Abstract
Synthesis of a novel 2′-deoxy-guanine carbocyclic nucleoside 4 constructed with spiro[2.4]heptane core structure in the aglycon moiety was carried out. Radical-mediated 5-exo-dig mode cyclization and following cyclopropanation proceeded efficiently to furnish the spiro alcohol 10. Subsequent Mitsunobu-type glycosylation between 13 and 14, deoxygenation of the 2′-hydroxyl group of 16 and deprotection of 17 gave the title compound 4. Compound 4 demonstrated moderate anti-HBV activity (EC50 value of 0.12 ± 0.02 μM) and no cytotoxicity against HepG2 cells was observed up to 100 μM.
Keywords: Anti-HBV, carbocyclic nucleoside, entecavir, spir, enatpeh]4.2[o radical-mediated cyclization
Introduction
The infection of Hepatitis B Virus (HBV)[1] into hepatocytes induces a serious hepatopathy which in turn leads to hepatitis B. Over 300 million people worldwide are chronically infected with HBV.[1, 2] Among the most frequently used drugs for the treatment of the diseases, entecavir (1, Figure 1) is considered as one of the best choices for chronic patients due to its lack of significant adverse effects.[3–5] Entecavir is a carbocyclic nucleoside having an exo-methylene function at the 5′-position. The exo-methylene moiety might be essential for the high anti-HBV activity because the potency of carbocyclic dG 2, which truncates the double bond, is ten times less than that of 1.[3] To combat the emergence of resistant virus, the development of more potent and less toxic novel anti-HBV agent is inevitable. In this context, we have initiated a structure-activity relationship study of exomethylenemodified entecavir and already disclosed the synthesis of 6″-fluiorinated entevavir and evaluation of its anti-HBV activity.[6]
Figure 1.
Structures of compounds 1–4.
Recently, a novel carbocyclic nucleoside, cyclopropyl-spirocarbocyclic adenosine 3 has been synthesized.[7] One of characteristic properties of 3 is that the carbocyclic ring adopts the 2′-endo (southern conformation) with anti-base disposition, which is the same sugar conformation of the deoxyribose moiety in DNA. In the course of our ongoing structure-activity relationship study of 1, we have chosen to synthesize the respective 2′-deoxyguanine derivative 4, which is recognized as an exomthylene-modified entecavir, and which is reported herein. Furthermore, evaluation of its anti-HBV activity and cytotoxicity will be also discussed.
Results and discussion
Chemistry
For the construction of the spiro[2.4]heptane structure of 4, Simmons-Smith cyclopropanation of exo-methylenecyclopentane 12a is most straightforward as described in the literature(Scheme 2).[7] Therefore, we prepared the key intermediate 12a. As mentioned above, we have already described the synthesis of 6″-fluorinated entecavir derivatives.[6] In the study, we have carried out a radical-mediated 5-exo-dig cyclization reaction of a 5-hexynyl radical (I or II) derived from 5 to lead to the desired (E)- and (Z)-anti-methylenecyclopentane 6 (combined yields; 69%) and its stereoisomer (E)-syn-7 (13%) (Scheme 1). This fact ledus to prepare the selenide 11 for the synthesis of 12a. As shown in Scheme 2, 12a was prepared from the enol ether 8. Thus, 8 obtained from d-ribose according to the reported procedure[8] was converted to silyl ether 9 in 94% yield by treatment with TIPSOTf and 2,6-lutidine. Electrophilic phenylselenenylation and subsequent DIBAL-H reduction of the generated aldehyde gave primary alcohol 10 as a mixture of diastereoisomers in 80% yield. The radical precursor 11 could be obtained by tritylation of 10 in 96% yield. When 11 was reacted with (Me3Si)3SiH (TTMSS) in the presence of Et3B at −50°C, 5-exo-dig mode cyclization of the incipient secondary carbon radical proceeded to give the desired anti-isomer 12a in 71% yield as a major isomer along with syn-isomer 12b (14%) after desilylation of the crude products using Bu4NF in the presence of AcOH. The depicted structures of 12a and 12b were assigned on the basis of its NOE experiments as illustrated in Scheme 2; OH-3/H-6 and H-3/TrOCH in 12a: H-3/H-6 and CH3-isopropylidene/TrOCH in 12b, respectively. The ratio of the stereoisomers 12a/12b was ca 5.1/1, which was consistent with the previous results (5.3/1) as shown in Scheme 1.[6] Cyclopropanation of 12a under Simmons-Smith condition proceeded efficiently by a treatment with CH2I2 and Et2Zn[7, 9] to form (4S, 5S, 6R, 7R)-4-hydroxy-5,6-isopropylidene-7-(trityloxymethyl)spiro[2.4]heptan-5,6-diol 13 in 90% yield.
