Abstract
A novel coronavirus disease, COVID-19, has created a global pandemic in 2020, posing an enormous challenge to healthcare systems and affected communities. COVID-19 is caused by severe acute respiratory syndrome (SARS)-coronavirus-2 (CoV-2) that manifests as bronchitis, pneumonia, or a severe respiratory illness. SARS-CoV-2 infects human cells via binding a “spike” protein on its surface to angiotensin-converting enzyme 2 (ACE2) within the host. ACE2 is crucial for maintaining tissue homeostasis and negatively regulates the renin-angiotensin-aldosterone system (RAAS) in humans. The RAAS is paramount for normal function in multiple organ systems including the lungs, heart, kidney, and vasculature. Given that SARS-CoV-2 internalizes via ACE2, the resultant disruption in ACE2 expression can lead to altered tissue function and exacerbate chronic diseases. The widespread distribution and expression of ACE2 across multiple organs is critical to our understanding of the varied clinical outcomes of COVID-19. This perspective review based on the current literature was prompted to show how disruption of ACE2 by SARS-CoV-2 can affect different organ systems.
Keywords: ACE2, complex disease, COVID-19, RAAS, SARS-CoV-2
INTRODUCTION
The novel severe acute respiratory syndrome (SARS) coronavirus, SARS-CoV-2, is responsible for the complex disease COVID-19 and is the source of a global pandemic in 2020, impacting nearly every country in the world. SARS-CoV-2 is closely related to SARS-CoV, which was identified as the virus responsible for the SARS outbreak in 2003. Like SARS-CoV, SARS-CoV-2 is a single-strand RNA that is composed of 30-kb nucleotides (1). These nucleotides encode four major structural proteins: the spike protein (S), membrane protein (M), an envelope protein (E), and a nucleocapsid protein (N) (1). Viral infections rely upon cellular entry to utilize the host’s machinery to replicate viral copies that are then released by the host (1). Viral entry into the host cells is primarily mediated by S proteins, which make the attachment of the virus to host cellular receptors and facilitate the fusion between host and viral membranes for successful viral entry into the host cell (2). Angiotensin-converting enzyme 2 (ACE2) is a membrane-bound enzyme, which both SARS-CoV and SARS-CoV-2 use as a receptor for host cellular entry (1, 3, 4). However, recent studies have shown that the S protein of SARS-CoV-2 interacts with the human ACE2 with a 10–20-fold higher affinity than SARS-CoV (5–7). This increased affinity to ACE2 may help explain SARS-CoV-2’s higher rate of transmission between individuals compared with SARS-CoV.
ACE2 internalization by SARS-CoV-2 is responsible for the reduction of ACE2 levels on the airway epithelial surface (3, 8). Symptomatic patients with SARS-CoV-2 infection are most often reported having fever, cough, nasal congestion, fatigue, and other signs of an upper respiratory tract infection, which can quickly develop into acute respiratory distress syndrome (ARDS) with a low survival rate (4). Although SARS-CoV-2 infection into host lung cells contributes largely to the severe symptoms in patients, it is noted by the Centers for Disease Control and Prevention that individuals with underlying medical conditions such as heart disease, diabetes, obesity, and asthma have a higher rate of infectivity and increased mortality from COVID-19 (9). It is important to note that ACE2 expression is not exclusive to the lungs but is present in most other tissues, including the nasal and oral mucosa, vasculature, kidney, heart, gastrointestinal (GI) tract, pancreas, and brain (10, 11). Furthermore, ACE2 is fundamental to the regulation of the renin-angiotensin-aldosterone system (RAAS), which is a major regulator of blood pressure as well as fluid and electrolyte homeostasis. In addition to being part of the circulatory system, the RAAS regulates various functions at the local level in an organ-specific manner (12), and the genes involved in the RAAS could provide some explanation to the variation and localization in expression of ACE2. However, an early meta-analysis study on the prevalence of ACE (I/D) genotype and COVID-19 failed to find a direct clinical relevance but did not rule out the influence of ACE genotype on rate of recovery and treatment of COVID-19 (13). Global collaborative efforts, such as the COVID-19 host genetics initiative (https://www.covid19hg.org/), are in progress to uncover the genetic determinants of susceptibility, severity, and outcomes of SARS-CoV-2 infection and disease. To help our understanding of the biology of SARS-CoV-2 infection, the varied clinical outcomes of COVID-19, and its potential long-term pathophysiological effects, we provide a perspective review on the distribution and expression of ACE2 across multiple organs and the consequential effects from altered ACE2-mediated pathways such as the RAAS.
LUNGS
SARS-CoV-2 infects well-differentiated cells from the lung apical surface through its binding of ACE2 in airway epithelia (3). It has been shown using single-cell RNA sequencing that ACE2 is expressed in 0.64% of all human lung cells and is most abundant in alveolar type II cells (AT2), what are known as the defenders of the integrity and function of the alveoli (14, 15). A gene ontology enrichment analysis comparing AT2 cells expressing ACE with AT2 cells not expressing ACE2 found that biological processes related to virus life cycle-related functions, such as replication and transmission, were significantly overrepresented in the ACE2-expressing cells. Specifically, caveolin (CAV2) and integrin (ITGB6) genes, which are components of the lipid raft within the epithelial plasma membrane that facilitates viral internalization, show upregulated expression in ACE2-expressing AT2 cells (16). Furthermore, several gene members of the endosomal sorting complex required for transport machinery within the lipid bilayer of the plasma membrane, which are associated with virus budding and release (17), were also enriched in ACE2-expressing AT2 cells. Together, this study suggests that the small population of ACE2-expressing AT2 cells in the lungs would be particularly targeted during SARS-CoV-2 infection.
