Abstract
Purpose
To evaluate vortex vein engorgement and choroidal vascular hyperpermeability in patients with polypoidal choroidal vasculopathy (PCV) using ultra-widefield indocyanine green angiography (ICGA).
Methods
This retrospective case control study included 51 patients with unilateral PCV, 7 patients with bilateral PCV, and 43 age-matched controls. The number of quadrants of vortex vein engorgement was evaluated in the middle phase of ICGA, which was classified as extended engorgement if the dilated choroidal vessels expanded to the macula. The area of choroidal vascular hyperpermeability was quantified stereographically from the late-phase ICGA and correlated with clinical and optical coherence tomography findings.
Results
Affected eyes had a larger choroidal hyperpermeability area and a thicker subfoveal choroid than eyes in the control group or fellow eyes (P < 0.001, P < 0.001). More quadrants with extended vortex vein engorgement were observed in affected eyes than in fellow eyes (P < 0.001). Significant differences were observed in the area of choroidal hyperpermeability, Haller layer thickness and greatest linear dimension according to the extended vortex vein engorgement in eyes with PCV (P < 0.001, P = 0.001, and P = 0.001, respectively). The area of choroidal hyperpermeability was significantly correlated with subfoveal choroidal thickness (P < 0.001, Pearson's correlation coefficient = 0.471).
Conclusions
Ultra-widefield ICGA results revealed that patients with PCV had vortex vein engorgement and an increased choroidal hyperpermeability area. The results from this study provide substantial information to clarify the pathogenesis and predict the prognosis in the patients with PCV.
Keywords: choroidal hyperpermeability, choroid, choroidal vasculature, polypoidal choroidal vasculopathy, ultra-widefield
Polypoidal choroidal vasculopathy (PCV), a subtype of exudative AMD, is characterized by a branching vascular network and polypoidal lesions that are detectable with indocyanine green angiography (ICGA).1 Choroidal vascular hyperpermeability is seen as multifocal hyperfluorescence in middle and late-phase ICGA. The frequency of choroidal vascular hyperpermeability in patients with PCV was reported from 34.7% to 59.3%, which suggests that choroidal vascular abnormalities may be involved in the pathogenesis of this disease.2,3 Using ICGA and a montage imaging technique, a previous study demonstrated that engorgement of the vortex vein is correlated with the presence of choroidal hyperpermeability in eyes with PCV.4
The pathogenesis of PCV remains unclear. However, recent studies have provided new insights into the pathogenesis of PCV, and imaging studies using optical coherence tomography (OCT) have suggested that PCV belongs to a spectrum of conditions characterized by a pachychoroid, which seems to be critical to its pathogenesis.5,6 The pachychoroid disease spectrum is considered as a group of conditions characterized by a thick choroid and retinal pigment epithelial changes, with or without corresponding retinal abnormalities. The term pachychoroid thus implies choroidal congestion and choroidal hyperpermeability manifested as choroidal thickening, dilated choroidal vessels, and other characteristic findings on ICGA. Several studies have also identified additional qualitative features including focal choroidal thickening, which is localized to the disease focus and attributable to pathologically dilated Haller layer veins (pachyvessels).7
Given the increasing use of ultra-widefield (UWF) ICGA to assess various retinal diseases, a thorough understanding of the appearance of the peripheral choroidal circulation and choroidal vascular changes is important. To our knowledge, quantitative assessment of choroidal vascular abnormalities using UWF ICGA remains limited.
Therefore, we investigated choroidal vascular abnormalities and features of posterior and peripheral retina in patients with PCV using UWF ICGA and analyzed the relationships between clinical findings and known prognostic factors.
Methods
Patients and Study Population
We retrospectively reviewed the medical records of patients with PCV and age-matched controls, from November 2018 to October 2019. This study was approved by the Internal Review Board of Yeungnam University Medical Center. Informed consent was obtained from all patients, and the study adhered to the tenets of the Declaration of Helsinki.
Inclusion criteria were subjects who were diagnosed with treatment-naïve PCV with subfoveal involvement at the initial visit. Contralateral eyes of epiretinal membrane patients or bilateral eyes of posterior vitreous detachment patients who wanted comprehensive ophthalmic examinations without other systemic diseases were included as an age-matched control group. Exclusion criteria included high myopia or hyperopia (greater than –6 or +3 diopters of refractive error), poor image quality, a history of anti-VEGF or photodynamic treatment, any other associated retinal pathology that may have irreversibly compromised or could likely compromise the visual acuity, or a history of any intraocular surgery.
All participants underwent comprehensive ophthalmic examinations including best-corrected visual acuity testing, slit-lamp biomicroscopy and ophthalmoscopic examination, including color fundus photography and spectral-domain OCT. All participants also underwent UWF fluorescein angiography and ICGA using an Optos California (Optos PLC, Dunfermline, UK) system. Spectral-domain OCT images were obtained with a Heidelberg Spectralis (Spectralis; Heidelberg Engineering, Heidelberg, Germany) system. Horizontal 6-mm line scans with a signal strength of 7 or more were used for the analysis. Subfoveal choroidal thickness, central retinal thickness, and pigmented epithelial detachment were calculated using the calipers tool provided in the built-in software. Haller's layer was defined as the layer containing the large choroidal vessels, followed by Sattler's layer with medium choroidal vessels. A major criterion to differentiate between Sattler's layer and Haller's layer is the hyperintense stroma caused by increased scattering by high density of melanocytes. The remaining choroidal vessels on the inner side of Sattler's layer consists of small vessels, including the choriocapillaris.
