This randomized clinical trial evaluates the effectiveness of a telephone cognitive behavioral therapy for insomnia vs education-only control in older adults with moderate to severe osteoarthritis pain.
Key Points
Question
What is the effectiveness of brief telephone cognitive behavioral therapy for insomnia (CBT-I) in reducing chronic insomnia symptoms among older adults with moderate to severe osteoarthritis pain?
Findings
In this randomized clinical trial of 282 participants 60 years and older across Washington state, 56.3% of participants receiving CBT-I remained in remission (Insomnia Severity Index score, ≤7) at 12 months compared with 25.8% of participants in the education-only control group.
Meaning
Telephone CBT-I may improve access to an individualized and effective treatment for chronic insomnia among older persons with osteoarthritis pain, including those in rural and medically underserved areas.
Abstract
Importance
Scalable delivery models of cognitive behavioral therapy for insomnia (CBT-I), an effective treatment, are needed for widespread implementation, particularly in rural and underserved populations lacking ready access to insomnia treatment.
Objective
To evaluate the effectiveness of telephone CBT-I vs education-only control (EOC) in older adults with moderate to severe osteoarthritis pain.
Design, Setting, and Participants
This is a randomized clinical trial of 327 participants 60 years and older who were recruited statewide through Kaiser Permanente Washington from September 2016 to December 2018. Participants were double screened 3 weeks apart for moderate to severe insomnia and osteoarthritis (OA) pain symptoms. Blinded assessments were conducted at baseline, after 2 months posttreatment, and at 12-month follow-up.
Interventions
Six 20- to 30-minute telephone sessions provided over 8 weeks. Participants submitted daily diaries and received group-specific educational materials. The CBT-I instruction included sleep restriction, stimulus control, sleep hygiene, cognitive restructuring, and homework. The EOC group received information about sleep and OA.
Main Outcomes and Measures
The primary outcome was score on the Insomnia Severity Index (ISI) at 2 months posttreatment and 12-month follow-up. Secondary outcomes included pain (score on the Brief Pain Inventory-short form), depression (score on the 8-item Patient Health Questionnaire), and fatigue (score on the Flinders Fatigue Scale).
Results
Of the 327 participants, the mean (SD) age was 70.2 (6.8) years, and 244 (74.6%) were women. In the 282 participants with follow-up ISI data, the total 2-month posttreatment ISI scores decreased 8.1 points in the CBT-I group and 4.8 points in the EOC group, an adjusted mean between-group difference of −3.5 points (95% CI, −4.4 to −2.6 points; P < .001). Results were sustained at 12-month follow-up (adjusted mean difference, −3.0 points; 95% CI, −4.1 to −2.0 points; P < .001). At 12-month follow-up, 67 of 119 (56.3%) participants receiving CBT-I remained in remission (ISI score, ≤7) compared with 33 of 128 (25.8%) participants receiving EOC. Fatigue was also significantly reduced in the CBT-I group compared with the EOC group at 2 months posttreatment (mean between-group difference, −2.0 points; 95% CI, −3.1 to −0.9 points; P = <.001) and 12-month follow-up (mean between-group difference, −1.8 points; 95% CI, −3.1 to −0.6 points; P = .003). Posttreatment significant differences were observed for pain, but these differences were not sustained at 12-month follow-up.
Conclusions and Relevance
In this randomized clinical trial, telephone CBT-I was effective in improving sleep, fatigue, and, to a lesser degree, pain among older adults with comorbid insomnia and OA pain in a large statewide health plan. Results support provision of telephone CBT-I as an accessible, individualized, effective, and scalable insomnia treatment.
