Abstract
Purpose
The purpose of this study was to investigate the cytoskeletal composition of myotendinous junctions (MTJs) in the human extraocular muscles (EOMs). Desmin and other major cytoskeletal proteins are enriched at the MTJs of ordinary myofibers, where they are proposed to be of particular importance for force transmission and required to maintain myofiber integrity.
Methods
EOM and limb muscle samples were analyzed with immunohistochemistry using antibodies against the intermediate filament proteins desmin, nestin, keratin 19, vimentin, and different myosin heavy chain (MyHC) isoforms. MTJs were identified by labeling with antibodies against laminin or tenascin.
Results
In contrast to MTJs in lumbrical muscle where desmin, nestin, and keratin 19 were always present, approximately one-third of the MTJs in the EOMs lacked either desmin and/or nestin, and all MTJs lacked keratin 19. Approximately 6% of the MTJs in the EOMs lacked all of these key cytoskeletal proteins.
Conclusions
The cytoskeletal protein composition of MTJs in human EOMs differed significantly from that of MTJs in limb muscles. These differences in cytoskeletal protein composition may indicate particular adaptation to meet the functional requirements of the EOMs.
Keywords: extraocular muscles (EOMs), desmin, nestin, keratin 19, vimentin, cytoskeletal protein, myotendinous junctions, force transmission
The myotendinous junction (MTJ) is the specialized site for force transmission from the intracellular contractile proteins of the myofiber to the extracellular connective tissue proteins of the tendon.1 The myofibers terminate at MTJs with digit-like projections surrounded by a basement membrane rich in laminin, tenascin, and collagen fibers on the tendon side.2,3 The muscle side of the MTJs is particularly rich in cytoskeletal intermediate filament proteins associated with force transmission from the thin filaments to the cell membrane. The cytoskeleton is particularly important for the establishment of series and parallel connections with the contractile proteins resulting in a cytoskeletal lattice through which force can be transmitted. Furthermore, intermediate filament proteins also link components of myofibrils to membrane protein complexes along the surface of the myofiber, enabling lateral transduction of force generated during muscle contraction.4
Desmin, the first muscle-specific protein detected during development, is the major intermediate filament protein in myofibers, linking adjacent Z-discs in neighboring myofibrils and connecting the most peripheral myofibrils to the costameres in the subsarcolemmal cytoskeleton.5 Desmin also provides a network to interconnect nuclei, mitochondria, and other cytoplasmic organelles, and is considered pivotal for maintenance of mature myofiber integrity upon loading and continued use. Lack of desmin targets highly used muscles working against high loads.6 Desmin is enriched at MTJs as well as neuromuscular junctions (NMJs) in limb muscle. Nestin and vimentin are two additional important intermediate filament proteins that are co-expressed with desmin during skeletal muscle development.7 Nestin expression is downregulated during muscle maturation and in adult muscle it is restricted to NMJs and MTJs8,9 as well as regenerating myofibers.10 Vimentin is widely expressed in skeletal myofibers in the early and middle stages of muscle development and becomes completely undetectable in fully developed myofibers.11 Keratin 19, another intermediate filament protein expressed in developing muscle but downregulated as myofibers mature, is present at both the Z-discs and the M-band of the most peripheral myofibrils linking them to the sarcolemma in mature muscle.4,12,13
Extraocular muscles (EOMs), consisting of four rectus and two oblique muscles, are highly specialized muscles responsible for the complex eyeball movements. In order to optimize perception and maintain stereo-vision in every gaze position, it is necessary to perfectly control and coordinate the delicate eyeball movements. The complexity of actions performed by the EOMs is reflected in their distinct myosin heavy chain (MyHC) composition, structural organization, innervation patterns, and physiological properties, that separate them from other skeletal muscles.14–16 Desmin was regarded as a ubiquitous muscle protein until we reported absence of desmin from a subgroup of normal and intact myofibers in the adult human EOMs.17,18 We initially reported absence of desmin in a subgroup of myofibers containing MyHC slow-tonic and MyHCI (MyHCsto/I)17 but we have recently shown that there is a complex relation between desmin content and innervation in the EOMs.18
In the present study, we investigated the intermediate filament protein composition with respect to desmin, nestin, keratin 19, and vimentin in relation to myofiber types at MTJs in the human EOMs. We found that there are important differences between MTJs of EOMs and limb muscles.
Material and Methods
Human Muscle Samples
Nine normal human samples were collected from the anterior portion of the lateral rectus, superior rectus, medial rectus, and inferior rectus muscles of 6 men with a mean age of 66 years (range = 42–83 years old) and lumbrical muscles of the hand from one man (75 years old) and one girl (13 years old) at autopsy, with the approval of the Regional Ethical Review Board in Umeå and was completed in accordance with the principles of the Declaration of Helsinki. The muscles were obtained from deceased individuals who, when alive, chose to donate their eyes and other tissues postmortem for transplantation and research purposes, according to Swedish law. All donors met the criteria for tissue donation for transplantation and none of the subjects was known to suffer from neuromuscular disease. The muscle samples were mounted on cardboard and rapidly frozen in propane chilled with liquid nitrogen, and stored at –80°C until used. Care was taken to keep the orientation of the EOM specimens in order to allow the longitudinal sections to include both the orbital and global layers. Serial longitudinal sections, 5 to 6 μm thick, were cut at –23°C in a cryostat (Reichert Jung; Leica, Heidelberg, Germany) and stored at –20°C until processed for immunohistochemistry. The smaller diameter of the myofibers in the orbital layer and the fact that the orbital layer does not extent all the way to the tendon allow the clear identification of the two layers in the longitudinal sections.
