Abstract
The COVID-19 pandemic is an ongoing threat to public health. Since the identification of COVID-19, the disease caused by SARS-CoV-2, no drugs have been developed to specifically target SARS-CoV-2. To develop effective and safe treatment options, a better understanding of cellular mechanisms underlying SARS-CoV-2 infection is required. To fill this knowledge gap, researchers require reliable experimental systems that express the host factor proteins necessary for the cellular entry of SARS-CoV-2. These proteins include the viral receptor, angiotensin-converting enzyme 2 (ACE2), and the proteases, transmembrane serine protease 2 (TMPRSS2) and furin. A number of studies have reported cell-type-specific expression of the genes encoding these molecules. However, less is known about the protein expression of these molecules. We assessed the suitability of primary human bronchial epithelial (HBE) cells maintained in an air-liquid interface (ALI) as an experimental system for studying SARS-CoV-2 infection in vitro. During cellular differentiation, we measured the expression of ACE2, TMPRSS2, and furin over progressive ALI days by reverse transcription-quantitative polymerase chain reaction (RT-qPCR), Western blot, and immunofluorescence staining. We also explored the effect of the fibrotic cytokine TGF-β on the expression of these proteins in well-differentiated HBE cells. Like ACE2, TMPRSS2 and furin proteins are localized in differentiated ciliated cells, as confirmed by immunofluorescence staining. These data suggest that well-differentiated HBE cells maintained in ALI are a reliable in vitro system for investigating cellular mechanisms of SARS-CoV-2 infection. We further identified that the profibrotic mediators, TGF-β1 and TGF-β2, increase the expression of furin, which is a protease required for the cellular entry of SARS-CoV-2.
Keywords: ACE2, COVID-19, furin, SARS-CoV-2, TGF-β, TMPRSS2
INTRODUCTION
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus was identified as the cause of coronavirus disease 2019 (COVID-19). Due to its rapid spread and the severity of the disease, by early 2020, COVID-19 became a pandemic and a serious threat to public health. Despite collaborative efforts by researchers, the root of individual susceptibility to SARS-CoV-2 infection and the severity of COVID-19 is not understood. It has been speculated that one of the major determinants of both susceptibility and severity could be the efficiency of viral entry into host cells. The entry of SARS-CoV-2 into host cells requires not only binding of the viral spike protein to the host cell receptor angiotensin-converting enzyme 2 (ACE2) but also cleavage of the viral spike (S) protein (1–5). Cleavage allows fusion of the viral membrane with the host cell membrane to complete viral entry. This cleavage occurs at a monobasic S1/S2 site that is processed by the transmembrane serine protease 2 (TMPRSS2) and at a multibasic S1/S2 site that is processed by the proprotein convertase furin (1, 4, 6–8). Although the monobasic S1/S2 site is found in both SARS-CoV-2 and its close relative SARS-CoV, the multibasic S1/S2 site is present only in SARS-CoV-2 (1, 8). After furin cleaves S protein, the resulting C-terminal fragment of the S1 protein binds neurophilin-1, which facilitates the entry of SARS-CoV-2 (7). Thus, this unique multibasic S1/S2 site may explain the high infectivity and severity of the disease caused by SARS-CoV-2 (4, 7–9). In addition, both susceptibility and severity of disease in COVID-19 appear to be correlated with expression levels of the host proteins that facilitate the cellular entry of SARS-CoV-2 (9–12). For example, the expression of the receptor ACE2 has been shown to be higher in males, who present a greater risk of developing the severe disease with SARS-CoV-2 infection (12). Further, in human lung cells, co-expression of these three proteins, namely, ACE2, TMPRSS2, and furin, is required for the cellular entry of SARS-CoV-2 (1, 6). Overexpression of the proteases, TMPRSS2 and furin, enhances the replication and cytopathology of SARS-CoV-2 in vitro (3). Therefore, the elevated expression of these proteins might lead to the more efficient entry of SARS-CoV-2 and can be linked to the increased susceptibility of individuals with underlying conditions to SARS-CoV-2. Thus, identification of the pathophysiologic conditions that are associated with increased expression of these cellular entry proteins could support the development of a therapeutic target or preventive strategy. This can be achieved using an appropriate in vitro model, in which ACE2, TMPRSS2, and furin proteins are co-expressed as a host cell assembly unit that facilitates cellular entry of SARS-CoV-2. RNA expression of these three genes has been detected in human bronchial brushes and lung and airway biopsies (13–16), thus suggesting that airway epithelial cells isolated from the proximal airway may be a good in vitro model for studying the host cell response to SARS-CoV-2 in vitro (17). Previous studies have identified the transcripts of these genes in multiple cell types in various organs, but little is known about the presence and regulation of the encoded proteins. Thus, spatial and temporal regulation of these genes and their encoded proteins remains to be characterized in vitro using airway epithelial cells isolated from the human proximal airway.