Trial Registration
Clinical Trials.gov Identifier: NCT02946957
Introduction
Insomnia, common among older adults, adversely affects daily function, quality of life, and health, and frequently occurs alongside other chronic conditions.1 Osteoarthritis (OA) affects 50% of older adults, more than half of whom complain of disturbed sleep, which makes OA-related insomnia a common comorbidity in older adults.2,3,4 Insomnia and chronic pain have reciprocal effects, each initiating, maintaining, and exacerbating one another.5,6,7,8,9
Cognitive behavioral therapy for insomnia (CBT-I), efficacious for individuals with comorbid conditions,10 is the first line of treatment for insomnia.11 It has been found to improve sleep in various populations with chronic pain and insomnia, with lesser benefits for pain and fatigue.12 However, CBT-I is not widely available, especially in medically underserved or health professional shortage areas (MU/HPSAs), and patients are rarely referred for treatment.13,14
The lack of scalable, accessible, and individualized treatment for insomnia has long been recognized.15,16,17,18 Web-based, telehealth, and mobile phone apps require supporting technology, whereas basic telephone services are almost universally available, even in MU/HPSAs. A few studies have reported that telephone CBT-I improved sleep, but these were limited by small sample sizes and inclusion of only patients from specialty insomnia clinics.19,20,21,22,23 The Osteoarthritis and Therapy for Sleep (OATS) study24 is, to our knowledge, the first large randomized trial to test telephone CBT-I, compared with attention control, in a statewide population of older adults with moderate to severe chronic OA pain and insomnia. Herein, we assess telephone CBT-I effectiveness for treating insomnia symptoms, as well as important comorbidities including pain, depression, and fatigue at 2 months posttreatment and 12-month follow-up.
Methods
Participants
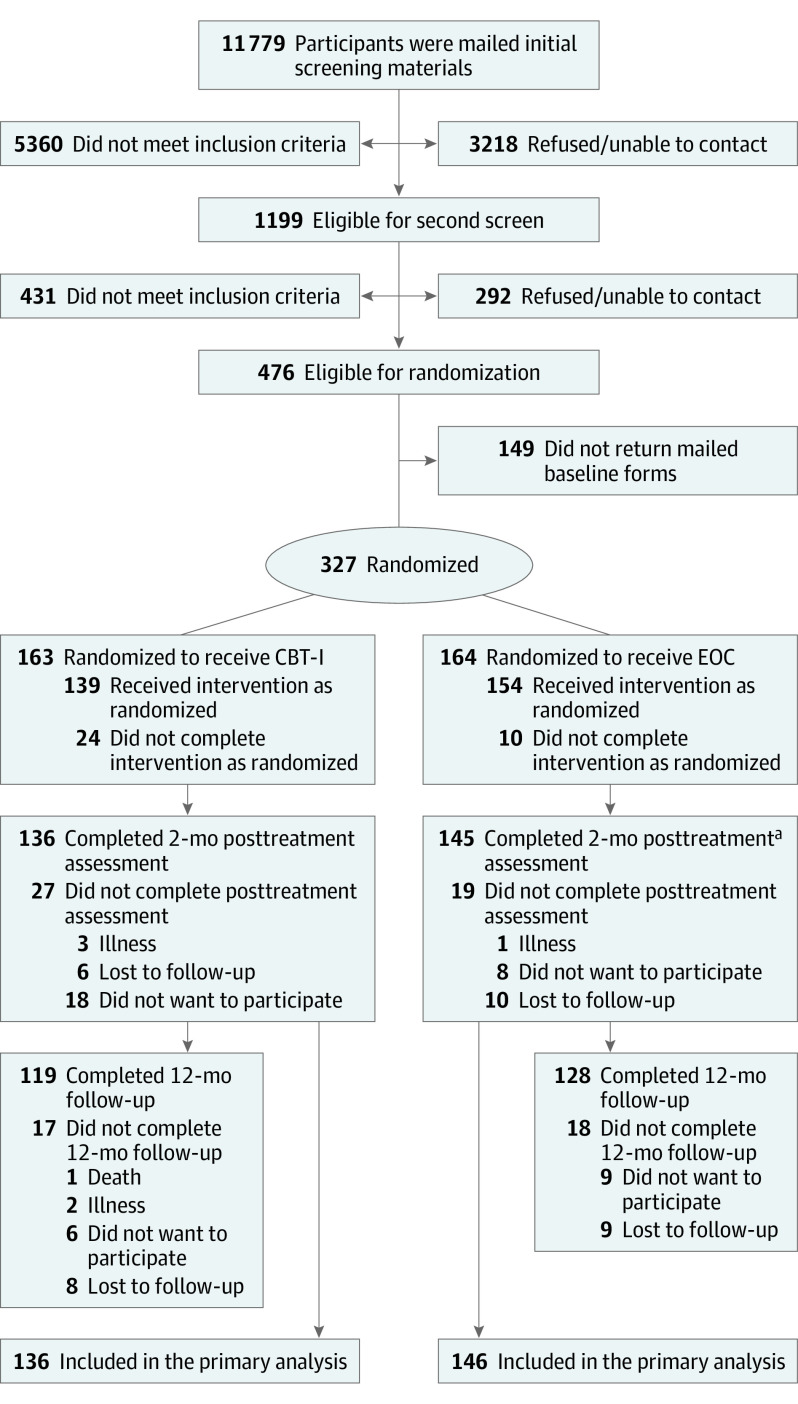
This study was conducted among Kaiser Permanente Washington (KPW) patients who were recruited September 2016 through December 2018. Participants were identified statewide using KPW electronic health records. Potential participants were 60 years and older, continuously enrolled at KPW for at least 1 year, and had a diagnosis of OA in the 3 years prior to screening. At initial telephone screening, eligible participants scored 11 or greater on the Insomnia Severity Index (ISI)25 with a sum score of 9 or greater on a 2-question Brief Pain Inventory-short form (BPI-sf). Double screening 3 weeks apart was used to recruit patients with chronic sleep and OA-related pain