UWF Image Acquisition and Quantification
The eyes of subjects were fully dilated and UWF fluorescein angiography and ICGA images were acquired. Simultaneous UWF fluorescein angiography and ICGA were performed after an intravenous injection of 5 mL of 10% sodium fluorescein and 25 mg of indocyanine green. The images were taken during the early (up to 2–3 minutes), middle (5–10 minutes after injection), and late (10–15 minutes after injection) phases of the angiogram including one or more vortex veins per quadrant to minimize the influence of the change in the brightness of the fluorescence caused by the tilt of the eye. They were transformed into stereographic projection images using prototype software from the manufacturer. The presence of vortex vein engorgement was determined by adjusting the brightness using imageJ software (National Institutes of Health, Bethesda, MD, USA; available at http://rsb.info.nih.gov/ij/index.html) in the middle phase image of UWF ICGA. The brightness of images was adjusted to the darkest and gradually brightened until the medium sized choroidal vessels in the intervortex space were visible (Supplementary Fig. S1). While gradually increasing the brightness, the vortex vein, which is brighter than the medium sized choroidal vessels in the intervortex space, was defined as engorged. Vortex vein engorgement was determined as present or not in each quadrant.4,8 The basis for this was that venous blood in each quadrant drains into its own vortex vein.4,8 Two trained retinal specialists (MS, AJ) counting the number of quadrants with vortex vein engorgement were masked with respect to the patient's clinical data. If the engorged vortex vein expanded to macular or optic disc areas, it was additionally defined as extended vortex vein engorgement (Fig. 1).
Figure 1.
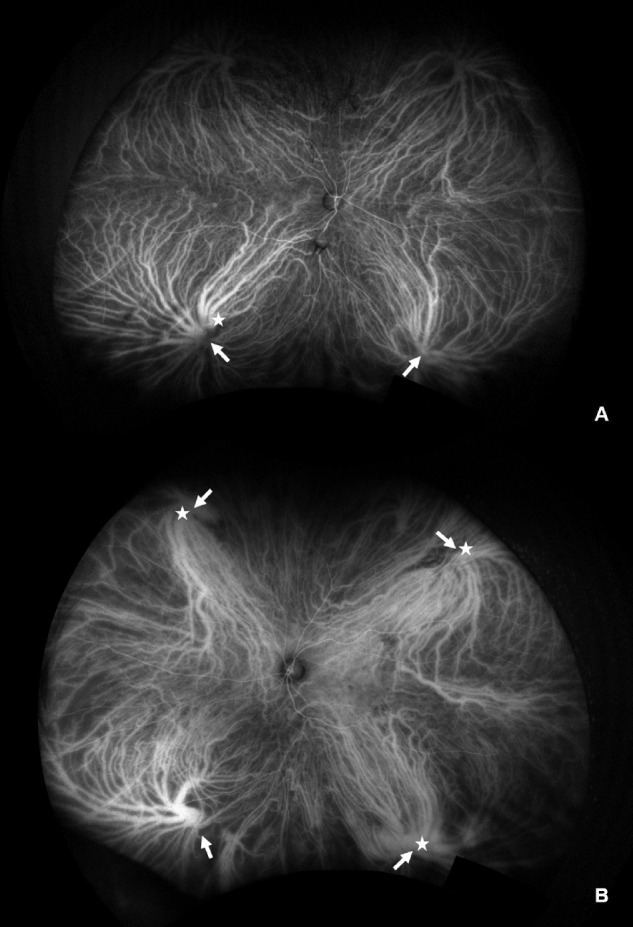
Representative images to count the engorged vortex veins. (A) A 61-year-old man with normal eye. There were two engorged vortex veins (arrows) and one of them expanded to the macula (star). (B) A 72-year-old man with polypoidal choroidal vasculopathy. There were four engorged vortex veins (arrows), only three of which expanded to the macula (stars).
The area of choroidal hyperpermeability defined as the region in which the contour of the choroidal vessels was not clearly observed with fluorescence brighter than the vortex vein ampulla in the posterior pole from the late phase image of ICGA. Two masked trained retinal specialists (MS, AJ) manually demarcated the outline of the hyperpermeable area using ImageJ software (Fig. 2). A recent version of the UWF software enables a region of interest to be automatically calculated as its real size (mm2) by correcting for nonlinear distortion. With these software tools, the size of a pixel can be defined by its location in the image and calculated using spherical trigonometry after it is projected back onto a sphere.9,10 Intergrader agreement was high for all annotations. For the number of quadrants with total and extended vortex vein engorgement, the kappa values were 0.90 and 0.93, respectively. For the area of choroidal hyperpermeability, intergrader agreement was high with an intraclass correlation coefficient of 0.869.
Figure 2.
