Abstract
Background:
Infection with rhinovirus (HRV) occurs following pediatric lung transplantation. Prospective studies documenting frequencies, persistence, and progression of HRV in this at-risk population are lacking.
Methods:
In the Clinical Trials in Organ Transplant in Children prospective observational study, we followed 61 lung transplant recipients for 2 years. We quantified molecular subtypes of HRV in serially collected nasopharyngeal (NP) and bronchoalveolar lavage (BAL) samples and correlated them with clinical characteristics.
Results:
We identified 135 community acquired respiratory infections (CARV) from 397 BAL and 480 NP samples. We detected 93 HRV events in 42 (68.8%) patients, 22 of which (23.4%) were symptomatic. HRV events were contiguous with different genotypes identified in 23 cases but symptoms were not preferentially associated with any particular species. Nine (9.7%) HRV events persisted over multiple successive samples for a median of 36 days (range 18 – 408 days). Three persistent HRV were symptomatic. When we serially measured forced expiratory volume in one second (FEV1) in 23 subjects with events, we did not observe significant decreases in lung function over 12 months post-HRV.
Conclusion:
In conjunction with our previous reports, our prospectively collected data indicate that molecularly heterogeneous HRV infections occur commonly following pediatric lung transplantation, but these infections do not negatively impact clinical outcomes.
Keywords: Lung Transplantation, Pediatrics, Human Rhinovirus (HRV), Community-Acquired Respiratory Virus (CARV), Forced Expiratory Volume (FEV1)
Background:
Lung transplantation is an accepted treatment option for end-stage pulmonary diseases. Outcomes for patients with lung transplantation remain suboptimal, with one-year and five-year survival of 80% and 54% respectively.1 There is a growing appreciation of the impact that community-acquired respiratory virus (CARV) infections have on lung transplant outcomes.2 CARV infection has been identified in 14–52%3–8 of lung transplant recipients (LTRs). Single center studies primarily in adults detected associations between CARV infection and acute cellular rejection (ACR) episodes and chronic lung allograft dysfunction/bronchiolitis obliterans syndrome (CLAD/BOS), but prospective analyses in pediatric lung transplant recipients have not been previously reported outside of this study.3,9–15
As molecular diagnostic tools have improved, human rhinovirus (HRV) has been identified as a common CARV infection among both LTRs and the general population.1,2,16,17 HRV accounts for 43–63% of CARV infections in LTRs, as both an asymptomatic and less frequently a symptomatic pathogen.4,7,13,16 Investigation into the role of HRV in LTRs is increasing, with studies demonstrating evidence of lower respiratory tract infection and association with CLAD.7,16,18–20 While HRV is more common in children21, studies examining HRV in pediatric LTRs are limited. One retrospective study found 13.8% of children developed a CARV infection after lung transplantation, with 21.8% being infected with HRV. This study probably underestimated the frequency of HRV because it was performed prior to modern molecular testing methods, it limited review to one year post-transplant and routine CARV monitoring was not performed.3
Previous reports demonstrated that HRV persists in immunocompromised patients as compared to immunocompetent patients.20 One cohort of 68 predominantly adult lung transplant patients reported HRV persisted in five cases.19,22 Although HRV is known to be a common post-transplant infection, the progression, persistence, and/or recurrence of individual HRV infections in pediatric LTRs has not been extensively explored.7,16 Herein, we report on our analysis incidence, persistence, and clinical manifestations of HRV in a multi-center, prospective study of pediatric LTRs.
Methods:
Study design
This NIH-funded Clinical Trials in Organ Transplant in Children (CTOTC-03) study was conducted from 2009 to 2013 at six institutions following Institutional Review Board (IRB) approval (NCT00891865). Microbiologic, radiologic, histologic, and clinical data were collected prospectively in this longitudinal cohort study. All samples for viral detection, including bronchoalveolar lavage (BAL) and/or nasopharyngeal (NP) swabs, were sent to the central study laboratory and batch tested so results were not available to the clinical teams. Sites were provided a COPAN brand plastic shaft minitip swab and red top COPAN UTM transport medium tube. Nucleic acid samples were extracted from the specimen using a Roche MagNA Pure Compact automated extractor and tested for respiratory viruses with the Luminex xTAG Respiratory Viral Panel. A research use–only panel was used, which included assays for the following viruses: influenza A, influenza A H1, influenza A H3, influenza B, respiratory syncytial viruses A and B, parainfluenza 1–4, enterovirus/rhinovirus, metapneumovirus, adenovirus, and coronaviruses OC43, 229E, NL63, and 229E. Samples with a mean fluorescence index (MFI) ≥1000 were considered positive. Samples with MFI 300 to 999 were retested and considered positive if the repeat MFI was ≥300. If the MFI of the repeat test was <300, the test was repeated a second time, and the sample was considered positive if the MFI of the second repeat test was ≥300.23 Specimens reported as enterovirus or rhinovirus were genotyped by sequencing a segment of the 5’ non-translated region of the viral genome as previously described.24 Briefly, Specimens containing picornavirus were reverse transcribed using a variable region of the 5’ non-coding region of the rhinovirus genome, yielding a 260 base pair fragment. This was then compared to a database containing the sequences of 101 classical rhinovirus species (groups A and B) as well as members of group C.