METHODS
Culture of primary human bronchial epithelial cells.
Primary human bronchial epithelial (HBE) cells were received from the Cystic Fibrosis Center Tissue Procurement and Cell Culture Core, under Protocol No. 03–1396 approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. Here, we used primary HBE cells from seven donors who had no smoking history or lung-related disease (male: 2, female: 5, Table 1) and followed the protocol as published previously (18–22). During expansion, cells were fed with Bronchial epithelial cell growth basal medium (BEBM, Lonza, Basel, Switzerland) supplemented with Bronchial epithelial growth medium bulletkit (BEGM, Lonza), 25 ng/mL epidermal growth factor (EGF), and 50 nM retinoic acid. HBE cells were used at passage 2 on Transwell permeable supports (Corning, Corning, NY). Upon reaching confluency in submerged culture, the apical medium was removed, exposing the cells to an air-liquid interface (ALI). During ALI culture, cells were basolaterally fed every other day with the media composed of a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM) (4.5 g/L glucose): BEBM supplemented with BEGM containing 0.5 ng/mL EGF and 50 nM retinoic acid.
Table 1.
Demographic characteristics of HBE- cell donors
| Donor No. | Age, yr | Sex | Smoking History | Ethnicity | BMI | Figure Panels |
|---|---|---|---|---|---|---|
| N1 | 23 | F | N | Caucasian | 26.2 | 4B |
| N2 | 59 | F | N | Hispanic | 27.9 | 4B |
| N3 | 32 | F | N | Hispanic | 30.8 | 4B, 4D |
| N4 | 21 | M | N | Caucasian | 30.2 | 1B, 1C, 2B, 4B, 4D |
| N5 | 35 | M | N | Caucasian | 31.4 | 1B, 1C, 2B, 3E, 3G 4B, 4D |
| N6 | 47 | F | N | Caucasian | 25.1 | 1B, 1C, 2A, 2B, 3A, 3B, 3D, 4D |
| N7 | 39 | F | N | Hispanic | 25.6 | 1B, 1C, 2B, 3F, 4D |
BMI, body mass index; F, female; M, male; N, never smoked.
Quantitative RT-qPCR.
We followed standard RT-qPCR protocol as previously performed in our laboratory (19–21). Primers used were previously published (19–21) or as follows: ACE2 FW 5′-GGACCCAGGAAATGTTCAGA-3′, ACE2 RV 5′-GGCTGCAGAAAGTGACATGA-3′; TMPRSS2 FW 5′-AGGTGAAAGCGGGTGTGAGG-3′, TMPRSS2 RV 5′-TGACCCTGAGTTCAAAGCCAT-3′; FURIN FW 5′-ACACCTGGTGGTACAGACCT-3′, FURIN RV 5′-ACTGTGGTCCAATTCTGGGC-3′.
Western blot analysis.
We followed the standard Western blot protocol as previously performed in our laboratory (19–21). Proteins were detected using primary antibodies as follows: ACE2 (AF933, R&D Systems, Minneapolis, MN), TMPRSS2 (ab92323, Abcam, Cambridge, UK), furin (ab3467, Abcam), and GAPDH (5174, Cell Signaling Technology, Danvers, MA). Protein bands were quantified using ImageJ software (National Institutes of Health, Bethesda, MD). Protein was normalized to GAPDH.
Immunofluorescence staining.
We followed standard immunofluorescence staining protocol as previously performed in our laboratory (19–21) using primary antibodies as follows: TMPRSS2 (ab92323), furin (ab3467), tubulin β-IV (T7941, Millipore Sigma, Burlington, MA), MUC5AC (MA5-12178, Thermo Fisher), ZO-1 (33–9100, Thermo Fisher), and E-cadherin (3195, Cell Signaling Technologies). Cell nuclei were counterstained with Hoechst (Thermo Fisher Scientific).