Antibodies and Immunofluorescence
The following monoclonal antibodies were used: D33 to detect desmin,17 MAB2736 and MAB1259 to detect nestin (R&D System, Abingdon, UK), and M0725 to detect vimentin (DAKO, Glostrup, Denmark). Rabbit polyclonal antibody ab15463 was used to detect keratin 19 (Abcam, Cambridge, UK). In addition, monoclonal antibodies against distinct MyHC isoforms were also used to identify the three major myofiber types in the EOMs, as previously reported18: BA-D519 against MyHCI, A4.7420 against MyHCIIa, and N2.26120,21 against MyHCI, MyHCIIa, and MyHCextraocular (MyHCeom) isoforms (The Developmental Studies Hybridoma Bank, Department of Biological Sciences, University of Iowa; Iowa City, IA, USA). MTJs were identified by either sheep polyclonal antibody PC128 against laminin2 (Binding Site Group, Birmingham, UK) or mouse monoclonal antibody ab88280 against tenascin22 (Abcam).
Because each EOM myofiber and its MTJ can be reliably studied only on a single longitudinal section, given the very small diameter of the myofibers in the EOMs, sections were processed for double or sequential immunostaining, as previously described.18 In brief, the tissue sections were air-dried, rehydrated in 0.01 M PBS, and then blocked with 5% normal donkey or goat serum for 15 minutes. Sections were then incubated with the first two primary antibodies, one against a cytoskeletal protein on the muscle side of the MTJs and another antibody against an extracellular matrix protein on the tendon side of the MTJs (desmin + laminin; nestin + laminin; vimentin + laminin; or keratin 19 + tenascin) at +4°C overnight. Thereafter, the sections were washed in PBS, additionally blocked with 5% normal serum for 15 minutes, and then incubated for 30 minutes at 37°C with the appropriate secondary antibodies (Supplementary Table S1).
Control sections were treated as above, except that the primary antibodies were omitted. No staining was observed in control sections. The sections were examined and photographed with a Spot camera (RT KE slider; Diagnostic Instruments, Inc., Sterling Heights, MI, USA) connected to a Nikon microscope (Eclipse, E800; Nikon, Tokyo, Japan).
After photographing, the third primary antibody (nestin or desmin) was applied respectively to the sections double labeled with the other antibodies (for example, the antibody against nestin was applied to a section treated with antibodies against desmin + laminin, in order to obtain a triple staining with desmin + laminin + nestin), for 60 minutes at 37°C, followed by incubation with the appropriate secondary antibody for 30 minutes at 37°C. The sections were examined and photographed again as above. Subsequently, sequential immunolabeling with primary antibodies against MyHCI (BA-D5), MyHCIIa (A4.74), and/or MyHCI + MyHCIIa + MyHCeom (N2.261) was performed on the aforementioned sections, adding one antibody at a time and taking photographs of the tissue section before adding the next MyHC antibody, followed by incubation with the appropriate secondary antibodies for 30 minutes at 37°C. Detailed information on the secondary antibodies used to detect each primary antibody is provided in Supplementary Table S1.
The images were processed using the Adobe Photoshop software (Adobe System, Inc., Mountain View, CA, USA).
Results
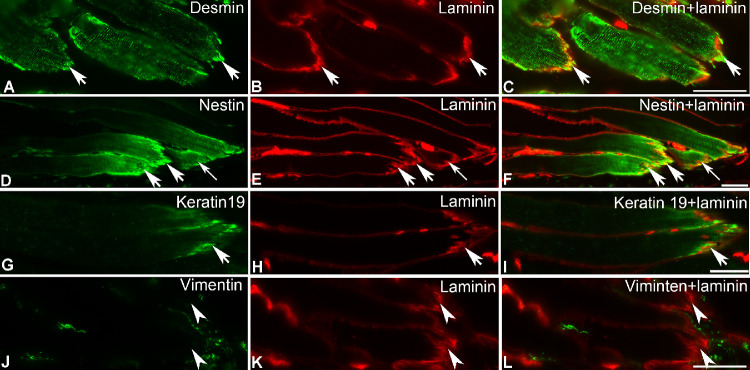
MTJs were identified by their typical appearance and labeling with antibodies against laminin or tenascin on the tendon side, in all muscle samples examined. In lumbrical muscles, desmin immunoreactivity was observed along the length of all myofibers and their MTJs. Higher desmin immunoreactivity was observed at the MTJs (Figs. 1A–C). Immunoreactivity with nestin was restricted to the myofiber region close to the MTJ and became stronger at the MTJ itself (Figs. 1D–F). Weak rather than strong labeling with nestin was occasionally found at MTJs (Figs. 1D–F). Immunolabeling with keratin 19 was also observed at the tip of the myofiber and it generally became stronger at the MTJs (Figs. 1G–I). Vimentin immunolabeling was not observed in myofibers of lumbrical muscle, nor at the MTJs, but it was sometimes present in a string on the tendon side of the MTJs (Figs. 1J–L).
Figure 1.
MTJs of lumbrical muscle doubled-labeled with antibodies (green) against desmin (A), nestin (D), keratin 19 (G), vimentin (J), and antibody against laminin (red; B, E, H, and K). The right column shows the merged images for each intermediate filament protein and laminin. Note the strong labeling at MTJs with the antibody against desmin (thick arrows in A and C), nestin (thick arrows in D and F), and keratin 19 (thick arrows in G and I). Notice the weak labeling with nestin at MTJs (thin arrows in D and F). Arrowheads (J–L) denote unlabeled MTJs with the antibody against vimentin on the muscle side of the MTJs. Bars = 50 µm.