Data collection was initiated pre-transplant and continued up to two years post-transplant or until either retransplantation or death. Scheduled study visits including clinical data collection occurred: pre-transplant, at transplant, and at post-transplant weeks 2, 4, 6, 8 and months 3, 6, 9, 12, 18 and 24. Additionally, data was collected during symptomatic episodes at unscheduled protocolized study visits. Methods of this study have been previously described in detail.23
Therapy Regimens
Patients underwent routine pre-transplant evaluation. Induction and standard immunosuppression were determined by the transplant center and followed International Pediatric Lung Transplant Collaborative guidelines.25 For induction, two centers used an IL-2 receptor antagonist (42 patients), two used rabbit anti-thymocyte globulin (rATG, 10 patients), one administered both an IL-2 receptor antagonist and rATG (6 patients), and one administered no induction therapy (3 patients). Triple drug maintenance immunosuppressive therapy was used in all patients, including tacrolimus, mycophenolate mofetil and prednisone, with standardized target ranges for tacrolimus based on time post-transplant. Induction and maintenance immunosuppression were performed according to standard practice as each participating institution.
Definitions
Respiratory virus detections were classified as different types of viral events according to the definitions below, which were based on relationship to other detections, duration of positivity and presence or absence of clinical manifestations. Viral events were classified independently by two members of the research team (EA, LDI). For purposes of analysis, BAL and NP samples taken within one week of each other were considered paired.
Isolated event –
Detection of viral nucleic acid in a BAL and/or NP sample.
Probable persistent event –
Detection of HRV of the same genotype in samples from 2 or more consecutive timepoints spanning a period of at least 14 days.
Possible persistent event –
Detection of a particular HRV genotype that followed prior detection of HRV of the same genotype, but with one or more samples that were negative for HRV present between. Periods of negative samples spanned at least two consecutive negative samples and at least one month.
Contiguous event –
Detection of HRV that followed detection of another respiratory virus including HRV of a different genotype without the presence of a negative test between events.
Lower respiratory tract infection (LRTI) –
An episode meeting the following requirements: 1) detection of a positive NP or BAL and the presence of shortness of breath, hypoxia, or a new infiltrate on chest x-ray; or 2) detection of a positive BAL and a cough with or without sputum.
Upper respiratory tract infection (URTI) –
An episode meeting the following requirements: 1) Detection of viral nucleic acid in a BAL and/or NP sample; 2) presence of fever, rhinorrhea, nasal congestion, or headache; and, 3) absence of LRTI symptoms.
Acute cellular rejection (ACR) –
Defined according to the ISHLT criteria.26
Forced Expiratory volume
Forced expiratory volume in one second (FEV1) was analyzed for short-term and long-term changes following HRV detection. FEV1 was evaluated using z-scores determined by the Global Lung Function Initiative (GLI) reference equations.27 Analysis was performed comparing the change of pre-event and post-event FEV1 z-scores of the same patient, as well as comparing HRV, non-HRV events, and patients with no events. Patients with both HRV and non-HRV infections were excluded from the analysis. HRV events starting less than 3 months post-transplant or if the pre-event FEV1 occurred less than 2 months post-transplant were excluded due to the lack of baseline value in early post-transplantation. One patient with infant PFT testing was excluded as it did not meet criteria for the GLI model. The most proximal FEV1 prior to infection that occurred at least 30 days prior to the first positive timepoint was used as the pre-event FEV1. Post-event FEV1 values were evaluated post-event at 1 to 2.5 months, 2.5 to 5 months, and 5 to 12 months. Patients with no events were evaluated at the first timepoint occurring more than 2 months post-transplant, and subsequent timepoints 2.5–5 months and 5–12 months following baseline. FEV1 values were not included in this analysis if they were associated with an episode of ACR, as determined by the International Society for Heart and Lung Transplantation criteria.
Statistical Analysis
Collected data were managed and stored by RHO Inc. (Chapel Hill, NC), with analyses performed at Cincinnati Children’s Hospital Medical Center with JMP (Version 14.0.0, Cary, NC). Association compared patients with one or more HRV events to those with no HRV events and to those with non-HRV viral events (including patients with both HRV and non-HRV events). Associations with continuous and nominal or ordinal variables were analyzed using t-tests and Fisher’s exact or chi squared tests, respectively. Concordance of samples at the same timepoint was determined using Cohen’s kappa. FEV1 values were analyzed by paired student’s t-test with corresponding 95% confidence intervals (CI). P-values of ≤ 0.05 were considered statistically significant.
Results
Sixty-one patients underwent lung transplantation; cystic fibrosis was the most common indication (29; 48%). Mean age at time of transplant was 11.3 years (range 9 months - 18 years). Additional demographic information is presented in Table 1.