symptoms. Exclusions included sleep disorder diagnosis other than insomnia (eg, sleep apnea), rheumatoid arthritis, active cancer or congestive heart failure, or a diagnosis of cognitive impairment.26 Participants were not excluded because of other chronic illnesses, including psychiatric disorders, or because they were taking prescription or over-the-counter sleep or pain medications. The University of Washington Institutional Review Board reviewed and approved the study protocol (Supplement 1), and this study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Verbal informed consent was obtained from participants at initial telephone screenings, and written informed consent was obtained when baseline assessment materials were sent. Detailed study methods have been published previously.24 Screening, eligibility, and study participation details are reported in Figure 1.
Figure 1. CONSORT Diagram.
CBT-I indicates cognitive behavioral therapy for insomnia; EOC, education-only control.
aOne participant in the EOC group did not provide 2-month posttreatment data but did provide 12-month follow-up data.
Randomization
The OATS trial used a permuted block randomization with random block sizes and patients randomly assigned to CBT-I or education-only control (EOC) groups within each block.24 Participants were told that the study compared 2 educational treatments for sleep problems in persons with OA pain, but they were not informed how groups differed.
Interventions
The CBT-I and EOC interventions both consisted of six 20- to 30-minute telephone sessions provided over 8 weeks. Group-specific materials were mailed to participants before the first session. Daily sleep diaries were kept by all participants.
Cognitive Behavioral Therapy for Insomnia
The CBT-I protocol was adapted from a standardized telephone CBT-I manual.23 Participants were given an in-bed restriction plan that initially matched the average sleep time reported in baseline diaries but was no fewer than 6.0 hours. The in-bed window was extended by 15 minutes when the sleep diary indicated an average 85% or greater sleep efficiency (time asleep divided by time in bed) the previous week. Stimulus control instructions strengthened the association between bed and sleep by reducing time spent in bed on nonsleep activities. Sleep hygiene education included information about improving bedtime routines and environment. Cognitive strategies included constructive worry and mindfulness techniques to reduce hyperarousal at night, along with education about age-related sleep changes and realistic sleep expectations.27 Intervention content details24 are available in eMethods 1 in Supplement 2.