Scheme 2.
Reagents and conditions: (a) TIPSOTf, 2,6-lutidine, CH2Cl2, 94%; (b) PhSeCl, THF; (c) DIBAL-H, THF, 80% for two steps; (d) TrCl, DMAP, i-Pr2NEt, CH2Cl2,96%; (e) TTMSS, Et3B, toluene, −50°C; (f) Bu4NF, AcOH, THF, 71% for 12a for two steps, 14% for 12b for two steps; (g) (for 12a) CH2I2, Et2Zn, CH2Cl2, 90%.
Scheme 1.
Radical-mediated 5-exo-dig cyclization of phenylsulfanylethynyl selenide 5.
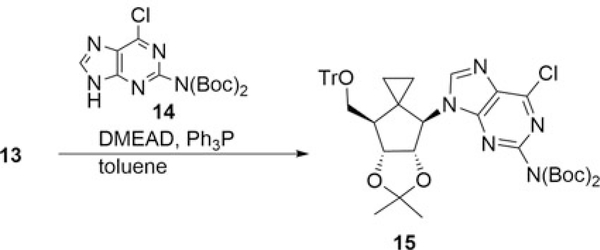
Introduction of the nucleobase to 13 was next examined (Scheme 3). Thus, when 13 was reacted with N2,N2-bis-Boc-2-amino-6-chloropurine 14[10, 11] under Mitsunobu conditions[12] using di(methoxyethyl)azodicarboxylate (DMEAD)[13] and Ph3P in THF, the desired SN2 product 15 was obtained in only trace amounts even after a prolonged reaction time (72 h). After modifying the reaction conditions in terms of solvent, reaction temperature and amount of reagents (14 or DMEAD), the isolated yield of 15 was slightly improved to 23% yield by using toluene as a solvent. Inspection by TLC of the reaction mixture revealed that 13 was consumed completely and unidentified side products were formed. Similar results were also observed in the C-glycosidation utilizing 6-chloropurine.[7] The depicted structure of 15 was confirmed by HMBC spectra withcorrelations between H-1′ and C-4, also H-1′ and C-8, respectively.
Scheme 3.
Mitsunobu reaction of 13; formation to the nucleoside 15.
Finally, conversion to the title compound 4 from 15 was carried out (Scheme 4). Treatment of 15 with aqueous TFA in THF and subsequent regioselective silylation of the resulting triol with 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane (TIPDSCl2) gave guanine ribonucleoside 16 in 56% yield in two steps. Compound 16 was converted to the respective 2′-O-phenoxythiocarbonate, which was subsequently subjected to radical reduction using TTMSS and AIBN in toluene at 100°C to give 2′-deoxyguanine nucleoside 17 in 76% yield. Desilylation of 17 was carried out using Bu4NF in the presence of AcOH in THF. The resulting crude product was purified by reverse phase HPLC (5% MeCN containing 0.1% of AcOH) to provide analytically pure 4 in 72% yield.
Scheme 4.
Reagents and conditions: (a) TFA, H2O; (b) TIPDSCl2, imidazole, DMF, 56% for two steps; (c) PhOC(S)Cl, DMAP, CH2Cl2; (d) TTMSS, AIBN, toluene, 100°C, 76% for two steps; (e) Bu4NF, AcOH, THF, 72%.
Antiviral evaluation
Evaluation of the anti-HBV activity of the novel cyclopropyl-spirocarbocyclic 2′-deoxyguanosine 4 synthesized in this study was conducted with HepG2 2.2.15 cells transfected with the HBV genome.[14] The result issummarized in Table 1. Compound 4 possessed moderate inhibitory activity, with EC50 value of 0.12 ± 0.02 μM. The potency of the antiviral activity was one hundred times less than that of entecavir 1. However, a lower CC50 value (>100 μM) was obtained in compared with that (CC50; 0.12 μM) of 1, resulting in an SI value (>833) of 4 that was four times less than that of 1 (SI; 3632). We have already reported the evaluation of the anti-HBV activity of 6″-fluoroentecavir.[6] The antiviral activity of the both of (E)-and (Z)-isomers retained the potent antiviral activity of entecavir with less cytotoxic against host cells, which led to the better SI values compared to the parent compound 1. The results suggested that a polarized exo-methylene moiety without alteration of the molecular shape of 1 meets the modification strategy of the entecavir. On the other hand, the antiviral evaluation conducted in this study suggested that the sterically encumbered circumstances around the cyclopropyl ring of 4, in which the methylene functionalities of the cycopropyl moiety stick out of the cyclopentane ring plane[7], leads to an unfavorable interaction with HBV RNA-dependent DNA polymerase.