Table 1:
Patient demographics
| All patients (n=61) | No events (n=11) | Non-HRV events (n=8) | HRV events (n=42) | ||
|---|---|---|---|---|---|
| n | n | n | n | P-value* | |
| Recipient sex | 1.00 | ||||
| Male | 25 (41.0) | 4 (36.4) | 4 (50.0) | 17 (40.5) | |
| Female | 36 (59.0) | 7 (63.6) | 4 (50.0) | 25 (59.5) | |
| Donor sex | 1.00 | ||||
| Male | 29 (48.3) | 6 (54.5) | 3 (37.5) | 20 (48.8) | |
| Female | 31 (51.7) | 5 (45.5) | 5 (62.5) | 21 (51.2) | |
| Race | 0.48 | ||||
| White | 50 (82.0) | 10 (90.9) | 7 (87.5) | 33 (78.6) | |
| Asian | 2 (3.3) | 0 (0.0) | 0 (0.0) | 2 (4.8) | |
| Black or African American | 6 (9.8) | 1 (9.1) | 1 (12.5) | 4 (9.5) | |
| Unknown/not reported | 3 (4.9) | 0 (0.0) | 0 (0.0) | 3 (7.1) | |
| Ethnicity | 0.14 | ||||
| Non-Hispanic/Non-Latino origin | 42 (68.9) | 7 (63.6) | 3 (37.5) | 32 (76.2) | |
| Hispanic/Latino origin | 5 (8.2) | 1 (9.1) | 2 (25.0) | 2 (4.8) | |
| Unknown/not reported | 14 (23.0) | 3 (27.3) | 3 (37.5) | 8 (19.0) | |
| Type of transplant | 0.03 | ||||
| Double lung | 56 (91.8) | 9 (81.8) | 6 (75.0) | 41 (97.6) | |
| Heart-lung | 5 (8.2) | 2 (18.2) | 2 (25.0) | 1 (2.4) | |
| Primary diagnosis | 0.28 | ||||
| Cystic fibrosis | 29 (47.5) | 4 (36.4) | 3 (37.5) | 22 (52.4) | |
| Non-cystic fibrosis | 32 (52.5) | 7 (63.6) | 5 (62.5) | 20 (47.6) | |
| Age at transplant | 0.70 | ||||
| Mean age | 12.15 | 11.96 | 11.08 | 12.41 | |
| Induction medication received | 0.55 | ||||
| Yes | 58 (95.1) | 11 (100.0) | 8 (100.0) | 39 (92.9) | |
| No | 3 (4.9) | 0 (0.0) | 0 (0.0) | 3 (7.1) | |
| Induction medication | 0.48 | ||||
| Basiliximab | 32 (52.5) | 7 (63.6) | 3 (37.5) | 22 (52.4) | |
| Daclizumab | 12 (19.7) | 1 (9.1) | 2 (25.0) | 9 (21.4) | |
| rATG | 14 (23.0) | 3 (27.3) | 3 (37.5) | 8 (19.0) | |
| None | 3 (4.9) | 0 (0.0) | 0 (0.0) | 3 (7.1) | |
HRV = human rhinovirus; rATG = rabbit anti-thymocyte globulin
P-value indicates association between patients with HRV events and patients with only non-HRV events or no events.
As reported previously23, 135 CARV infections were identified in 50 patients with the majority identified as HRV. 116 enterovirus/rhinovirus timepoints were identified, sequenced, and defined as isolated, possible persistent, or probable persistent as described above for a total of 93 HRV events (69%) and one enterovirus event. Other CARV identified are listed in Table 2 and have been described in detail previously.23 These 50 patients had an average of 2.70 CARV events (range 1 – 9) during the study period, and 34 (68%) had two or more CARV events.
Table 2:
CARV infections and symptomatology
| CARV type | LRTI+URTI (n=1) | LRTI (n=18) | URTI (n=18) | Asymptomatic (n=98) |
|---|---|---|---|---|
| HRV (n=93) | 1 (1.1%) | 9 (9.7%) | 12 (12.9%) | 71 (76.3%) |
| A (n=43; 46.2%) | 1 | 7 | 6 | 29 |
| B (n=14; 15.1%) | - | 1 | 1 | 12 |
| C (n=26; 28.0%) | - | 1 | 5 | 20 |
| Unsequenced (n=10; 10.8%) | - | - | - | 10 |
| Adenovirus (n=4) | - | 1 | - | 3 |
| Human coronavirus (n=8) | - | 2 | 3 | 3 |
| Influenza (n=4) | - | 1 | - | 3 |
| hMPV (n=4) | - | 1 | - | 3 |
| PIV (n=13) | - | 3 | - | 10 |
| RSV (n=8) | - | 1 | 2 | 5 |
| Enterovirus (EDV68) (n=1) | - | - | 1 | - |
CARV = community acquired respiratory virus; URTI = upper respiratory tract infection; LRTI = lower respiratory tract infection; HRV = human rhinovirus; hMPV = human metapneumovirus; PIV = parainfluenza virus; RSV = respiratory syncytial virus
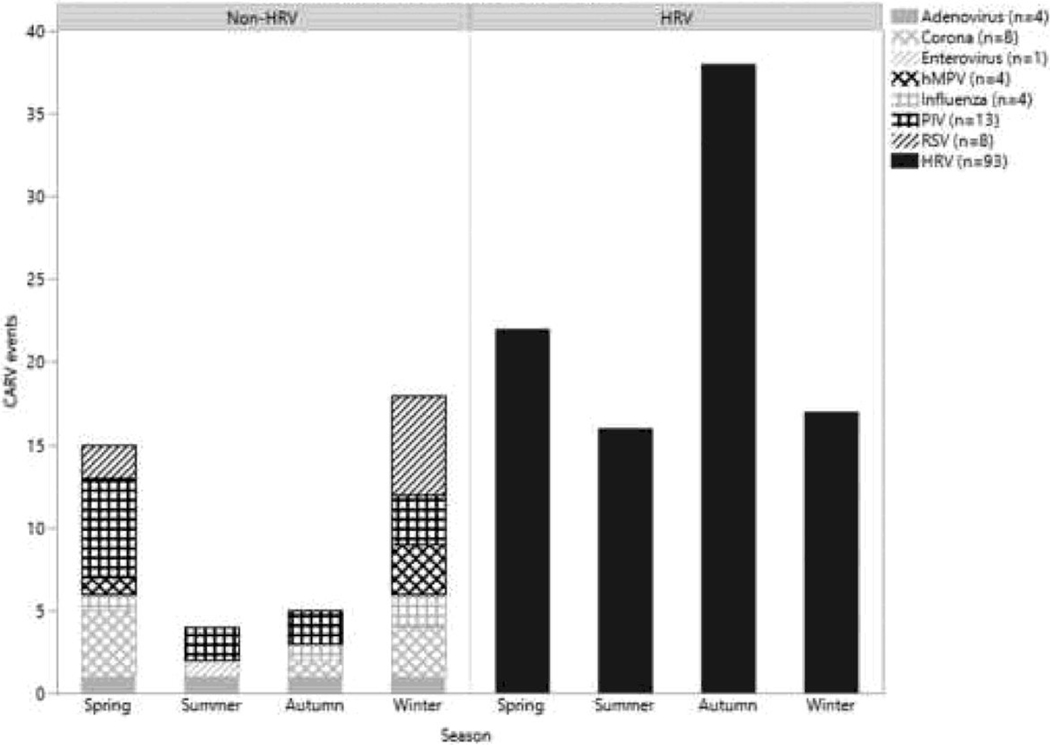
Ninety-three HRV events were detected in 42 patients (68.8%). HRV occurred most often in autumn months (38; 40.4%), while the non-HRV CARV infections were most commonly detected in the winter season (18; 42.9%) (Figure 1). Reported HRV events occurred a median of 288 days post-transplant (range = 0 – 784 days) with the majority identified in the first year (59.1%) as expected given the predominance of scheduled visits in the early post-transplant period. Thirteen (14.0%) HRV events occurred in the first 30 days, and 36 (38.7%) occurred in the first 6 months post-transplant (Figure 2). Similarly, non-HRV events occurred a median of 258.5 days post-transplant (range = 0 – 713 days), with six (14.3%) occurring in the first 30 days and 17 (40.5%) occurring in the first 6 months.