MTJs in EOMs
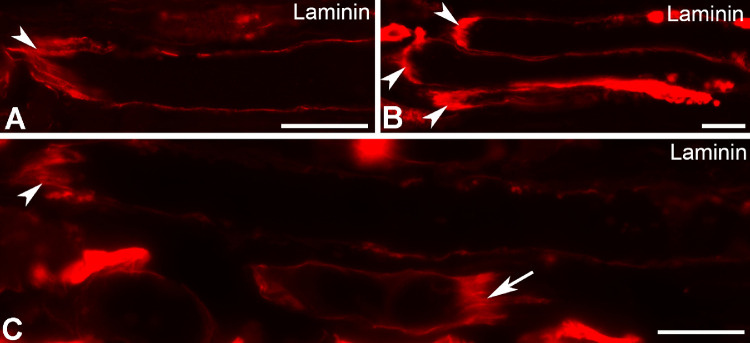
The longitudinal sections of EOMs investigated in the present study comprised approximately the most anterior one fourth of each muscle. MTJs were encountered in all muscle sections examined, in both the global and the orbital layers, with higher frequency in the global layer. The MTJs were generally found at the myofiber end facing the tendon (Figs. 2A–C), as in limb muscles. However, occasional structures with similar morphology to that of typical MTJs were also found at the opposite end of the myofiber, that is, the end facing away from the tendon (Fig. 2C), and were myo-endomysial junctions, as previously described.23–25
Figure 2.
MTJs in the human EOMs labeled with the antibody against laminin. The arrowheads (A–C) denote MTJs facing the tendon. Arrow (C) denotes MTJ facing away from the tendon. Bars = 20 µm.
A total of 493 MTJs were examined in 9 human EOM specimens. Heterogeneous labeling patterns with antibodies against intermediate filament proteins were noted, in contrast to the consistent labeling patterns described above and previously reported for the MTJs of limb muscles.8,9 In the EOM samples studied, approximately 85% of MTJs were present in myofibers containing MyHCIIa, and the rest were present in myofibers containing MyHCI.
Desmin
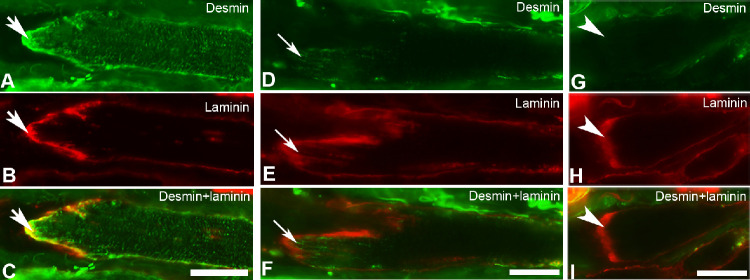
The vast majority of the MTJs (85%) examined in muscle sections treated with the antibody against desmin were labeled with this antibody (Fig. 3) and these MTJs were observed in myofibers containing either MyHCIIa or MyHCI, in both the global and the orbital layers. The number of labeled MTJs varied among specimens within and between subjects. The immunoreactivity for desmin at MTJs varied from strong to weak. More than half (68%) of the MTJs labeled with desmin in these muscle sections showed stronger immunoreactivity than that observed in the remaining length of the myofiber present in the longitudinal section, irrespective of whether the tip of the myofiber was strongly or weakly labeled with this antibody (Figs. 3A–F). Approximately 28% of MTJs labeled with desmin showed identical levels of immunoreactivity as the remaining part of the myofiber present in the section (not shown). Decreased immunoreactivity with desmin was sporadically (approximately 4%) observed in the folds of MTJs of myofibers containing desmin along the remaining of their length (not shown).
Figure 3.
MTJs in EOMs double-immunolabeled with antibodies against desmin (green in A, D, and G) and laminin (red in B, E, and H). Merged images for desmin and laminin are shown in C, F, and I. Immunolabeling with desmin is increased at MTJs in myofibers containing desmin (thick arrows in A–C) or present in the proximity of the MTJs in myofibers lacking desmin in the remaining of their length (thin arrows in D–F). Note the absence of desmin at MTJs in myofibers lacking desmin in the remaining of their length (arrowheads in G–I). Bars = 20 µm.
Fifteen percent of the total number of MTJs in muscle sections treated with the antibody against desmin were unlabeled with this antibody (Figs. 3G–I) and were found in both the global (54.5%) and the orbital (45.5%) layers. Immunoreactivity with the antibody against desmin was generally absent along the remaining length of the myofibers displaying the unlabeled MTJs (Figs. 3G–I). MTJs unlabeled with the antibody against desmin were observed mostly in myofibers containing MyHCIIa (71%), but also in myofibers containing MyHCI (29%). There was no apparent relation between the MyHC isoform content of myofibers and the levels of desmin immunoreactivity at their MTJs.
Nestin
Immunolabeling with the antibody against nestin was present in a proportion of myofibers in the human EOMs, as previously reported17 and in contrast to myofibers in limb muscle, which are unstained with this antibody. The immunolabeling intensity with the antibody against nestin in these myofibers was generally weaker than that observed with desmin, in a given myofiber, as previously described.17
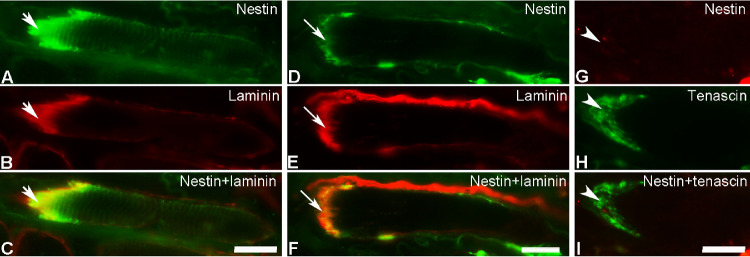
The vast majority of MTJs (91%) in the human EOM sections treated with the antibody against nestin showed immunoreactivity with this antibody in both the global and the orbital layers, irrespective of whether the myofibers were labeled or unlabeled along the remaining of their length (Figs. 4A–F). The remaining proportion of MTJs in these muscle sections was unlabeled with the antibody against nestin (Figs. 4G–I). The proportion of nestin labeled MTJs varied between different specimens and between subjects. The MTJs labeled with the antibody against nestin were mostly found in myofibers containing MyHCIIa (77%), less in myofibers containing MyHCI (23%).
Figure 4.