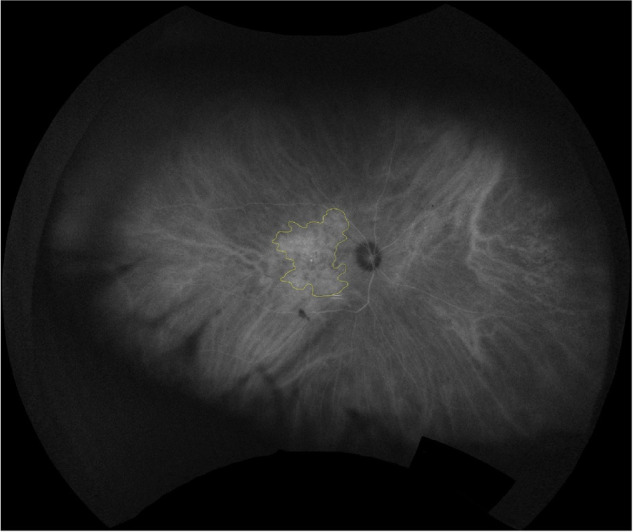
Area of choroidal hyperpermeability. Late phase indocyanine green angiography (ICGA) shows choroidal hyperpermeability area with multifocal hyperfluorescence. The outline of the hyperpermeable area after stereographic projection is demarcated.
Statistical Analysis
Statistical analysis was performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). The Mann–Whitney U test and Fisher's exact test were used to compare PCV and control groups. The Wilcoxon signed rank test and Fisher's exact test were applied for a comparison of the affected eye and unaffected fellow eye. The Kruskal–Wallis test was used to evaluate the number of quarters with extended vortex vein engorgement and associated factors such as best-corrected visual acuity, area of choroidal hyperpermeability, subfoveal choroidal thickness, central retinal thickness, greatest linear dimension, polyp number, and largest pigmented epithelial detachment height. Univariate and multivariate linear regression analyses were carried out to determine associations between the area of choroidal hyperpermeability and various ocular factors. Pearson's correlation analysis was applied to correlate the area of choroidal hyperpermeability and subfoveal choroidal thickness. All P values of less than 0.05 were considered statistically significant.
Results
Baseline Characteristics
In total, 65 eyes of 58 Korean patients with PCV and 43 eyes of an age-matched control were included in this study. The mean age was 68.3 ± 9.2 years in the PCV group and 64.7 ± 10.8 years in the control group (P = 0.061). The distributions of affected eyes were 32 (49.2%) in the right eye and 33 (50.8%) in the left eye in the PCV group, and 18 (41.9%) in the right eye and 25 (58.1%) in the left eye in the control group (P = 0.452). The spherical equivalent was +0.28 ± 1.60 diopter in the PCV group and +0.24 ± 0.97 diopter in the control group (P = 0.564). The mean subfoveal choroidal thickness was significantly thicker in the PCV group than the control group (428.22 ± 71.08 vs. 253.35 ± 60.53 µm; P < 0.001). The mean central retinal thickness was significantly thicker in the PCV group than in the control group (354.77 ± 129.44 vs. 247.16 ± 60.80 µm; P < 0.001). All 65 PCV eyes showed various degrees of hypercyanescence caused by choroidal hyperpermeability in the posterior pole. The area of choroidal hyperpermeability was 20.97 ± 13.91 mm2 in the PCV group and 7.40 ± 6.72 mm2 in the control group, and the difference between the two groups was significant (P < 0.001). All PCV eyes exhibited engorgement of the vortex vein in two or more quadrants on ICGA, whereas such engorgement was detected in only 25 eyes (58%), in only one quadrant, among the 43 eyes in the age-matched control group (P < 0.001). Additionally, all PCV eyes had one or more extended engorged vortex veins, whereas only nine of the control eyes (21%) had an extended engorged vortex vein in only one quadrant (P < 0.001) (Table 1).
Table 1.
Demographic and Baseline Characteristics of Patients With Polypoidal Choroidal Vasculopathy and Normal Controls
| Baseline Patients Demographics | |||
|---|---|---|---|
| Polypoidal Choroidal Vasculopathy | Control | ||
| Parameters | (n = 58 Patients, 65 Eyes) | (n = 43 Patients, 43 Eyes) | P Value |
| Age (years) | 68.32 ± 9.20 | 64.65 ± 10.78 | 0.061* |
| Male/female | 44 (67.7%)/21 (32.3%) | 22 (51.2%)/21 (48.8%) | 0.085† |
| Affected eye (OD/OS) | 32 (49.2%)/33 (50.8%) | 18 (41.9%)/25 (58.1%) | 0.452† |
| Spherical equivalent (diopter) | 0.28 ± 1.60 | 0.24 ± 0.97 | 0.564 |
| Subfoveal choroidal thickness (µm) | 428.22 ± 71.08 | 253.35 ± 60.53 | <0.001* |
| Central retinal thickness (µm) | 354.77 ± 129.44 | 247.16 ± 60.80 | <0.001* |
| Area of choroidal hyperpermeability (mm2) | 20.97 ± 13.91 | 7.40 ± 6.72 | <0.001* |
| Area of choroidal hyperpermeability of fellow eye (mm2) | 9.65 ± 6.64 | ||
| Greatest linear dimension (µm) | 2686 ± 906 | ||
| Polyp number | 1.65 ± 0.74 | ||
| Quadrants with one or more engorged vortex veins | <0.001† | ||
| 0 quadrants | 0 | 18 (42%) | |
| 1 quadrants | 0 | 25 (58%) | |
| 2 quadrants | 7 (11%) | 0 (0%) | |
| 3 quadrants | 37 (57%) | 0 (0%) | |
| 4 quadrants | 21 (32%) | 0 (0%) | |
| Quadrants with one or more extended engorged vortex vein | <0.001† | ||
| 0 quadrants | 0 (0%) | 34 (79%) | |
| 1 quadrants | 11 (17%) | 9 (21%) | |
| 2 quadrants | 44 (68%) | 0 (0%) | |
| 3 quadrants | 10 (15%) | 0 (0%) | |
| 4 quadrants | 0 (0%) | 0 (0%) | |
Values are presented as mean ± SD unless indicated otherwise.