Figure 1:
Seasonality of CARV events separated by HRV (right) and non-HRV (left) events. Seasons are divided by month, with Spring March 1 – May 31, Summer June 1 – August 31, Autumn September 1 – November 30, and Winter December 1 – February 28. hMPV = human metapneumovirus; PIV = parainfluenza virus; RSV = respiratory syncytial virus; HRV = human rhinovirus.
Figure 2:
Human rhinovirus (HRV) event occurrence post-transplant, presented as the number of new events per month.
Patients with HRV had on average 2.21 HRV events during the study period (range 1 – 5 events), and 30 patients (71.4%) had more than one HRV event. HRV sequencing found 43 events were HRV species A (HRVA), 14 were species B (HRVB), and 26 were species C (HRVC). In 10 events, a genotype was not determined. Genotype of HRV varied widely within species, with 30 HRVA genotypes, eight HRVB genotypes, and 21 HRVC genotypes identified. The most common genotype identified was the HRVA genotype R68 (5 events) (Table 3). Twenty-three (24.4%) HRV events were from different genotypes but occurred contiguously with other HRV events without negative samples between events. Additionally, one HRV event was preceded by another non-HRV event, and five HRV events were followed by another non-HRV event without negative samples between events. Two HRV events occurred concurrently with a non-HRV event: one each with PIV and coronavirus.
Table 3:
Human rhinovirus genotype
| HRV Species | Genotype (number of events) |
|---|---|
| HRVA (n=43) | R1B (1), R2 (1), R9 (2), R10 (2), R11 (1), R12 (1), R15 (1), R20 (2), R21 (2), R22 (1), R24 (1), R25 (2), R28 (1), R29 (1), R33 (1), R36 (1), R41 (1), R44 (1), R46 (2), R49 (1), R53 (2), R58 (2), R60 (1), R61 (2), R65 (1), R66 (1), R68 (5), R81 (1), R82 (1), W48 (1) |
| HRVB (n=14) | R3 (1), R7(3), R14 (1), R27 (2), R52 (4), R83 (1), R91 (1), R97 (1) |
| HRVC (n=26) | W4 (1), W6 (1), W7 (1), W8 (1), W9 (1), W10 (2), W12 (1), W19 (1), W20 (1), W23 (2), W24 (2), W25 (2), W31 (1), W32 (1), W33 (1), W36 (2), W37 (1), W38 (1), W41 (1), W46 (1), W50 (1) |
| Undetermined | (10) |
Symptomatic HRV infection was identified in 22 of 93 events (23.7%) with 12 URTI, 9 LRTI and one event progressing from URTI to LRTI. By comparison, non-HRV events were symptomatic in 15 of 42 events (35.7%) with 6 URTI and 9 LRTI. Symptomatic HRV were further assessed based on the HRV species with HRVA accounting for six (50.0%) of the 12 URTI and seven (77.8%) of the nine LRTI (Table 2). However, we did not detect a significant association between HRVA and symptomatic events when compared to either HRVB (p=0.20) or HRVC (p=0.43), or when compared to HRVB and HRVC combined (p=0.22). Additionally, we did not detect a significant association between symptomatology of HRVA and HRVC combined compared to HRVB (p=0.22). In HRV events in which a BAL was obtained, a positive BAL sample was associated with the presence of symptoms (p=0.03). Symptomatic HRV events occurred in each season (7 winter, 6 fall, 5 spring, 4 summer), and accounted for the highest proportion of events in winter (33% winter, 17% fall, 24% spring, 25% summer). The most common symptoms with URTI were cough (10 events), followed by rhinorrhea (9), nasal congestion (6), fever (1) and headache (1). LRTI signs and symptoms included cough (10), followed by shortness of breath (5), new infiltrate on chest x-ray (2), rhinorrhea (2), fever (1), headache (1), and new supplemental oxygen requirement (1). One isolated HRV event was identified concurrently with ACR.