Both ACE2 and COVID-19 are associated with the overall susceptibility to acute respiratory distress syndrome (ARDS). In terms of ARDS pathogenesis, the lung’s initial response to injury, termed the exudative phase of ARDS (phase I), exhibits alveolar epithelial inflammation. This is due to the innate immune cell-mediated damage of the alveolar endothelial and epithelial barriers that results in the accumulation of protein-rich edema fluid within the pulmonary interstium and alveoli (18). As a result, there is impaired surfactant function and pulmonary fluid clearance resulting in the increased surface tension throughout the lung, alveolar collapse, and eventually a significant reduction of oxygen in the blood (hypoxemia) (19). In the proliferative phase of ARDS (phase II), the repair process is initiated. Tissue homeostasis is restored by expanding resident fibroblasts, forming a provisional matrix, and proliferating airway progenitor cells and AT2 cells, which then differentiate into type I alveolar epithelial cells. Finally, the fibrotic phase of ARDS (phase III) does not occur in many patients but is linked to prolonged mechanical ventilation and increased mortality. Phase III exhibits extensive cell basement membrane damage and delayed reepithelialization of the lung tissue resulting in the development of interstitial and intra-alveolar fibrosis (18).
The clinical state of acute respiratory distress syndrome (ARDS) is characterized by hypoxemia and chest images that suggest bilateral pulmonary effusion in the absence of heart failure (20). Supportive treatments such as supplemental oxygen and mechanical ventilation are effective, yet the mortality rate associated with ARDS is ∼30% (21). In the progression of ARDS, ACE2 plays a major role (20). It has been previously reported that during the occurrence of ARDS, high expression levels of ACE2 along with angiotensin II (ANG II) and angiotensin II type 1 receptor are responsible for the progression in lung injury (22). Whereas, ACE2 and angiotensin II type 2 receptor are shown to improve ARDS symptoms (22). Since SARS-CoV-2 uses ACE2 for cell entry, we can speculate that angiotensin I and ANG II degradation by ACE2 will be downregulated, and as a result, ACE, ANG II, and angiotensin II type 1 receptor levels will exacerbate the promotion of lung injury.
In general, patients with COVID-19 who developed ARDS are older with an increase in comorbidities and mortality (20). A recent meta-analysis of 46,248 patients with COVID-19 reported that compared with nonsevere patients, patients with severe disease have more hypertension, chronic respiratory disease, and cardiovascular disease (23). An additional study with 44,000 patients with COVID-19 also reported that severe disease was linked to comorbities such as hypertension, chronic respiratory disease, and cardiovascular disease, as well as diabetes mellitus and cancer (24). Many of these comorbidities are characterized by shifts in the ACE/ACE2 regulatory balance (20).
Significant efforts are being made to find the best therapeutic approaches to prevent the progression of SARS-CoV-2 lung infection to the final and fatal stage of ARDS. One of the major approaches is utilizing ACE2 inhibitors. ACE2 degrades angiotensin II to angiotensin (1–7), which bind to the G-protein-coupled Mas receptors (MasR) expressed on the surface of bronchial smooth muscle cells, alveolar epithelium, and angiotensin II type 2 receptor (AT2). Angiotensin (1–7)/MasR then exerts anti-inflammatory effects with less infiltrates of lymphocytes and neutrophils, hence reducing perivascular and peribronchial inflammation, and subsequently preventing fibrosis (25). Nonetheless, it would be fatal to completely eliminate ACE2 receptors since experimental studies have shown that ACE2 deficiency obtained through deletion or inhibition can result in hypertension, cardiac hypertrophy induced by increased angiotensin II, and enhanced susceptibility to heart failure, and in the lungs, it facilitates the progression of inflammatory and a hypercoagulation process (26–28). Due to this action, the use of soluble recombinant ACE2 has been suggested (29). If used in excess, however, recombinant ACE2 can bind competitively with SARS-CoV-2 and neutralize the virus as well as rescue the ACE2 cellular activity that negatively regulates the RAAS to protect the lungs from injury as well as to slow the viral entry into the cells, hence decreasing viral spread (29–31).
ORAL CAVITY AND TONGUE
Considering the fact that SARS-CoV-2 infection symptoms are largely respiratory, the primary method of transmission of COVID-19 is through liquid droplets generated out of oral and nasal cavities. Although no severe symptoms from positive COVID-19 patients involve the oral cavity, researchers are investigating the likelihood of SARS-CoV-2 infection within the oral cavity (11). RNA-seq profiles from two public databases including The Cancer Genome Atlas (TCGA) and Functional Annotation of The Mammalian Genome Cap Analysis of Gene Expression (FANTOM5 CAGE) that were then identified and confirmed with single-cell transcriptomics from an independent data show that the ACE2 is expressed in mucosa of the oral cavity and is enriched in oral epithelial cells. Out of the 32 different oral sites evaluated, the tongue epithelium, the fungiform, and the surrounding papillae were found to be the regions with the highest level of ACE2 expression. These findings suggest that the oral mucosa may be a possibly high-risk path for SARS-CoV-2 infection (11). Studies in mice show that angiotensin II is present in oral tissue and in taste buds, thus allowing it to be rapidly degraded by the ACE2 enzyme. The ACE receptor is also involved in short-term regulation of taste sensitivity through a local RAAS enzyme and plays a functional role in homeostatic pathways both systemically and locally (32).
Two interesting clinical symptoms often associated with milder cases of COVID-19 include ageusia (loss of taste) and anosmia (loss of smell) (33, 34). To assess olfactory and gustatory disorders induced by SARS-CoV-2, an international multicenter study involving 12 hospitals evaluated 417 patients with confirmed SARS-CoV-2 infection (35). The study utilized questionnaires on olfactory and gustatory changes, based on the National Health and Nutrition Survey and the short version of the Questionnaire of Negative Declarations of Olfactory Disorders. Briefly, the report showed that 85.6% and 88.0% of the patients reported olfactory and gustatory disorders, respectively.
Despite high ACE2 expression, why the oral cavity shows limited COVID-19 symptoms warrants further investigation. It will be of interest to know if blocking ACE2/SARS-CoV-2 interaction resolves anosmia and ageusia in infected patients. Currently, the only treatment for anosmia and ageusia in COVID-19 is application of nasal steroids. Of note, the olfactory and gustatory dysfunctions are common symptoms at the beginning of the infection and gradually disappear (34).
Many studies have shown lower rates of SARS-CoV-2 infection in children when compared with in adults (36). In addition, children are also shown to have fewer and less severe symptoms when compared with adults. ACE2 gene expression was measured in the nasal cavity since the nasal epithelium is one of the first sites of infection with SARS-CoV-2 (36). Among a cohort of 305 patients aged 4–60 yr, older children (10–17 yr old; n = 185), young adults (18–24 yr old; n = 46), and adults (≥25 yr old; n = 29) all showed higher levels of ACE2 in the nasal epithelium when compared with the younger children (4–9 yr old; n = 45) (36). In addition, there was a significant trend toward a higher change in ACE2 expression with advancing age.