Student t-test.
χ2 test.
Comparison Between Affected and Unaffected Eyes in Unilateral PCV
In a comparison of affected and unaffected fellow eyes from the 51 patients with unilateral PCV, affected eyes had a significantly larger area of choroidal hyperpermeability than unaffected fellow eyes (21.10 ± 14.03 vs. 9.75 ± 4.80 mm2; P < 0.001). Additionally, subfoveal choroidal thickness was significantly thicker in affected eyes than unaffected fellow eyes, especially in the Haller layer, whereas choriocapillaris with the Sattler layer did not differ significantly between both eyes (subfoveal choroidal thickness, 429.69 ± 66.37 vs. 387.31 ± 58.59 µm [P < 0.001]; Haller layer, 328.02 ± 51.21 vs. 299.69 ± 57.28 µm [P < 0.001]; choriocapillaris with Sattler layer, 101.67 ± 44.20 vs. 87.63 ± 32.10 µm [P = 0.062]). No significant difference was observed in the number of quadrants with engorged vortex veins between both eyes; the concordance between them was 84% (P = 0.132). In contrast, there was a significant difference in the number of quadrants with extended engorged vortex veins between affected and unaffected eyes (P < 0.001) (Table 2).
Table 2.
Indocyanine Green Angiographic Features and Subfoveal Choroidal Thickness of the Affected Eyes and Unaffected Fellow Eyes
| 51 Patients | |||
|---|---|---|---|
| Parameter | Affected Eye | Unaffected Fellow Eye | P Value |
| Area of choroidal hyperpermeability (mm2) | 21.10 ± 14.03 | 9.75 ± 4.80 | <0.001* |
| Subfoveal choroidal thickness (µm) | 429.69 ± 66.37 | 387.31 ± 58.59 | <0.001* |
| Choriocapillary with Sattler layer | 101.67 ± 44.20 | 87.63 ± 32.10 | 0.062* |
| Haller layer | 328.02 ± 51.21 | 299.69 ± 57.28 | <0.001* |
| Engorged vortex vein | 0.132† | ||
| 1 quadrant | 0 (0%) | 2 (3.9%) | |
| 2 quadrants | 5 (9.8%) | 5 (9.8%) | |
| 3 quadrants | 28 (54.9%) | 28 (54.9%) | |
| 4 quadrants | 18 (35.3%) | 16 (31.4%) | |
| Extended engorged vortex vein | <0.001† | ||
| None | 0 (0%) | 2 (3.9%) | |
| 1 quadrant | 8 (15.7%) | 27 (52.9%) | |
| 2 quadrants | 35 (68.6%) | 22 (43.1%) | |
| 3 quadrants | 8 (15.7%) | 0 (0%) | |
| 4 quadrants | 0 (0%) | 0 (0%) | |
Values are presented as mean ± SD unless indicated otherwise.
Paired t-test.
χ2 test.
Extended Engorged Vortex Vein and Associated Factors
The number of extended engorged vortex veins did not differ significantly with regard to visual acuity, central retinal thickness, polyp number, or largest pigmented epithelial detachment height (P = 0.095, P = 0.057, P = 0.877, and P = 0.300, respectively). However, the Haller layer thickness differed significantly with regard to the number of extended engorged vortex veins (subfoveal choroidal thickness, P = 0.815; choriocapillaris with Sattler layer, P = 0.039; Haller layer, P = 0.001). Moreover, the area of choroidal hyperpermeability and greatest linear dimension differed significantly according to the number of quadrants with extended engorged vortex veins (P < 0.001 and P = 0.001, respectively) (Table 3).
Table 3.
Changes of Several Factors According to the Number of Extended Engorged Vortex Vein
| Extended Engorged Vortex Vein | |||||
|---|---|---|---|---|---|
| 1 Quadrant | 2 Quadrants | 3 Quadrants | 4 Quadrants | ||
| Variable Parameter | (n = 11) | (n = 44) | (n = 10) | (n = 0) | P Value |
| BCVA (logMAR) | 0.97 ± 0.50 | 0.36 ± 0.53 | 0.70 ± 0.48 | N/A | 0.095 |
| Area of choroidal hyperpermeability (mm2) | 14.18 ± 6.51 | 19.23 ± 12.81 | 36.10 ± 14.84 | N/A | <0.001* |
| Subfoveal choroidal thickness (µm) | 419.55 ± 107.60 | 432.16 ± 60.89 | 420.40 ± 71.50 | N/A | 0.815 |
| Choriocapillary with Sattler layer | 144.09 ± 74.60 | 96.93 ± 38.95 | 74.90 ± 34.52 | N/A | 0.039 |
| Haller layer | 275.45 ± 54.17 | 335.23 ± 44.33 | 345.50 ± 51.56 | N/A | 0.001* |
| Central retinal thickness (µm) | 286.55 ± 100.94 | 381.45 ± 139.63 | 312.40 ± 59.20 | N/A | 0.057 |
| Greatest linear dimension (µm) | 1893 ± 551 | 2755 ± 837 | 3255 ± 998 | N/A | 0.001* |
| Polyp number | 1.73 ± 0.79 | 1.61 ± 0.72 | 1.70 ± 0.82 | N/A | 0.877 |
| Largest PED height (µm) | 202.36 ± 126.09 | 293.11 ± 192.31 | 246.10 ± 161.67 | N/A | 0.300 |
Values are presented as mean ± SD unless indicated otherwise.
