Abstract
Background.
Rises in the incidence of bacterial infections, such as infective endocarditis (IE), have been reported in conjunction with the opioid crisis. However, recent trends for IE and other serious infections among persons with substance use disorders (SUDs) are unknown.
Methods.
Using the Premier Healthcare Database, we identified hospitalizations from 2012 through 2017 among adults with primary discharge diagnoses of bacterial infections and secondary SUD diagnoses, using International Classification of Diseases, Clinical Modification Ninth and Tenth Revision codes. We calculated annual rates of infections with SUD diagnoses and evaluated temporal trends. Blood and cardiac tissue specimens were identified from IE hospitalizations to describe the microbiology distribution and temporal trends among hospitalizations with and without SUDs.
Results.
Among 72 481 weighted IE admissions recorded, SUD diagnoses increased from 19.9% in 2012 to 39.4% in 2017 (P < .0001). Hospitalizations with SUDs increased from 1.1 to 2.1 per 100 000 persons for IE, 1.4 to 2.4 per 100 000 persons for osteomyelitis, 0.5 to 0.9 per 100 000 persons for central nervous system abscesses, and 24.4 to 32.9 per 100 000 persons for skin and soft tissue infections. For adults aged 18–44 years, IE-SUD hospitalizations more than doubled, from 1.6 in 2012 to 3.6 in 2017 per 100 000 persons. Among all IE-SUD hospitalizations, 50.3% had a Staphylococcus aureus infection, compared with 19.4% of IE hospitalizations without SUDs.
Conclusions.
Rates of hospitalization for serious infections among persons with SUDs are increasing, driven primarily by younger age groups. The differences in the microbiology of IE hospitalizations suggest that SUDs are changing the epidemiology of these infections.
Keywords: substance use, bacterial infection, injection drug use, opioid, endocarditis
Drug overdose deaths have increased considerably across the United States, primarily driven by an epidemic of opioid-involved overdose deaths [1]. While the risks of bloodborne virus transmission are well known, people with substance use disorders (SUDs), particularly those who inject drugs, are also at increased risk for serious bacterial infections associated with significant morbidity and mortality [2–6]. For example, a recent study estimated that people who inject drugs were 16 times more likely to develop invasive methicillin-resistant Staphylococcus aureus (MRSA) infections [7]. While use of sterile injection equipment (including needles, water, cottons) is an effective means of reducing transmission of bloodborne pathogens, even sterile needles can be contaminated with skin or environmental flora during injection and cause local or systemic bacterial or fungal infections [8].
The spectrum of infections caused by SUDs range from skin and soft tissue infections (SSTIs), the most common reason for hospital admission and emergency department visits among this population [9, 10], to infective endocarditis (IE) [11]. Recent studies have reported an increase in incidence of IE among persons with SUDs, especially in younger populations [12–14]. Other serious complications of injection drug use include osteomyelitis and abscesses of the central nervous system (CNS) and adjacent structures, such as epidural abscesses [5, 6, 15].
The incidence of drug overdose deaths has increased in recent years [1]. A previous multicenter study suggested that the incidence of serious infections related to SUDs has also increased from 2009 to 2013 [6]. However, an analysis of more recent national trends has not been conducted. In this study, we describe trends in hospitalizations due to serious bacterial infections among persons with SUDs and characterize the microbiology of these infections from electronic health records.
METHODS
We conducted a retrospective cohort study using the Premier Healthcare Database (PHD), a comprehensive electronic healthcare database from approximately 800 private and academic hospitals, representing approximately 20% of US inpatient discharges. The PHD contains data on sociodemographics, comorbidities, procedures, medications, patient charges and costs, and outcomes that are based on International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] and International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM]) codes for diagnoses and procedures. Additionally, the PHD contains microbiology laboratory data from approximately 200 hospitals, including specimen identification, test name, test day of service and time, and result and sensitivity data [16].
We identified hospitalizations among persons aged ≥18 years from 2012 through 2017 with primary discharge diagnoses of selected bacterial infections using ICD-9-CM and ICD-10-CM diagnostic codes (Supplementary Table 1). We analyzed 4 diagnosis groups separately: IE, CNS abscess (including intracranial, intraspinal, and epidural abscesses), osteomyelitis, and SSTIs.
We described characteristics of patients with selected infections, including age, sex, race, insurance, region, length of stay, death, secondary diagnoses of interest, and total hospital costs to treat patients during hospital encounters. Total costs represent the sum of fixed costs and variable costs, where fixed costs included all overhead costs while variable costs included in-hospital services including procedures, room and board, services provided by hospital staff, and pharmacy costs. Deaths included deaths that occurred in the inpatient setting and discharges to hospice. Secondary diagnoses of interest included human immunodeficiency virus infection; viral hepatitis B, C, or D virus infection; and SUDs. We identified secondary SUD diagnoses among hospitalizations of serious infections using ICD-9 and ICD-10 diagnosis codes derived from the Clinical Classifications Software category 661 for “substance-related disorders” [17] (Supplementary Table 2), excluding alcohol, nicotine, and cannabis. Among hospitalizations for patients with SUDs, substances were classified as opioids, cocaine, amphetamines, hallucinogens, or other/unspecified drugs. SUD hospitalizations may include diagnosis codes for more than 1 substance type.