Immunoreactivity with the antibody against nestin (green in A and D; red in G) at MTJs labeled with antibodies against laminin (red in B and E) or tenascin (green in H) in EOMs. Merged images for desmin and either laminin or tenascin are shown in C, F, and I. Strong immunolabeling with the antibody against nestin was present at MTJs in myofibers containing nestin (thick arrows in A–C) and in myofibers lacking nestin (thin arrows in D–F) in the remaining of their length. Note the absence of nestin in MTJs of myofibers completely lacking nestin in the remaining of their length (arrowheads in G–I). Bars = 20 µm.
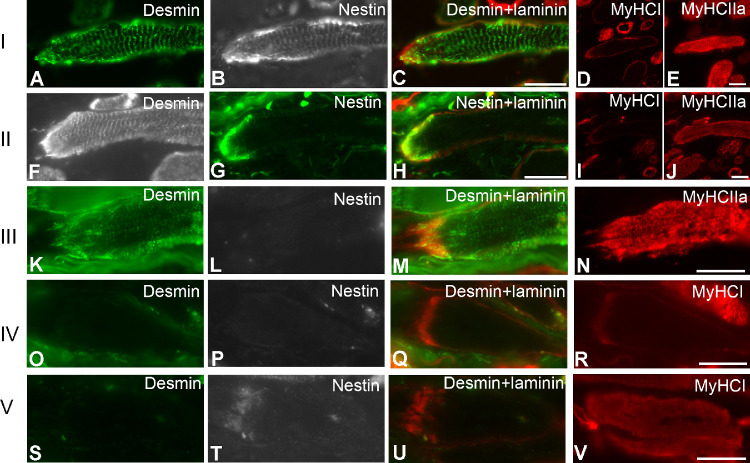
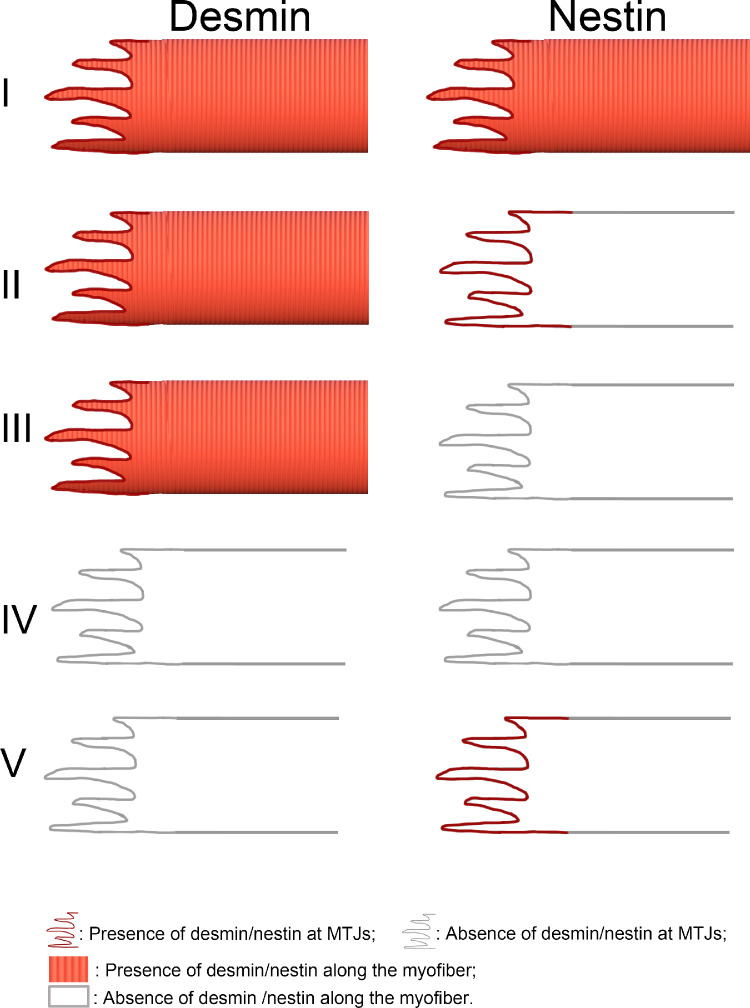
We asked the question whether there was a correlation between desmin and nestin at the MTJs in the EOMs. Taking into account the presence/absence of desmin or nestin along the myofibers seen in each muscle section treated with both antibodies, five distinct patterns of immunolabeling were observed (Figs. 5, 6): (I) co-expression of desmin and nestin at the MTJs and along the myofibers (approximately 68%; Figs. 5A–E; Fig. 6I); (II) co-expression of desmin and nestin only at the MTJs and presence of desmin but absence of nestin along the myofiber (approximately 15%; Figs. 5F–J; Fig. 6II); (III) presence of desmin at the MTJs and along the myofiber but absence or very weak immunolabeling for nestin in approximately 6% of MTJs and the remaining portion of the myofiber (Figs. 5K–N; Fig. 6III); (IV) absence of both desmin and nestin in 6% of MTJs and along the myofibers (Figs. 5O–R; Fig. 6IV); and (V) absence/weak immunolabeling with desmin but presence of nestin at the MTJs only (approximately 5%) and absence of desmin and nestin along the myofiber (Figs. 5S–V; Fig. 6V).
Figure 5.
Five (I–V) different patterns of immunostaining with antibodies against desmin (green in A, K, O, and S; and gray in F) or nestin (gray in B, L, P, and T; and green in G) at MTJs in myofibers containing MyHCIIa (A–R) and MyHCI (S–V) in the EOMs. Merged images of desmin and laminin are shown in C, M, Q, and U. Merged image of nestin and laminin is shown in H. Bars = 20 µm.
Figure 6.
Schematic illustration of the major patterns of immunoreactivity regarding desmin and nestin at MTJ and in the remaining portion of the myofiber present in the muscle sections of the human EOMs. I: Desmin and nestin were present in both MTJs and the remaining portion of the myofibers. II: Desmin and nestin were present at MTJs but nestin was lacking in the remaining portion of the myofibers. This pattern is similar to that of myofibers and their MTJs in skeletal muscle in general. III: Desmin was present in both the MTJs and along the myofibers whereas nestin was totally absent. IV: Desmin and nestin were absent at MTJs and along the remaining of the myofibers. V: Only nestin was present at the MTJs whereas neither desmin nor nestin were present in the remaining portion of the myofiber present in the muscle section.