Education-Only Control
The EOC protocol followed principles of credible behavioral attention control conditions for insomnia clinical trials.28 The EOC intervention was designed to control for nonspecific treatment effects (eg, social support and education). Sessions were conducted in an informative, supportive, nondirective format, with education relevant to living with chronic OA (eMethods 2 in Supplement 2). There was no instruction in CBT-I principles and no homework prescribed except to keep a sleep diary and read the educational booklet.
Study Coaches, Training, and Treatment Fidelity
All participants had telephone contact with a study interventionist (hereafter known as a coach) 6 times over 8 weeks. Coaches included an MS-level psychologist, a PhD nurse, and a PhD social worker. All coaches delivered both manual-based interventions. Coaches received 1-day trainings for each intervention condition led by experts in CBT-I (S.M.M.) and OA pain education (M.T.). Telephone sessions were recorded.24
Training included review by a coauthor (S.M.M.) of all 6 recordings for 3 pilot cases (2 CBT-I and 1 EOC) for each coach. Thereafter, 2 sessions for each participant (1 randomly selected and 1 chosen by either the coach or reviewer) were reviewed to maintain treatment fidelity and ensure no cross-treatment contamination. Fidelity reviews were discussed during weekly team meetings.
Measures
Blinded assessments included a primary sleep outcome and secondary outcome measures of pain, depression, and fatigue. Assessments were collected at baseline (before randomization), posttreatment (2 months), and follow-up (12 months).
Baseline Characteristics
Participants self-reported age, sex, race, education, marital status, and duration of sleep and pain disturbances. Medications (antidepressant, opioid, and sedative/hypnotic) and Charlson Comorbidity Index score were derived from electronic health records. An MU/HPSA residence was determined from participant zip codes.
Treatment Evaluations
At the end of the first intervention session and at posttreatment assessment, participants completed intervention suitability, acceptability, and perceived efficacy surveys.28,29 Items included: (1) Does this treatment and its rationale make sense to you?; (2) How acceptable do you consider this treatment?; (3) How suitable is this treatment for improving your quality of life despite having OA?; (4) How effective do you expect this treatment to be?; (5) How well do you think you will be able to adhere to this treatment program?; and (6) How would you rate the quality of your working relationship with your OA coach? Items were rated on a 1-7 scale with a higher score indicating better assessment.
Primary Sleep Outcome
The primary outcome was score on the ISI,25,30 a 7-item questionnaire assessing insomnia severity, at 2 months posttreatment and 12-month follow-up. Items are rated 0 to 4 (total score range, 0-28), with a score of 15 or greater indicating moderate to severe insomnia. A score of 10 or greater is considered optimal for detecting insomnia cases in community samples, and a 6-point within-group reduction is a clinically meaningful change.31 An ISI score of 7 or less indicates insomnia remission.30
Secondary Outcomes
Arthritis pain intensity and interference with activities were measured using an 11-item BPI-sf.32 The BPI-sf rates pain severity (4 items; BPI-Severity) and interference (7 items; BPI-Interference) from 0 to 10, with higher scores reflecting greater pain and activity interference.
Depression was measured using the 8-item Patient Health Questionnaire (PHQ-8).33 The PHQ-8 rates frequency of occurrence of 8 depressive symptoms on a 4-point scale. Scores of 5 to 9 indicate mild or subclinical depression, and scores of 10 or greater indicate depression.34,35
Fatigue was measured using the Flinders Fatigue Scale (FFS),36 a 7-item self-reported questionnaire measuring fatigue level in a variety of situations. A higher score indicates worse fatigue. Cronbach α for the ISI, BPI-Interference, BPI-Severity, PHQ-8, and FFS were 0.87, 0.93, 0.88, 0.83, and 0.91, respectively.
