Abstract
The FVIII activity in patients treated with several extended half-life FVIII (EHL-FVIII) agents different when various activated partial thromboplastin time (APTT) reagents were used. The present study examined the difference in clot waveform analysis (CWA) findings and FVIII activity when various APTT reagents and CWA were used. The CWA including FVIII activity was measured using 12 APTT reagents, and the FIX activation based on a small amount of tissue factor assay (sTF/FIX) were examined in reference plasma (RP), EHL-FVIII (Jivi®) and Kovaltry®. The 3 APTT reagents were associated with high variation in the peak time and height in the CWA when analyzing low concentrations of FVIII. The peak time and height could not be measured with one APTT reagent, and there were marked differences in the CWA findings between Jivi® and Kovaltry® among APTT reagents. Several APTT reagents showed a markedly lower FVIII activity with Jivi® than with Kovaltry®. In the FVIII assay, the peak time measured with sTF/FIX did not differ markedly between Jivi® and Kovaltry®; however, the FVIII activity in Jivi® (as measured by the peak height) tended to be higher than in Kovaltry®. The CWA findings for monitoring Jivi® varied for monitoring Jivi® depending on the APTT reagents used, and sTF/FIX assay may be able to measure the EHL-FVIII.
Keywords: CWA, APTT reagent, sTF/FIX assay, FVIII, EHL-FVIII
Introduction
The activated partial thromboplastin time (APTT) is a clotting time assay that is useful for screening hemophilia1 and the presence of inhibitors (e.g. lupus anticoagulant2 and FVIII inhibitor3) and for monitoring unfractionated heparin treatment.4 The development of automatic optical coagulation analyzers has made it easy to perform multiple inexpensive assays as routine assays.
An optical coagulation analyzer can demonstrate the clot reaction curve of APTT. An MDA system was first used to present the clot reaction curve for detecting the biphasic form in a case of disseminated intravascular coagulation (DIC).5 Such an analysis of the APTT is called a clotting waveform analysis (CWA), and its utility for examining the coagulation factor FVIII (FVIII) activity in patients with hemophilia A has also been reported.6,7 A new FVIII assay using a small amount of tissue factor and coagulation factor FIX (sTF/FIX) has also reported useful for measuring FVIII levels, which are activated via the extrinsic pathway.8 Although routine APTT assays are based on the peak time of CWA-APTT, the peak height of CWA-APTT is considered to more closely reflect the physiological clotting ability than the peak time of CWA-APTT.9
Prophylactic treatment with FVIII concentrate is preferred to prevent bleeding and joint damage in children with severe hemophilia.10,11 Extended half-life FVIII (EHL-FVIII), which reduces the numbers of injections, would substantially improve the treatment options for hemophilia A patients.11,12 The attachment of polyethylene glycol (PEG) has been considered an effective method for prolonging the half-life of recombinant FVIII.13,14 However, it was recently reported that the FVIII activity in patients treated with EHL-FVIII, including PEG-FVIII, varied among various APTT reagents,15 suggesting that some APTT reagents may be not useful for monitoring hemophilia A patients treated with EHL-FVIII. The relationship between various APTT reagents and normal half-life-(NHL-) and EHL-FVIII has been described in several reports using a routine APTT assay based on the peak times of a CWA and chromogenic substrate assay.16–18 However, few reports have described this relationship using the peak height of an APTT-CWA including a sTF/FIX assay.9 An APTT-CWA may be able to reveal the differences among various APTT reagents, as the differences in APTT-CWA results may be greater in patients with lower concentrations of FVIII than in those with higher concentrations.
In the present study, APTT and FVIII activity assays using 12 APTT reagents were performed to evaluate the FVIII concentration in reference plasma (RP; Instrumentation Laboratory, Bedford, MA, USA), Kovaltry® (NHL-FVIII), and Jivi® (EHL-FVIII; Bayer, Leverkusen, Germany). In addition, the APTT and FVIII activities were also evaluated by a CWA, including a sTF/FIX assay.
Materials and Methods
APTT Reagents
A, APTT-SP; B, APTT-SS; C, STA Cephascreen; D, Coagpia APTT-N; E, APTT PSL; F, C.K. Prest; G, APTT-SLA; H, TC APTT; I, APTT FS; J, APTT FSL; K, PTT ACT; L, STA PTT A.
[Abbreviations] HemosIL APTT-SP; APTT-SP and HemosIL SynthASil; APTT-SS (Instrumentation Laboratory, Bedford, MA, USA), STA Cephascreen; STA Cephascreen, D, STA PTT Automate; STA PTT A and C.K. Prest (Diagnostica Stago S.A.S., Asnières-sur-Sreine, France), Coagpia APTT-N; APTT-N (SEKISUI MEDICAL CO., LTD., Tokyo Japan), Thrombocheck APTT-SLA; APTT-SLA and Thrombocheck APTT; TC APTT (Sysmex Corporation, Kobe, Japan) and Dade® Actin ® FS Activated PTT Reagent; APTT FS, Dade® Actin ® FSL Activated PTT Reagent; APTT FSL, Dade® Actin ® FSL Activated Cephaloplastin Reagent; APTT ACT and Pathromtin ® SL; APTT PSL (Siemens Healthcare Diagnostics Products GmbH, Malvern, PA, USA).