Table 1.
Anti-HBV activity and cytotoxicity of 4 and entecavir (1).
| Compound | EC50 [μM] | EC50 [μM](HepG2) | SI |
|---|---|---|---|
| 4 | 0.12 ± 0.02 | >100 | >833 |
| Entecavir (1) | 0.0038 | 13.8 ± 8.3 | 3632 |
Conclusion
A novel carbocyclic 2′-deoxyguanosine 4 constructing with a spiro[2.4]heptane core structure in the aglycon moiety has been synthesized on the basis of radical-mediated 5-exo-dig mode cyclization as a key step. Compound 4 showed anti-HBV activity, with an EC50 of 0.12 ± 0.02 μM, which is one hundred times less potent than that of 1. However, cytotoxicity for host HepG2 cells was not observed up to 100 μM, which led to an SI value of >833. Evaluation of the antiviral activity of 4 suggested that sterically encumbered circumstances around the exo-methylenemoiety of entecavir gave rise to an adverse effect for inhibitory activity for HBV DNA polymerase. This study may serve as the basis for further modification of entecavir to identify more potent and less toxic anti-HBV agents.
Experimental
General methods:
NMR (1H and13C) spectra were recorded with a Jeol JNM AL-400 or Jeol JNM ECA-500 instruments (1H, 400 or 500 MHz,13C. 125 MHz). Chemical shifts were reported relative to Me4Si. Mass spectra (MS) were taken in FAB mode with m-nitrobenzyl alcohol as a matrix. Column chromatography was carried out on silica gel (Micro Bead Silica Gel PSQ 100B, Fuji Silysia Chemical Ltd.) or neutral silica gel (Silica Gel 60N, Kanto Chemical Co., Inc.). Thin-layer chromatography (TLC) was performed on precoated silica gel plate F254. THF was distilled from benzophenone ketyl.
Triisopropyl(((S)-1-((4R,5R)-5-(2-methoxyvinyl)-2,2-dimethyl-1,3-dioxolan-4-yl)prop-2-yn-1-yl)oxy)silane (9):
To a solution of 8 (6.9 g, 32.6 mmol) and 2,6-lutidine (10.8 mL, 97.7 mmol) in CH2Cl2 (80 mL) was added TIPSOTf (10.7 mL, 39.1 mmol) at 0°C. The resulting mixture was stirred at rt for 50 min. The mixture was partitioned between aq. saturated NaHCO3 and CH2Cl2. Column chromatography on neutral silica gel (hexane/AcOEt = 7/1) of the organic layer gave a diastereomeric mixture (ca. 3:1) of 9 (11.24 g, 94%) as an oil: [α]D25 +23.9 (c 1.56, CHCl3);1H NMR (400 MHz, CD2Cl2) δ 1.03–1.20 (m, 27.93H), 1.13 (s, 3.99H), 1.46 (s, 3H), 1.47 (s, 0.99H), 2.47 (d, J = 2.4 Hz, 0.33H), 2.52 (d, J = 2.4 Hz, 1H), 3.54 (s, 3H), 3.59 (s, 0.99H), 4.11 (dd, J = 6.4 and 4.2 Hz, 1H), 4.16 (dd, J = 6.8 and 4.8 Hz, 0.33H), 4.49 (dd, J = 4.4 and 2.4 Hz, 0.33H), 4.52 (dd, J = 5.2 and 2.4 Hz, 1H), 4.58 (dd, J = 8.8 and 6.8 Hz, 1H), 4.67 (dd, J = 8.8 and 6.4 Hz, 0.33H), 5.02 (dd, J = 12.8 and 8.8 Hz, 1H), 5.14–5.19 (m, 0.33H), 6.06 (dd, J = 6.4 and 1.2 Hz, 0.33H), 6.52 (d, J = 12.8 Hz, 1H);13C NMR (125 MHz, CD2Cl2) δ 12.7, 12.8, 17.9, 18.2, 18.3, 25.3, 25.4, 27.2, 27.5, 56.4, 60.2, 63.7, 64.1, 71.2, 74.7, 75.1, 76.8, 81.3, 81.4, 83.5, 83.7, 88.1, 102.5, 108.5, 108.6, 149.9, 152.0; FAB-MS m/z 368 (M+). HRMS (FAB+): calcd for C20H36O4Si 368.2383, Found 368.2381 [M++H].