Statistical Analysis
Analyses of primary and secondary outcomes were conducted with complete-case data (n = 282) following intent-to-treat principles. Randomized participants were included in the analysis if they had at least 1 outcome measure at either posttreatment or follow-up. The study was designed to have 80% power to detect an expected difference of 1.5 points corresponding to an effect size of 0.30 on the primary outcome (ISI score).24
Summaries of baseline patient characteristics by treatment group were computed, and standardized differences between treatment groups were examined to identify potential imbalances.37 For the primary outcome, adjusted mean differences in ISI change scores between the CBT-I and EOC groups from baseline to 2 months posttreatment and 12-month follow-ups were estimated in a single model using generalized estimating equations with working independence and robust standard errors to account for correlation between repeated measures within individuals. The analysis model included change in ISI score at each follow-up as the dependent variable, as well as baseline ISI score (analysis of covariance), treatment group, time (2 months posttreatment or 12-month follow-up), and an interaction between treatment group and time as the primary independent variables. Adjustments for baseline age, pain, depression, fatigue, and use of opioids, hypnotics, and antidepressants were included per a priori specification. To account for observed imbalances between treatment groups in complete-case data at baseline, additional adjustments were included for race, education, and the Charlson Comorbidity Index.
The same analytic approach was used for all continuous secondary outcomes and post hoc analyses for PHQ-8 and FFS without sleep-related items, with the addition of adjustment for baseline ISI score. A comparable post hoc subgroup analysis was performed on participants (n = 160) with severe baseline pain and insomnia (BPI-sf score, ≥5.0 and ISI score, ≥15, respectively).
For all outcomes, in addition to estimating the difference in the change between treatment groups at 2 months posttreatment and 12-month follow-up, we also estimated the proportion of individuals who were considered treatment responders (30% reduction in scores at both time points).38 These estimates were obtained with logistic regression for repeated measures fit with generalized estimating equations using working independence and robust standard errors. The functional form of the model, including adjustment variables, was identical to that described above for the analysis of change. We also performed a post hoc analysis examining the proportion of insomnia remitters at both time points (total ISI score, ≤7).30
Sensitivity analyses were performed using multiple imputation of missing outcome data to relax the assumption that outcomes were missing completely at random in favor of the assumption that data are missing at random.39 In addition to variables included in the complete-case models, the imputation models included information on self-reported duration of sleep problems, number of days of pain in the last year, and MU/HPSA and marital status. Ten imputed data sets were created using imputation by chained equations,39 and each imputed data set was analyzed separately with the same methodology that was used in the complete-case analyses. The corresponding estimates were combined using Rubin rules.40 Analyses were conducted with SAS, version 9.4 (SAS Institute Inc), for Microsoft Windows and IVEware: Imputation and Variance Estimation Software, version 0.3 (University of Michigan), for Microsoft Windows. For all analyses, a 2-sided P < .05 was regarded as statistically significant.
Results
A total of 327 participants (mean (SD) age, 70.2 (6.8) years; 244 [74.6%] women) were randomly assigned to the 2 experimental conditions (Table 1). Because 12-month response rates were lower than initially projected (76% vs 85%), we increased the randomized sample from the originally planned 270 before examining study results.
Table 1. Baseline Characteristics By Intervention Group Among the Full Randomized Sample (N = 327).