We used sampling weights provided by PHD to extrapolate results to nationwide hospitalizations with primary diagnosis codes for selected infections. Annual rates of hospitalizations were calculated by dividing the weighted number of hospitalizations by US Census population estimates for each year. Among hospitalizations with SUD diagnoses, we described the proportion with at least 1 opioid-related diagnosis code and also characterized length of stay, deaths, and hospitalization costs. To detect any discrepancies in coding due to the ICD-9 to ICD-10 transition, we initially analyzed temporal trends within 2 discrete time periods: the beginning of the study period (1 January 2012) up to the ICD-9/ICD-10 transition (30 September 2015) and from 1 October 2015 through 31 December 2017 (Supplementary Table 3). Descriptive statistics included means for continuous variables and frequencies for categorical variables as appropriate. We evaluated trends in the rates of primary IE, CNS abscess, osteomyelitis, and SSTI hospitalizations with secondary diagnoses of SUDs (IE-SUD, CNS-SUD, osteomyelitis-SUD, and SSTI-SUD, respectively) from 2012 through 2017. We tested for the presence of temporal trends via weighted regression using generalized estimating equations, including clustering of the data within hospital provider. To determine whether increased awareness and coding of SUDs could be responsible for an increase in the incidence of SUD diagnoses, we examined rates of hospitalizations with 2 separate control outcomes, leukemia or thyroid disorders, not expected to be associated with SUDs.
To assess whether microbiology of infections changed, we evaluated IE hospitalizations with available laboratory data. We described the distribution of positive cultures from blood and cardiac tissue specimens, compared the distributions among IE-SUD and non-SUD–related IE hospitalizations, and described temporal trends of positive cultures among all IE hospitalizations. For IE hospitalizations with all negative results among blood and cardiac tissue specimens, we characterized patients who had been transferred from another healthcare facility using the point-of-origin code provided by PHD. This code has been previously verified and is comparable to physician-documented history [18]. These data do not contain direct personal identifiers, and institutional review board approval was not required. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
The annual rates and outcomes of patients hospitalized with each group of selected infections are presented in Table 1. There were 72 481 weighted admissions with a primary diagnosis of IE recorded; of those, 20 784 (28.7%) included SUD diagnoses. From 2012 through 2017, the overall rate of IE and CNS abscess hospitalizations did not change (P = .0958 and P = .3315, respectively) and the overall rates of osteomyelitis and SSTI hospitalizations decreased (P = .0139 and P = .0022, respectively). The proportion of IE hospitalizations with a diagnosis of SUD increased from 19.9% to 39.4% (P < .0001). Among 242 651 osteomyelitis hospitalizations, prevalence of SUDs increased from 8.2% to 16.6% (P < .0001). Prevalence of SUDs among 48 742 CNS abscess admissions increased from 15.4% in 2012 to 23.8% in 2017 (P < .0001), and prevalence of SUDs among patients hospitalized for SSTIs increased from 6.8% to 9.4% (P < .0001). Among all infection hospitalizations with SUDs, 58.6% had ≥1 discharge diagnosis code related to opioids, while 40.8% had ≥1 code related to cocaine, 18.0% related to amphetamines, 0.2% related to hallucinogens, and 55.1% related to other drugs or unspecified drug use. Some hospitalizations had discharge diagnosis codes for more than 1 substance type, and thus categories are not mutually exclusive.
Table 1.