Keratin 19
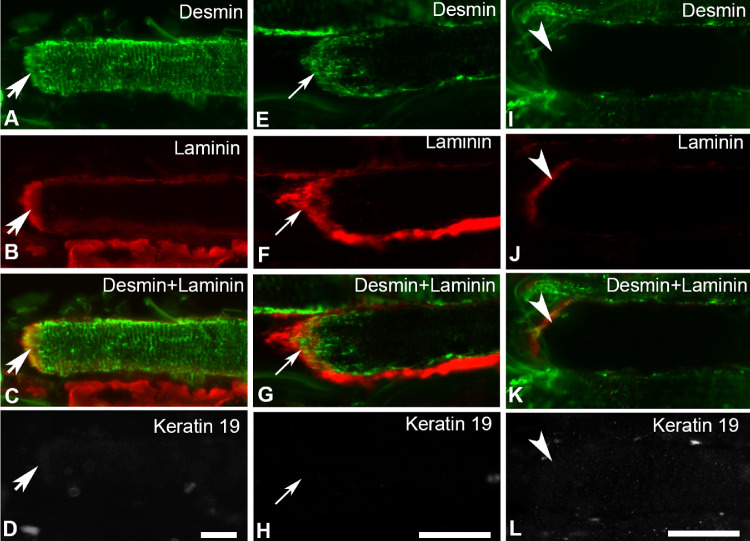
The vast majority of myofibers was unlabeled with the antibody against keratin 19 in the EOM samples examined (not shown). At MTJs, keratin 19 immunolabeling was always absent regardless of whether it was absent or occasionally present along the myofiber length (not shown). Double immunolabeling with keratin 19 and desmin showed that keratin 19 was absent at MTJs and in the remaining parts of the myofibers irrespective of whether desmin was present or absent at MTJs or along the myofibers (Fig. 7).
Figure 7.
Immunolabeling with antibodies against desmin (green in A, E, and I), laminin (red in B, F, and J) and keratin 19 (gray in D, H, and L) in EOMs. Merged images of desmin and laminin are shown in C, G, and K. Keratin 19 was absent from MTJs and the remaining parts of the myofibers containing desmin along their length (thick arrows in A–D) or only in the vicinity of the MTJs (thin arrows in E–H), or in myofibers completely lacking desmin (arrowheads in I–L). Bars = 20 µm.
Vimentin
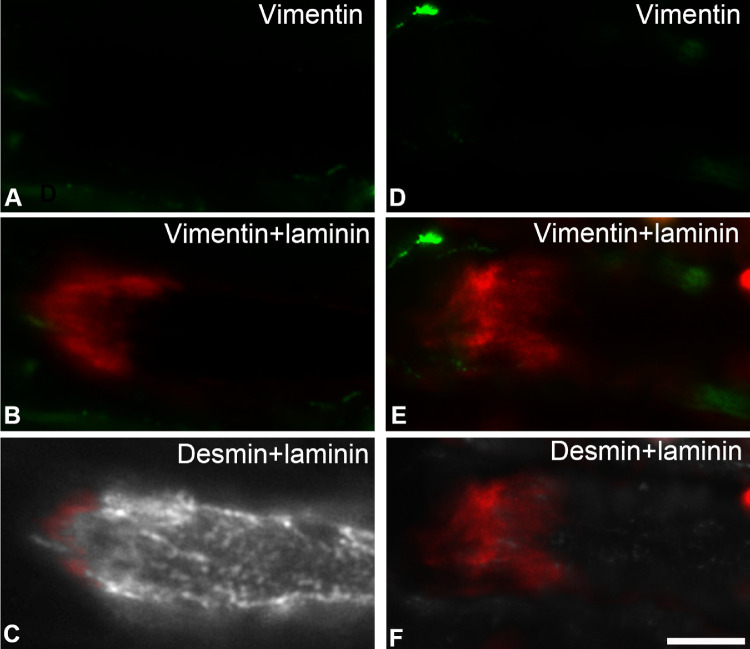
Immunolabeling with the antibody against vimentin was not observed on the muscle side of MTJs but was readily observed as strings on the tendon side of most MTJs, in both the global and orbital layers (not shown). Immunolabeling for vimentin was present in the connective tissue, blood vessels, and nerves, as described previously.17 Vimentin labeling was absent from the myofibers, with the notable exception of extremely sporadic myofibers (less than a total of 10 myofibers in all specimens examined) that were labeled weakly with this antibody (not shown). Sequential immunolabeling with vimentin and desmin confirmed that MTJs were always unlabeled with the antibody against vimentin, irrespectively whether myofibers were labeled or unlabeled with the antibody against desmin (Fig. 8).
Figure 8.
Immunolabeling with antibodies against vimentin (green in A, B, D, and E) and desmin (gray in C and F) at MTJs identified with the antibody against laminin (red in B, C, E, and F). Note that vimentin was absent from the MTJs and along the myofibers either containing (C) or lacking (F) desmin. Bar = 20 µm.
Discussion
The present study was the first to thoroughly investigate the distribution of the key intermediate filament proteins desmin, nestin, keratin 19, and vimentin at MTJs in the human EOMs and to compare immunolabeling patterns with those of the lumbrical muscles. In lumbrical muscle, desmin was present both at NMJs and along the length of the myofibers, nestin, and keratin 19 were present at MTJs only, whereas vimentin was absent both at the MTJs and in myofibers, as previously reported in skeletal muscle in general.4,8–10 In contrast, in the human EOMs: (i) a subgroup of MTJs lacked either desmin or nestin; (ii) some MTJs lacked both desmin and nestin; (iii) keratin 19 was absent from all MTJs; and (iv) the majority of the MTJs in the EOMs contained desmin and nestin, but still differed from those in skeletal muscle in general by lacking keratin 19.