The samples, RP (Instrumentation Laboratory), Kovaltry®, and Jivi® were diluted to 1.0, 0.50, 0.10, 0.05, 0.01, 0.005 and 0.001 IU with FVIII-deficient plasma (Instrumentation Laboratory).
The APTT in RP, Kovaltry®, and Jivi® (FVIII, 1.0, 0.50, 0.10, 0.05, 0.01, 0.005 and 0.001 IU) was measured using the above mentioned APTT reagents and an ACL-TOP® system (Instrumentation Laboratory). Three types of curves are shown on the monitor of an ACL-TOP® system. One is a curve showing the changes in absorbance observed while measuring the APTT, corresponding to the fibrin formation (FF). The second is the first derivative peak (1st DP) of the absorbance, corresponding to the coagulation velocity. The third is the second derivative peak (2nd DP) of the absorbance, corresponding to the coagulation acceleration. In addition, we calculated the peak time and height of FF, the 1st DP and the 2nd DP (Supplementary Figure 1). As a limitation, although there was little evidence for various APTT reagents in ACL-TOP instrument, all assays using various APTT reagents were conducted with a CWA using only one instrument as ACL-TOP, due to the fact that most other instruments could not performe CWA.
The FVIII (1.0, 0.50, 0.10, 0.05, 0.01, and 0.005 IU) activity was measured by the one-stage clotting method using each APTT reagent in an ACL-TOP system, with the chromogenic substrate method using a RevohemTM FVIII chromogenicsystem (HYPHEN BioMed, Neuville-sur-Oise, France) using a CS-5100 device (Sysmex), or with the sTF/FIX method with an ACL-TOP system.8 The sTF/FIX assay was performed using a C.K.Prest, FIX (Nonacog Alfa; Pfizer, Tokyo, Japan) and 2,000-fold-diluted HemosIL RecombiPlasTin 2G, (Instrumentation Laboratory) with an ACL-TOP® system8 (Supplementary Figure 2).
Statistical Analyses
All assays were performed 8 times. The “error” indicated when the peak height or time could not be measured. The data are expressed as the median (25th-75th percentiles). The significance of differences between groups was examined using the Mann-Whitney U-test. P values of <0.05 were considered to indicate statistical significance. All of the statistical analyses were performed using the Stat-Flex software program (version 6; Artec Co Ltd, Osaka, Japan).
Results
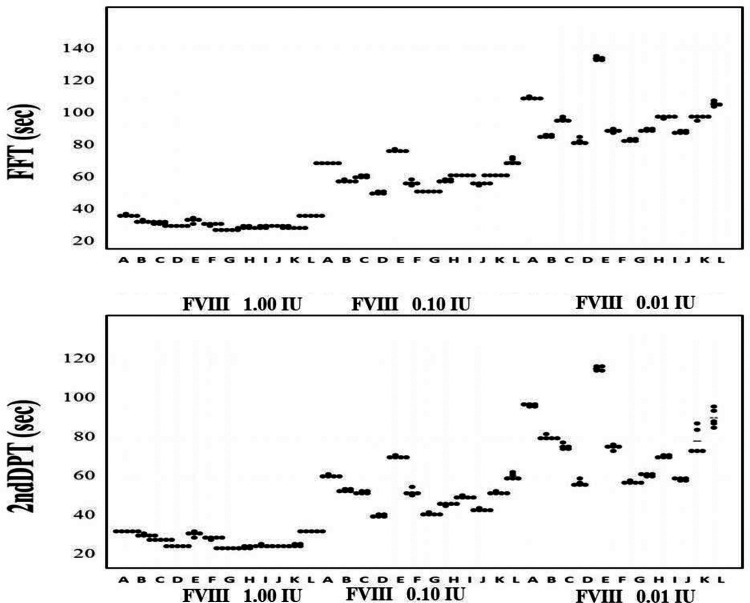
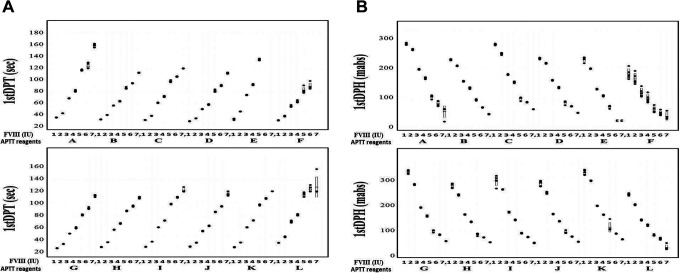
Although there were no significant differences in the APTT (FF or 2nd DP time) with 1.00 IU of FVIII among various APTT reagents (A-L), the differences in APTT measured with APTT reagents A-L increased in RP from 0.1 to 0.01 IU of FVIII. The differences in the peak time tended to be larger in the 2nd DP than in the FF (Figure 1). The relationship between the FVIII concentration from 0.001 to 1.00 IU RP and the FF peak time, 1st DP time, or 1st DP height on the APTT-CWA is shown in Figure 2A and 2B and Supplemental Table 1-A, 1-B, and 1-C. When using APTT reagent E, the peak time of FF could not be measured with 0.001 or 0.005 IU of FVIII, and the variation coefficient in 0.001 and 0.005 IU of FVIII was high with APTT reagents A, F, I, and L. The range of the FF peak time with 0.001 to 1.00 IU of FVIII was the longest with APTT reagent A and the pattern of the FF peak time was similar with APTT reagents B, C, G, H, J and K. The time and height of the 1st DP when using the APTT reagents, with the exception of APTT reagent E, was able to be measured with 0.001 to 1.00 IU of FVIII, and the variation coefficient of the peak time and height with 0.001 and 0.005 IU of FVIII was particularly high with APTT reagents A, F and L (Figure 2A and 2B). The range of the 1st DP time from 0.001 to 1.00 IU of FVIII was the longest with APTT reagents A and L and shortest with APTT reagent F, and the range of the 1st DP height was the longest with APTT reagents G, I and K.