Annual Rates and Outcomes of Patients Hospitalized With Selected Bacterial and Fungal Infections as Primary Diagnosis, Premier Healthcare Database, 2012–2017
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|---|
| Infective endocarditis: all hospitalizations | ||||||
| Weighted N | 12 415 | 12 081 | 11 600 | 11 686 | 11 760 | 12 939 |
| Rate per 100 000 persons | 5.3 | 5.1 | 4.8 | 4.8 | 4.8 | 5.3 |
| Substance use disordersa N (%) | 19.9 | 23.8 | 26.4 | 29.7 | 32.6 | 39.4 |
| Infective endocarditis: hospitalizations with substance use diagnoses | ||||||
| Opioidb (%) | 55.6 | 58.2 | 54.9 | 62.4 | 67.4 | 72.4 |
| Length of stay, mean, days | 14.3 | 15.3 | 15.9 | 14.9 | 15.3 | 15.1 |
| Diedc (%) | 5.8 | 3.7 | 3.5 | 3.6 | 3.5 | 2.1 |
| Hospitalization costs, mean | US$30 515 | US$32 038 | US$36 765 | US$32 695 | US$35 860 | US$33 610 |
| Osteomyelitis: all hospitalizations | ||||||
| Weighted N | 39 246 | 39 623 | 40 537 | 40 014 | 47 458 | 35 773 |
| Rate per 100 000 persons | 16.7 | 16.7 | 16.9 | 16.5 | 19.4 | 14.5 |
| Substance use disordersa N (%) | 8.2 | 9.0 | 11.1 | 11.2 | 10.5 | 16.6 |
| Osteomyelitis: hospitalizations with substance use diagnoses | ||||||
| Opioidb (%) | 46.4 | 48.8 | 47.1 | 52.5 | 56.4 | 60.4 |
| Length of stay, mean, days | 11.5 | 11.9 | 10.8 | 10.5 | 12.3 | 11.6 |
| Diedc (%) | 0.9 | 1.0 | 0.8 | 0.3 | 0.8 | 0.4 |
| Hospitalization costs, mean | US$20 706 | US$21 432 | US$19 366 | US$20 551 | US$24 815 | US$22 607 |
| CNS abscess: all hospitalizations | ||||||
| Weighted N | 7801 | 7722 | 7480 | 7831 | 8764 | 9144 |
| Rate per 100 000 persons | 3.3 | 3.3 | 3.1 | 3.2 | 3.6 | 3.7 |
| Substance use disordersa N (%) | 15.4 | 15.9 | 17.5 | 18.9 | 19.6 | 23.8 |
| CNS abscess: hospitalizations with substance use diagnoses | ||||||
| Opioidb (%) | 50.5 | 56.7 | 47.8 | 52.4 | 60.8 | 59.1 |
| Length of stay, mean, days | 17.2 | 13.8 | 15.2 | 14.6 | 15.2 | 14.7 |
| Diedc (%) | 2.3 | 1.6 | 1.3 | 0.8 | 2.3 | 1.8 |
| Hospitalization costs, mean | US$36 675 | US$28 785 | $33 190 | US$31 515 | US$31 151 | US$30 064 |
| SSTI: all hospitalizations | ||||||
| Weighted N | 845 300 | 822 250 | 804 036 | 797 066 | 862 065 | 860 161 |
| Rate per 100 000 persons | 360.1 | 347.0 | 335.9 | 329.0 | 352.1 | 349.2 |
| Substance use disordersa N (%) | 6.8 | 7.8 | 8.5 | 9.0 | 8.7 | 9.4 |
| SSTI: hospitalizations with substance use diagnoses | ||||||
| Opioidb (%) | 48.9 | 47.7 | 47.6 | 49.1 | 54.7 | 53.8 |
| Length of stay, mean, days | 4.7 | 4.7 | 4.8 | 4.7 | 4.8 | 4.8 |
| Diedc (%) | 0.4 | 0.4 | 0.5 | 0.4 | 0.5 | 0.5 |
| Hospitalization costs | US$8433 | US$8212 | US$8964 | US$9137 | US$9946 | US$10 254 |
Abbreviation: CNS, central nervous system; SSTI, skin and soft tissue infection.
Includes opioids, cocaine, amphetamines, hallucinogens, or other/unspecified drugs (Supplementary Materials).
Proportion of hospitalizations of selected infections with at least 1 opioid-related diagnosis code (Supplementary Materials).
In-hospital death or discharge to hospice.
Table 2 describes rates of hospitalizations for selected infections with SUD diagnoses by age group. Rates for each group of infections increased across the study period, primarily in younger age groups. Among all age groups, the rate of hospitalizations with diagnoses of SUDs increased from 1.1 to 2.1 per 100 000 persons for IE hospitalizations, 1.4 to 2.4 per 100 000 persons for osteomyelitis hospitalizations, 0.5 to 0.9 hospitalizations per 100 000 persons for CNS abscess hospitalizations, and 24.4 to 32.9 per 100 000 persons for SSTI hospitalizations. The rates of hospitalizations for persons with thyroid disease or leukemia and who also had SUD diagnoses were unchanged (0.3 and 0.2 hospitalizations per 100 000 persons, respectively).
Table 2.