Concordance of Sampling between BAL and NP Specimens
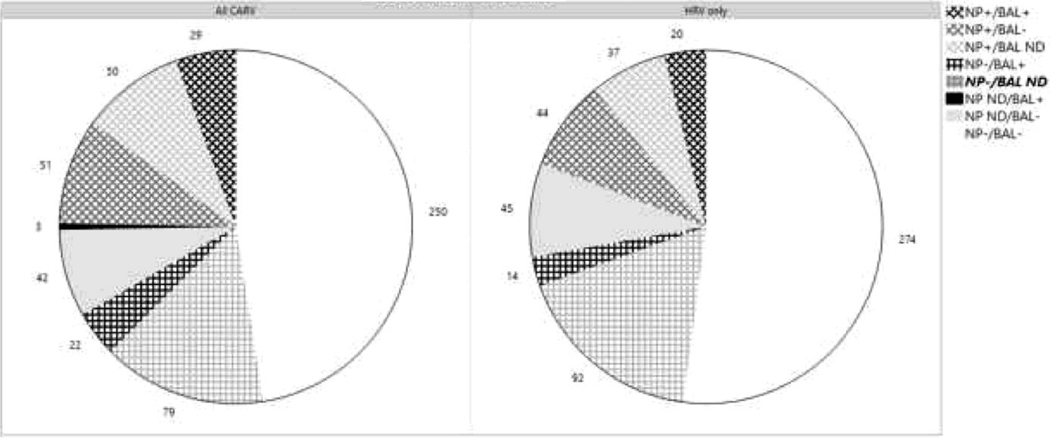
We evaluated CARV events in 480 NP and 397 BAL specimens by the central laboratory (Figure 3). 130 NP (27.0%) and 54 BAL (13.6%) samples were positive, identifying 135 CARV events in 50 patients (81.9%). CARV infections were identified through 352 paired samples, 29 of which were positive for the same CARV event (Cohen’s Kappa = 0.33; 95% CI[0.21–0.45]). Discordance occurred between NP and BAL samples with 51 NP+/BAL- and 22 BAL+/NP-. Additionally, 50 NP had no concordant BAL, and 3 BAL occurred in the absence of NP. 250 paired samples were negative for all viruses.
Figure 3:
Positivity of samples for all CARV events (left) and HRV-only events (right). NP and BAL samples are positive (+) or negative (−) when paired, or indicated as not done (N/D) when no paired sample was performed. HRV = human rhinovirus; NP = nasopharyngeal swab; BAL = bronchoalveolar lavage.
For 115 HRV event samples with NP, BAL or both (Figure 3), concordance of NP and BAL samples occurred at 20 (17.4%) timepoints (kappa = 0.43; CI[0.30–0.56]), similar to that reported for CARV overall. However, 36.6% of NP positive for HRV did not have a corresponding BAL for comparison. Discordance occurred between NP and BAL samples with 44 NP+/BAL- and 14 BAL+/NP-.
Delineation of HRV Events by Sequencing Reveals Persistence
We classified HRV events by their course and duration of positivity. Events were classified as isolated in 76 (81.7%) cases, probable persistent in nine (9.7%), and possible persistent in two (2.2%). Six HRV events (6.4%) were considered unclassified and excluded from this analysis as a genotype was not determined. Of the six unclassified events, five had no additional samples within 6 months. One event could have been considered possible persistent had the same genotype as a subsequent event been identified but was excluded as genotype could not be determined.
Further analysis was performed to describe the nine events classified as probable persistent HRV. Successive events occurred over a median interval of 36 days (range = 18 days – 408 days) and were identified in a median of 2 timepoints (range = 2 – 8). Most persistent HRV events identified were detected in the early post-transplant period, with five persistent HRV events beginning within one week post-transplant (range 0 days – 617 days). Persistent HRV events were HRVA (6, 66.6%), HRVB (2, 22.2%), and HRVC (1, 11.1%).
Persistent HRV was associated with symptomatic LRTI in three cases, with the patients initially asymptomatic at first recovery of HRV. Patients then became symptomatic 6, 27, and 63 days following initial HRV detection, respectively.
Forced expiration volume before, during and after HRV
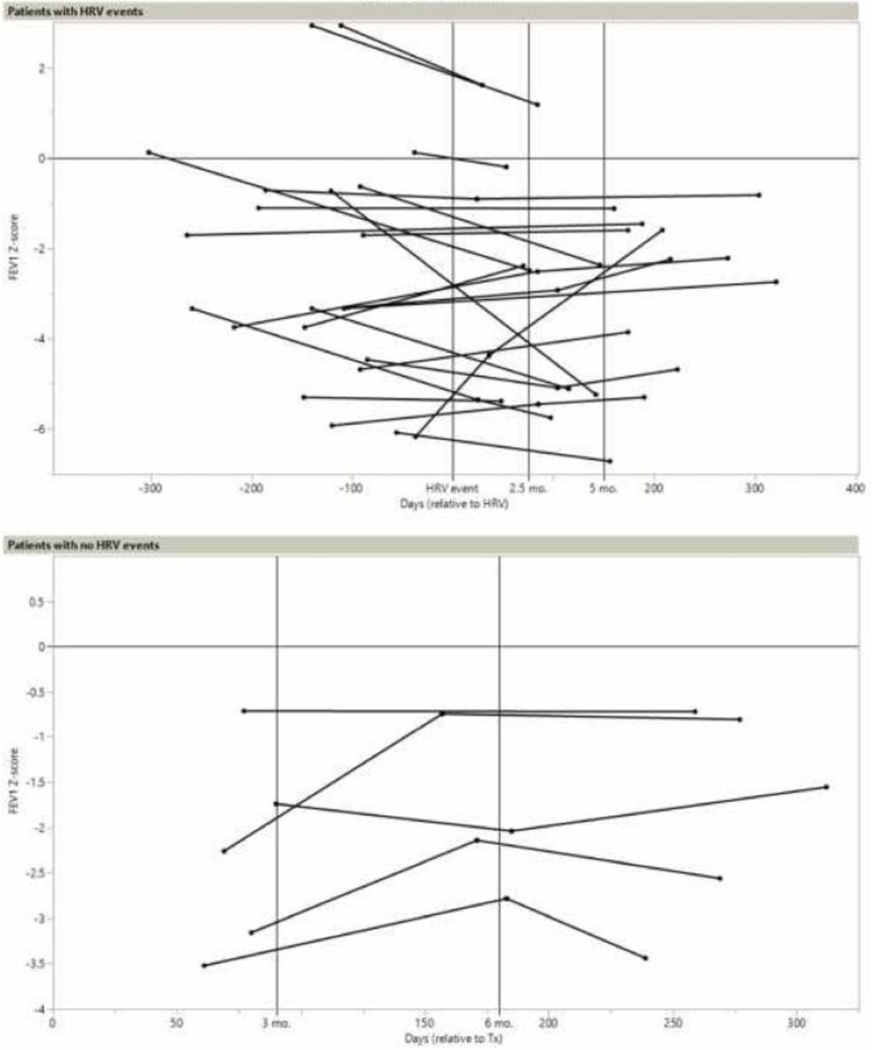
We tested for relationships between changes to FEV1 (based on criteria described in the methods) and HRV events in 14/42 patients who had 22 events (Figure 4). Pre-event FEV1 values were collected a mean of 138 days prior to the event (range 37 – 302 days). FEV1 changes are reported as positive if the z-score increased over time. Seven patients had FEV1 values between 1 and 2.5 months post-event. z-scores increased in two patients and decreased in five patients (mean −0.11; CI[−1.36 to +1.14]). Between 2.5 and 5 months post-event, nine patients had FEV1 with three increasing and six decreasing (mean −1.29; CI[−2.69 to +0.11]). Finally, at 5 to 12 months post-event, eight patients reported FEV1 increases and five showed decreases (mean +0.53; CI[−0.36 to +1.41]). Two of nine patients with probable persistent HRV met criteria for inclusion in FEV1 analysis, each with one FEV1 post-event. One showed a decrease in FEV1 z-score of −0.012 at 160 days following final positivity with HRV, while the z-score decreased in the other by −4.505 