Lack of Desmin at MTJs
The intermediate filament proteins desmin and nestin are highly expressed at MTJs in skeletal muscles.8,9,17,26 The fundamental functional role of desmin at MTJs has been proposed to be related to mechanical behavior at the junctional site, where myofibers are exposed to greatest mechanical stress and may easily be damaged.9,26 Approximately 15% of MTJs in the human EOMs showed absence of desmin whereas the majority (85%) of the examined MTJs had desmin. The present results suggest that desmin is not of crucial importance for the stability of MTJs in the human EOMs, given that these myofibers and their MTJs had normal appearance. Furthermore, MTJs show a similar morphology in control and in desmin knockout mice, indicating that desmin might not play the functional role that it has been assigned previously.8 Given a number of particular properties of the EOMs, our results may be interpreted as that lateral force transmission may be of particular importance in myofibers lacking desmin at MTJs. Eye movements are very different from movements across a bone joint, as the eyeballs are not rigidly attached to anything and have a minimal weight. Thus, there is no weight lifting involved in muscle movement and lower force is generated during EOM muscle contraction as compared to limb muscle.27,28 On the other hand, lateral force transmission across the muscle cell membrane may put different requirements on the myofibers of the EOMs, given that they are surrounded by a very rich connective tissue bed and, in addition, a large number of myofibers have tapered ends, terminating within the muscle itself.24,29–32 We recently demonstrated enriched desmin immunoreactivity in the subsarcolemma of EOM myofibers in both human17,18 and zebrafish,33 with weak or lack of desmin immunoreactivity inside the myofibers. Such strong immunoreactivity for desmin is typically seen at NMJs or MTJs but not seen subsarcolemmally in limb muscles.17,18 The high concentration of desmin in the subsarcolemmal region may partially provide the required sarcolemmal strength and the connections to the underlying contractile apparatus, to ensure lateral force transmission in the EOMs.34
Lack of Desmin at MTJs is Most Likely not Related to Palisade Endings in EOMs
The palisade endings are found in the close vicinity of MTJs, exclusively in myofibers containing MyHCsto/I in the global layer of EOMs.35–37 In the present study, we could not study the palisade endings as the tendon end of the muscle had been cut very close to the end of the myofibers and the typical morphology of palisade endings could not be ascertained. However, we have previously established that desmin is always present in myofibers containing MyHCsto/I found in the close vicinity of MTJs.
In other words, our previous studies indicate that most likely myofibers containing MyHCsto/I and which may have received palisade endings, contain desmin.18 In the current study, approximately 96% of the myofibers labeled with the antibody against desmin along their length were also labeled at their MTJs. Altogether, the evidence available suggests that the absence of desmin at MTJs in the EOMs is not likely associated with the presence of palisade endings.
Lack of Desmin, Nestin, Keratin 19, and Vimentin at MTJs
Desmin, nestin, keratin 19, and vimentin belong to different classes of intermediate filaments present in myofibers of skeletal muscles and have different temporal expression patterns during the course of development.4 These intermediate filament proteins interact closely with each other during development, as illustrated by colocalization of desmin, nestin, and/or vimentin.7,38 However, in mature limb muscle, desmin is continually expressed throughout the full length of the myofibers, whereas nestin is only expressed at the NMJs and MTJs of the myofibers,8,9 and vimentin is not present in the myofibers.39 Similarly, keratin 19 is transiently expressed in developing skeletal muscle13 and although it has been shown to be localized at both M-band and Z-disk in the close vicinity of the sarcolemma in mature skeletal muscle, keratin 19 is only present in trace amounts in myofibers, and it is hard to detect beyond the MTJ.40
In the human EOMs, nestin was detected in the vast majority of MTJs and it is tempting to speculate whether the presence of nestin, to some extent, may compensate for the lack of desmin at MTJs. The present study did not address this question, but because the other two patterns (lack of both desmin and nestin or presence of desmin and absence of nestin) were observed in approximately the same proportion of MTJs, it is fair to suggest that the presence of nestin and desmin are independent of each other at MTJs in the human EOMs. Furthermore, 6% of MTJs had neither desmin nor nestin, and no MTJs examined had keratin 19 or vimentin, indicating that these MTJs lack the fundamental cytoskeletal organization proposed to be required for force transmission. The lack of all major intermediate filament proteins at MTJs in the EOMs represents a paradigm shift questioning the proposed roles of these proteins as necessary to maintain integrity and provide mechanical strength to the myofibers.4
In summary, our results provide strong evidence that approximately one-third of the MTJs in the human EOMs lacked desmin and/or nestin and all MTJs lacked keratin 19. Approximately 6% of MTJs lacked all the cytoskeletal proteins examined. The present findings are in line with our previous reports that lack of desmin in myofibers is not compensated by nestin in human EOMs,17 nor in EOMs of control or desmin knock out mice.41 We propose that the particular cytoskeletal organization of the MTJs may suggest adaptation to meet the functional requirements of the human EOMs, which include significant lateral force transmission, very rich connective tissue bed surrounding each individual myofiber, and myofibers that do not run the full length of the muscle.