Rates of Hospitalizations for Selected Infections, Thyroid Disorders, and Leukemia With Substance Use Disorder Diagnoses, Premier Healthcare Database, 2012–2017
| Year | |||||||
|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||
| Infective endocarditis | |||||||
| 18–44 years | Weighted N | 1803 | 2048 | 2328 | 2579 | 3002 | 4089 |
| Rate per 100 000 persons | 1.6 | 1.8 | 2.1 | 2.3 | 2.6 | 3.6 | |
| 45–64 years | Weighted N | 614 | 755 | 696 | 830 | 783 | 972 |
| Rate per 100 000 persons | 0.7 | 0.9 | 0.8 | 1.0 | 0.9 | 1.2 | |
| 65+ years | Weighted N | 60 | 39 | 43 | 62 | 45 | 38 |
| Rate per 100 000 persons | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Osteomyelitis | |||||||
| 18–44 years | Weighted N | 1332 | 1590 | 2076 | 2141 | 2229 | 2884 |
| Rate per 100 000 persons | 1.2 | 1.4 | 1.8 | 1.9 | 2.0 | 2.5 | |
| 45–64 years | Weighted N | 1639 | 1691 | 2104 | 1983 | 2361 | 2750 |
| Rate per 100 000 persons | 2.0 | 2.1 | 2.5 | 2.4 | 2.8 | 3.3 | |
| 65+ years | Weighted N | 252 | 269 | 330 | 359 | 396 | 308 |
| Rate per 100 000 persons | 0.6 | 0.6 | 0.7 | 0.8 | 0.8 | 0.6 | |
| Central nervous system abscess | |||||||
| 18–44 years | Weighted N | 525 | 537 | 605 | 703 | 784 | 1036 |
| Rate per 100 000 persons | 0.5 | 0.5 | 0.5 | 0.6 | 0.7 | 0.9 | |
| 45–64 years | Weighted N | 604 | 566 | 682 | 717 | 834 | 1047 |
| Rate per 100 000 persons | 0.7 | 0.7 | 0.8 | 0.9 | 1.0 | 1.3 | |
| 65+ years | Weighted N | 72 | 84 | 84 | 62 | 96 | 97 |
| Rate per 100 000 persons | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | |
| Skin and soft tissue infection | |||||||
| 18–44 years | Weighted N | 32 620 | 36 859 | 38 270 | 41 093 | 43 061 | 47 111 |
| Rate per 100 000 persons | 29.3 | 33.0 | 34.1 | 36.4 | 37.9 | 41.4 | |
| 45–64 years | Weighted N | 22 276 | 24 072 | 26 405 | 26 708 | 28 011 | 29 079 |
| Rate per 100 000 persons | 27.2 | 29.4 | 32.0 | 32.1 | 33.5 | 34.9 | |
| 65+ years | Weighted N | 2466 | 3183 | 3691 | 4101 | 4211 | 4743 |
| Rate per 100 000 persons | 5.9 | 7.4 | 8.3 | 8.9 | 8.9 | 9.6 | |
| Thyroid disease | |||||||
| 18–44 years | Weighted N | 292 | 331 | 371 | 389 | 361 | 343 |
| Rate per 100 000 persons | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | |
| 45–64 years | Weighted N | 245 | 402 | 355 | 368 | 305 | 310 |
| Rate per 100 000 persons | 0.3 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | |
| 65+ years | Weighted N | 115 | 78 | 79 | 103 | 56 | 91 |
| Rate per 100 000 persons | 0.3 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | |
| Leukemia | |||||||
| 18–44 years | Weighted N | 153 | 167 | 179 | 167 | 243 | 192 |
| Rate per 100 000 persons | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | |
| 45–64 years | Weighted N | 210 | 227 | 267 | 253 | 289 | 235 |
| Rate per 100 000 persons | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | |
| 65+ years | Weighted N | 125 | 118 | 140 | 91 | 87 | 130 |
| Rate per 100 000 persons | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.3 | |
Includes opioids, cocaine, amphetamines, hallucinogens, or other/unspecified drugs (Supplementary Materials).
Overall, the rate of non-SUD–related IE hospitalizations without SUD diagnoses decreased from 4.2 hospitalizations per 100 000 persons in 2012 to 3.2 hospitalizations per 100 000 persons in 2017. Figure 1 compares temporal trends of IE-SUD hospitalizations by age group. Among patients aged 18–44 years, the rate of IE-SUD hospitalizations more than doubled from 1.6 per 100 000 persons in 2012 to 3.6 per 100 000 persons in 2017, resulting in approximately 4089 hospitalizations in 2017. In contrast, non-SUD–related IE hospitalizations for patients aged 18–44 years decreased slightly (Figure 1). Rates of non-SUD hospitalizations for CNS abscess, osteomyelitis, and SSTIs were also stable or decreased slightly over the study period (data not shown).
Figure 1.
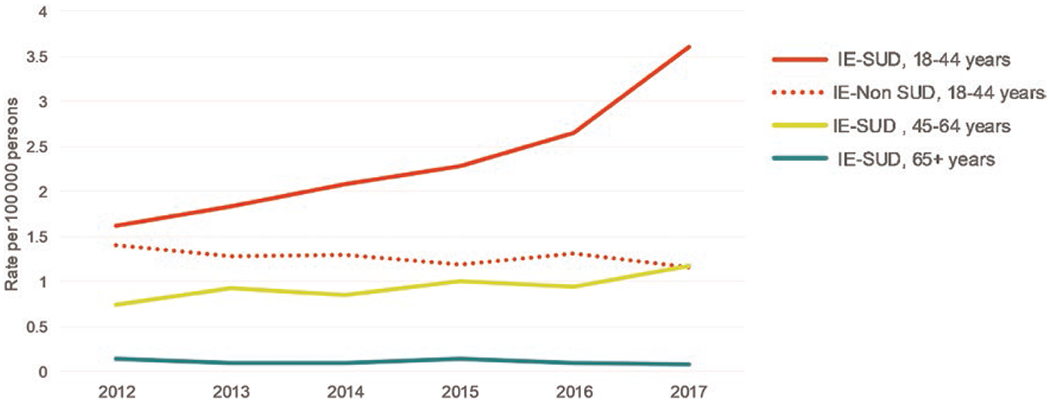
Weighted infective endocarditis hospitalizations with and without substance use disorder diagnoses, Premier Healthcare Database, 2012–2017. Includes opioids, cocaine, amphetamines, hallucinogens, or other/unspecified drugs (see Supplementary Materials). Abbreviations: IE-SUD, infective endocarditis hospitalization with substance use disorder diagnoses; IE-Non-SUD, infective endocarditis hospitalization without substance use disorder diagnoses.
