lopez-Knowles E., Ferree S., Cowens J.W. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105(19):1504–1511. doi: 10.1093/jnci/djt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GS5-01 Residual cancer burden after neoadjuvant therapy and long-term survival outcomes in breast cancer: a multi-center pooled analysis. https://www.abstractsonline.com/pp8/#!/7946/presentation/1923 [Internet]. [cited 2020 Jan 20]. Available from:
- 25.Chang J.C., Makris A., Gutierrez M.C., Hilsenbeck S.G., Hackett J.R., Jeong J. Gene expression patterns in formalin-fixed, paraffin-embedded core biopsies predict docetaxel chemosensitivity in breast cancer patients. Breast Canc Res Treat. 2008;108(2):233–240. doi: 10.1007/s10549-007-9590-z. [DOI] [PubMed] [Google Scholar]
- 26.Yardley D.A., Peacock N.W., Shastry M., Burris H.A., Bechhold R.G., Hendricks C.B. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with HER2-negative breast cancer: correlation of pathologic complete response with the 21-gene recurrence score. Breast Canc Res Treat. 2015;154(2):299–308. doi: 10.1007/s10549-015-3613-y. [DOI] [PubMed] [Google Scholar]
- 27.Pease A.M., Riba L.A., Gruner R.A., Tung N.M., James T.A. Oncotype DX® recurrence score as a predictor of response to neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26(2):366–371. doi: 10.1245/s10434-018-07107-8. [DOI] [PubMed] [Google Scholar]
- 28.Kantor O., Barrera E., Kopkash K., Pesce C., Barrera E., Winchester D.J. Are we overtreating hormone receptor positive breast cancer with neoadjuvant chemotherapy? Role of OncotypeDx® for hormone receptor positive patients undergoing neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26(10):3232–3239. doi: 10.1245/s10434-019-07555-w. [DOI] [PubMed] [Google Scholar]
- 29.Pivot X., Mansi L., Chaigneau L., Montcuquet P., Thiery-Vuillemin A., Bazan F. In the era of genomics, should tumor size be reconsidered as a criterion for neoadjuvant chemotherapy? Oncol. 2015;20(4):344–350. doi: 10.1634/theoncologist.2014-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bear H.D., Wan W., Robidoux A., Rubin P., Limentani S., White R.L. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol. 2017;115(8):917–923. doi: 10.1002/jso.24610. [DOI] [PMC free article] [PubMed] [Google Scholar]