Figure 1.
The FFT and 2nd DPT with various APTT reagents and various concentrations of FVIII. [APTT reagents] A, APTT-SP; B, APTT-SS; C, STA Cephascreen; D, Coagpia APTT-N; E, APTT PSL; F, C.K. Prest; G, APTT-SLA; H, TC APTT; I, APTT FS; J, APTT FSL; K, PTT ACT; L, STA PTT A. [Abbreviations] HemosIL APTT-SP; APTT-SP and HemosIL SynthASil; APTT-SS (Instrumentation Laboratory), STA Cephascreen; STA Cephascreen, D, STA PTT Automate; STA PTT A and C.K. Prest (Diagnostica Stago S.A.S), Coagpia APTT-N; APTT-N (SEKISUI MEDICAL CO., LTD.), Thrombocheck APTT-SLA; APTT-SLA and Thrombocheck APTT; TC APTT (Sysmex Corporation) and Dade® Actin® FS Activated PTT Reagent; APTT FS, Dade® Actin ® FSL Activated PTT Reagent; APTT FSL, Dade® Actin® FSL Activated Cephaloplastin Reagent; APTT ACT and Pathromtin ® SL; APTT PSL (Siemens Healthcare Diagnostics Products GmbH). FFT, fibrin formatin time; 2ndDPT, 2nd derivative peak time; FVIII, coagulation factor FVIII.
Figure 2.
The 1st DPT (A), and 1st DPH (B) and FVIII concentrations with various APTT reagents. [FVIII concentrations] 1, 1.00 IU; 2, 0.50 IU; 3, 0.10 IU; 4, 0.05 IU; 5, 0.01 IU; 6, 0.005 IU and 7, 0.001 IU. [APTT reagents] A, APTT-SP; B, APTT-SS; C, STA Cephascreen; D, Coagpia APTT-N; E, APTT PSL; F, C.K. Prest; G, APTT-SLA; H, TC APTT; I, APTT FS; J, APTT FSL; K, PTT ACT; L, STA PTT A. [Abbreviations] HemosIL APTT-SP; APTT-SP and HemosIL SynthASil; APTT-SS (Instrumentation Laboratory), STA Cephascreen; STA Cephascreen, D, STA PTT Automate; STA PTT A and C.K. Prest (Diagnostica Stago S.A.S), Coagpia APTT-N; APTT-N (SEKISUI MEDICAL CO., LTD.), Thrombocheck APTT-SLA; APTT-SLA and Thrombocheck APTT; TC APTT (Sysmex Corporation) and Dade® Actin® FS Activated PTT Reagent; APTT FS, Dade® Actin ® FSL Activated PTT Reagent; APTT FSL, Dade® Actin® FSL Activated Cephaloplastin Reagent; APTT ACT and Pathromtin ® SL; APTT PSL (Siemens Healthcare Diagnostics Products GmbH). FFT, fibrin formation time; FVIII, coagulation factor FVIII.
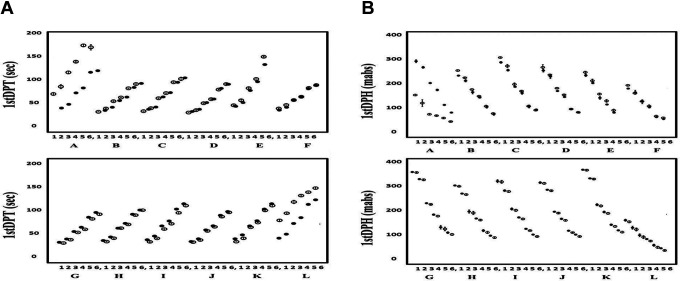
The relationships between the FVIII concentration and the 1st DP time or height of the APTT-CWA in 0.005 to 1.00 IU of Kovaltry® or Jivi® are shown in Figure 3A and 3B, and Supplemental Tables 2A and 2B. The 1st DP time could not be measured with APTT reagent E and was longer in Jivi® than in Kovaltry® when APTT reagents A and L were used. When other APTT reagents were used, the 1st DP time was prolonged in a dose-dependent manner as the FVIII concentration decreased in Jivi® or Kovaltry®. The 1st DP height was unable to be measured with APTT reagent E, and it was lower in Jivi® than in Kovaltry® with APTT reagent A. When other APTT reagents were used, the 1st DP height was reduced in a dose-dependent manner as the FVIII concentration decreased in Jivi® or Kovaltry®. The 1st DP height tended to be higher in Jivi® than in Kovaltry® when other APTT reagents were used.