Because the oral cavity serves as an entrance and an exit for the viral particles, use of saliva as a noninvasive screening tool for early detection and diagnosis of disease has gained attention (37). Collection of saliva is considered an alternative to the use of nasopharyngeal and oropharyngeal swabs that are not only a discomfort to the patient but also a hazard to healthcare workers. Saliva samples can be provided safely, with minimal exposure to healthcare workers, by asking the patient to carefully expectorate into a sterile bottle that is then sealed and submitted for testing (37). In a recent study, 11 out of 12 patients who confirmed positive with SARS-CoV-2 showed viral presence in saliva, therefore suggesting its utility for COVID-19 detection (37). One of the first studies to assess the viability and utility of saliva samples for screening of COVID-19 was conducted in Australia between March 25 and April 1, 2020 (38). Nasopharyngeal swabs were collected from all 622 patients, and 522 patients provided nasopharyngeal swabs plus saliva. The results obtained in this study showed that 39 out of 622 [6.3%; 95% confidence interval (CI) = 4.6%–8.5%] patients presented as PCR-positive according to nasopharyngeal swabs, and 33 out of those 39 positive patients (84.6%; 95% CI = 70.0%–93.1%) also had SARS-CoV-2 detected in their saliva. Considering the emergence and severity of the COVID-19 pandemic, an accurate, fast, noninvasive, and effective diagnosis is necessary to detect the infection, and saliva is a viable alternative. In addition, ACE2 expression was found to be higher in the minor salivary glands than the lungs, indicating that saliva samples could potentially be a better diagnostic for SARS-CoV-2 infection (39).
VASCULATURE
One of the major ACE2-expressing cell types includes vascular endothelial cells, thus it is crucial to investigate the effects of SARS-CoV-2 infection on the vasculature (8, 11, 40). ACE2 metabolizes angiotensin II to create angiotensin (1–7), which leads to the activation of endothelial nitric oxide synthase (eNOS) in blood vessels via the ANG II type I receptor (45). eNOS is mainly responsible for nitric oxide production that has an important role in vasodilation. ACE2 and angiotensin (1–7) are beneficial to the vasculature by protecting endothelial function and inhibiting inflammatory response (46). Loss of ACE2 leads to nitric oxide imbalance and vascular dysfunction (47). Nitric oxide imbalance decreases the bioavailability of nitric oxide used by smooth muscle to promote vasodilation. As the virus replicates, expression of surface ACE2 is also downregulated, which decreases its vasodilatory effect in the vasculature (48). Prolonged contraction of arteries results in the dysfunction and increased inflammation, leading to significant cardiovascular damage. Use of a soluble form of ACE2 as a competitive inhibitor of SARS-CoV-2 binding may provide a tool to prevent viral infection while keeping the vascular functions intact (49, 50).
There is an emergence of a new symptom referred to as “COVID toes.” This term refers to chilblain-like skin lesions that are primarily observed on the fingers or toes of younger patients (51). Typically, these painful red or purplish lesions would be usually caused by extended exposure to cold or damp temperatures. However, there have been many new cases suggesting an impaired vasculature due to COVID-19 infection. The lesions appeared at a time when infection rates of COVID-19 were high and led to the suspicion that SARS-CoV-2 was the cause. Early studies with a combined total of 86 patients having these lesions were not able to determine a distinct role for COVID-19 (51–53). However, a current hypothesis is that these lesions are a late manifestation of COVID-19 infection and associated with vasculature-based complications (52).
HEART AND KIDNEYS
Patients with hypertension in combination with kidney disease are at a high risk for COVID-19-associated mortality (44). Both the heart and kidney help maintain fluid and electrolyte homeostasis and control blood pressure. Both organs express high levels of ACE2, thus making them attractive targets for SARS-CoV-2 infection (54). Single-cell RNA-seq data from human hearts showed that more than 7.5% of myocardial cells are positive for ACE2 (40). In addition, single-cell RNA-seq data from humans have shown that high levels of ACE2 were found in renal proximal tubule cells (40). The physiological role of the ACE2 in the heart and kidney is to regulate the functions of the RAAS. In brief, the RAAS is responsible for angiotensin I (ANG I) conversion to angiotensin II (ANG II) by the angiotensin-converting enzyme (ACE) (55). Angiotensin II is responsible for increase in sympathetic activity, electrolyte reabsorption, water retention, and aldosterone secretion. ANG II also induces vasoconstriction to increase blood pressure and renal perfusion (55). An “alternative” arm of the RAAS exists where angiotensin (1–7) is produced by ACE2 and has the opposite effect of angiotensin II, and blood pressure decreases by increasing sodium excretion, resulting in vasodilation (55). Genetic analysis has shown that hypomethylation and overexpression of ACE2 are found in lupus patients and may result in an increased viral infection of SARS-CoV-2 in this clinical population (56).
According to the Pneumonitis Diagnosis and Treatment Program for New Coronavirus Infection (Trial Version 4), elderly patients aged >60 years with underlying cardiovascular disease such as hypertension and coronary heart disease are more likely to develop severe symptoms when infected with SARS-CoV-2 (57). One study has shown that among patients who are severely affected by COVID-19 and hospitalized, 58% had hypertension, 25% had heart disease, and 44% had arrhythmia (14). Patients infected with SARS-CoV-2 who were admitted to the intensive care unit (ICU) had a significantly higher systolic blood pressure (145 mmHg) when compared with those who were not treated in the ICU (122 mmHg) (14). Furthermore, a significant increase in myocardial injury biomarkers such as creatine kinase-MB and high-sensitivity cardiac troponin I levels has been reported in patients with COVID-19 admitted to the ICU when compared with patients who were not admitted to the ICU (14). Early on in the pandemic, reports indicated that among individuals who have died from COVID-19 in China, only 11.8% of those patients did not have any underlying cardiovascular disease (57). According to the mortality data from China, 35% of the patients were hypertensive, and 17% of the patients had a history of coronary heart diseases (57).