BCVA = best-corrected visual acuity; logMAR = logarithm of the minimal angle of resolution; PED = pigment epithelial detachment
One-way analysis of variance.
Area of choroidal hyperpermeability: 1 Q vs 2 Q : 0.691, 1 Q vs 3 Q : <0.001†, 2 Q vs 3 Q : 0.001†.
Choriocapillary with Sattler layer : 1 Q vs 2 Q : <0.010,† 1 Q vs 3 Q : 0.003,† 2 Q vs 3 Q : 0.532.
Haller layer : 1 Q vs 2 Q : 0.001,† 1 Q vs 3 Q : 0.004,† 2 Q vs 3 Q : 0.913.
Greatest linear dimension: 1 Q vs 2 Q : <0.009,† 1 Q vs 3 Q : 0.001,† 2 Q vs 3 Q : 0.266.
Bonferroni-corrected post hoc Mann–Whitney tests for between group comparison (P < 0.017 significant).
Area of Choroidal Hyperpermeability and Associated Factors
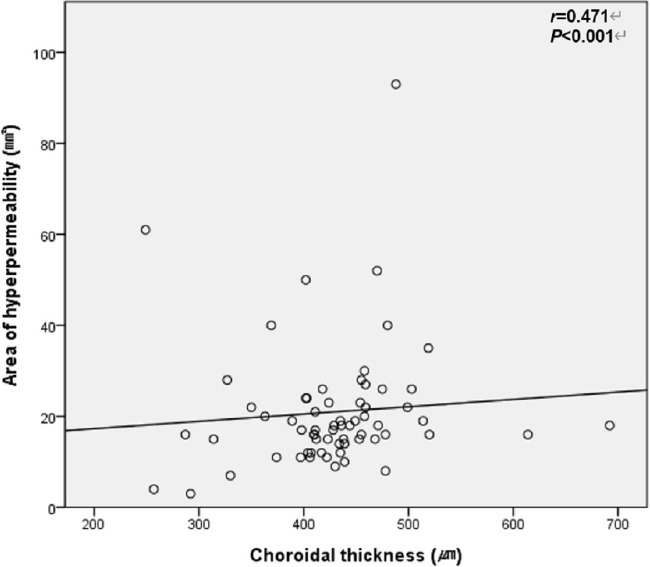
Univariate linear regression revealed that the area of choroidal hyperpermeability was significantly correlated with the number of extended engorged vortex veins and greatest linear dimension (P < 0.001 and P = 0.002, respectively). Multivariate linear regression demonstrated that the area of choroidal hyperpermeability was significantly correlated with the number of quadrants with extended engorged vortex veins, but was not correlated with greatest linear dimension (P < 0.001 and P = 0.823, respectively) (Table 4). In the Pearson correlation analysis, the area of choroidal hyperpermeability was significantly correlated with subfoveal choroidal thickness in the PCV group (correlation coefficient = 0.471; P < 0.001) (Fig. 3) and control group (correlation coefficient = 0.391; P = 0.010) (Fig. 4).
Table 4.
Linear Regression Analysis of Choroidal Hyperpermeability Area
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factors | B | P Value | B | P Value |
| Age (years) | 0.107 | 0.577 | ||
| Spherical equivalent (Diopter) | 0.439 | 0.690 | ||
| Intraocular pressure (mmHg) | −2.052 | 0.229 | ||
| BCVA (LogMAR) | 3.797 | 0.248 | ||
| Extended Engorged Vortex Vein | 10.768 | <0.001* | 10.768 | <0.001* |
| Subfoveal Choroidal Thickness (µm) | −0.015 | 0.549 | ||
| Choriocapillary with Sattler Layer | −0.063 | 0.073 | ||
| Haller Layer | 0.029 | 0.381 | ||
| Central retinal thickness (µm) | −0.001 | 0.965 | ||
| Greatest linear dimension (µm) | 0.003 | 0.002* | −0.028 | 0.823 |
| Polyp number | 0.295 | 0.901 | ||
| Largest PED height (µm) | 0.008 | 0.421 | ||
BCVA = Best corrected visual acuity; logMAR = logarithm of the minimal angle of resolution; PED = pigment epithelial detachment.
Linear regression.
Figure 3.
Correlation between choroidal thickness and area of choroidal hyperpermeability in patients with PCV. The Pearson correlation analysis was used (P < 0.05 significant; r = Pearson correlation coefficient).
Figure 4.
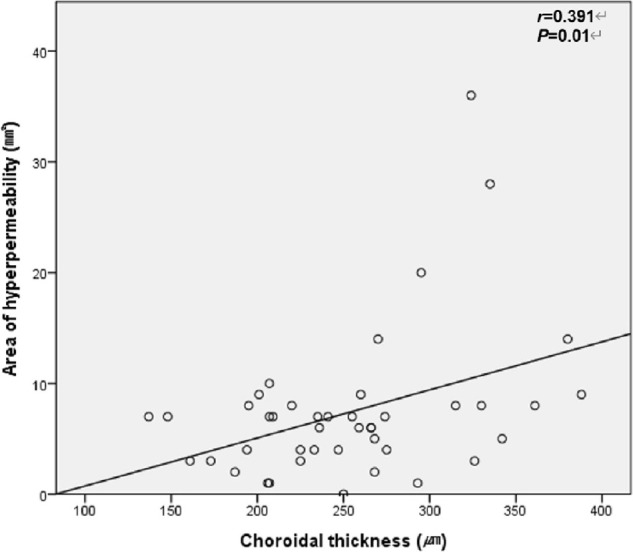
Correlation between choroidal thickness and area of choroidal hyperpermeability in Normal patients. The Pearson correlation analysis was used (P < 0.05 significant; r = Pearson correlation coefficient).