Data analysis.
Data were analyzed using GraphPad Prism (San Diego, CA) using a nonparametric, one-way Kruskal–Wallis test with a Dunn’s multiple comparison test.
RESULTS AND DISCUSSION
Using primary HBE cells cultured in an ALI, here we assessed the utility of this in vitro system as a model for infection with SARS-CoV-2. In this study, we used primary HBE cells from seven donors. HBE cells were seeded on transwells and maintained in submerged conditions until confluence and then switched to ALI culture conditions, indicated as ALI day 0 (Fig. 1A). On ALI day 0, the confluent epithelium is composed solely of undifferentiated basal cells. During the subsequent course of 14 days in ALI culture, the basal cells undergo differentiation to a pseudostratified epithelium (Fig. 1A, Fig. 3). Although some culture techniques and medium compositions for differentiation of HBE cells require up to 28 days in ALI, we used a previously developed method in which HBE cultures are well-differentiated by 14 days in ALI (see methods) (18, 22). As biochemically and morphologically characterized, this well-differentiated pseudostratified epithelium recapitulates the cellular constituency and architecture of the intact human airway (18–20, 22–24). We harvested HBE cells on ALI days 0, 7, and 14 to detect the expression of ACE2, TMPRSS2, and furin by RT-qPCR and Western blot analysis to determine the temporal development of these three host factors during differentiation of HBE cells (Fig. 1A). To ensure the proper differentiation of the HBE cells over the course of 14 days in ALI culture, we detected cell-type markers specific to differentiated HBE cells (Figs. 1B, 2, and 3). To ensure the proper pseudostratified architecture of the epithelium, we also detected adherens junction protein, E-cadherin and tight junction protein, ZO-1 (20) (Fig. 3C). During differentiation, mRNA expression of ACE2 and TMPRSS2 increased, whereas mRNA expression of FURIN remained constant (Fig. 1C). Similarly, in protein expression detected by Western blot analysis, ACE2 and TMPRSS2 were barely detectable on ALI day 0 but prominently detectable on ALI day 14 (Fig. 2A). These data suggest little or no expression of either protein in undifferentiated basal cells but prominent expression of both ACE2 and TMPRSS2 in well-differentiated HBE cells on ALI day 14 (Fig. 2). Furin protein was prominently detectable over the course of ALI days, suggesting that furin protein is abundantly expressed in both undifferentiated basal and differentiated cells (Fig. 2). In well-differentiated HBE cells, ACE2 has been shown to be localized in ciliated cells (25). Using immunofluorescence (IF) staining, we visualized the cellular localization of TMPRSS2 and furin proteins in well-differentiated HBE cells on ALI day 14 (Fig. 3). TMPRSS2 and furin proteins were localized to the apical surface of the pseudostratified cell layer. As shown by costaining with the ciliated-cell marker tubulin β-IV, TMPRSS2 and furin colocalized in ciliated cells (Fig. 3, D and E). Furthermore, as shown by costaining with the goblet-cell marker MUC5AC, TMPRSS2 and furin rarely colocalized in goblet cells (Fig. 3, F and G). Together, our data show that well-differentiated HBE cells maintained in ALI culture reliably express transcripts of ACE2, TMPRSS2, and FURIN as well as their encoded proteins, indicating that the ALI culture of HBE cells is a reliable in vitro model to study cellular mechanisms of SARS-CoV-2 infection.
Figure 1.

In human bronchial epithelial (HBE) cells during air-liquid interface (ALI) culture, mRNA expression of angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) increases during differentiation. A: outline of the time-course experiment during ALI culture. Created with BioRender.com. B: from ALI day 0 to day 14, TP63 (basal-cell marker) remains unchanged, whereas MUC5AC (goblet-cell marker), FOXJ1, and TEKT1 (ciliated-cell markers) increase [median + interquartile range (IQR), n = 4], *P < 0.05, **P < 0.01. C: ACE2 and TMPRSS2 significantly increase, whereas FURIN remains unchanged (median + IQR, n = 4), **P < 0.01.
Figure 3.