In the kidney, ACE2 is highly expressed in the epithelium. Loss of ACE2 or internalization of the ACE2 in the kidney can lead to an increase in sodium reabsorption, increasing blood volume, and consequently increasing blood pressure and kidney damage (8). Internalization of the ACE2 by SARS-CoV-2 can alter the ratio between angiotensin II to angiotensin (1–7), therefore exacerbating both cardiovascular and renal damage, leading to increased morbidity and mortality from COVID-19 (27). Furthermore, increased circulating levels of ANG II has implications on other organ systems, such as the vasculature, resulting in vasodilation and increased blood pressure (as mentioned earlier). Early data on kidney function have shown that patients who are admitted to the hospital for COVID-19 have an increase in protein in the urine (proteinuria) and blood in the urine (hematuria) (14). Patients with a severe clinical course for COVID-19 show higher circulating renin, ANG II, and aldosterone compared with those with mild COVID-19 clinical complications (58). Moreover, acute kidney injury (AKI) occurs in nearly 36.6% of SARS-CoV-2-infected patients (59). The prevalence of AKI increases largely among ventilated patients. Autopsied patients with COVID-19 tested for SARS-CoV-2 viral load indicate a presence of the viral genome in kidneys, particularly in the renal tubule cells and glomeruli cells (60). Both the tubular cells and the glomeruli cells are responsible for maintaining homeostasis by regulating the filtration function of kidneys. In addition, SARS-CoV-2 viral particles have also been found in urine samples from patients with COVID-19 (61).
GASTROINTESTINAL TRACT
Gastrointestinal epithelium is the binding site of ACE2 in the gastrointestinal (GI) organ system (62). In vitro RNA-sequencing analyses of gut organoids, three-dimensional (3-D) cell cultures that incorporate key features of intestinal tissue, have shown that the highest expression of ACE2 is located at the brush border of mature intestinal enterocytes, particularly colocalized with the protein villin, a tissue-specific marker for intestinal epithelium (63, 64). The distinct presence of ACE2 in the gastrointestinal epithelium suggests a necessary function; however, few studies have investigated its role in this system.
Deficiency of ACE2 in intestinal epithelial cells has been linked to altered gut microbiota composition, dietary amino acid malabsorption, intestinal barrier dysfunction, and increased susceptibility to colitis, therefore, suggesting its potential role as a regulator of intestinal inflammation (65). Loss of gastrointestinal barrier integrity and gut dysbiosis are strongly linked to increased susceptibility to enteric infection; it is speculated that loss of ACE2 due to SARS-CoV-2 infection may contribute to conditions such as gastrointestinal discomfort, diarrhea, and other GI symptoms connected with COVID-19 (14, 65). However, more studies are needed to validate this link. In addition, ACE2 deficiency produced via SARS-CoV-2 infection may negatively impact gastrointestinal barrier function, leading to an increased risk for opportunistic infection and exacerbated metabolic syndrome. Moreover, SARS-CoV-2 viral particles have been observed in the feces of patients with COVID-19 (14, 66). It is important that clinicians and healthcare providers be informed of the clinical implications of fecal particle transmission to establish appropriate precautions.
PANCREAS
Diabetes mellitus (DM) has been recognized as a distinctive comorbidity of COVID-19. In a study of 1,099 confirmed COVID-19 patients, 173 patients with severe disease had a higher frequency of DM, as compared with the nonsevere disease patients (67). A small study with 39 patients suggested that COVID-19 may enhance the incidence of induced DM. In addition, a larger study including 72,314 cases of COVID-19 showed a higher mortality rate in patients with DM (66).
The role of ACE2 has been related to patients with DM plus COVID-19. Until recently, the link between DM and ACE2 has been limited. However, with the availability of large datasets summarized from genome-wide association studies (GWAS), researchers can connect thousands of specific genetic variations to individual diseases. The GWAS data can be used in Mendelian randomization phenome-wide association studies (PheWAS) to efficiently screen for potential determinants of disease risk. Indeed, PheWAS has been used to examine the diseases and traits causally linked to increased ACE2 expression in the lungs. With this approach, it is noted that DM is causally linked to increased ACE2 expression (68). Furthermore, a population‐based, prospective epidemiological association study consisting of over 28,000 individuals revealed that furin, the protease that facilitates viral entry into the human cell, is elevated in patients with DM (69). Therefore, these large database-type studies indicate that patients with DM may indeed be more susceptible to SARS-CoV-2 infection.
In the pancreas, ACE2 is expressed at the site of insulin production. Deficiency of ACE2 in obese mice was observed to reduce β-cell mass and proliferation in tandem with a significantly reduced pancreatic islet area (70). Loss of ACE2 has been linked to decreased insulin secretion and alterations in glucose tolerance (43). It was previously demonstrated that immunostaining for ACE2 was strong in the pancreatic islets and weak in the exocrine cells, suggesting that the SARS coronavirus may enter islet cells using ACE2, and, therefore, may damage islets, which may cause acute insulin-dependent DM (71). Although further evidence is needed, pancreatic damage may also be present in patients with COVID-19, possibly contributing to worse outcomes of patients with DM. Defining the characteristics and extent of the bidirectional relationship between diabetes and COVID-19 for both those with preexisting diabetes as well as new-onset, COVID-19-related diabetes is the collaborative goal of a Global Registry of COVID-19-related diabetes, CoviDiab (http://covidiab.e-dendrite.com/).
BRAIN
Expression of ACE2 within the brain occurs specifically in the glial cells, neurons, and spinal fluid. Deletion of ACE2 in mice was observed to be associated with a deterioration of cognitive function elicited through enhanced reactive oxygen species generation and a decrease in brain-derived neurotrophic factor, a potent nerve growth factor necessary for the proliferation and differentiation of sensory and sympathetic neurons (72). ACE2 in the brain is also responsible for baroreflex regulation, and when ACE2 is inhibited or locally deleted, a decrease in baroreflex sensitivity is detected (73). These findings corroborate studies linking ACE2 deficiency to neurogenic hypertension—an increase in blood pressure due to a dysregulation of neurally driven sympathetic and endocrine mechanisms (74, 75).