Discussion
The results of the current study revealed that all PCV eyes had more engorged vortex vein, more extended engorged vortex vein, and a larger area of choroidal vascular hyperpermeability compared with the age-matched control group. In patients with unilateral PCV, the number of extended engorged vortex veins was significantly greater in the affected eyes than in the unaffected fellow eyes, even if there was no difference in that of engorged vortex veins between them. Also, affected eyes had larger area of choroidal hyperpermeability. The number of quadrants with extended engorged vortex veins was correlated with the area of choroidal hyperpermeability.
In the present study, vortex vein engorgement was observed more frequently in eyes with PCV compared with the control group. Vortex vein engorgement in the fellow eye of unilateral PCV was associated with engorgement of the affected eye, and there was a concordance between the two eyes. Interestingly, in the patients with unilateral PCV, the affected eyes had the engorged vortex veins extending to the macula more commonly than the unaffected eyes. Binocular concordance of vortex vein engorgement in patients with unilateral PCV suggests that outflow changes through vortex veins may be included as a predisposing factor in the etiology in these patients. The previous study has proposed that multiple choroidal veins converging on the ampulla of vortex veins may buffer the ocular pulse pressure like superior sagittal sinus buffering intracranial pressure increases in the brain.11 Because the choroidal vessel dilation in the posterior pole is far from the ampulla of vortex veins, it is difficult to expect that the dilated veins in the posterior pole modulates the pressure through the vortex vein outflow. Consequently, the regional venous changes including abnormally increased pressure on the choriocapillaris may help explain the observed vortex vein engorgement extending to the posterior pole in the affected eyes of patients with unilateral PCV.
It is widely known that choroidal vascular hyperpermeability has been observed more frequently in eyes with PCV than in eyes with typical exudative AMD. However, the prevalence of choroidal vascular hyperpermeability varies widely in eyes with either PCV (9.8%–50.0%) or typical exudative AMD (1.9%–37.5%).2,3,12–19 This variation may arise from an evaluation of choroidal vascular hyperpermeability based on qualitative image analysis. To our knowledge, this study is the first to analyze quantitatively the area of choroidal vascular hyperpermeability from UWF ICGA in patients with PCV, in an attempt to gain insights into its pathogenesis.
It is generally accepted that the presence of choroidal vascular hyperpermeability is associated with pathologic conditions. Choroidal vascular hyperpermeability has been reported to remain even after fluorescein leakage has resolved in eyes with active central serous chorioretinopathy.20 Additionally, choroidal thickening associated with vortex vein congestion has been observed in PCV eyes.4 Previous studies have also reported multifocal choroidal hyperpermeability and dilatation of choroidal veins have been observed in late-phase ICGA of eyes with PCV.3,21 One study reported multifocal choroidal hyperpermeability in 12 of 122 eyes (9.8%) with PCV; choroidal venous dilation was detected on ICGA in all 12 eyes.3 Together, these findings support the hypothesis that choroidal changes may be a primary etiology in these disease entities. Choroidal hyperpermeability and dilated choroidal vessels indicate a pathophysiologic disturbance in choroidal circulation. This disturbance is believed to result from hypertension of choroidal circulation, which increases extravasation of fluid and protein-bound indocyanine green from the choriocapillaris or large choroidal vessels into the surrounding choroid.
We also analyzed the association between the area of choroidal hyperpermeability and vortex vein engorgement. The area of choroidal hyperpermeability was significantly correlated with the number of quadrants with extended engorged vortex veins in eyes with PCV. The number of quadrants with engorged vortex veins and extended engorged vortex veins differed significantly between PCV and normal control groups. The larger area of choroidal hyperpermeability in eyes with PCV likely results from choroidal vessel dilation, with blood outflow congestion as a potential contributor to the pathogenesis of PCV. Elevated hydrostatic pressure resulting from choroidal hyperpermeability affects the increased extravascular volume within the choroid and vortex vein engorgement in PCV. Additionally, the elevated hydrostatic pressure may also correlate with dilated choroidal vessels and retinal pigment epithelium detachment among less adherent layers, causing retinal pigment epithelium tear or breakthrough vitreous hemorrhage in eyes with PCV.8,22–26
In our study, the mean subfoveal choroidal thickness was significantly thicker in eyes with PCV compared with normal controls. Subfoveal choroidal thickness was also increased in eyes affected with PCV compared with the fellow eye in patients with unilateral PCV. These results are similar to those of a previous study, which reported that subfoveal choroidal thickness was greater in eyes with PCV owing to choroidal congestion.27,28 This increase in choroidal thickness was considered to be associated with increased ocular perfusion pressure and engorgement of the vortex vein.29 However, one cohort study including more than 300 eyes with PCV found that the distribution of the mean subfoveal choroidal thickness had bimodal peaks at 170 and 390 µm. In these eyes, pachyvessels and related choroidal changes were associated topographically with sites of branching vascular network ingrowth, which suggests that pachychoroid features underlie the pathogenesis of PCV lesions, even in eyes with normal or subnormal choroid thickness.7,30–32 Although choroidal changes are possibly involved in the pathogenesis of PCV, it remains unclear whether PCV with varying choroidal thickness has similar clinical characteristics and progression.33–38 In the current study, the Haller layer thickness differed significantly according to the number of extended engorged vortex veins. Although the changes in the choroid may be focal in PCV eyes with a thin/subnormal choroid, most of the eyes with a thickened subfoveal choroid showed global dilatation of Haller vessels.30 Previous studies have suggested the hypothesis that a Haller layer with dilated vessels and thinning of the inner choroid vessel may be relevant to the mechanism of the pachychoroid disease spectrum, including PCV.30,39–41 Moreover, we found that, as the number of extended engorged vortex veins increased, the thickness of choriocapillaris with a Sattler layer tended to decrease. Loss of the choriocapillaris may induce a relatively ischemic condition, leading to overexpression of angiogenic factors and expansion of the Haller vessel volume. Additionally, the decreased buffer effect of choriocapillaris may produce damage to overlying tissues, contributing to retinal pigment epithelium changes or a focal break in Bruch's membrane. Therefore, the extended engorged vortex vein seems to be more involved in the etiology.