2-((4S,5R)-2,2-Dimethyl-5-((S)-1-((triisopropylsilyl)oxy)prop-2-yn-1-yl)- 1,3-dioxolan-4-yl)-2-(phenylselanyl)ethan-1-ol (10):
To a solution of 9 (ca. 3:1 of diastereomeric mixture, 7.9 g, 19.2 mmol) in THF (100 mL, containing 400 μL of H2O) was added a solution of PhSeCl (3.87 g, 20.2 mmol) in THF (40 mL) at −80°C. The resulting yellow solution was stirred at same temperature for 1 h. After stirring the mixture for 30 min at rt, the reaction mixture was partitioned between aqueous saturated NaHCO3 and AcOEt. After evaporation of all of the volatiles, the residue was used for next step without further purification. To a solution of above residue in anhydrous THF (100 mL) was added DIBAL-H (1.01 mol/L in toluene, 57 mL, 57.6 mmol) at −80°C and the reaction mixture was stirred for 2 h at same temperature. The resulting mixture was partitioned between 0.5 M HCl and AcOEt. After the organic layer was filtered through a celite pad, the filtrate was evaporated and purified by column chromatography on silica gel (hexane/AcOEt = 6/1) to give 10 (7.85 g, 80%, ca. 10:1 of diastereomeric mixture) as an oil: Physical data for major isomer of 10: [α]D24 +9.3 (c 1.61, CHCl3);1H NMR (400 MHz, CDCl3) δ 1.02–1.06 (m, 18H), 1.11–1.20 (m, 3H), 1.40 (s, 3H), 1.58 (s, 3H), 2.36 (t, J = 6.0 Hz, 1H), 2.54 (d, J = 2.0 Hz, 1H), 3.67–3.73 (m, 1H), 3.77–3.82 (m, 1H), 3.84–3.89 (m, 1H), 4.26 (dd, J = 8.0 and 6.0 Hz, 1H), 4.49 (t, J = 6.0 Hz, 1H), 5.06 (dd, J = 8.4 and 2.0 Hz, 1H), 7.23–7.31 (m, 3H), 7.59–7.61 (m, 2H);13C NMR (125 MHz, CDCl3) δ 12.9, 18.16, 18.21, 24.8, 26.8, 46.4, 62.4, 63.6, 75.2, 78.8, 79.7, 83.1, 108.6, 127.6, 127.9, 129.1, 134.9; FAB-MS m/z 513 (M++H). Anal. Calcd for C25H40O4SeSi: C, 58.69; H, 7.88, Found: C, 58.46; H, 7.91.
(1S)-1-((4R,5S)-2,2-Dimethyl-5-(1-phenylselanyl-2-trityloxyethyl)-1,3-dioxolan-4-yl)prop-2-yn-1-yl)oxy)triisopropylsilane (11):
A solution of 10 (ca. 10:1 of diastereomeric mixture, 5.3 g, 10.35 mmol), DMAP (1.4 g, 11.39 mmol), TrCl (4.35 g, 15.53 mmol) and i-Pr2NEt (5.4 mL, 31.05 mmol) in 1,2-dichloromethane (80 mL) was heated at 70°C for 24 h. The resulting mixture was partitioned between aqueous saturated NaHCO3 and CH2Cl2. Column chromatography on silica gel (hexane/AcOEt = 7/1) of the organic layer gave 11 (7.49 g, 96%, ca. 10:1 of diastereomeric mixture) as a foam: Physical data for major isomer of 11; [α]D25 + 44.3 (c 0.26, CHCl3);1H NMR (400 MHz, CDCl3) δ 0.97–1.13 (m, 21H), 1.40 (s, 3H), 1.51 (s, 3H), 2.52 (d, J = 2.4 Hz, 1H), 3.42 (dd, J = 9.2 and 4.4 Hz, 1H), 3.54 (t, J = 9.2 Hz, 1H), 3.83–3.88 (m, 1H), 4.22 (dd, J = 8.8 and 6.8 Hz, 1H), 4.93 (dd, J = 6.8 and 2.8 Hz, 1H), 5.31 (dd, J = 8.8 and 2.4 Hz, 1H), 7.09–7.12 (m, 2H), 7.14–7.16 (m, 1H), 7.19–7.29 (m, 9H), 7.31–7.36 (m, 2H), 7.36–7.47 (m, 6H);13C NMR (125 MHz, CDCl3) δ 12.3, 13.0, 18.0, 18.1, 18.27, 18.35, 24.4, 26.1, 44.2, 62.1, 65.2, 75.0, 75.8, 79.7, 83.5, 86.7, 108.5, 126.9, 127.7, 128.9, 129.9, 132.8, 143.9; FAB-MS m/z 754 (M++H). Anal. Calcd for C44H54O4SeSi: C, 70.09; H, 7.22; Found: C, 70.10; H, 7.08.