Neurons within the brain have been shown to be targeted by SARS-CoV (76). In vivo studies in mice that are transgenic for ACE2 demonstrate that SARS-CoV enters the brain through the olfactory bulb, allowing infection to spread quickly throughout the brain. This causes a large amount of neuronal infection leading to death of the mice (76). Patients infected with SARS-CoV-2 have reported an altered sense of smell (see previous section), which could be related to the virus entering through the cribriform plate of the ethoid bone that protects the olfactory bulb, a neural structure responsible for transmitting smells from a person’s nose to the brain (42).
Analyzing patients who have been infected with SARS-CoV-2 has shown that the virus has a profound effect on brain tissue. Autopsies from deceased patients who succumbed to COVID-19 indicate brain tissue edema and partial neuronal degeneration (77). A study consisting of 214 patients with COVID-19 shows that ∼36% exhibit different types of neurovascular symptoms that were categorized as central nervous system symptoms, peripheral nervous system symptoms, and musculoskeletal symptoms. Based on the guidelines from the American Thoracic Society for community-acquired pneumonia (78), patients with COVID-19 in severe condition were more likely to exhibit neurological symptoms compared with patients in noncritical condition. Interestingly, a case of viral encephalitis caused by SARS-CoV-2 has been reported from a hospital in Beijing. The virus was identified in cerebrospinal fluid, indicating that the virus has the capability to affect the nervous system (79).
COVID-19 TREATMENT
Because ACE2 is considered the “entry door” for the viral infection, it has quickly become the primary target for extensive therapeutic studies. Patients already infected with SARS-CoV-2 exhibit an overall downregulation in ACE2 due to internalization (8). As we know, ACE2 receptor expression is vital for maintaining tissue integrity and preventing injury. Restoring diminished ACE2 levels helps alleviate and reverse tissue damage (48). Recent studies examining the applicability of recombinant ACE2 have shown promising results for potential clinical application in the treatment of patients with COVID-19 suffering from ARDS (80, 81). However, it should be noted that restoration of ACE2 may increase the likelihood of SARS-CoV-2 reinfection for individuals who have not acquired immunity upon first exposure (48). For this reason, here we will review two novel prophylactic treatments and a therapeutic treatment, both targeting the interaction between SARS-CoV-2 and ACE2 for disease prevention.
One method currently being investigated for treating SARS-CoV-2 is the use of fusion inhibitors (7, 82). This treatment involves lipopeptide targeting of the heptad repeat 1 (HR1) domain of SARS-CoV-2 and disrupting viral membrane fusion that is critical for entry into the host cell. This approach has previously been designed and utilized in the prevention of fusion/entry of other HR1-HR2 domain-mediated viruses, including SARS-CoV. Recent studies have examined another potential therapeutic utilizing modified antibodies, with sequence homology similar to that of the ACE2 receptor, to target the S1 domain