In well-differentiated human bronchial epithelial (HBE) cells, transmembrane serine protease 2 (TMPRSS2) and furin are detected in ciliated cells. On air-liquid interface (ALI) day 14, HBE cells exhibit a pseudostratified architecture and prominent expression of tubulin β-IV (ciliated-cell marker, A), MUC5AC (goblet-cell marker, B), and junctional proteins ZO-1 and E-cadherin (C). TMPRSS2 (D and F) and furin (E and G) localize to the apical cell surfaces and co-localize in ciliated cells. A–G: DNA counterstained with Hoechst; scale bars = 20 µm.
Figure 2.

In human bronchial epithelial (HBE) cells during air-liquid interface (ALI) culture, angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) proteins increase during differentiation. A: representative blots indicate progressive increases in ACE2 and TMPRSS2 proteins during differentiation. Acetylated tubulin (ciliated-cell marker) increases during differentiation. Protein lysates from two independent wells are shown for each ALI day. B: the abundance of each protein was determined from four donors [arbitrary units (A.U.), normalized to GAPDH, median + IQR, n = 4], *P < 0.05, **P < 0.01.
We next tested the extent to which expression of ACE2, TMPRSS2, and furin could be modulated by the profibrotic cytokine TGF-β. In the lung, TGF-β can either play a beneficial or detrimental role depending on its balance with antagonizing cytokines and the pathophysiological context (26–28). For example, TGF-β plays a beneficial role in promoting repair in response to injury. However, in acute respiratory distress syndrome (ARDS), TGF-β plays detrimental roles, including reduction of alveolar fluid clearance, increased epithelial permeability, and resulting pulmonary fibrosis (27, 29, 30). During viral infections, TGF-β can either suppress or enhance viral replication. TGF-β suppresses the replication of human immunodeficiency virus (HIV) in monocytes and macrophages (31) and the RNA replication of hepatitis C virus (HCV) in hepatocytes (32), whereas TGF-β enhances the replication of the respiratory syncytial virus (RSV) in HBE cells (33). In patients infected with SARS-CoV-2, the level of TGF-β is higher in plasma from patients with severe disease (34), suggesting that TGF-β might contribute to the disease severity in COVID-19. In COVID-19, severity and fatality are often linked to the development of ARDS, which in turn shows a detrimental effect by TGF-β (35). Here, we tested if TGF-β has the potential to modulate the efficiency of viral infection by the regulation of host cell proteins that are involved in viral infection.
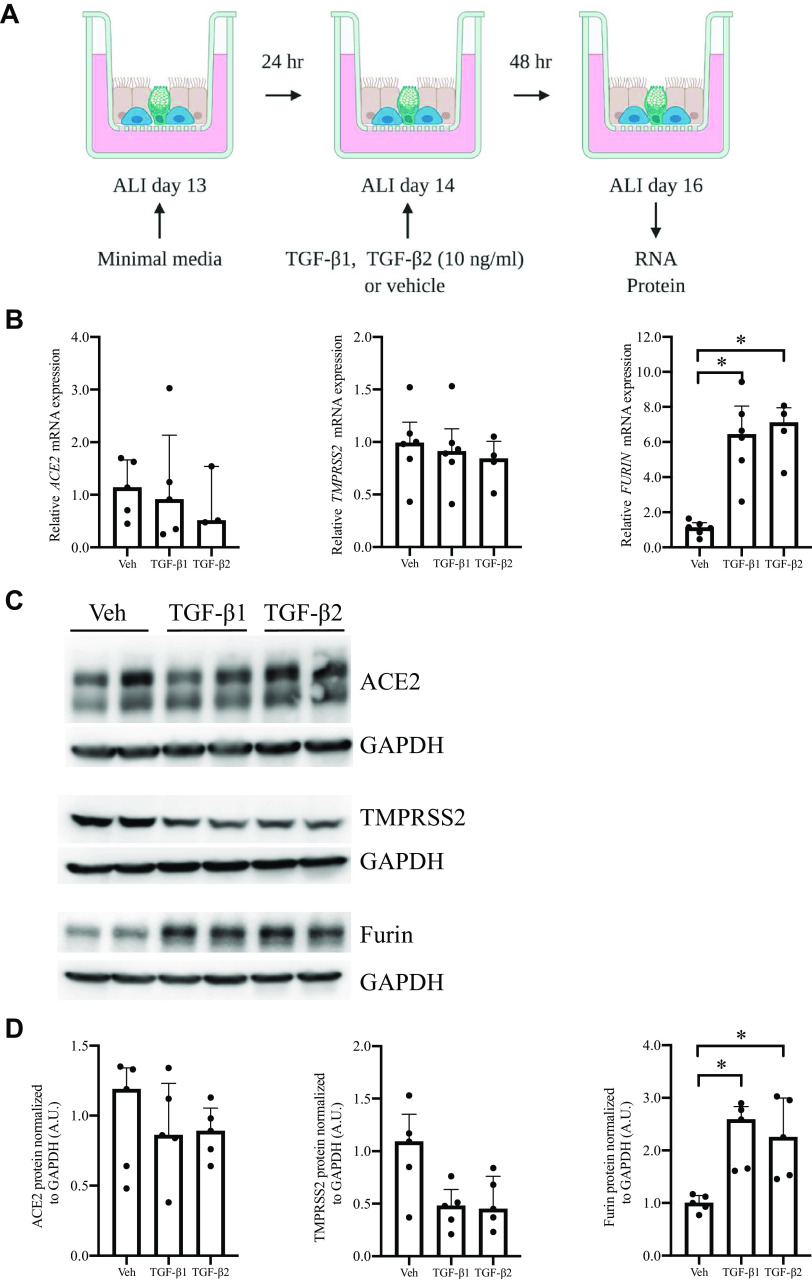