Radical-mediated 5-exo-dig cyclization of 11: formation of 12a and 12b:
To a solution of a mixture of 11 (ca. 10:1 of diastereomeric mixture, 7.4 g, 9.80 mmol) and (Me3Si)3SiH (TTMSS) (6.05 mL, 19.62 mmol) in toluene (200 mL) was added a freshly opened Et3B (1.0 mol/L in THF, 19.62 mL, 19.62 mmol) at −50°C under positive pressure of dry Ar (contained a trace amount of O2). The resulting mixture was stirred at the same temperature for 65 h. Furthermore, Et3B (19.62 mL, 19.62 mmol) was added after 24 h and 48 h, respectively. After evaporation of all of the volatiles, the residue was roughly purified by column chromatography on silica gel (hexane/Et2O = 10/1) to give the crude products containing the cyclized product. This crude mixture was used for the next step without further purification. To a solution of the residue and AcOH (560 μL, 9.8 mmol) in THF (100 mL) was added Bu4NF (1.0 mol/L in THF, 19.6 mL, 19.6 mmol) at 0°C. The resulting mixture was stirred at rt for 24 h. The mixture was partitioned between aqueous saturated NaHCO3 and AcOEt. Column chromatography on silica gel (hexane/AcOEt = 4/1) of the organic layer gave 12a (3.1 g, 71%, foam) and 12b (610 mg, 14%, foam), respectively. Physical data for 12a: [α]D25 −74.5 (c 0.19, CHCl3);1H NMR (400 MHz, CDCl3) δ 1.32 (s, 3H), 1.40 (s, 3H), 2.31 (d, J = 10.4 Hz, 1H), 2.67 (br-s, 1H), 3.11–3.18 (m, 2H), 4.44 (d, J = 5.2 Hz, 1H), 4.60–4.64 (m, 2H), 5.15 (s, 1H), 5.36 (s, 1H), 7.21–7.26 (m, 4H), 7.27–7.31 (m, 6H), 7.37–7.39 (m, 5H);13C NMR (125 MHz, CDCl3) δ 24.8, 26.5, 49.6, 65.8, 73.8, 79.1, 81.1, 87.0, 110.5, 110.6, 127.1, 127.8, 128.6, 143.7, 152.7; FAB-MS m/z 443 (M++H). HRMS (FAB+): calcd for C29H31O4 443.2222, Found 443.1772 [M++H]. Physical data for 12b: [α]D25 +1.0 (c 1.51, CHCl3);1H NMR (400 MHz, CDCl3) δ 1.25 (s, 3H), 1.34 (s, 3H), 2.26 (d, J = 10.8 Hz, 1H), 4.26–4.52 (m, 1H), 3.30 (dd, J = 8.8 and 5.6 Hz, 1H), 3.52 (t, J = 8.8 Hz, 1H), 4.16–4.20 (m, 1H), 4.54 (t, J = 5.8 Hz, 1H), 4.69 (s, 1H), 4.83 (t, J = 5.8 Hz, 1H), 5.10 (s, 1H), 7.21–7.25 (m, 3H), 7.27–7.31 (m, 6H), 7.48–7.50 (m, 6H);13C NMR (125 MHz, CDCl3) δ 24.9, 25.9, 43.0, 60.1, 73.1, 78.2, 86.6, 106.3, 110.3, 126.9, 127.7, 128.8, 144.2, 150.4; FAB-MS m/z 443 (M++H). HRMS (FAB+): calcd for C29H31O4 443.2222, Found 443.1465 [M++H].