Comprehensive tables that include characteristics of hospitalizations for each group of serious infections by year are available in Supplementary Table 3. The proportion of white patients and Medicaid patients among all hospitalizations increased over the study period. Among all IE, OM, and CNS abscess hospitalizations, the percentage of patients aged 18–44 years increased over the study period, while the percentage of SSTI hospitalizations increased in patients aged ≥65 years. Among all infection hospitalizations except for SSTI, mean length of stay and median costs were higher in hospitalizations with SUD diagnoses: 15.2 vs 11.0 days and $19 320 vs $17 125 for IE hospitalizations, 11.5 vs 7.6 days and $12 910 vs $10 824 for osteomyelitis hospitalizations, and 15.1 vs 10.9 days and $22 073 vs $20 067 for CNS abscess hospitalizations. IE, OM, and CNS abscess patients with SUDs were also more likely to have a length of stay >20 days (26% vs 12%, 15% vs 4%, and 22% vs 11%, respectively). Among SSTI SUD and non-SUD hospitalizations, the mean length of stay was 4.8 days compared with 5.2 days, and median costs were $9232 compared with $10 054, respectively.
Among 17 063 unweighted IE hospitalizations, 4120 hospitalizations (24%) occurred in facilities with available microbiology test result data. Of these, there were 3149 hospitalizations with culture results from blood or cardiac tissue specimens, 1826 hospitalizations (58%) with at least 1 positive culture, and 1323 hospitalizations (42%) with negative culture results from blood or cardiac tissue specimens. Of the hospitalizations with negative culture results, 522 (40%) were transferred from another healthcare facility or clinic, 771 (58%) were transferred from a nonhealthcare facility (including possible clinic referral or transfer from an ambulatory surgery center), and 30 (2%) had an unknown point of origin.
The distribution of microorganisms identified from IE hospitalizations with positive cultures are described in Table 3. Approximately half of IE-SUD hospitalizations had a positive culture for S. aureus compared with 19.4% of non-SUD–related IE hospitalizations. Viridans streptococci were most common among non-SUD–related IE hospitalizations (29.4%). While gram-negative and fungal/mycobacterial infections were less common overall, significantly more were found in IE-SUD hospitalizations (21.6%) compared with non-SUD–related IE hospitalizations (8.6%). Among all positive S. aureus cultures, 45% were methicillin resistant (MRSA). Figure 2 describes the temporal trends of microorganisms identified among IE hospitalizations. Overall, the prevalence of S. aureus increased from approximately 24% of IE hospitalizations with positive cultures in 2012 to 39% in 2017. Conversely, prevalence of viridans streptococci decreased from 27% of IE hospitalizations with positive cultures in 2012 to 20% in 2017. For both IE-SUD and non-SUD–related IE hospitalizations, prevalence of S. aureus increased and prevalence of viridans streptococci decreased (data not shown).
Table 3.
Distribution of Microorganisms Among Infective Endocarditis Hospitalizations With Positive Cultures, Premier Healthcare Database, 2012-2017
| Microorganism | Infective Endocarditis Hospitalization With SUD Diagnoses (n) |
%a | Infective Endocarditis Hospitalization Without SUD Diagnoses (n) |
%a |
|---|---|---|---|---|
| Gram-positive cultures | ||||
| Staphylococcus aureus | 235 | 50.3 | 263 | 19.4 |
| Other staphylococci | 31 | 6.6 | 188 | 13.8 |
| Viridans group streptococci | 84 | 18.0 | 400 | 29.4 |
| Other streptococci | 13 | 2.8 | 107 | 7.9 |
| Enterococcus faecalis | 48 | 10.3 | 246 | 18.1 |
| Enterococcus faecium | 1 | 0.2 | 20 | 1.5 |
| Other enterococci | 4 | 0.9 | 21 | 1.5 |
| Other gram-positive | 23 | 4.9 | 84 | 6.2 |
| Gram-negative cultures | ||||
| Pseudomonas spp. | 20 | 4.3 | 9 | 0.7 |
| Serratia spp. | 18 | 3.9 | 3 | 0.2 |
| Stenotrophomonas maltophilia | 7 | 1.5 | 4 | 0.3 |
| Enterobacter spp. | 6 | 1.3 | 5 | 0.4 |
| Escherichia coli | 0 | 0.0 | 13 | 1.0 |
| Klebsiella spp. | 0 | 0.0 | 13 | 1.0 |
| Other gram-negative | 24 | 5.1 | 42 | 3.1 |
| Other | ||||
| Candida spp. | 23 | 4.9 | 21 | 1.5 |
| Other fungal/mycobacterial infection | 3 | 0.6 | 7 | 0.5 |
| Other positive cultureb | 10 | 2.1 | 47 | 3.5 |
Abbreviation: SUD, substance use disorder.