of the spike protein of SARS-CoV-2, effectively preventing viral interaction and attachment to host cells (41, 77, 83). Finally, four human-origin monoclonal antibodies have a neutralization effect on the cells infected with SARS-CoV-2. Moreover, these antibodies show a therapeutic effect in vivo through a transgenic mouse model, displaying inhibitory and neutralizing effects of the antibodies (84). Collectively, these treatments highlight the significance of ACE2 in SARS-CoV-2 infection and pathogenesis (Fig. 1).
Figure 1.
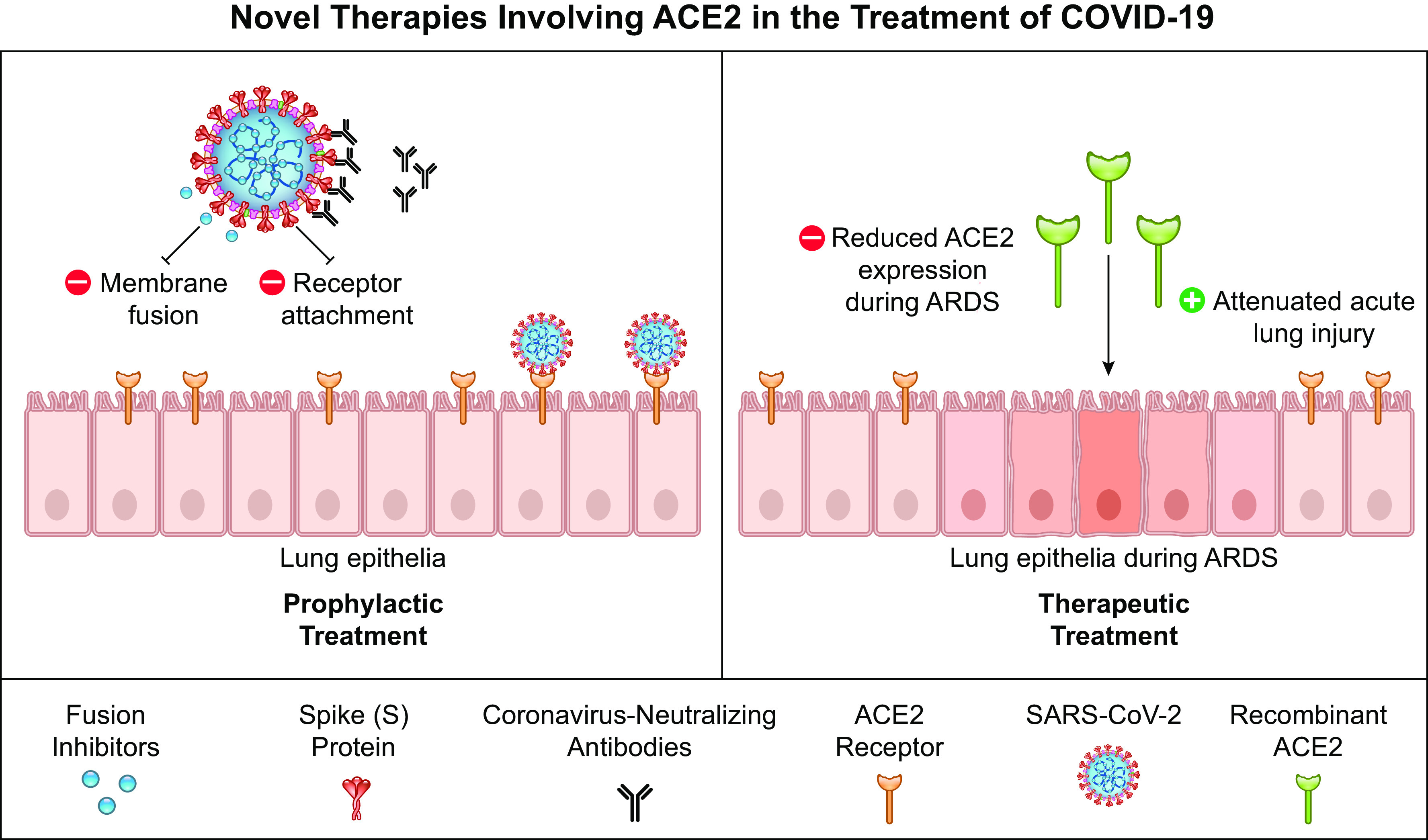
Novel therapies involving ACE2 in the treatment of COVID-19. Novel therapeutics have been proposed that target/utilize ACE2’s role as the entry receptor for SARS-CoV-2. Prophylactic treatments involve the use of fusion inhibitors that disrupt the viral membrane union and coronavirus-neutralizing antibodies that target spike proteins to prevent ACE2 attachment and interaction with the host cell. For therapeutic treatments, recombinant ACE2 has proven beneficial in attenuating lung injury, which may reduce severe symptoms exhibited in many patients with COVID-19. ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
CONCLUSIONS
The expression of ACE2 is widespread throughout many tissues and cell types, and the SARS-CoV-2 infection can invade multiple cell types and organ systems. Moreover, due to the diverse functions of ACE2, it is important to relate the effect of SARS-CoV-2-induced ACE2 disruption to the various symptoms that are observed in patients with COVID-19. It is also important to consider the long-term effect of the association between ACE2 and SARS-CoV-2 infection, as observed in the 2002–2003 SARS pandemic. Affected patients of the SARS pandemic show altered lipid and glucose metabolism, cardiovascular abnormalities, and an increased susceptibility to lung infections (85). Therefore, we can surmise that there may be long-term comorbidities for patients who recover from the SARS-CoV-2. The clinical treatment used to control the disease may further alter the long-term consequences of COVID-19. Based on the current knowledge, any therapeutic option targeting ACE2 needs to consider both systemic and local effects of its functions (Fig. 2). Collectively, the cross and counter effects by every organ system in the body must be taken into consideration when proposing new therapeutic strategies to combat this unprecedented and formidable viral threat.
Figure 2.
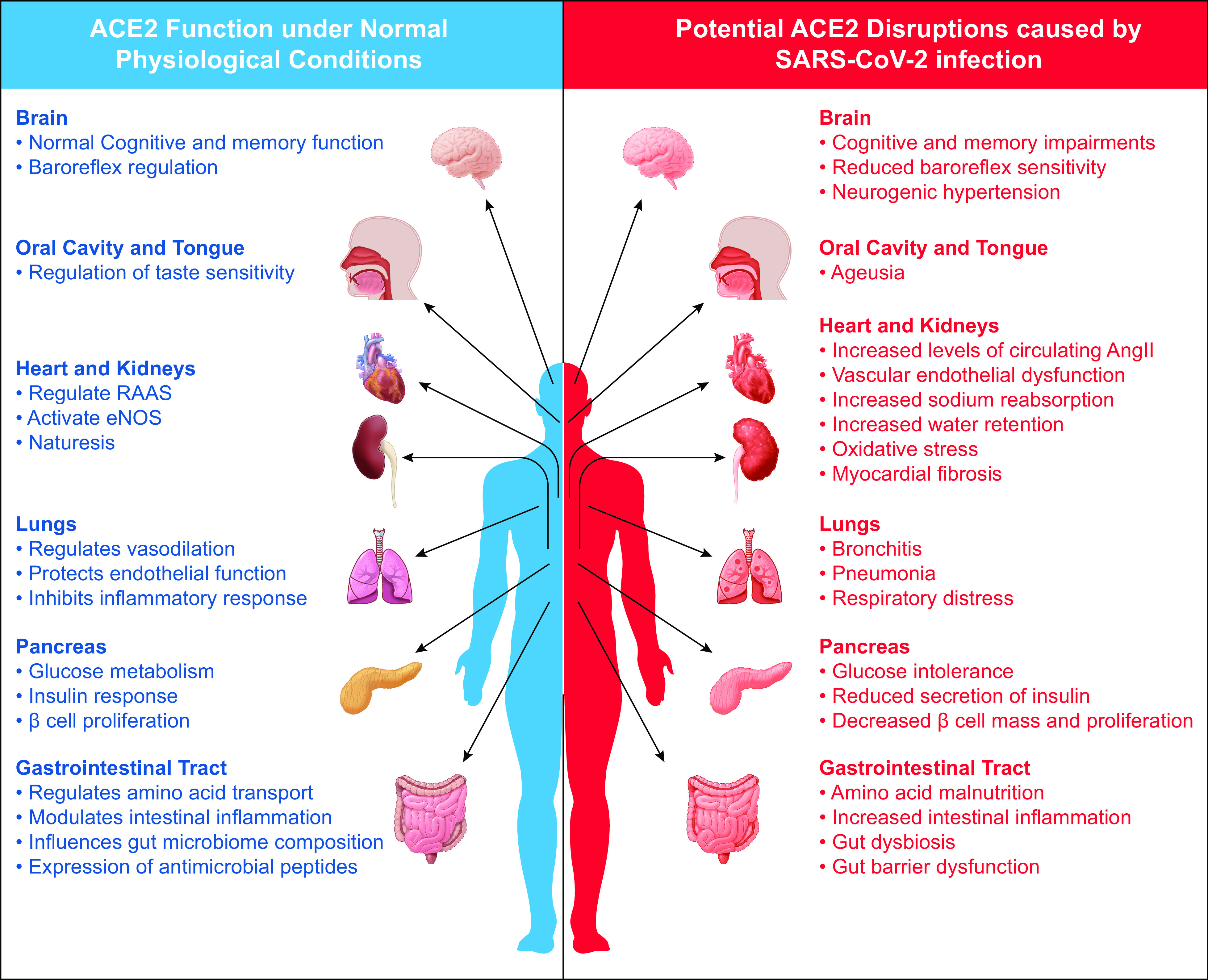
An organ review of normal ACE2 function versus potential pathophysiological consequences of ACE2 disruption caused by SARS-CoV-2 infection. ACE2 is present in various tissues, and its expression is vital to normal physiological functions. ACE2 disruption initiated through the binding of SARS-CoV-2 may have short- and long-term pathophysiological consequences to numerous organ systems that utilize ACE2 for proper function. ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
GRANTS
This work was supported by Grants from NIH P40OD021331 (to L.G.K.), deArce-Koch Memorial Endowment funds (to R.C.), NSF NOA-AGEP 1432878 (to J.M.E.), Fulbright Foreign Student Program: E0610013, IIE: PS00280384 (to E.W.W.), and NIH R01CA219144-05S1 (to A.A.A.).