Changes of MyHC Isoforms Along EOM Myofibers
In contrast to limb muscles in which myofibers typically contain one or two MyHC isoforms uniformly along their length,42 the myofibers in the human EOMs contain a mixture of up to five different MyHC isoforms in a single myofiber.14,21 Three major myofiber groups are found in the human EOMs taking into account their MyHC composition: myofibers containing MyHCI, myofibers containing MyHCIIa, and myofibers containing MyHCeom, but lacking MyHCI and MyHCIIa.14,43 MyHCeom is present in EOMs of most mammals,44,45 and the human MyHCeom has been detected at both the gene and the protein levels.14,21,45
A particularly interesting finding of the current study was that almost all MTJs examined in the EOMs were present at the distal end of myofibers containing either MyHCI or MyHCIIa. MTJs were hardly ancountered in myofibers containing MyHCeom but lacking MyHCI and MyHCII. This finding provided indirect evidence of heterogeneity in MyHC composition along myofiber length in the human EOMs, and fits previous findings that MyHC content of individual myofibers varies along the myofiber length, both in human EOMs14 and in other species.46,47 The expression of MyHCeom has been shown to be limited to the endplate zone of singly innervated myofibers, which contain MyHCIIa at their proximal and distal portions in both the global and the orbital layers, in the EOMs of rabbits48,49 and rats.46
Supplementary Material
Acknowledgments
Supported by grants from the Swedish Research Council (2018-02401; Stockholm, Sweden), County Council of Västerbotten in cooperation with Umeå University (Centrala ALF, Spjutspets medel; Umeå, Sweden), Ögonfonden (Stockholm, Sweden), Kronprinsessan Margaretas Arbetsnämnd för Synskadade (Valdemarsvik, Sweden).
Disclosure: J.-X. Liu, None; F. Pedrosa Domellöf, None
References
- 1. Benjamin M, Ralphs JR.. Tendons and ligaments–an overview. Histol Histopathol . 1997; 12(4): 1135–1144. [PubMed] [Google Scholar]
- 2. Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol . 1996; 8(5): 618–624. [DOI] [PubMed] [Google Scholar]
- 3. Chiquet M, Fambrough DM.. Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J Cell Biol . 1984; 98(6): 1926–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henderson CA, Gomez CG, Novak SM, Mi-Mi L, Gregorio CC.. Overview of the muscle cytoskeleton. Compr Physiol . 2017; 7(3): 891–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schultheiss T, Lin ZX, Ishikawa H, Zamir I, Stoeckert CJ, Holtzer H.. Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J Cell Biol . 1991; 114(5): 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Z, Colucci-Guyon E, Pinçon-Raymond M, et al.. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol . 1996; 175(2): 362–366. [DOI] [PubMed] [Google Scholar]
- 7. Sjöberg G, Jiang WQ, Ringertz NR, Lendahl U, Sejersen T.. Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three-dimensional fluorescence digital imaging microscopy. Exp Cell Res . 1994; 214(2): 447–458. [DOI] [PubMed] [Google Scholar]
- 8. Carlsson L, Li Z, Paulin D, Thornell L.. Nestin is expressed during development and in myotendinous and neuromuscular junctions in wild type and desmin knock-out mice. Exp Cell Res . 1999; 251(1): 213–223. [DOI] [PubMed] [Google Scholar]
- 9. Vaittinen S, Lukka R, Sahlgren C, et al.. Specific and innervation-regulated expression of the intermediate filament protein nestin at neuromuscular and myotendinous junctions in skeletal muscle. Am J Pathol . 1999; 154(2): 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaittinen S, Lukka R, Sahlgren C, et al.. The expression of intermediate filament protein nestin as related to vimentin and desmin in regenerating skeletal muscle. J Neuropathol Exp Neurol . 2001; 60(6): 588–597. [DOI] [PubMed] [Google Scholar]
- 11. Small JV, Furst DO, Thornell LE.. The cytoskeletal lattice of muscle cells. Eur J Biochem . 1992; 208(3): 559–572. [DOI] [PubMed] [Google Scholar]
- 12. Stone MR, O'Neill A, Lovering RM, et al.. Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J Cell Sci . 2007; 120(Pt 22): 3999–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kosmehl H, Langbein L, Katenkamp D.. Transient cytokeratin expression in skeletal muscle during murine embryogenesis. Anat Anz . 1990; 171(1): 39–44. [PubMed] [Google Scholar]
- 14. Kjellgren D, Thornell LE, Andersen J, Pedrosa-Domellöf F.. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci . 2003; 44(4): 1419–1425. [DOI] [PubMed] [Google Scholar]
- 15. Fischer MD, Budak MT, Bakay M, et al.. Definition of the unique human extraocular muscle allotype by expression profiling. Physiol Genomics . 2005; 22(3): 283–291. [DOI] [PubMed] [Google Scholar]
- 16. Sadeh M. Extraocular muscles. In: Engel AG, Franzini-Armstrong C, eds. Myology. New York: McGraw-Hill; 1994: 119–127. [Google Scholar]
- 17. Janbaz AH, Lindstrom M, Liu JX, Pedrosa Domellöf F. Intermediate filaments in the human extraocular muscles. Invest Ophthalmol Vis Sci . 2014; 55(8): 5151–5159. [DOI] [PubMed] [Google Scholar]
- 18. Liu JX, Pedrosa Domellöf F. Complex correlations between desmin content, myofiber types, and innervation patterns in the human extraocular muscles. Invest Ophthalmol Vis Sci . 2020; 61(3): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiaffino S, Gorza L, Sartore S, et al.. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil . 1989; 10(3): 197–205. [DOI] [PubMed] [Google Scholar]
- 20. Maggs AM, Taylor-Harris P, Peckham M, Hughes SM.. Evidence for differential post-translational modifications of slow myosin heavy chain during murine skeletal muscle development. J Muscle Res Cell Motil . 2000; 21(2): 101–113. [DOI] [PubMed] [Google Scholar]
- 21. Liu JX, Eriksson PO, Thornell LE, Pedrosa-Domellöf F.. Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem . 2002; 50(2): 171–184. [DOI] [PubMed] [Google Scholar]