Percentage of hospitalizations with at least 1 positive culture (N = 1826). Hospitalizations may have more than 1 positive culture resulting in the total exceeding 100%.
Rare organisms or insufficient information to classify.
Figure 2.
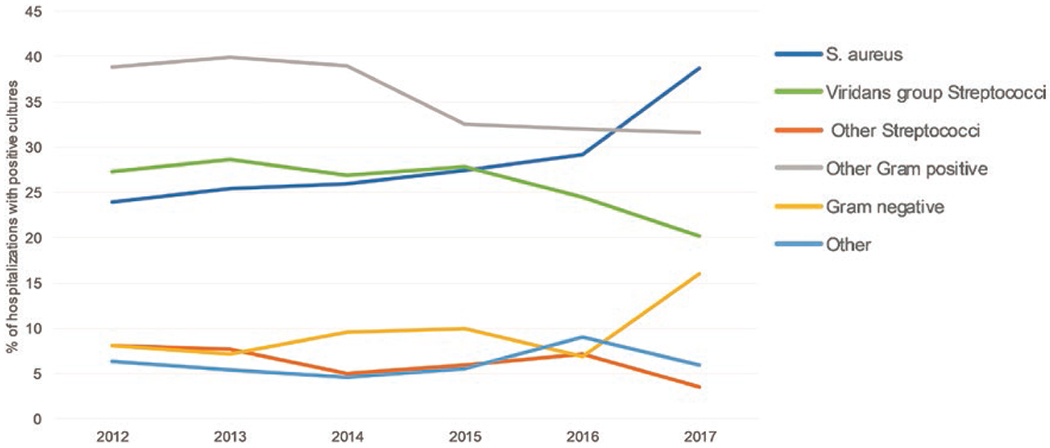
Trends of microorganisms among infective endocarditis hospitalizations with positive cultures, Premier Healthcare Database, 2012–2017 (N = 1826). Abbreviation: S. aureus, Staphylococcus aureus.
DISCUSSION
While the overall rates of hospitalizations for IE, CNS abscess, osteomyelitis, and SSTIs remained stable or decreased between 2012 and 2017, the proportion of admissions with SUD diagnoses increased significantly among all serious infection hospitalizations. Most striking were increases in rates of hospitalizations with SUD diagnoses among patients aged 18–44 years, for whom the estimated rate of IE-SUD hospitalizations more than doubled from 1.6 in 2012 to 3.6 in 2017 per 100 000 people. These data also provide insight into the microbiology of IE among hospitalizations with and without SUD diagnoses using laboratory data from more than 200 US hospitals. The differences in IE-SUD and non-SUD–related IE hospitalizations, including length of stay, microbiology, as well as the significant increase in IE-SUD hospitalizations over the study period, suggest that SUDs are changing the epidemiology of these infections.
The incidence of drug overdose deaths has been rising over the past few years [1], and communities are reporting shifts in behavior toward an increase in the use of injection as the route of drug delivery [19, 20]. In parallel, the incidence of infections such as endocarditis and invasive MRSA in patients with SUDs or who inject drugs has been increasing [7]. The finding that S. aureus and viridans streptococci were the most common pathogens among IE-SUD patients suggests that additional emphasis on strategies to prevent infections caused by inadvertent injection of skin and mouth flora could be important for prevention of endocarditis [8].
Current efforts to prevent infections among SUD patients are focused on prevention and treatment of underlying SUDs and injection drug use in the community [21]. Increased awareness of harm reduction principles and education regarding the risks related to substance use during clinical treatment for infections are complementary strategies for addressing the substantial and rising burden of these acutely life-threatening diseases. A recent report from the Centers for Disease Control and Prevention advocates for routine care of persons who inject drugs to include advice on hand hygiene, not injecting into skin that has not been cleaned, and not using any equipment contaminated by reuse, saliva, soil, or water [22]. Proposals to provide comprehensive care that addresses both substance use treatment and prevention of infectious complications have included reducing barriers to medication-assisted treatment prescribing, conducting demonstration projects that focus on cotreatment in locations that routinely have SUD clients, and developing a subspecialty that combines infectious diseases and addiction treatment expertise [23–25].
This analysis has many strengths. Approximately 800 US hospitals were included for each year of the study, including both community and teaching hospitals from both rural and urban areas. Using PHD weights, we extrapolated our results to produce national estimates for primary diagnoses. We included 6 years of data, allowing for trend analyses, and provided updated recent national estimates up to 2017. Our ability to use the PHD to approximate results by Collier et al [6], who estimated serious infection hospitalization rates using the Nationwide Inpatient Sample, adds to the validity of this study (data not shown).