(3aS,4S,6R,6aR)-2,2-Dimethyl-6-(trityloxymethyl)tetrahydrospiro[cyclopenta[d][1,3]dioxole-5,1′-cyclopropan]-4-ol (13):
A mixture of 12a (928 mg, 2.09 mmol) and Et2Zn (1.0 mol/L in hexane, 10.49 mL, 10.49 mmol) in CH2Cl2 (21 mL) was stirred at 0°C for 15 min. To the mixture was added CH2I2 (1.69 mL, 20.98 mmol). The resulting mixture was stirred further 2 h at rt. The resulting mixture was partitioned between aqueous saturated NH4Cl and CH2Cl2. Column chromatography on silica gel (hexane/AcOEt = 4/1) of the organic layer gave 13 (858 mg, 90%) as a foam: [α]D25 −62.6 (c 0.27, CHCl3);1H NMR (400 MHz, CDCl3) δ 0.24–0.29 (m, 1H), 0.33–0.40 (m, 4H), 0.87–0.92 (m, 1H), 1.36 (s, 3H), 1.49 (s, 3H), 1.70 (t, J = 4.4 Hz, 1H), 2.28 (d, J = 10.4 Hz, 1H), 3.10 (d, J = 4.4 Hz, 2H), 4.29–4.33 (m, 1H), 4.53 (d, J = 6.0 Hz, 1H), 4.65 (t, J = 6.0 Hz, 1H), 7.22–7.25 (m, 3H), 7.28–7.32 (m, 6H), 7.40–7.43 (m, 6H);13C NMR (125 MHz, CDCl3) δ 1.7, 8.8, 24.4, 26.3, 26.4, 49.8, 64.0, 72.6, 79.5, 81.9, 87.3, 110.7, 127.0, 127.8, 128.7, 143.8; FAB-MS m/z 495 (M++K). Anal. Calcd for C30H32O4•H2O: C, 75.92; H, 7.22; Found: C, 75.68; H, 6.94.
Mitsunobu reaction of 13: formation of 15:
To a mixture of 13 (1.09 g, 2.39 mmol), Ph3P (1.25 g, 4.77 mmol) and Boc-protected 2-amino-6-chloropurine 14 (1.77 g, 4.77 mmol) in toluene (48 mL) was added DMEAD (1.12 g, 4.77 mmol) at 0°C and the mixture was stirred at rt for 3 h. The mixture was partitioned between brine and Et2O. Column chromatography on silica gel (hexane/AcOEt = 2/1) of the organic layer gave 15 (450 mg, 23%) as a foam: [α]D24 −7.5 (c 0.35, CHCl3);1H NMR (400 MHz, CDCl3) δ 0.12–0.17 (m, 1H), 0.60–0.70 (m, 2H), 0.89–0.91 (m, 1H), 1.30 (s, 3H), 1.42 (s, 18H), 1.57 (s, 3H), 2.46–2.50 (m, 1H), 2.97 (dd, J = 9.6 and 4.0 Hz, 1H), 3.18 (dd, J = 9.6 and 6.4 Hz, 1H), 4.50 (dd, J = 6.4 and 2.8 Hz, 1H), 4.71 (d, J = 3.6 Hz, 1H), 5.01 (d, J = 6.4 and 3.6 Hz, 1H), 7.21–7.31 (m, 8H), 7.39–7.45 (m, 7H), 8.03 (s, 1H);13C NMR (125 MHz, CDCl3) δ 5.0, 12.9, 14.1, 22.6, 24.8, 26.2, 27.1, 27.9, 31.6, 49.9, 63.4, 68.2, 82.1, 83.5, 87.4, 112.3, 127.2, 128.0, 128.7, 130.0, 143.5, 144.9, 150.4, 151.2, 151.7, 152.8; HMBC H-1 (δ 4.71)/C-4 (δ 152.8), H-1/C-8 (δ 144.9). FAB-MS m/z 809 (M++H). HRMS (FAB+): calcd for C45H51ClN5O7 808.3477, Found 808.3408 [M++H].
Deprotection of 15 and subsequent silylation of the resulting diol: formation of 16:
To a solution of 15 (790 mg, 0.98 mmol) in THF (3 mL) was added 50% TFA (20 mL). The resulting suspension was stirred at rt for 76 h. After evaporation of all of the volatiles, the residue was treated with saturated NH3 in MeOH (30 mL) at rt. for 12 h. After evaporation of all of the volatiles, the residue was dried in vacuo. To a solution of the residue and imidazole (200 mg, 2.94 mmol) in dry DMF (20 mL) was added TIPDSCl2 (310 μL, 0.98 mmol) dropwise at 0°C and the mixture was stirred for 1h at same temperature. The reaction mixture was diluted with water (50 mL) and extracted with AcOEt and, then CHCl3. Column chromatography on silica gel (AcOEt/80% MeOH = 40/1) of the combined organic layer gave 16 (304 mg, 56%) as a solid: [α]D24 −7.7 (c 0.43, CHCl3);1H NMR (400 MHz, DMSO-d6) δ−0.25 to −0.20 (m, 1H), 0.51–0.59 (m, 2H), 0.64–0.70 (m, 1H), 0.98–1.07 (m, 28H), 2.29–2.33 (m, 1H), 3.48 (dd, J = 12.0 and 4.0 Hz, 1H), 3.70 (dd, J = 12.0 and 4.0 Hz, 1H), 4.13–4.17 (m, 1H), 4.31 (dd, J = 8.4 and 5.2 Hz, 1H), 4.48 (d, J = 4.0 Hz, 1H), 5.11 (d, J = 5.2 Hz, 1H), 6.40 (br-s, 2H), 7.59 (s, 1H), 10.56 (br-s, 1H);13C NMR (125 MHz, DMSO-d6) δ 6.9, 9.0, 12.0, 12.5, 12.7, 12.9, 16.9, 17.0, 17.1, 17.3, 17.4, 17.5, 21.6, 47.6, 58.3, 62.9, 72.0, 74.6, 116.1, 135.7, 151.5, 153.6, 156.8; FAB-MS m/z 550 (M++H). HRMS (FAB+): calcd for C25H44N5O5Si2 550.2881, Found 550.2895 [M++H].