Figure 3.
The 1st DP time (A) and 1st DPH (B), and FVIII concentration in Kovaltry® and Jivi® measured with various APTT reagents. Closed circle, Kovaltry®; open circle, Jivi®; [FVIII concentrations] 1, 1.00 IU; 2, 0.50 IU; 3, 0.10 IU; 4, 0.05 IU; 5, 0.01 IU; 6, 0.005 IU. [APTT reagents] A, APTT-SP; B, APTT-SS; C, STA Cephascreen; D, Coagpia APTT-N; E, APTT PSL; F, C.K. Prest; G, APTT-SLA; H, TC APTT; I, APTT FS; J, APTT FSL; K, PTT ACT; L, STA PTT A. [Abbreviations] HemosIL APTT-SP; APTT-SP and HemosIL SynthASil; APTT-SS (Instrumentation Laboratory), STA Cephascreen; STA Cephascreen, D, STA PTT Automate; STA PTT A and C.K. Prest (Diagnostica Stago S.A.S), Coagpia APTT-N; APTT-N (SEKISUI MEDICAL CO., LTD.), Thrombocheck APTT-SLA; APTT-SLA and Thrombocheck APTT; TC APTT (Sysmex Corporation) and Dade® Actin® FS Activated PTT Reagent; APTT FS, Dade® Actin ® FSL Activated PTT Reagent; APTT FSL, Dade® Actin® FSL Activated Cephaloplastin Reagent; APTT ACT and Pathromtin ® SL; APTT PSL (Siemens Healthcare Diagnostics Products GmbH). 1stDPT, 1st derivative peak time: FVIII, coagulation factor FVIII.
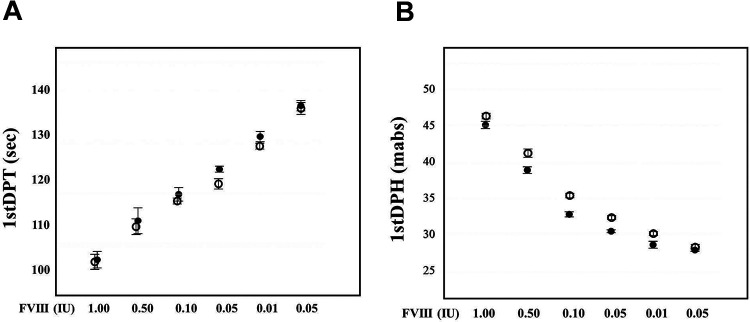
In the sTF/FIX assay, the 1st DP time was shortened in a dose-dependent manner, and the 1st DP height increased in a dose-dependent manner from 0.005 to 1.00 IU (Supplemental Figure 3A and 3B and Supplemental Table 3). The 1st DP time of Jivi® and Kovaltry® was shortened in a dose-dependent manner from 0.005 to 1.00 IU of FVIII and few differences were observed between Jivi® and Kovaltry® (Figure 4 A and Supplemental Table 3). The 1st DP height of Jivi® and Kovaltry® was increased in a dose-dependent manner from 0.005 to 1.00 IU of FVIII but it tended to be higher in Jivi® than in Kovaltry® (Figure 4B and Supplemental Table 3).
Figure 4.
The 1st DPT (A) and 1st DPH (B) of the sTF/FIX assay with FVIII reagents of various concentrations. Closed circle, Kovaltry®; open circle, Jivi®; 1st DPT, first derivative peak time; 1st DPH, first derivative peak height.
In FVIII assays, a routine clotting assay showed an error in 0.5% Kovaltry® using APTT reagents E and F, and in 0.005 IU Jivi® using APTT reagents A, E, F and L (Table 1). The FVIII activity in Jivi® was markedly lower than that in Kovaltry® when APTT reagents A, F and L were used. The FVIII assay by the 1st DP time and height showed an error in 0.005 IU Kovaltry® when APTT reagent E was used and in 0.005 IU Jivi® when APTT reagents A, E and L were used (Tables 1 and 2). The FVIII activity, as measured by the 1st DP time and 1st DP height, was markedly lower in Jivi® than in Kovaltry® when APTT reagents A, F and L and APTT reagents A and L, were used respectively. When APTT reagents other than A and L were used, the results of the FVIII assay by the 1st DP height tended to be higher than those of the chromogenic FVIII assay. The results of the FVIII assay by the 1st DP time of sTF/FIX showed no significant difference between Jivi® and Kovaltry®; however, the results of the 1st DP height assay showed that the FVIII activity tended to be higher in Jivi® than in Kovaltry® (Table 3).
Table 1.