Several limitations should be noted. First, we relied on ICD-9-CM and ICD-10-CM discharge codes to identify diagnoses. These administrative data are collected primarily for billing purposes and adapted for research, and there may be misclassification in SUD codes. For example, ICD-10 codes that relate to “use” of opioids might be used for patients without an opioid use disorder but who are using opioids as prescribed. However, ICD-10 “use” codes were only found in approximately 1% of hospitalizations categorized as having SUDs in this study. Additionally, causal inferences cannot be obtained solely from diagnosis codes given during the same encounter. Infections could have been acquired from nondrug use-related exposures.
Coding practices may vary by facility and may have changed over the study period, particularly during the transition to ICD-10 [26] and as awareness of the opioid crisis has continued to increase in recent years. However, in a qualitative assessment, we did not observe substantial shifts in trends following the ICD-10 transition (see Supplementary Table 3). Additionally, our secondary analysis of non-SUD–related IE hospitalizations among patients aged 18–44 years showed no inverse trend. If increased coding of SUDs was entirely driving these results, we would expect a decrease in the rate of non-SUD-related IE of approximately half to account for the doubled rates of SUD-related hospitalizations in this age group, but the rate declined only slightly (1.4 to 1.2 per 100 000 persons). In addition, we found rates of leukemia and thyroid diseases hospitalizations, which are not likely to be associated with SUDs, to be relatively stable over this time, which also suggests that there is not a general trend to increase SUD coding during the study period. A further complication of reliance on administrative coding is underreporting of illicit drug use, with studies showing that 30%−50% of SUDs are not appropriately coded [27–29]. In a recent study, drug use was not recorded at the time of infection, but within 6 months, for more than half of SUD-related infections identified [30]. By including only inpatient diagnoses recorded during the same encounter, our study likely underestimates the burden of SUD-related infections. Finally, we could not determine routes of drug administration due to the lack of specific ICD-CM codes for injection drug use. We conducted a secondary analysis that was limited to substances more likely to be injected (opioids, cocaine, and amphetamines) and found a 2.5-fold increase in IE-SUD hospitalizations over the study period compared with a 2-fold increase when our more sensitive definition was used.
We used only the primary diagnosis code to identify patients within the 4 disease groups, and these syndromes may represent different facets of the same infection. Although this increases our positive predictive value of the coded definition, and validation studies have suggested good positive predictive value specifically for SSTI and osteomyelitis [31], it would likely underestimate infection burden in the United States. Therefore, our estimates, while similar to those from a previous study that used only primary codes [6], likely represent a minimum burden for these disease groups. In a sensitivity analysis that included diagnosis codes present on admission in any position for IE hospitalizations, we found an almost 4-fold increase in hospitalizations; however, increases in associated SUDs were comparable across IE definitions (data not shown).
Our analyses only included deaths that occurred in a hospital or hospice, which might not capture all deaths due to these infections. Finally, our microbiology analyses were limited to data available in PHD, which may not be representative of all IE hospitalizations in this study. Additionally, among the 43% of hospitalizations with microbiology data that showed negative culture results, 40% were transferred from another healthcare facility and may not be new IE hospitalizations. Some of these patients may have previously received antimicrobial medications, resulting in negative cultures (eg, treated at another facility if transferred or self-treated at home).
CONCLUSIONS
Our analyses have important clinical, economic, and public health implications. National rates of hospitalizations for serious bacterial infections among persons with SUDs continue to increase, driven primarily by younger age groups. These data highlight an important correlate of the ongoing opioid crisis and indicate the threat posed to recent progress in reducing the impact of invasive bacterial diseases such as S. aureus infection [32]. Interventions and health communications aimed at reducing the risk of bacterial infections as a complication of substance misuse should be added to preventive care for persons with SUDs [22].