DISCLOSURES
No conflicts of interest, financial, or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
U.M.A., L.G.K., A.A.A., J.M.E., E.W.W., R.S.R., S.A-M.H., K.B.S., and R.C. conceived and designed research; U.M.A., L.G.K., A.A.A., J.M.E., E.W.W., R.S.R., S.A-M.H., and K.B.S. analyzed data; U.M.A., L.G.K., A.A.A., J.M.E., E.W.W., R.S.R., S.A-M.H., K.B.S., and R.C. interpreted results of experiments; U.M.A., L.G.K., A.A.A., J.M.E., E.W.W., R.S.R., S.A-M.H., K.B.S., and R.C. prepared figures; U.M.A., L.G.K., A.A.A., J.M.E., E.W.W., R.S.R., S.A-M.H., K.B.S., and R.C. drafted manuscript; U.M.A., L.G.K., A.A.A., J.M.E., E.W.W., R.S.R., S.A-M.H., K.B.S., and R.C. edited and revised manuscript; U.M.A., L.G.K., A.A.A., J.M.E., E.W.W., R.S.R., S.A-M.H., K.B.S., and R.C. approved final version of manuscript.
ACKNOWLEDGMENTS
This review is the outcome of the Training Leaders Course, a professional development series for graduate and postdoctoral trainees supported by the Department of Physiology and Pharmacology at The University of Toledo College of Medicine and Life Sciences.
REFERENCES
- 1.Luan J, Lu Y, Jin X, Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun 526: 165–169, 2020. doi: 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J 16: 69, 2019. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB, Jr.. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79: 14614–14621, 2005. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukassen S, Lorenz Chua R, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, Hennig BP, Kreuter M, Conrad C, Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J 39: e105114, 2020. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature 579: 265–269, 2020. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S, Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30: 343–355, 2020. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318: H1084–H1090, 2020. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaherty GT, Hession P, Liew CH, Lim BC, Leong TK, Lim V, Sulaiman LH. COVID-19 in adult patients with pre-existing chronic cardiac, respiratory and metabolic disease: a critical literature review with clinical recommendations. Trop Dis Travel Med Vaccines 6: 16, 2020. doi: 10.1186/s40794-020-00118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 26: 1270–1277, 2005. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 12: 8, 2020. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 13.Hatami N, Ahi S, Sadeghinikoo A, Foroughian M, Javdani F, Kalani N, Fereydoni M, Keshavarz P, Hosseini A. Worldwide ACE (I/D) polymorphism may affect COVID-19 recovery rate: an ecological meta-regression. Endocrine 68: 479–484, 2020. doi: 10.1007/s12020-020-02381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med 202: 756–759, 2020. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Liu DX, Tam JP. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem Biophys Res Commun 369: 344–349, 2008. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pincetic A, Leis J. The mechanism of budding of retroviruses from cell membranes. Adv Virol 2009: 6239691–6239699, 2009. doi: 10.1155/2009/623969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 377: 1904–1905, 2017. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 19.Luks AM, Freer L, Grissom CK, McIntosh SE, Schoene RB, Swenson ER, Hackett PH. COVID-19 lung injury is not high altitude pulmonary edema. High Alt Med Biol 21: 192–193, 2020. doi: 10.1089/ham.2020.0055. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Li S, Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad Med J 96: 403–407, 2020. doi: 10.1136/postgradmedj-2020-137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet 388: 2416–2430, 2016. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 94: 91–95, 2020. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balla M, Merugu GP, Patel M, Koduri NM, Gayam V, Adapa S, Naramala S, Konala VM. COVID-19, modern pandemic: a systematic review from front-line health care providers' perspective. J Clin Med Res 12: 215–229, 2020. doi: 10.14740/jocmr4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76: 14–20, 2020. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res 118: 1313–1326, 2016. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76: 14–20, 2020. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122: 717–728, 2010. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Wang F, Shen C, Peng W, Li D, Zhao C, Li Z, Li S, Bi Y, Yang Y, Gong Y, Xiao H, Fan Z, Tan S, Wu G, Tan W, Lu X, Fan C, Wang Q, Liu Y, Zhang C, Qi J, Gao GF, Gao F, Liu L. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 368: 1274–1278, 2020. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci 61: 2738–2743, 2004. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Yuan K, Phuong HT, Park BM, Kim SH. Angiotensin-(1-5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides 86: 33–41, 2016. doi: 10.1016/j.peptides.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Shigemura N, Takai S, Hirose F, Yoshida R, Sanematsu K, Ninomiya Y. Expression of renin-angiotensin system components in the taste organ of mice. Nutrients 11: 2251, 2019. doi: 10.3390/nu11092251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayin İ, Yaşar KK, Yazici ZM. Taste and smell impairment in COVID-19: an AAO-HNS anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg 163: 473–479, 2020. doi: 10.1177/0194599820931820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, Babudieri S, Petrocelli M, Serra A, Bussu F, Ligas E, Salzano G, De Riu G. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck 42: 1252–1258, 2020. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, , et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277: 2251–2261, 2020. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323: 2427, 2020. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sri Santosh T, Parmar R, Anand H, Srikanth K, Saritha M. A review of salivary diagnostics and its potential implication in detection of Covid-19. Cureus 12: e7708, 2020. doi: 10.7759/cureus.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a non-invasive specimen for detection of SARS-CoV-2. J Clin Microbiol 58: e00776-20, 2020. doi: 10.1128/JCM.00776-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baghizadeh Fini M. Oral saliva and COVID-19. Oral Oncol 108: 104821, 2020. doi: 10.1016/j.oraloncology.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14: 185–192, 2020. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alhenc-Gelas F, Drueke TB. Blockade of SARS-CoV-2 infection by recombinant soluble ACE2. Kidney Int 97: 1091–1093, 2020. doi: 10.1016/j.kint.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11: 995–998, 2020. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 43.Batlle D, Jose Soler M, Ye M. ACE2 and diabetes: ACE of ACEs? Diabetes 59: 2994–2996, 2010. doi: 10.2337/db10-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and cardiovascular disease. Circulation 141: 1648–1655, 2020. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 45.Yagil Y, Yagil C. Hypothesis: ACE2 modulates blood pressure in the mammalian organism. Hypertension 41: 871–873, 2003. doi: 10.1161/01.HYP.0000063886.71596.C8. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y-H, Zhang Y-H, Dong XF, Hao QQ, Zhou XM, Yu QT, Li SY, Chen X, Tengbeh AF, Dong B, Zhang Y. ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm Res 64: 253–260, 2015. doi: 10.1007/s00011-015-0805-1. [DOI] [PubMed] [Google Scholar]