Concentrations of FVIII in Kovaltry® as Determined by Clotting (With Various APTT Reagents) and Chromogenic Substrate Methods.
| Kovaltry® | X | Methods | A | B | C | D | E | F | G | H | I | J | K | L | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FVIII(IU) | 1.00 | 1.02 | APTT | 0.92 | 1.05 | 0.89 | 1.00 | 0.86 | 0.80 | 1.00 | 0.79 | 0.84 | 1.08 | 0.72 | 0.86 |
| 1st DPT | 0.88 | 1.10 | 0.88 | 1.02 | 0.66 | 0.96 | 0.98 | 0.80 | 0.70 | 1.10 | 0.72 | 0.99 | |||
| 1st DPH | 1.02 | 1.20 | 1.02 | 1.24 | 0.99 | 1.00 | 1.16 | 1.00 | 1.00 | 1.10 | 1.10 | 1.02 | |||
| 0.50 | 0.45 | APTT | 0.45 | 0.50 | 0.46 | 0.50 | 0.44 | 0.43 | 0.52 | 0.40 | 0.40 | 0.50 | 0.34 | 0.47 | |
| 1st DPT | 0.44 | 0.54 | 0.48 | 0.52 | 0.38 | 0.46 | 0.49 | 0.42 | 0.34 | 0.54 | 0.34 | 0.51 | |||
| 1st DPH | 0.50 | 0.58 | 0.49 | 0.62 | 0.50 | 0.52 | 0.60 | 0.50 | 0.51 | 0.54 | 0.64 | 0.52 | |||
| 0.10 | 0.09 | APTT | 0.10 | 0.11 | 0.10 | 0.11 | 0.13 | 0.10 | 0.12 | 0.09 | 0.01 | 0.11 | 0.01 | 0.01 | |
| 1st DPT | 0.08 | 0.12 | 0.10 | 0.12 | 0.09 | 0.10 | 0.11 | 0.09 | 0.09 | 0.11 | 0.16 | 0.10 | |||
| 1st DPH | 0.11 | 0.10 | 0.11 | 0.12 | 0.13 | 0.12 | 0.12 | 0.11 | 0.12 | 0.11 | 0.13 | 0.16 | |||
| 0.05 | 0.48 | APTT | 0.05 | 0.05 | 0.05 | 0.05 | 0.06 | 0.05 | 0.07 | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 | |
| 1st DPT | 0.05 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | |||
| 1st DPH | 0.05 | 0.05 | 0.05 | 0.06 | 0.05 | 0.06 | 0.05 | 0.05 | 0.06 | 0.05 | 0.06 | 0.05 | |||
| 0.01 | 0.009 | APTT | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| 1st DPT | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| 1st DPH | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| 0.005 | 0.004 | APTT | 0.005 | 0.005 | 0.005 | 0.005 | error | error | 0.006 | 0.005 | 0.005 | 0.005 | 0.006 | 0.005 | |
| 1st DPT | 0.005 | 0.006 | 0.005 | 0.006 | error | 0.005 | 0.005 | 0.004 | 0.005 | 0.005 | 0.004 | 0.005 | |||
| 1st DPH | 0.005 | 0.005 | 0.006 | 0.006 | error | 0.007 | 0.005 | 0.005 | 0.005 | 0.005 | 0.006 | 0.005 | |||
X, chromogenic substrate method; A, 2ndDPT; B-L, FFT; 1stDPT, first derivative peak time; FVIII, coagulation factor FVIII. 1stDPH, first derivative peak height;
APTT, activated partial thromboplastin time; 2nd DPT, second derivative peak time; [APTT reagents] A, APTT-SP; B, APTT-SS; C, STA Cephascreen; D, Coagpia APTT-N; E, APTT PSL; F, C.K. Prest; G, APTT-SLA; H, TC APTT; I, APTT FS; J, APTT FSL; K, PTT ACT; L, STA PTT A.
Table 2.
Concentrations of FVIII in Jivi®, as Determined by Clotting (With Various APTT Reagents) and Chromogenic Substrate Methods.