Supplementary Material
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2018; 67:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy EL, DeVita D, Liu H, et al. Risk factors for skin and soft-tissue abscesses among injection drug users: a case-control study. Clin Infect Dis 2001; 33:35–40. [DOI] [PubMed] [Google Scholar]
- 3.Dahlman D, Håkansson A, Kral AH, Wenger L, Ball EL, Novak SP. Behavioral characteristics and injection practices associated with skin and soft tissue infections among people who inject drugs: a community-based observational study. Subst Abus 2017; 38:105–12. [DOI] [PubMed] [Google Scholar]
- 4.Phillips KT, Stein MD. Risk practices associated with bacterial infections among injection drug users in Denver, Colorado. Am J Drug Alcohol Abuse 2010; 36:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandrasekar PH, Narula AP. Bone and joint infections in intravenous drug abusers. Rev Infect Dis 1986; 8:904–11. [DOI] [PubMed] [Google Scholar]
- 6.Collier MG, Doshani M, Asher A. Using population based hospitalization data to monitor increases in conditions causing morbidity among persons who inject drugs. J Commun Health 2018; 43:598–603. [DOI] [PubMed] [Google Scholar]
- 7.Jackson KA, Bohm MK, Brooks JT, et al. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs—six sites, 2005–2016. MMWR Morb Mortal Wkly Rep 2018; 67:625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larney S, Peacock A, Mathers BM, Hickman M, Degenhardt L. A systematic review of injecting-related injury and disease among people who inject drugs. Drug Alcohol Depend 2017; 171:39–49. [DOI] [PubMed] [Google Scholar]
- 9.Bassetti S, Hoffmann M, Bucher HC, Fluckiger U, Battegay M. Infections requiring hospitalization of injection drug users who participated in an injection opiate maintenance program. Clin Infect Dis 2002; 34:711–3. [DOI] [PubMed] [Google Scholar]
- 10.Palepu A, Tyndall MW, Leon H, et al. Hospital utilization and costs in a cohort of injection drug users. CMAJ 2001; 165:415–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med 2005; 353:1945–54. [DOI] [PubMed] [Google Scholar]
- 12.Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med. 2018; 170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deo SV, Raza S, Kalra A, et al. Admissions for infective endocarditis in intravenous drug users. J Am Coll Cardiol 2018; 71:1596–7. [DOI] [PubMed] [Google Scholar]
- 14.Njoroge LW, Al-Kindi SG, Koromia GA, ElAmm CA, Oliveira GH. Changes in the association of rising infective endocarditis with mortality in people who inject drugs. JAMA Cardiol 2018; 3:779–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamohan P, Berger JR. Spinal epidural abscess. Curr Infect Dis Rep 2014; 16:436. [DOI] [PubMed] [Google Scholar]
- 16.Premier Healthcare Database white paper: data that informs and performs. 2018; Available at: https://learn.premierinc.com/white-papers/premier-healthcaredatabase-whitepaper. Accessed 29 July 2018.
- 17.HCUP CCS. Healthcare cost and utilization project (HCUP), 2017. Available at: www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed 11 June 2019.
- 18.Prabaker KK, Hayden MK, Weinstein RA, Lin MY; CDC Prevention Epicenter Program. Use of the point of origin code from a universal billing form, UB-04, to efficiently identify hospitalized patients admitted from other health care facilities. Am J Infect Control 2012; 40:659–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philadelphia sentinel community site (SCS) drug use patterns and trends. 2017. Available at: https://ndews.umd.edu/sites/ndews.umd.edu/files/philadelphia-scs-drug-use-patterns-and-trends-2017-final.pdf. Accessed 2 May 2019.
- 20.Chicago metro sentinel community site (SCS) drug use patterns and trends. 2018. https://ndews.umd.edu/sites/ndews.umd.edu/files/SCS-Report-2018-Chicago-FINAL.pdf. Accessed 2 May 2019.
- 21.National Opioids Crisis. Help, resources and information, 2018. Available at: https://www.hhs.gov/opioids. Accessed 9 May 2019.
- 22.Hartnett KP, Jackson KA, Felsen C, et al. Bacterial and fungal infections in persons who inject drugs—Western New York, 2017. MMWR Morb Mortal Wkly Rep 2019; 68:583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infectious diseases and opioid use disorder (OUD), 2018. https://www.idsociety.org/globalassets/idsa/topics-of-interest/opioid/id-and-the-opioid-epidemic-policy-brief_3-19-2018-updated.pdf?_ga=2.193293550.21244946.1521734567-1708005240.1512422793. Accessed 20 November 2019.
- 24.Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Springer SA, Korthuis PT, Del Rio C. Integrating treatment at the intersection of opioid use disorder and infectious disease epidemics in medical settings: a call for action after a National Academies of Sciences, Engineering, and Medicine Workshop. Ann Intern Med 2018; 169:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Official CMS industry resource for the ICD-10 transition, 2019. Available at: https://www.cms.gov/medicare/coding/icd10. Accessed 22 August 2019.
- 27.Holt SR, Ramos J, Harma MA, et al. Prevalence of unhealthy substance use on teaching and hospitalist medical services: implications for education. Am J Addict 2012; 21:111–9. [DOI] [PubMed] [Google Scholar]
- 28.Wood DM, Conran P, Dargan PI. ICD-10 coding: poor identification of recreational drug presentations to a large emergency department. Emerg Med J 2011; 28:387–9. [DOI] [PubMed] [Google Scholar]
- 29.Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug-related infections are missed: a population-based cohort study of inpatient and emergency department settings. Clin Infect Dis 2018; 68:1166–75. [DOI] [PubMed] [Google Scholar]
- 30.Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug-related infections are missed: a population-based cohort study of inpatient and emergency department settings. Clin Infect Dis 2019; 68: 1166–75. [DOI] [PubMed] [Google Scholar]
- 31.Wiese AD, Griffin MR, Stein CM, et al. Validation of discharge diagnosis codes to identify serious infections among middle age and older adults. BMJ Open 2018; 8:e020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kourtis AP, Hatfield K, Baggs J, et al. ; Emerging Infections Program MRSA Author Group. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep 2019; 68:214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