- 22. Gullberg D, Velling T, Sjöberg G, et al.. Tenascin-C expression correlates with macrophage invasion in Duchenne muscular dystrophy and in myositis. Neuromuscul Disord . 1997; 7(1): 39–54. [DOI] [PubMed] [Google Scholar]
- 23. Patel TJ, Lieber RL.. Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev . 1997; 25: 321–363. [PubMed] [Google Scholar]
- 24. McLoon LK, Rios L, Wirtschafter JD.. Complex three-dimensional patterns of myosin isoform expression: differences between and within specific extraocular muscles. J Muscle Res Cell Motil . 1999; 20(8): 771–783. [DOI] [PubMed] [Google Scholar]
- 25. Huijing P. Muscular force transmission: a unified, dual or multiple system? A review and some explorative experimental results. Arch Physiol Biochem . 1999; 107(4): 292–311. [DOI] [PubMed] [Google Scholar]
- 26. Tidball J. Desmin at myotendinous junctions. Exp Cell Res . 1992; 199: 206–212. [DOI] [PubMed] [Google Scholar]
- 27. Frueh BR, Gregorevic P, Williams DA, Lynch GS.. Specific force of the rat extraocular muscles, levator and superior rectus, measured in situ. J Neurophysiol . 2001; 85(3): 1027–1032. [DOI] [PubMed] [Google Scholar]
- 28. Croes SA, von Bartheld CS.. Measurement of contractile force of skeletal and extraocular muscles: effects of blood supply, muscle size and in situ or in vitro preparation. J Neurosci Methods . 2007; 166(1): 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davidowitz J, Philips G, Breinin GM.. Organization of the orbital surface layer in rabbit superior rectus. Invest Ophthalmol Vis Sci . 1977; 16(8): 711–729. [PubMed] [Google Scholar]
- 30. Mayr R, Gottschall J, Gruber H, Neuhuber W.. Internal structure of cat extraocular muscle. Anat Embryol (Berl) . 1975; 148(1): 25–34. [DOI] [PubMed] [Google Scholar]
- 31. Meijer HJ, Baan GC, Huijing PA.. Myofascial force transmission is increasingly important at lower forces: firing frequency-related length-force characteristics of rat extensor digitorum longus. Acta Physiol (Oxf) . 2006; 186(3): 185–195. [DOI] [PubMed] [Google Scholar]
- 32. McLoon LK, Vicente A, Fitzpatrick KR, Lindström M, Pedrosa Domellöf F. Composition, architecture, and functional implications of the connective tissue network of the extraocular muscles. Invest Ophthalmol Vis Sci . 2018; 59(1): 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dennhag N, Liu JX, Nord H, von Hofsten J, Pedrosa Domellöf F. Absence of desmin in myofibers of the zebrafish extraocular muscles. Transl Vis Sci Technol . 2020; 9(10): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bloch RJ, Gonzalez-Serratos H.. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev . 2003; 31(2): 73–78. [DOI] [PubMed] [Google Scholar]
- 35. Ruskell G. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J Neurocytol . 1978; 7(6): 693–708. [DOI] [PubMed] [Google Scholar]
- 36. Alvarado-Mallart R, Pincon-Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope. Tissue Cell . 1979; 11(3): 567–584. [DOI] [PubMed] [Google Scholar]
- 37. Blumer R, Maurer-Gesek B, Gesslbauer B, et al.. Palisade endings are a constant feature in the extraocular muscles of frontal-eyed, but not lateral-eyed, animals. Invest Ophthalmol Vis Sci . 2016; 57(2): 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bignami A, Dahl D.. Early appearance of desmin, the muscle-type intermediate filament protein, in the rat embryo. J Histochem Cytochem . 1984; 32(5): 473–476. [DOI] [PubMed] [Google Scholar]
- 39. Franke WW, Schmid E, Osborn M, Weber K.. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci USA . 1978; 75(10): 5034–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ursitti JA, Lee PC, Resneck WG, et al.. Cloning and characterization of cytokeratins 8 and 19 in adult rat striated muscle. Interaction with the dystrophin glycoprotein complex. J Biol Chem . 2004; 279(40): 41830–41838. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez M, Liu J, Parkkonen K, Li Z, Pedrosa Domellöf F. The cytoskeleton in the extraocular muscles of desmin knockout mice. Invest Ophthalmol Vis Sci . 2018; 59(12): 4847–4855. [DOI] [PubMed] [Google Scholar]
- 42. Schiaffino S, Reggiani C.. Fiber types in mammalian skeletal muscles. Physiol Rev . 2011; 91(4): 1447–1531. [DOI] [PubMed] [Google Scholar]
- 43. Liu JX, Perdrosa Domellöf F. A novel type of multiterminal motor endplate in human extraocular muscles. Invest Ophthalmol Vis Sci . 2018; 59(1): 539–548. [DOI] [PubMed] [Google Scholar]
- 44. Sartore S, Mascarello F, Rowlerson A, et al.. Fibre types in extraocular muscles: a new myosin isoform in the fast fibres. J Muscle Res Cell Motil . 1987; 8(2): 161–172. [DOI] [PubMed] [Google Scholar]
- 45. Mascarello F, Toniolo L, Cancellara P, Reggiani C, Maccatrozzo L.. Expression and identification of 10 sarcomeric MyHC isoforms in human skeletal muscles of different embryological origin. Diversity and similarity in mammalian species. Ann Anat . 2016; 207: 9–20. [DOI] [PubMed] [Google Scholar]
- 46. Rubinstein NA, Hoh JF.. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci . 2000; 41(11): 3391–3398. [PubMed] [Google Scholar]
- 47. Rubinstein NA, Porter JD, Hoh JF.. The development of longitudinal variation of Myosin isoforms in the orbital fibers of extraocular muscles of rats. Invest Ophthalmol Vis Sci . 2004; 45(9): 3067–3072. [DOI] [PubMed] [Google Scholar]
- 48. Briggs MM, Schachat F.. The superfast extraocular myosin (MYH13) is localized to the innervation zone in both the global and orbital layers of rabbit extraocular muscle. J Exp Biol . 2002; 205(Pt 20): 3133–3142. [DOI] [PubMed] [Google Scholar]
- 49. Lucas CA, Rhee HSM, Hoh JFY.. Changes in myosin heavy chain isoforms along the length of orbital fibers in rabbit extraocular muscle. Invest Ophthalmol Vis Sci . 2018; 59(3): 1178–1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.