| Jivi® | X | Methods | A | B | C | D | E | F | G | H | I | J | K | L | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FVIII(IU) | 1.00 | 1.01 | APTT | 0.22 | 1.06 | 0.96 | 1.01 | 0.83 | 0.41 | 1.08 | 0.85 | 0.91 | 1.10 | 0.82 | 0.08 |
| 1st DPT | 0.10 | 1.30 | 1.10 | 1.20 | 0.56 | 0.54 | 1.10 | 0.98 | 1.10 | 1.30 | 1.02 | 0.08 | |||
| 1st DPH | 0.04 | 1.88 | 1.20 | 1.46 | 1.24 | 1.34 | 1.30 | 1.26 | 1.16 | 1.34 | 1.70 | 0.13 | |||
| 0.50 | 0.43 | APTT | 0.09 | 0.54 | 0.50 | 0.51 | 0.46 | 0.19 | 0.54 | 0.44 | 0.44 | 0.50 | 0.38 | 0.05 | |
| 1st DPT | 0.04 | 0.62 | 0.56 | 0.56 | 0.28 | 0.26 | 0.54 | 0.51 | 0.56 | 0.07 | 0.51 | 0.04 | |||
| 1st DPH | 0.02 | 0.92 | 0.62 | 0.72 | 0.64 | 0.64 | 0.70 | 0.62 | 0.56 | 0.70 | 0.88 | 0.04 | |||
| 0.10 | 0.11 | APTT | 0.02 | 0.11 | 0.11 | 0.10 | 0.11 | 0.08 | 0.11 | 0.10 | 0.10 | 0.10 | 0.10 | 0.02 | |
| 1st DPT | 0.01 | 0.13 | 0.11 | 0.11 | 0.07 | 0.09 | 0.12 | 0.10 | 0.13 | 0.12 | 0.11 | 0.01 | |||
| 1st DPH | 0.003 | 0.18 | 0.13 | 0.14 | 0.18 | 0.14 | 0.17 | 0.16 | 0.16 | 0.14 | 0.16 | 0.1.2 | |||
| 0.05 | 0.04 | APTT | 0.01 | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.07 | 0.05 | 0.05 | 0.04 | 0.05 | 0.01 | |
| 1st DPT | 0.002 | 0.06 | 0.05 | 0.05 | 0.04 | 0.04 | 0.05 | 0.05 | 0.06 | 0.06 | 0.05 | 0.004 | |||
| 1st DPH | 0.001 | 0.08 | 0.06 | 0.07 | 0.08 | 0.08 | 0.08 | 0.08 | 0.07 | 0.07 | 0.07 | 0.06 | |||
| 0.01 | 0.007 | APTT | 0.003 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | error | |
| 1st DPT | error | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.001 | |||
| 1st DPH | error | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.001 | |||
| 0.005 | 0.002 | APTT | error | 0.005 | 0.005 | 0.006 | error | error | 0.007 | 0.005 | 0.005 | 0.005 | 0.005 | error | |
| 1st DPT | error | 0.006 | 0.005 | 0.006 | error | 0.004 | 0.006 | 0.005 | 0.006 | 0.006 | 0.005 | error | |||
| 1st DPH | error | 0.006 | 0.006 | 0.007 | error | 0.008 | 0.007 | 0.007 | 0.008 | 0.007 | 0.008 | error | |||
X, chromogenic substrate method; A, 2ndDPT; B-L, FFT; 1stDPT, first derivative peak time; FVIII, coagulation factor FVIII. 1stDPH, first derivative peak height;
APTT, activated partial thromboplastin time; 2nd DPT, second derivative peak time; [APTT reagents] A, APTT-SP; B, APTT-SS; C, STA Cephascreen; D, Coagpia APTT-N; E, APTT PSL; F, C.K. Prest; G, APTT-SLA; H, TC APTT; I, APTT FS; J, APTT FSL; K, PTT ACT; L, STA PTT A.
Table 3.
FVIII Concentrations in Kovaltry® and Jivi® as Determined by 1st DPT and 1st DPH in the sTF/FIX Assay.
| FVIII | 1.00 IU | 0.50 IU | 0.10 IU | 0.05 IU | 0.01 IU | 0.005 IU | |
|---|---|---|---|---|---|---|---|
| 1stDPT | Kovaltry® | 0.88 IU | 0.43 IU | 0.11 IU | 0.05 IU | 0.01 IU | 0.006 IU |
| Jivi® | 0.91 IU | 0.48 IU | 0.13 IU | 0.07 IU | 0.02 IU | 0.007 IU | |
| 1st DPH | Kovaltry® | 1.15 IU | 0.50 IU | 0.10 IU | 0.05 IU | 0.01 IU | 0.006 IU |
| Jivi® | 1.52 IU | 0.76 IU | 0.16 IU | 0.08 IU | 0.02 IU | 0.08 IU |
1st DP, first derivative peak; 1st DPT, 1st DP time; 1st DPH, 1st DP height; FVIII, coagulation factor FVIII.
Discussions
Two methods are used to measure the FVIII activity: the one-stage method, which used an APTT assay, and the two-stage method, which use a chromogenic substrate assay.19,20 In addition, the APTT-CWA consists of a peak time which uses a routine APTT and a peak height which so far has only been rarely reported.9 Thereafter, an examination to determine the peak height of APTT-CWA is required in the future. Recently, the presence of marked inter-laboratory variability in the results obtained using APTT peak time-based assays, which can be intensified in a reagent-specific manner depending on the EHL-FVIII used, has attracted attention.15–18 The use of a chromogenic substrate assay, which tends to show less variability, especially with EHL-FVIII, is therefore recommended.19,20 However, the one-stage APTT peak time-based method is widely used and more easily performed in small laboratories than the chromogenic substrate assay.
In the present study of the peak time and height using a CWA, for the examination of RP, there were no significant differences in the APTT among the APTT reagents with 1 IU FVIII; however, the variability in the peak time and height of CWA among APTT reagents increased with low concentrations of FVIII. In particular, the APTT could not be measured with 0.001 or 0.005 IU of RP when using APTT reagent E in this CWA of the ACL-TOP system, suggesting that the reagent, which consists of soybean lecithin and silica, may not be useful for diagnosing patients with severe hemophilia in this CWA. The FF peak time of the CWA is used as the routine APTT. The height of the 1st DP measured using APTT reagents (other than E) was able to be used to measure low levels of FVIII concentration-dependently, indicating that the height of the 1st DP shows an equivalent or superior ability for measuring low FVIII concentrations to the peak time of FF (Supplemental Table 1).