Well-differentiated HBE cells were exposed to either TGF-β1 or TGF-β2 (Fig. 4A). Neither elicited an effect on either mRNA or protein expression of ACE2 (Fig. 4, B, C, and D). Neither TGF-β1 nor TGF-β2 affected TMPRSS2 mRNA expression, but both led a trend toward the reduction of TMPRSS2 protein (Fig. 4, B, C, and D). This reduced detection of TMPRSS2 protein suggests that TGF-β could potentially lead to the secretion or degradation of TMPRSS2 (36). Interestingly, both TGF-β1 and TGF-β2 significantly increased the expression of furin mRNA and protein (Fig. 4, B, C, and D). At 24 and 48 h, FURIN mRNA expression was increased by TGF-β1 and TGF-β2, with the maximum induction observed at 48 h (TGF-β1: 5.91 ± 0.69, n = 5, TGF-β2: 4.79 ± 0.61, n = 3, Fig. 4B). At 48 and 72 h, furin protein was increased by TGF-β1 and TGF-β2, with the maximum induction detected at 48 h (TGF-β1: 2.30 ± 0.28, n = 5, TGF-β2: 2.24 ± 0.34, n = 5, Fig. 4, B and C). These data indicate that in well-differentiated HBE cells, both TGF-β1 and TGF-β2 induce furin expression. Furin is a member of the evolutionarily ancient family of proprotein convertases (37), and its enzyme activity plays a critical role in a variety of pathophysiological processes, including viral infections. As a host protease, furin could not only facilitate viral entry but also modulate innate immunity (37).
Figure 4.
In well-differentiated human bronchial epithelial (HBE) cells in air-liquid interface (ALI) culture, TGF-β1 and TGF-β2 induce expression of furin mRNA and protein. A: on ALI day 14, well-differentiated cells were exposed to either TGF-β1 or TGF-β2 for 48 h. TGF-β1 and TGF-β2 induce FURIN mRNA expression (B, median + IQR, n = 3–5, *P < 0.05) and furin protein as indicated by representative blots (C) and as determined from five donors (D) (A.U., normalized to GAPDH, median + IQR, n = 5, *P < 0.05).
TGF-β has been suggested as a target for COVID-19 therapies to potentially abrogate pathologic conditions in the lung during disease progression (38). In patients with COVID-19, the severity of the disease is often associated with ARDS, which is in turn associated with increased pulmonary edema and fibrosis attributable to the increased levels of active TGF-β (38). Here we suggest that activation of the TGF-β pathways could also potentially enhance the infectivity of SARS-CoV-2 by increasing cellular expression of furin, although further studies with live SARS-CoV-2 virus are required. The regulation of furin by TGF-β has been shown in rat synoviocytes, where furin is both increased by TGF-β1 and correlated with increased production of mature and active TGF-β1 (39). This previous report suggests activation of a positive feedback loop between furin and TGF-β1, resulting in amplified pathology. In addition, furin is indicated as a target gene of canonical TGF-β signaling pathway (40). If furin and TGF-β do play critical roles at multiple stages in COVID-19, including susceptibility to infection with SARS-CoV-2 and severity of the disease, they could be promising targets for therapy.