2-Amino-9-((6aR,8S,9aS)-2,2,4,4-tetraisopropyltetrahydro-6H-spiro[cyclopenta[f][1,3,5,2,4]trioxadisilocine-7,1′-cyclopropan]-8-yl)-1,9-dihydro-6H-purin-6-one (17):
To a suspension of 16 (260 mg, 0.47 mmol), DMAP (63 mg, 0.52 mmol) and i-Pr2NEt (206 μL, 1.18 mmol) in CH2Cl2 (10 mL) was added PhOC(S)Cl (163 μL, 1.18 mmol) at 0°C. The resulting orange solution was stirred for 1 h at same temperature. The mixture was partitioned between freshly prepared aqueous saturated NaHCO3 and CH2Cl2. The inorganic layer was further washed with AcOEt and the combined organic layer was evaporated to dryness. A solution of the crude product, TTMSS (290 μL, 0.94 mmol) and AIBN (39 mg, 0.24 mmol) in toluene (10 mL) was heated at 100°C for 1 h. The resulting mixture was purified by column chromatography on silica gel (AcOEt/80% MeOH = 40/1) to give 17 (191 mg, 76% for two steps) as a solid:[α]D23 −9.6 (c 0.28, CHCl3);1H NMR (400 MHz, DMSO-d6) δ−0.27 to −0.22 (m, 1H), 0.55–0.63 (m, 2H), 0.64–0.69 (m, 1H), 1.00–1.06 (m, 28H), 2.02–2.06 (m, 1H), 2.28–2.32 (m, 2H), 3.41 (dd, J = 12.4 and 3.6 Hz, 1H), 3.73 (dd, J = 12.4 and 3.6 Hz, 1H), 4.55 (dd, J = 16.8 and 8.0 Hz, 1H), 4.73–4.77 (m, 1H), 6.37 (br, 2H), 7.60 (s, 1H), 10.54 (br, 1H);13C NMR (125 MHz, DMSO-d6) δ 7.3, 8.9, 12.0, 12.4, 12.7, 12.8, 16.9, 17.01, 17.04, 17.29, 17.31, 17.5, 24.9, 38.9, 51.5, 54.9, 57.8, 71.0, 116.1, 135.6, 151.3, 153.5, 156.8; FAB-MS m/z 534 (M++H). HRMS (FAB+): calcd for C25H44N5O4Si2 534.2932, Found 534.2930 [M++H].
2-Amino-9-((4S,6S,7R)-6-Hydroxy-7-(hydroxymethyl)spiro[2.4]heptan-4-yl)-1,9-dihydro-6H-purin-6-one (4):
To a solution of a mixture of 17 (66 mg, 0.12 mmol) and AcOH (15 μL, 0.27 mmol) in THF (4 mL) was added Bu4NF (1.0 mol/L in THF, 270 μL, 0.27 mmol) at room temperature, and the mixture was stirred for 24 h at same temperature. After evaporation of all of the volatiles, the residue was purified by reverse phase HPLC (5% MeCN containing 0.1% of AcOH, 20 mL/min, Rt = 16.9 min) to give 4 (26 mg, 72%) as a solid; [α]D23 +13.9 (c 0.73, MeOH);1H NMR (400 MHz, DMSO-d6) δ −0.25 to −0.15 (m, 1H), 0.58–0.67 (m, 2H), 0.74–0.77 (m, 1H), 1.81–1.85 (m, 1H), 2.03–2.09 (m, 1H), 2.24–2.35 (m, 1H), 3.36–3.51 (m, 2H), 4.61 (t, J = 4.8 Hz, 1H), 4.70 (t, J = 7.2 Hz, 1H), 4.87 (d, J = 4.0 Hz, 1H), 6.40 (br, 2H), 7.85 (s, 1H), 10.54 (br, 1H);13C NMR (125 MHz, DMSO-d6) δ 6.7, 13.8, 26.2, 40.6, 53.7, 57.8, 59.9, 71.3, 116.1, 136.5, 151.3, 153.4, 156.8; FAB-MS m/z 292 (M++H). HRMS (FAB+): calcd for C13H18N5O3 292.1410, Found 292.1412 [M++H].