In the comparison between Kovaltry® and Jivi®, the 1st DP time was longer in Jivi® than in Kovaltry® when using APTT reagents A and L. APTT reagent A consists of a synthetic phospholipid and colloidal silica, and APTT reagent L consists of cephalin and celite. A PEG moiety on EHL-FVIII appears to interact with silica-based APTT reagents, leading to over- or under-recovery.15,21 However, the phospholipid of an APTT reagent may also contribute to any subsequent variability in the APTT. In APTT reagents other than A, E and L, the 1st DP time was prolonged in a dose-dependent manner as the FVIII concentration of Jivi® or Kovaltry® decreased. The 1st DP height in the APTT-CWA showed similar results to the 1st DP time; however, the 1st DP height in Jivi® was not decreased in comparison to Kovaltry® when APTT reagent L was used. The 1st DP height tended to be higher in Jivi® than in Kovaltry®. The peak 1st DP height in the APTT-CWA may reflect a thrombin burst,22 which may better reflect the physiological clotting activity than the 1st DP time in the APTT-CWA. These findings suggest that the 1st DP height in the APTT-CWA may reflect the actual hemostatic ability more closely than the 1st DP time. The sTF/FIX assay8 can measure low levels of FVIII in Jivi® and Kovaltry® and showed a similar pattern to the APTT-CWA, suggesting that the sTF/FIX assay without activation of contact factor8,9 may be useful for monitoring hemophilic patients treated with Jivi®. The inability to measure very low concentrations of FVIII activity suggests that APTT reagents E and F may not be useful in the monitoring severe hemophilia in a CWA with the ACL-TOP system. However, the precise reason why APTT reagent F, which consists of cephalin from rabbit brain and ellagic acid, was not able to measure very low concentrations of FVIII activity remains unclear. The discrepancy between Kovaltry® and Jivi® suggests that APTT reagents A and L may not be useful in hemophilic patients being treated with PEG-FVIII, possibly due to the presence of celite, which is found in APTT reagent L.
The FVIII activity determined based on the 1st DP height may be more accurate than that determined based on the FF time of the APTT-CWA. The FVIII activity measured by the sTF/FIX assay tended to be higher in Jivi® than in Kovaltry®. As there are little reports for monitoring hemophilic using sTF/FIX assay in hemophilic patients, further study using the sTF/FIX assay for monitoring hemophilic patients treated with Kovaltry® or Jivi® will be required. The difference in the 1st DP time and height in the sTF/FIXassay suggests that the physiological clotting activity evaluated by the 1st DP height might be different from that evaluated by the 1st DP time and that the physiological clotting activity with Jivi® might be different from that with Kovaltry®. The modified sTF/FIX assay using platelet-rich plasma instead of commercial phospholipid is not affected by APTT reagents,23 suggesting that this assay might be useful in a clinical setting for monitoring hemophilic patients treated with EHL-FVIII.
In conclusion, APTT reagents A, E and L are not recommended for monitoring hemophilia treatment with Jivi® as PEG-FVIII in this CWA of the ACL-TOP system. The FVIII activity varied when using the peak time or height of the CWA-APTT, chromogenic assay, or sTF/FIX assay, suggesting that further studies will be needed to ensure the adequate evaluation of the physiological coagulation ability in a clinical setting.
Supplemental Material
Supplemental Material, sj-pdf-1-cat-10.1177_1076029620976913 for The Evaluation of APTT Reagents in Reference Plasma, Recombinant FVIII Products; Kovaltry® and Jivi® Using CWA, Including sTF/7FIX Assay by Hideo Wada, Katsuya Shiraki, Takeshi Matsumoto, Kohshi Ohishi, Hideto Shimpo, Yumi Sakano, Hiroko Nishii and Motomu Shimaoka in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was performed as a doctor-led research “Monitoring for EHL-FVIII using new clot wave assay.” which was partially supported by a grant from Bayer AG. This work was supported in part by a Grant-in-Aid from the Rare/ Intractable Disease Project of Japan from Japan Agency for Medical Research and Development, AMED.