| Baseline characteristic | Participants, No. (%) | Standardized difference | |
|---|---|---|---|
| CBT-I group (n = 163) | EOC group (n = 164) | ||
| Age, mean (SD), y | 70.1 (7.1) | 70.4 (6.5) | −0.04 |
| Sex | |||
| Male | 39 (23.9) | 44 (26.8) | 0.07 |
| Female | 124 (76.1) | 120 (73.2) | |
| Race | |||
| White | 0.33 | ||
| Non-Hispanic | 143 (87.7) | 123 (75.0) | |
| Hispanic | 16 (9.8) | 31 (18.9) | |
| Non-White | 4 (2.5) | 10 (6.1) | |
| Educational level | |||
| No college/some college | 77 (47.2) | 92 (56.1) | −0.18 |
| College graduate or higher | 86 (52.8) | 72 (43.9) | |
| Marital status | |||
| Married/living with partner | 107 (65.6) | 108 (65.8) | −0.01 |
| Single/divorced/living alone/unknown | 56 (34.4) | 56 (34.1) | |
| Living in medically underserved or health professional shortage area | 105 (64.4) | 109 (66.5) | −0.04 |
| Charlson Comorbidity Index score | |||
| 0 | 106 (65.0) | 97 (59.2) | 0.14 |
| 1 | 28 (17.2) | 29 (17.7) | |
| ≥2 | 29 (17.8) | 38 (23.2) | |
| When sleep problem started | |||
| In the past y | 22 (13.5) | 18 (11.0) | 0.08 |
| >1 y ago | 141 (86.5) | 146 (89.0) | |
| Pain in past 6 mo, No. of d | |||
| No d/less than half of the d | 24 (14.7) | 28 (17.1) | −0.06 |
| At least half of the d or more | 139 (85.3) | 136 (82.9) | |
| Antidepressants use | 23 (14.0) | 28 (17.1) | −0.08 |
| Opioid use | 35 (21.5) | 29 (17.7) | 0.10 |
| Sedative/hypnotic use | 8 (4.9) | 10 (6.1) | −0.05 |
| Blessed Short Orientation-Memory-Concentration Test score, mean (SD) | 2.1 (1.9) | 2.4 (2.1) | 0.16 |
| Insomnia Severity Index score, mean (SD) | 15.5 (3.3) | 15.5 (3.3) | 0.003 |
| Brief Pain Inventory-short form score, mean (SD) | |||
| Total | 9.7 (3.0) | 9.4 (3.0) | 0.09 |
| Severity subscale | 4.7 (1.6) | 4.6 (1.5) | 0.04 |
| Interference subscale | 5.0 (1.8) | 4.8 (1.8) | 0.12 |
| Patient Health Questionnaire, depression scale score, mean (SD) | 6.6 (3.7) | 7.2 (3.7) | −0.18 |
| Flinders Fatigue Scale | 14.2 (5.8) | 14.1 (5.0) | −0.02 |
Abbreviations: CBT-I, cognitive behavioral therapy for insomnia; EOC, education-only control.
Treatment Discontinuation and Adherence
Study retention was 83% and 88% at 2 months and 73% and 78% at 12 months for CBT-I vs EOC, respectively (Figure 1). Most participants completed all 6 CBT-I (130 of 163 [80.0%]) or EOC (147 of 164 [89.6%]) sessions. The MS-level coach delivered the most treatment sessions (267 of 327 [81.6%]). There were no significant differences between treatment conditions in number of telephone sessions attended, session length (median, 23.4 and 23.0 minutes for CBT-I and EOC, respectively), or distribution of CBT-I vs EOC participants among coaches.
Baseline Characteristics
Baseline characteristics by group for the full OATS sample are presented in Table 1. The CBT-I and EOC recipients were similar in age, sex, and MU/HPSA and marital status. Average baseline insomnia, fatigue, and pain scores were comparable. Small differences were observed in medication use, duration of sleep problems, and number of days of pain in the prior 6 months. Moderate differences in participant race, education, and comorbidity score were observed, where participants in the CBT-I group vs EOC group were more frequently White (143 of 163 [87.7%] vs 123 of 164 [75.0%]), more likely to be college graduates (86 of 163 [52.8%] vs 72 of 164 [43.9%]), and less likely to have a Charlson Comorbidity Index score of 1 or greater (57 of 163 [35.0%] vs 67 of 164 [40.9%]).