at 142 days after final positivity. The duration of these HRV events was 18 and 27 days, respectively. FEV1 changes in HRV events were compared to 4/25 patients who had four non-HRV CARV infections and 5/11 patients with no events. When compared with patients with no events, FEV1 z-scores did not significantly differ between 2.5–5 months (p=0.06) and 5–12 months (p=0.92) post-event. When compared to FEV1 changes of non-HRV CARV infections, there were no significant differences at 5–12 months (p=0.87). Only one FEV1 value was available for non-HRV CARV infections from 1–2.5 and 2–5.5 months post-event, so analysis was not performed (Table 4).
Figure 4:
Forced expiratory volume (FEV1) z-scores in patients with and without HRV events. Days in patients with HRV events are relative to the time of the HRV event at day 0 and in patients without HRV event are relative to transplant at day 0.
Table 4:
Change in FEV1 z-score before and after HRV infection
| 1–2.5 months | 2.5–5 months | 5–12 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | n | change in z-score | Median days post-event | n | change in z-score | Median days post-event | n | change in z-score | Median days post-event | |
| HRV | 22 | 7 | −0.11 (−1.36 – 1.14) | 36d | 9 | −1.29 (−2.69 – 0.11) | 97d | 13 | 0.53 (−0.36 – 1.41) | 190d |
| Non-HRV | 4 | 1 | −1.72 | 63d | 1 | −1.58 | 98d | 4 | 0.40 (−0.16 – 0.96) | 185d |
| No Event | 5 | - | - | - | 4 | 0.74 (−0.48 – 1.96) | 93d | 5 | 0.46 (−0.28 – 1.21) | 189d |
P-values were found to be non-significant for all comparable timepoints between HRV and non-HRV values, and between HRV values and patients with no events.
Discussion
This study represents the largest and longest prospective multi-institutional surveillance of pediatric LTRs to delineate the features of HRV post-transplant. First, HRV occurred in almost 70% of pediatric patients over the entire two-year period. Nearly half of the cohort presented with two or more distinct HRV events. While most HRV events were asymptomatic, we identified cases of both URTI and LRTI from HRV. Often, HRV events had a complex presentation, with nine cases of HRV persisting over multiple timepoints. Contiguous events with two different HRV detected in serial samples occurred on 23 occasions, involving multiple HRV events of different genotypes which could only be detected based on viral sequencing. The availability of new molecular techniques for identifying HRV has allowed us to recognize that HRV can not only persist over longer periods of time but can quickly follow other HRV events in a continuum of contiguous illnesses adding to the information available to care for this population with complex clinical presentations. Our study further describes the epidemiology of HRV in the pediatric lung transplantation population while describing identified cases of persistent HRV.
Previous studies found HRV to be a common virus in both the general pediatric population and in LTRs. In the era of molecular testing, HRV has been reported to comprise between 42% and 63% of CARV infections, which is lower than what was found in this cohort (69.1%).4,13 The higher incidence may be related to the number of scheduled study visits with sample collection, as well as improvements in molecular detection. Additionally, pediatric patients have historically been found to have more colds than adults and exposure to infection through school or daycare may increase risk of HRV.28 This study highlights the high incidence of HRV, particularly in the pediatric population. While HRV was most often identified early post-transplant and in the autumn months, it was identified through all seasons and up to 2 years post-transplant. HRV was found to be symptomatic in only 23.4% of cases, both as URTI and LRTI. Although infrequent (9.6% of HRV events), HRV was found with LRTI in some cases.