Our study had several limitations. First, our sample size was relatively small, and it was taken from a single institution. Further studies with a larger sample size are needed to validate our findings and explore the implications of choroidal vascular hyperpermeability in patients with PCV. Second, we performed a manual analysis of choroidal thickness from a single scan of the subfoveal area; this may not be representative of the entire choroidal vascular structure. Third, we used subjective methods to demarcate the area of choroidal hyperpermeability and count the quadrants with engorged vortex veins. We attempted to overcome this limitation by using independent observers who were masked to the disease status of eyes and other patient information and found that the strength of agreement was relatively good.
In conclusion, patients with PCV have engorged vortex veins and an increased area of choroidal hyperpermeability on the macula compared with normal controls. In patients with unilateral PCV, both eyes had a higher number of quadrants with engorged vortex veins, but the number of quadrants with extended engorged vortex veins was observed more often only in the affected eye with PCV. Also, the number of quadrants with extended engorged vortex veins was correlated with the choroidal hyperpermeability area, suggesting outflow congestion as a potential contributor to the pathogenesis of PCV. These findings suggest that extended engorged vortex veins contribute to PCV development, because they lead to the actual expansion of the choroidal hyperpermeability area. Further longitudinal studies are required to reveal associations between the extended engorged vortex vein and the prognosis or treatment response of PCV.
Supplementary Material
Acknowledgments
Supported by a 2018 Yeungnam University Research Grant. This funding source had no role in the study design, collection, analysis, interpretation of data, or writing of the manuscript.
Disclosure: A. Jeong, None; J. Lim, None; M. Sagong, None
References
- 1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B.. Idiopathic polypoidal choroidal vasculopathy (IPCV). 1990. Retina. 2012; 32(Suppl 1): 1–8. [PubMed] [Google Scholar]
- 2. Jirarattanasopa P, Ooto S, Nakata I, et al.. Choroidal thickness, vascular hyperpermeability, and complement factor H in age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2012; 53(7): 3663–3672. [DOI] [PubMed] [Google Scholar]
- 3. Sasahara M, Tsujikawa A, Musashi K, et al.. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006; 142(4): 601–607. [DOI] [PubMed] [Google Scholar]
- 4. Chung SE, Kang SW, Kim JH, Kim YT, Park DY.. Engorgement of vortex vein and polypoidal choroidal vasculopathy. Retina. 2013; 33(4): 834–840. [DOI] [PubMed] [Google Scholar]
- 5. Warrow DJ, Hoang QV, Freund KB.. Pachychoroid pigment epitheliopathy. Retina. 2013; 33(8): 1659–1972. [DOI] [PubMed] [Google Scholar]
- 6. Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015; 35(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 7. Dansingani KK, Balaratnasingam C, Naysan J, Freund KB.. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 2016; 36(3): 499–516. [DOI] [PubMed] [Google Scholar]
- 8. Pang CE, Shah VP, Sarraf D, Freund KB.. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol. 2014; 158(2): 362–371.e2. [DOI] [PubMed] [Google Scholar]
- 9. DICOM Standards Committee. Digital Imaging and Communications in Medicine (DICOM) Supplement 173: Wide Field Ophthalmic Photography Image Storage SOP Classes, 2015. Available at https://www.dicomstandard.org/News/progress/docs/sups/sup173.pdf. Accessed XXXX. [Google Scholar]
- 10. Sagong M, van Hemert J, Olmos de Koo LC, Barnett C, Sadda SR. Assessment of accuracy and precision of quantification of ultra-widefield images. Ophthalmology. 2015; 122(4): 864–866. [DOI] [PubMed] [Google Scholar]
- 11. Spaide RF. Choroidal blood flow: review and potential explanation for the choroidal venous anatomy including the vortex vein system. Retina. 2020; 40(10): 1851–1864. [DOI] [PubMed] [Google Scholar]
- 12. McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA.. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009; 50(10): 4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu J, Leng X, Hu Y, et al.. The features of inflammation factors concentrations in aqueous humor of polypoidal choroidal vasculopathy. PLoS One. 2016; 11(1): e0147346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yanagisawa S, Sakurada Y, Miki A, Matsumiya W, Imoto I, Honda S.. The association of elastin gene variants with two angiographic subtypes of polypoidal choroidal vasculopathy. PLoS One. 2015; 10(3): e0120643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar S, Nakashizuka H, Jones A, et al.. Proteolytic degradation and inflammation play critical roles in polypoidal choroidal vasculopathy. Am J Pathol. 2017; 187(12): 2841–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho HJ, Kim HS, Jang YS, et al.. Effects of choroidal vascular hyperpermeability on anti–vascular endothelial growth factor treatment for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013; 156(6): 1192–1200.e1. [DOI] [PubMed] [Google Scholar]