Acknowledgments
A Health and Labor Sciences Research Grant [Practical Research on Hepatitis (Research on the innovative development and the practical application of new drugs for hepatitis B)] is gratefully acknowledged.
References
- 1.Lai CL; Ratziu V; Yuen M-F; Poynard T Viral hepatitis B. Lancet 2003, 362, 2089–2094. [DOI] [PubMed] [Google Scholar]
- 2.Li J; Meng A-P; Guan X-L; Li J; Wu Q; Deng S-P; Su X-J; Yang RY Anti-hepatitis B virus lignans from the root of Streblus asper. Bioorg. Med. Chem. Lett 2013, 23, 2238–2244. [DOI] [PubMed] [Google Scholar]
- 3.Innaimo SF; Seifer M; Bisacchi GS; Stabdring DN; Zahler R; Colonno RJ Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob. Agents Chemother 1997, 41, 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamanaka G; Wilson T; Innaimo S; Bisacchi GS; Egli P; Rinehart JK; Zahler R;Colonno RJ Metabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virus. Antimicrob. Agents Chemother 1999, 43, 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Will H; Reiser W; Weimer T; Pfaff E; Büscher M; Sprengel R; Cattaneo R; Schaller H Replication strategy of human hepatitis B virus. J. Virol 1987, 61, 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumamoto H; Fukano M; Nakano T; Iwagami K; Takeyama C; Kohgo S; Imoto S;Amano M; Kuwata-Higashi N; Aoki M; Abe H; Mitsuya H; Fukuhara K; Haraguchi K Diastereoselective synthesis of 6″- (Z)- and (E)-fluoro analogues of anti-hepatitis B virus agent Entecavir and its evaluation of the activity and toxicity profile of the diastereomers. J. Org. Chem 2016, 81, 2827–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadthula S; Rawal RK; Sharon A; Wu D; Korba B; Chu CK Synthesis and antiviral activity of cyclopropyl-spirocarbocyclic adenosine, (4R,5S,6R,7R)-4-(6-amino-9H-purin-9-yl)-7-(hydroxymethyl)spiro[2.4]heptane-5,6-diol against hepatitis C virus. Bioorg. Med. Chem. Lett 2011, 21, 3982–3985. [DOI] [PubMed] [Google Scholar]
- 8.Marco-Contelles J; Destabel C; Galleno P; Chiara JL; Bernabé M A new synthetic approach to the carbocyclic core of cyclopentane-type glycosidase inhibitors: Asymmetric synthesis of aminocyclopentitols via free radical cycloisomerization of enantiomerically pure alkyne-tethered oxime ethers derived from carbohydrates. J. Org. Chem 1996, 61, 1354–1362. [Google Scholar]
- 9.Lee JA; Moon HR; Kim HO; Kim KR; Lee KM; Kim BT; Hwang KJ; Chun MW; Jacobson KA; Jeong LS Synthesis of novel apio carbocyclic nucleoside analogues as selective A3 adenosine receptor agonists. J. Org. Chem 2005, 70, 5006–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey S; Garner P Synthesis of tert-butoxycarbonyl (Boc)-protected purines. J. Org. Chem 2000, 65, 7697–7699. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher S; Shahani VM; Lough AJ; Gunning PT Concise access to N9-mono-, N2-mono- and N2,N9-di-substituted guanines via efficient Mitsunobu reaction. Tetrahedron 2010, 66, 4621–4632. [Google Scholar]
- 12.Mitsunobu O The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. Synthesis 1981, 1–28. [Google Scholar]
- 13.Sugimura T; Hagiya K Di-2-methoxyethyl azodicarboxylate (DMEAD): An inexpensive and separation-friendly alternative reagent for the mitsunobu reaction. Chem. Lett 2007, 36, 566–567. [Google Scholar]
- 14.Sells MA; Chen ML; Acs G Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. PNAS. 1987, 84, 1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]