Treatment Evaluations
Most participants rated treatments favorably (≥5 points on a 7-point scale) on the 6 treatment perception ratings (CBT-I range, 75.9%-99.3%; EOC, 69.0%-97.9%). Average summed treatment perceptions improved between session 1 and 2 months posttreatment for both groups (mean, 5.9 vs 6.1 points for CBT-I and 5.6 vs 5.8 points for EOC).
Primary Sleep Outcomes
In complete-case analyses (n = 282), from baseline to 2 months posttreatment the average raw score ISI decreased 8.1 points in the CBT-I group and 4.8 points in the EOC group, an adjusted between-group difference of −3.5 points (95% CI, −4.4 to −2.6 points; P < .001). Group differences were sustained at 12-month follow-up (P < .001; Table 2). In post hoc analyses, MU/HPSA status had no significant treatment interaction with ISI score. Baseline characteristics for complete-case participants can be found in eTable 1 in Supplement 2.
Table 2. Primary and Secondary Outcome Results By Treatment Condition in Complete-Case Analysis (n = 282).
| Outcome | Mean (SD) score | Adjusted mean difference (95% CI)a | Pairwise P valueb | |
|---|---|---|---|---|
| CBT-I group (n = 136) | EOC group (n = 146) | |||
| Insomnia Severity Index | ||||
| Baseline | 15.3 (3.2) | 15.5 (3.2) | 1 [Reference] | NA |
| Month 2 (posttreatment) | 7.2 (4.0) | 10.7 (4.3) | −3.51 (−4.43 to −2.59) | <.001 |
| Month 12 (follow-up) | 7.7 (4.5) | 10.8 (4.5) | −3.04 (−4.12 to −1.96) | <.001 |
| Brief Pain Inventory-short form (Severity) | ||||
| Baseline | 4.6 (1.6) | 4.6 (1.5) | 1 [Reference] | NA |
| Month 2 (posttreatment) | 3.6 (1.9) | 3.9 (1.7) | −0.37 (−0.73 to −0.01) | .05 |
| Month 12 (follow-up) | 3.4 (1.7) | 3.5 (1.9) | −0.06 (−0.46 to 0.33) | .75 |
| Brief Pain Inventory-short form (Interference) | ||||
| Baseline | 5.0 (1.8) | 4.7 (1.8) | 1 [Reference] | NA |
| Month 2 (posttreatment) | 3.3 (2.0) | 3.7 (2.1) | −0.51 (−0.93 to −0.10) | .02 |
| Month 12 (follow-up) | 3.1 (2.2) | 3.2 (2.2) | −0.19 (−0.68 to 0.30) | .45 |
| Patient Health Questionnaire for depression | ||||
| Baseline | 6.4 (3.6) | 7.2 (3.7) | 1 [Reference] | NA |
| Month 2 (posttreatment) | 3.7 (3.2) | 5.1 (3.5) | −1.06 (−1.70 to −0.42) | .001 |
| Month 12 (follow-up) | 3.6 (3.6) | 4.7 (3.5) | −0.64 (−1.40 to 0.12) | .10 |
| Flinders Fatigue Scale | ||||
| Baseline | 14.1 (5.8) | 14.1 (5.1) | 1 [Reference] | NA |
| Month 2 (posttreatment) | 8.8 (5.7) | 10.9 (6.1) | −1.97 (−3.07 to −0.88) | <.001 |
| Month 12 (follow-up) | 7.9 (5.9) | 10.2 (5.8) | −1.84 (−3.08 to 0.61) | .003 |
Abbreviations: CBT-I, cognitive behavioral therapy for insomnia; EOC, education-only control; NA, not applicable.
Means adjusted for baseline values of relevant outcome, age, race, educational level, Charlson Comorbidity Index score, and drug (antidepressant, opioid, and sedative/hypnotic) use.
Pairwise P values for CBT-I minus EOC. P values correspond to a 2-sided test of the null hypothesis that the difference between the 2 means is zero.