ORCID iD: Hideo Wada  https://orcid.org/0000-0001-9021-8633
https://orcid.org/0000-0001-9021-8633
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Matsumoto T, Nogami K, Shima M. A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int J Hematol. 2017;105(2):174–183. [DOI] [PubMed] [Google Scholar]
- 2. Tokutake T, Baba H, Shimada Y, et al. Exogenous magnesium chloride reduces the activated partial thromboplastin times of lupus anticoagulant-positive patients. PLoS One. 2016;11(6):e0157835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsumoto T, Wada H, Nishioka Y, et al. Frequency of abnormal biphasic aPTT clot waveforms in patients with underlying disorders associated with disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2006;12(2):185–192. [DOI] [PubMed] [Google Scholar]
- 4. Byun JH, Jang IS, Kim JW, Koh EH. Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy. Blood Res. 2016;51(3):171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toh CH, Samis J, Downey C, et al. Biphasic transmittance waveform in the APTT coagulation assay is due to the formation of a Ca(++)-dependent complex of C-reactive protein with very-low-density lipoprotein and is a novel marker of impending disseminated intravascular coagulation. Blood. 2002;100(7):2522–2529. [DOI] [PubMed] [Google Scholar]
- 6. Shima M.: Understanding the hemostatic effects of recombinant factor VIIa by clot wave form analysis. Semin Hematol. 2004;41(1):125–131. [DOI] [PubMed] [Google Scholar]
- 7. Shima M, Matsumoto T, Fukuda K, et al. The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII: C). Thromb Haemost. 2002;87:436–441. [PubMed] [Google Scholar]
- 8. Matsumoto T, Wada H, Toyoda H, Hirayama M, Yamashita Y, Katayama N. Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab: comment. J Thromb Haemost. 2018;16(6):1665–1666. [DOI] [PubMed] [Google Scholar]
- 9. Wada H, Matsumoto T, Ohishi K, Shiraki K, Shimaoka M. Update on the clot waveform analysis. Clin Appl Thromb Hemost. 2020 doi: 10.1177/1076029620912027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Hemophilia Foundation. MASAC recommendation concerning prophylaxis (regular administration of clotting factor concentrate to prevent bleeding). Accessed January 10, 2014 http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=57&contentid=1007
- 11. MJ Manco-Johnson, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. [DOI] [PubMed] [Google Scholar]
- 12. Di Minno G, Cerbone AM, Coppola A, et al. Longer-acting factor VIII to overcome limitations in haemophilia management: the PEGylated liposomes formulation issue. Haemophilia. 2010;16(1):2–6. [DOI] [PubMed] [Google Scholar]
- 13. Chapman AP, Antoniw P, Spitali M, West S, Stephens S, King DJ. Therapeutic antibody fragments with prolonged in vivo half-lives. Nat Biotechnol. 1999;17:780–783. [DOI] [PubMed] [Google Scholar]
- 14. Mei B, Pan C, Jiang H, et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia a treatment. Blood. 2010;116:270–279. [DOI] [PubMed] [Google Scholar]
- 15. Gu JM, Ramsey P, Evans V, et al. Evaluation of the activated partial thromboplastin time assay for clinical monitoring of PEGylated recombinant factor VIII (BAY 94-9027) for haemophilia A. Haemophilia. 2014;20(4):593–600. [DOI] [PubMed] [Google Scholar]
- 16. Peyvandi F, Kenet G, Pekrul I, Pruthi RK, Ramge P, Spannagl MJ. Laboratory testing in hemophilia: impact of factor and non-factor replacement therapy on coagulation assays. Thromb Haemost. 2020;18(6):1242–1255. [DOI] [PubMed] [Google Scholar]
- 17. Gray E, Kitchen S, Bowyer A, et al. Laboratory measurement of factor replacement therapies in the treatment of congenital haemophilia: a United Kingdom Haemophilia Centre Doctors’ Organisation guideline. Haemophilia. 2020;26(1):6–16. [DOI] [PubMed] [Google Scholar]
- 18. Jeanpierre E, Pouplard C, Lasne D, et al. French Study Group on the Biology of Hemorrhagic Diseases (the BIMHO group). Factor VIII and IX assays for post-infusion monitoring in hemophilia patients: guidelines from the French BIMHO group (GFHT). Eur J Haematol. 2020;105:103–115. [DOI] [PubMed] [Google Scholar]
- 19. Adcock DM, Strandberg K, Shima M, Marlar RA. Advantages, disadvantages and optimization of one-stage and chromogenic factor activity assays in haemophilia A and B. Int J Lab Hematol. 2018;40(6):621–629. [DOI] [PubMed] [Google Scholar]
- 20. Kitchen S, Blakemore J, Friedman KD, et al. A computer-based model to assess costs associated with the use of factor VIII and factor IX one-stage and chromogenic activity assays. J Thromb Haemost. 2016;14(4):757–764. [DOI] [PubMed] [Google Scholar]
- 21. Dodt J, Hubbard AR, Wicks SJ, et al. Potency determination of factor VIII and factor IX for new product labelling and postinfusion testing: challenges for caregivers and regulators. Haemophilia. 2015;21(4):543–549. [DOI] [PubMed] [Google Scholar]
- 22. Salvagno GL, Berntorp E. Thrombin generation testing for monitoring hemophilia treatment: a clinical perspective. Semin Thromb Hemost. 2010;36(7):780–790. [DOI] [PubMed] [Google Scholar]
- 23. Wada H, Shiraki K, Matsumoto T, Ohishi K, Shimpo H, Shimaoka M. Effects of platelet and phospholipids on clot formation activated by a small amount of tissue factor. Thromb Res. 2020;193:146–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cat-10.1177_1076029620976913 for The Evaluation of APTT Reagents in Reference Plasma, Recombinant FVIII Products; Kovaltry® and Jivi® Using CWA, Including sTF/7FIX Assay by Hideo Wada, Katsuya Shiraki, Takeshi Matsumoto, Kohshi Ohishi, Hideto Shimpo, Yumi Sakano, Hiroko Nishii and Motomu Shimaoka in Clinical and Applied Thrombosis/Hemostasis