- 17. Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S.. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013; 155(2): 305–313.e1. [DOI] [PubMed] [Google Scholar]
- 18. Miyake M, Tsujikawa A, Yamashiro K, et al.. Choroidal neovascularization in eyes with choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014; 55(5): 3223–3230. [DOI] [PubMed] [Google Scholar]
- 19. Kim JH, Chang YS, Lee TG, Kim CG.. Choroidal vascular hyperpermeability and punctate hyperfluorescent spot in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015; 56(3): 1909–1915. [DOI] [PubMed] [Google Scholar]
- 20. Iida T, Kishi S, Hagimura N, Shimizu K.. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999; 19(6): 508–512. [DOI] [PubMed] [Google Scholar]
- 21. Koizumi H, Yamagishi T, Yamazaki T, Kawasaki R, Kinoshita S.. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2011; 249(8): 1123–1128. [DOI] [PubMed] [Google Scholar]
- 22. Nakashizuka H, Mitsumata M, Okisaka S, et al.. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(11): 4729–4737. [DOI] [PubMed] [Google Scholar]
- 23. Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N.. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002; 86(10): 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takahashi A, Ooto S, Yamashiro K, et al.. Pachychoroid geographic atrophy: clinical and genetic characteristics. Ophthalmol Retina. 2018; 2(4): 295–305. [DOI] [PubMed] [Google Scholar]
- 25. Baek J, Lee JH, Jung BJ, Kook L, Lee WK.. Morphologic features of large choroidal vessel layer: age-related macular degeneration, polypoidal choroidal vasculopathy, and central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2018; 256(12): 2309–2317. [DOI] [PubMed] [Google Scholar]
- 26. Sonoda S, Sakamoto T, Otsuka H, et al.. Responsiveness of eyes with polypoidal choroidal vasculopathy with choroidal hyperpermeability to intravitreal ranibizumab. BMC Ophthalmol. 2013; 13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao XY, Xia S, Luo MY, Wang EQ, Chen YX.. The occurrence, characteristics, management, and prognosis of retinal pigment epithelium tears in patients with polypoidal choroidal vasculopathy: a retrospective study of 397 patients. Retina. 2020; 40(3): 477–489. [DOI] [PubMed] [Google Scholar]
- 28. Chung SE, Kang SW, Lee JH, Kim YT.. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118(5): 840–845. [DOI] [PubMed] [Google Scholar]
- 29. Kim SW, Oh J, Kwon SS, Yoo J, Huh K.. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011; 31(9): 1904–1911. [DOI] [PubMed] [Google Scholar]
- 30. Rishi P, Rishi E, Mathur G, Raval V.. Ocular perfusion pressure and choroidal thickness in eyes with polypoidal choroidal vasculopathy, wet-age-related macular degeneration, and normals. Eye (Lond). 2013; 27(9): 1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee WK, Baek J, Dansingani KK, Lee JH, Freund KB.. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina. 2016; 36(Suppl 1): S73–S82. [DOI] [PubMed] [Google Scholar]
- 32. Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB.. Pachychoroid disease. Eye (Lond). 2019; 33(1): 14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sho K, Takahashi K, Yamada H, et al.. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121(10): 1392–1396. [DOI] [PubMed] [Google Scholar]
- 34. Lim TH, Laude A, Tan CS.. Polypoidal choroidal vasculopathy: an angiographic discussion. Eye (Lond). 2010; 24(3): 483–490. [DOI] [PubMed] [Google Scholar]
- 35. Goldman DR, Freund KB, McCannel CA, Sarraf D.. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: a report of 10 eyes. Retina. 2013; 33(1): 48–55. [DOI] [PubMed] [Google Scholar]
- 36. Uyama M, Wada M, Nagai Y, et al.. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002; 133(5): 639–648. [DOI] [PubMed] [Google Scholar]
- 37. Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA.. Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol. 2010; 55(6): 501–515. [DOI] [PubMed] [Google Scholar]
- 38. Al-Rashaed S. Idiopathic polypoidal choroidal vasculopathy in a young man: case report and literature review. Middle East Afr J Ophthalmol. 2008; 15(2): 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang YC, Wu WC.. Polypoidal choroidal vasculopathy in Taiwanese patients. Ophthalmic Surg Lasers Imaging. 2009; 40(6): 576–581. [DOI] [PubMed] [Google Scholar]
- 40. Byeon SH, Lee SC, Oh HS, Kim SS, Koh HJ, Kwon OW.. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008; 52(1): 57–62. [DOI] [PubMed] [Google Scholar]
- 41. Balaratnasingam C, Lee WK, Koizumi H, Dansingani K, Inoue M, Freund KB.. Polypoidal choroidal vasculopathy: a distinct disease or manifestation of many? Retina. 2016; 36(1): 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.