GRANTS
This work was supported in part by NIH Grants R01HL148152, T32HL007118, P30ES000002, and P30DK065988; Parker B. Francis Foundation; FUJIFILM Corporation; and The COVID-19 Acceleration Award by the Harvard T.H. Chan School of Public Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.O., J.A.M., and J-A.P. conceived and designed research; M.J.O., J.A.M., C.M., and M.M. performed experiments; M.J.O. and J.A.M. analyzed data; M.J.O., J.A.M., P.K., and J-A.P. interpreted results of experiments; M.J.O. and J.A.M. prepared figures; M.J.O., J.A.M., and J-A.P. drafted manuscript; M.J.O., J.A.M., C.M., P.K., and J-A.P. edited and revised manuscript; M.J.O., J.A.M., C.M., M.M., P.K., and J-A.P. approved final version of manuscript.
REFERENCES
- 1.Hoffmann M, Kleine-Weber H, Pohlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell 78: 779–784.e5, 2020. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182: 429–446.e14, 2020. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA 117: 11727–11734, 2020. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bestle D, Heindl MR, Limburg H, Van Lam van T, Pilgram O, Moulton H, Stein DA, Hardes K, Eickmann M, Dolnik O, Rohde C, Klenk HD, Garten W, Steinmetzer T, Bottcher-Friebertshauser E. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance 3, 2020. doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Anton-Plagaro C, Shoemark DK, Simon-Gracia L, Bauer M, Hollandi R, Greber UF, Horvath P, Sessions RB, Helenius A, Hiscox JA, Teesalu T, Matthews DA, Davidson AD, Collins BM, Cullen PJ, Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370: 861–865, 2020. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181: 281–292e286, 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, , et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January. retrospective cohort study. BMJ 369: m1443–2020, March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323: 2427, 2020. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, Billheimer D, Kraft M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol, 146: 80–88.e8, 2020. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 41: 1810–1817, 2020. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradding P, Richardson M, Hinks TSC, Howarth PH, Choy DF, Arron JR, Wenzel SE, Siddiqui S. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma-implications for COVID-19. J Allergy Clin Immunol, 146: 208–211, 2020. doi: 10.1016/j.jaci.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukassen S, Lorenz Chua R, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, Hennig BP, Kreuter M, Conrad C, Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. Embo J, 39, 2020. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Rostami MR, Leopold PL, Mezey JG, O’Beirne SL, Strulovici-Barel Y, Crystal RG. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med 202: 219–229, 2020. doi: 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, lung-network@humancellatlas.org HCALBNEa, , et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035.e19, 2020., and Network HCALB. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderheiden A, Ralfs P, Chirkova T, Upadhyay AA, Zimmerman MG, Bedoya S, Aoued H, Tharp GM, Pellegrini KL, Manfredi C, Sorscher E, Mainou B, Lobby JL, Kohlmeier JE, Lowen AC, Shi PY, Menachery VD, Anderson LJ, Grakoui A, Bosinger SE, Suthar MS. Type I and Type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol 94, 2020. doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 14: 104–112, 1996. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 19.Lan B, Mitchel JA, O’Sullivan MJ, Park CY, Kim JH, Cole WC, Butler JP, Park J-A. Airway epithelial compression promotes airway smooth muscle proliferation and contraction. Am J Physiol Lung Cell Mol Physiol, 315: L645–L652, 2018. doi: 10.1152/ajplung.00261.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchel JA, Das A, O’Sullivan MJ, Stancil IT, DeCamp SJ, Koehler S, Ocaña OH, Butler JP, Fredberg JJ, Nieto MA, Bi D, Park J-A. In primary airway epithelial cells, the unjamming transition is distinct from the epithelial-to-mesenchymal transition. Nat Commun 11: 5053, 2020. doi: 10.1038/s41467-020-18841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Sullivan MJ, Mitchel JA, Das A, Koehler S, Levine H, Bi D, Nagel ZD, Park JA. Irradiation induces epithelial cell unjamming. Front Cell Dev Biol 8: 21, 2020. doi: 10.3389/fcell.2020.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton DJ, Gray T, Nettesheim P, Howard M, Koo JS, Sheehan JK. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L1118–1128, 2000. doi: 10.1152/ajplung.2000.278.6.L1118. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak A, Tilley AE, Shaykhiev R, Wang R, Crystal RG. Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome? Am J Respir Cell Mol Biol 44: 465–473, 2011. doi: 10.1165/rcmb.2009-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross AJ, Dailey LA, Brighton LE, Devlin RB. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol 37: 169–185, 2007. doi: 10.1165/rcmb.2006-0466OC. [DOI] [PubMed] [Google Scholar]