With molecular typing, we were able to assess the potential impact of HRV species on symptomatic infection. Our study found HRVA species more commonly in symptomatic HRV events although this was not statistically significant. The study was underpowered to address this question due to the number pediatric LTRs performed. The clinical impact of different HRV species is still being assessed, but studies in other populations demonstrate variation in disease severity may exist between species.29–32 Recognizing these changes through genotyping of HRV provides another potential tool in future studies to assess association between CARV (symptomatic or asymptomatic) and allograft function to further guide clinical decision making.
Notably, nine HRV events were persistent over multiple timepoints. These were most often found to begin shortly after transplant. Length of persistent HRV was variable but was found in some cases to last for more than one year. Similar to previous studies, a minority of HRV events were persistent. However, a higher proportion of the pediatric LTR population had persistent HRV compared to a predominantly adult population.19,22 These persistent events were primarily asymptomatic, but three initially asymptomatic cases developed LRTI over the course of HRV recovery suggesting a period of incubation prior to symptom onset. This highlights the unpredictability of symptom development in HRV. As multiple patients developed symptoms after initial positivity, vigilance for persistent viral infections is necessary to quickly and efficaciously respond to changing clinical presentations.
Our analysis of FEV1 in HRV patients, while limited by the early post-transplant occurrence of HRV prior to baseline FEV1 determination, did not show significant changes to short-term or long-term values following HRV infection. Both increases and decreases in FEV1 occurred at similar frequencies at 1–2.5 months post-event, and in the majority of patients analyzed FEV1 improved 5–12 months post-HRV. Changes over time were not significantly different when compared to patients with no events at 2.5–5 or 5–12 month intervals. Other studies have also found no significant association between HRV and FEV1 decline13; however, a future study with a larger sample size could further evaluate the relationship between HRV, including HRV species, and allograft function.
The limitations of our study have been reported previously23. In addition to limitations previously described, some HRV events may have been missed later in the study period due to the increasing intervals between routine testing. However, this study represents the most comprehensive monitoring investigation of respiratory viruses that has been performed in pediatric LTRs to date. Outcomes concerning this cohort were well reported in a previous manuscript and did not find association between either CARV overall or any HRV and outcomes including BOS, death and listing for retransplantation.23 The limited number of persistent HRV events (n=9) precluded specific analysis with regards to outcomes such as BOS. While the identification of HRV genotype added to our ability to differentiate events, we did not explore HRV viral load levels and kinetics to assess for an association between viral load and symptomatology. Instead, we considered HRV to be positive or negative based on mean fluorescence index values. Future studies may benefit from considering the relationship of viral load in persistent HRV to symptomatology, especially as the course of illness in several patients indicated development of symptoms over time with progression from URTI to LRTI in at least one episode.
In our cohort of pediatric LTRs, we identified HRV as the most common CARV infection found HRV events throughout the first two years post-transplant. While most HRV events were asymptomatic, a minority of HRV events presented with symptoms of both URTI and LRTI, and progression of symptoms occurred with persistent HRV. Our prospectively collected data indicate that molecularly heterogeneous HRV infections occur commonly following pediatric lung transplantation, but these infections do not negatively impact clinical outcomes and were not associated with significant short-term or long-term changes in FEV1.
Acknowledgements:
This research was performed as a project of the Clinical Trials in Organ Transplantation in Children, a collaborative clinical research project headquartered at the National Institute of Allergy and Infectious Diseases. The work was supported by Grant U01 AI077810 “Viral Triggers of Alloimmunity and Autoimmunity in Pediatric Lung Transplantation” from the Division of Allergy, Immunology and Transplantation of the National Institutes of Health.
Data Sharing Statement:
The data supporting this publication will be available at ImmPort (immport.org) under study accession SDY960-Viral Triggers of Alloimmunity and Autoimmunity in Pediatric Lung Transplantation.32
Abbreviations:
- LTR
lung transplant recipient
- CARV
community acquired respiratory virus
- NP
nasopharyngeal
- BAL
bronchoalveolar lavage
- FEV1
forced expiratory volume
- LRTI
lower respiratory tract infection
- URTI
upper respiratory tract infection
- BOS
bronchiolitis obliterans syndrome
- CLAD
Chronic lung allograft dysfunction
- ISHLT
International Society of Heart and Lung Transplantation
- rATG
rabbit anti-thymocyte globulin
- CF
cystic fibrosis
- ACR
acute rejection
- PIV
parainfluenza virus
- hMPV
human metapneumovirus
- RSV
respiratory syncytial virus
- HRV
human rhinovirus
- HRVA
human rhinovirus species A
- HRVB
human rhinovirus species B
- HRVC
human rhinovirus species C
Footnotes
Disclosures
None of the authors have any disclosures.