- 47.Rabelo LA, Todiras M, Nunes-Souza V, Qadri F, Szijarto IA, Gollasch M, Penninger JM, Bader M, Santos RA, Alenina N. Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS One 11: e0150255, 2016. doi: 10.1371/journal.pone.0150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaduganathan M, Vardeny O, Michel T, McMurray JJ, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 382: 1653–1659, 2020. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Espinosa de los Monteros-Mena A, Gonzalez-Pineda A, Reyes-Zapata H. Neonatal manifestations of congenital hypothyroidism. Bol Med Hosp Infant Mex 39: 742–747, 1982. [PubMed] [Google Scholar]
- 50.Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181: 905–913 e907, 2020. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain-like acral lesions during the Covid-19 pandemic (“Covid Toes”): histologic, immunofluorescence and immunohistochemical study of 17 cases. J Am Acad Dermatol 83: 870–875, 2020. doi: 10.1016/j.jaad.2020.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol 59: 739–743, 2020. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccolo V, Neri I, Filippeschi C, Oranges T, Argenziano G, Battarra VC, Berti S, Manunza F, Fortina AB, Di Lernia V, Boccaletti V, De Bernardis G, Brunetti B, Mazzatenta C, Bassi A. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 34: e291–e293, 2020. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simoes ES, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 107: 154–162, 2016. doi: 10.1016/j.phrs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 55.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol 4: 1201–1228, 2014. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawalha AH, Zhao M, Coit P, Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol 215: 108410, 2020. doi: 10.1016/j.clim.2020.108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 17: 259–260, 2020. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villard O, Morquin D, Molinari N, Raingeard I, Nagot N, Cristol JP, Jung B, Roubille C, Foulongne V, Fesler P, Lamure S, Taourel P, Konate A, Maria AT, Makinson A, Bertchansky I, Larcher R, Klouche K, Le Moing V, Renard E, Guilpain P. The plasmatic aldosterone and C-reactive protein levels, and the severity of Covid-19: the Dyhor-19 study. J Clin Med 9: 2315, 2020. doi: 10.3390/jcm9072315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium, Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schroder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med 46: 1339–1348, 2020. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532: 107–110, 2002. doi: 10.1016/S0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 63.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RB, van Schayck JP, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJ, Schippers D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science 369: 50–54, 2020. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB, Diamond MS, Ciorba MA, Whelan SP, Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol 5: eabc3582, 2020. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duvallet C, Gibbons SM, Gurry T, Irizarry RA, Alm EJ. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun 8: 1784, 2017. doi: 10.1038/s41467-017-01973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang A, Tong ZD, Wang HL, Dai YX, Li KF, Liu JN, Wu WJ, Yuan C, Yu ML, Li P, Yan JB. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis 26: 1337–1339, 2020. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, , et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med 382: 1708–1720, 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rao S, Lau A, So H-C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of 2019-nCov: a Mendelian randomization analysis. medRxiv 43, 1416–1426, 2020. doi: 10.2337/dc20-0643. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez C, Rysä J, Almgren P, Nilsson J, Engström G, Orho-Melander M, Ruskoaho H, Melander O. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med 284: 377–387, 2018. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shoemaker R, Yiannikouris F, Thatcher S, Cassis L. ACE2 deficiency reduces beta-cell mass and impairs beta-cell proliferation in obese C57BL/6 mice. Am J Physiol Endocrinol Metab 309: E621–E631, 2015. doi: 10.1152/ajpendo.00054.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J-K, Lin S-S, Ji X-J, Guo L-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47: 193–199, 2010. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang XL, Iwanami J, Min LJ, Tsukuda K, Nakaoka H, Bai HY, Shan BS, Kan-No H, Kukida M, Chisaka T, Yamauchi T, Higaki A, Mogi M, Horiuchi M. Deficiency of angiotensin-converting enzyme 2 causes deterioration of cognitive function. NPJ Aging Mech Dis 2: 16024, 2016. doi: 10.1038/npjamd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem 107: 1482–1494, 2008. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept 2012: 1–8, 2012. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res 113: 1087–1096, 2013. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82: 7264–7275, 2008. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420–422, 2020. [Erratum in Lancet Respir Med 8:420–422, 2020]. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77: 683, 2020. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 94:55–58, 2020. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care 21: 305, 2017. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xia S, Zhu Y, Liu M, Lan Q, Xu W, Wu Y, Ying T, Liu S, Shi Z, Jiang S, Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol 17: 765–767, 2020. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lei C, Qian K, Li T, Zhang S, Fu W, Ding M, Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat Commun 11: 2070, 2020. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus A, van Kuppeveld FJ, Haagmans BL, Grosveld F, Bosch BJ. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 11: 2251, 2020. [Erratum in Nat Commun 11:2511, 2020]. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, Xu L, Li X, Liu H, Yin P, Li K, Zhao J, Li Y, Wang X, Li Y, Zhang Q, Xu G, Chen H. Altered lipid metabolism in recovered sars patients twelve years after infection. Sci Rep 7: 9110, 2017. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