- 25.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB. Jr.. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79: 14614–14621, 2005. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med 31: S258–264, 2003. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- 27.Frank JA, Matthay MA. TGF-beta and lung fluid balance in ARDS. Proc Natl Acad Sci U S A 111: 885–886, 2014. doi: 10.1073/pnas.1322478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morty RE, Konigshoff M, Eickelberg O. Transforming growth factor-beta signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 607–613, 2009. doi: 10.1513/pats.200908-087RM. [DOI] [PubMed] [Google Scholar]
- 29.Overgaard CE, Schlingmann B, Dorsainvil White S, Ward C, Fan X, Swarnakar S, Brown LA, Guidot DM, Koval M. The relative balance of GM-CSF and TGF-beta1 regulates lung epithelial barrier function. Am J Physiol Lung Cell Mol Physiol 308: L1212–1223, 2015. doi: 10.1152/ajplung.00042.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters DM, Vadasz I, Wujak L, Wygrecka M, Olschewski A, Becker C, Herold S, Papp R, Mayer K, Rummel S, Brandes RP, Gunther A, Waldegger S, Eickelberg O, Seeger W, Morty RE. TGF-beta directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc Natl Acad Sci USA 111: E374–383, 2014. doi: 10.1073/pnas.1306798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poli G, Kinter AL, Justement JS, Bressler P, Kehrl JH, Fauci AS. Transforming growth factor beta suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J Exp Med 173: 589–597, 1991. doi: 10.1084/jem.173.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murata T, Ohshima T, Yamaji M, Hosaka M, Miyanari Y, Hijikata M, Shimotohno K. Suppression of hepatitis C virus replicon by TGF-beta. Virology 331: 407–417, 2005. doi: 10.1016/j.virol.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 33.McCann KL, Imani F. Transforming growth factor beta enhances respiratory syncytial virus replication and tumor necrosis factor alpha induction in human epithelial cells. J Virol 81: 2880–2886, 2007. doi: 10.1128/JVI.02583-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrati C, Sacchi A, Bordoni V, Cimini E, Notari S, Grassi G, Casetti R, Tartaglia E, Lalle E, D'Abramo A, Castilletti C, Marchioni L, Shi Y, Mariano A, Song JW, Zhang JY, Wang FS, Zhang C, Fimia GM, Capobianchi MR, Piacentini M, Antinori A, Nicastri E, Maeurer M, Zumla A, Ippolito G. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19). Cell Death Differ 27: 3196–3207, 2020. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito A, Horie M, Nagase T. TGF-β signaling in lung health and disease. Int J Mol Sci 19: 2460, 2018. doi: 10.3390/ijms19082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chi M, Shi X, Huo X, Wu X, Zhang P, Wang G. Erratum to dexmedetomidine promotes breast cancer cell migration through Rab11-mediated secretion of exosomal TMPRSS2. Ann Transl Med 8: 1040–1040, 2020. doi: 10.21037/atm-2020-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun E, Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin Transl Immunol 8: e1073, 2019. doi: 10.1002/cti2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W. A potential treatment of COVID-19 with TGF-beta blockade. Int J Biol Sci 16: 1954–1955, 2020. doi: 10.7150/ijbs.46891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanchette F, Day R, Dong W, Laprise MH, Dubois CM. TGFbeta1 regulates gene expression of its own converting enzyme furin. J Clin Invest 99: 1974–1983, 1997. doi: 10.1172/JCI119365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng L, Dang TA, Schunkert H. Genetics links between transforming growth factor beta pathway and coronary disease. Atherosclerosis 253: 237–246, 2016. doi: 10.1016/j.atherosclerosis.2016.08.029. [DOI] [PubMed] [Google Scholar]