Disclosure:
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Thabut G, Mal H. Outcomes after lung transplantation. J Thorac Dis. 2017;9(8):2684–2691. doi: 10.21037/jtd.2017.07.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vu DL, Bridevaux PO, Aubert JD, Soccal PM, Kaiser L. Respiratory viruses in lung transplant recipients: A critical review and pooled analysis of clinical studies. Am J Transplant. 2011;11(5):1071–1078. doi: 10.1111/j.1600-6143.2011.03490.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M, Worley S, Arrigain S, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis. 2009;11(4):304–312. doi: 10.1111/j.1399-3062.2009.00397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peghin M, Hirsch HH, Len, et al. Epidemiology and Immediate Indirect Effects of Respiratory Viruses in Lung Transplant Recipients: A 5-Year Prospective Study. Am J Transplant. 2017;17(5):1304–1312. doi: 10.1111/ajt.14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milstone AP, Brumble LM, Barnes J, et al. A single-season prospective study of respiratory viral infections in lung transplant recipients. Eur Respir J. 2006;28(1):131–137. doi: 10.1183/09031936.06.00105505 [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Husain S, Chen MH, et al. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89(8):1028–1033. doi: 10.1097/TP.0b013e3181d05a71 [DOI] [PubMed] [Google Scholar]
- 7.Bridevaux PO, Aubert JD, Soccal PM, et al. Incidence and outcomes of respiratory viral infections in lung transplant recipients: A prospective study. Thorax. 2014;69(1):32–38. doi: 10.1136/thoraxjnl-2013-203581 [DOI] [PubMed] [Google Scholar]
- 8.Magnusson J, Westin J, Andersson LM, et al. Viral respiratory tract infection during the first postoperative year is a risk factor for chronic rejection after lung transplantation. Transplant Direct. 2018;4(8):e370. doi: 10.1097/TXD.0000000000000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soccal PM, Aubert JD, Bridevaux PO, et al. Upper and Lower Respiratory Tract Viral Infections and Acute Graft Rejection in Lung Transplant Recipients. Clin Infect Dis. 2010;51(2):163–170. doi: 10.1086/653529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. J Hear Lung Transplant. 2002;21(5):559–566. doi: 10.1016/S1053-2498(01)00405-3 [DOI] [PubMed] [Google Scholar]
- 11.Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5(8):2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peghin M, Los-Arcos I, Hirsch HH, et al. Community-acquired Respiratory Viruses Are a Risk Factor for Chronic Lung Allograft Dysfunction. Clin Infect Dis. 2019;69(7):1192–1197. doi: 10.1093/cid/ciy1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayah DM, Koff JL, Leard LE, Hays SR, Golden JA, Singer JP. Rhinovirus and other respiratory viruses exert different effects on lung allograft function that are not mediated through acute rejection. Clin Transplant. 2013;27(1). doi: 10.1111/ctr.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins P, McNeil K, Kermeen F, et al. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med. 2008;178(8):876–881. doi: 10.1164/rccm.200711-1657OC [DOI] [PubMed] [Google Scholar]
- 15.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory Viral Infections Are a Distinct Risk for Bronchiolitis Obliterans Syndrome and Death. Am J Respir Crit Care Med. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359oc [DOI] [PubMed] [Google Scholar]
- 16.Ambrosioni J, Bridevaux PO, Aubert JD, Soccal P, Wagner G, Kaiser L. Role of rhinovirus load in the upper respiratory tract and severity of symptoms in lung transplant recipients. J Clin Virol. 2015;64:1–5. doi: 10.1016/j.jcv.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 17.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J Med Virol. 2006;78(5):644–650. doi: 10.1002/jmv.20588 [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Mallory GB, Schecter MG, et al. Long-term impact of respiratory viral infection after pediatric lung transplantation. Pediatr Transplant. 2010;14(3):431–436. doi: 10.1111/j.1399-3046.2010.01296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser L, Aubert JD, Pache JC, et al. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med. 2006;174(12):1392–1399. doi: 10.1164/rccm.200604-489OC [DOI] [PubMed] [Google Scholar]
- 20.Gerna G, Piralla A, Rovida F, et al. Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J Med Virol. 2009;81(8):1498–1507. doi: 10.1002/jmv.21548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110(1):145–160. doi: 10.1017/S0950268800050779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapparel C, Cordey S, Junier T, et al. Rhinovirus Genome Variation during Chronic Upper and Lower Respiratory Tract Infections. PLoS One. 2011;6(6):1–8. doi: 10.1371/journal.pone.0021163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweet SC, Chin H, Conrad C, et al. Absence of evidence that respiratory viral infections influence pediatric lung transplantation outcomes: results of the CTOTC-03 study. Am J Transplant. 2019. doi: 10.1111/ajt.15505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2(10). doi: 10.1371/journal.pone.0000966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldfarb S, Benden C, Sweet S, et al. ISHLT Monograph Series: Pediatric Lung Transplantation; 2013. [Google Scholar]
- 26.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Hear Lung Transplant. 2007;26(12):1229–1242. doi: 10.1016/j.healun.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 27.Cooper BG, Stocks J, Hall GL, et al. The global lung function initiative (GLI) network: Bringing the world’s respiratory reference values together. Breathe. 2017. doi: 10.1183/20734735.012717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winther B, Gwaltney JM, Mygind N, Hendley JO. Viral-Induced Rhinitis. Am J Rhinol. 1998;12(1):17–20. [DOI] [PubMed] [Google Scholar]
- 29.Calvo C, Casas I, García-García ML, et al. Role of rhinovirus C respiratory infections in sick and healthy children in Spain. Pediatr Infect Dis J. 2010;29(8):717–720. doi: 10.1097/INF.0b013e3181d7a708 [DOI] [PubMed] [Google Scholar]
- 30.Lee WM, Lemanske RF, Evans MD, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186(9):886–891. doi: 10.1164/rccm.201202-0330OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X, Schneider E, Jain S, et al. Rhinovirus viremia in patients hospitalized with community-acquired pneumonia. J Infect Dis. 2017;216(9):1104–1111. doi: 10.1093/infdis/jix455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochkov YA, Gern JE. Clinical and molecular features of human rhinovirus C. Microbes Infect. 2012;14(6):485–494. doi: 10.1016/j.micinf.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SDY960 “Viral Triggers of Alloimmunity and Autoimmunity in Pediatric Lung Transplantation”. In. NIAID, trans:ImmPort (immport.org). 2018. [Google Scholar]