Secondary Outcomes
Fatigue outcomes paralleled those observed for ISI score. From baseline to 2 months posttreatment, the FFS decreased 5.3 points in the CBT-I group and 3.1 points in the EOC group, an adjusted mean difference of −2.0 points (95% CI, −3.1 to −0.9 points; P < .001), a difference sustained at 12-month follow-up (P = .003; Table 2). When the single sleep-related item was removed from the FFS, results were unchanged.
Short-term between-group differences were observed in change scores for pain (BPI-Severity and BPI-Interference) and depression (PHQ-8) at 2 months posttreatment (Table 2) but were not significant at 12-month follow-up. When the single sleep-related item was removed from the PHQ-8, the posttreatment difference did not exceed chance expectation.
Sensitivity and Imputed Data Analyses
Imputed analyses did not yield appreciable differences from the primary and secondary adjusted complete-case analyses. For example, the posttest adjusted mean difference in ISI score for the full sample was −3.36 (95% CI, −4.26 to −2.46), compared with −3.51 (95% CI, −4.43 to −2.59) for the complete-case analyses (eTables 2 and 3 in Supplement 2).
Responder Analyses
For the ISI and FFS, the likelihood of clinically meaningful treatment response from baseline to follow-up, defined as a 30% or greater reduction in symptom severity,38 was substantially greater in the CBT-I group than in the EOC group (Table 3). The adjusted odds ratio (AOR) comparing the proportion with clinically meaningful improvement in ISI score from baseline for CBT-I vs EOC exceeded 4 at both 2 months posttreatment (AOR, 4.9 [95% CI, 2.8-8.8]) and 12-month follow-up (AOR, 5.0 [95% CI, 2.8-9.0]). The AOR for clinically meaningful improvement in fatigue from baseline among CBT-I vs EOC recipients at 2 months posttreatment was 3.0 (95% CI. 1.8-5.0) and at 12-month follow-up was 2.8 (95% CI, 1.6-4.9). The AORs also showed CBT-I recipients were more likely than EOC recipients to have a clinically meaningful improvement in BPI-sf pain scores at 2 months posttreatment but not at 12-month follow-up (Table 3). There was a small effect at both time points in the PHQ-8, which was not sustained when the insomnia item was removed.
Table 3. Participants With ≥30% Improvement From Baseline for Insomnia, Pain, Depression, and Fatigue at Posttreatment and Follow-up (n = 282).
| Measurement | Group | Posttreatment (2 mo) | Follow-up (12 mo) | ||||
|---|---|---|---|---|---|---|---|
| % | Odds ratio (95% CI)a | P valueb | % | Odds ratio (95% CI) | P value | ||
| Insomnia Severity Index | CBT-I | 81 | 4.93 (2.78-8.76) | <.001 | 80 | 4.97 (2.75-8.96) | <.001 |
| EOC | 49 | 47 | |||||
| Brief Pain Inventory–short form (Severity) | CBT-I | 40 | 2.08 (1.22-3.54) | .01 | 40 | 1.20 (0.69-2.06) | .52 |
| EOC | 28 | 39 | |||||
| Brief Pain Inventory–short form (Interference) | CBT-I | 55 | 2.16 (1.28-3.64) | .004 | 62 | 1.44 (0.83-2.47) | .19 |
| EOC | 38 | 54 | |||||
| Patient Health Questionnaire for depression | CBT-I | 61 | 1.89 (1.13-3.16) | .02 | 69 | 1.87 (1.08-3.25) | .03 |
| EOC | 48 | 57 | |||||
| Flinders Fatigue Scale | CBT-I | 65 | 2.95 (1.76-4.97) | <.001 | 71 | 2.80 (1.59-4.92) | <.001 |
| EOC | 38 | 44 | |||||
Abbreviations: CBT-I, cognitive behavioral therapy for insomnia; EOC, education-only control.
Adjusted odds ratios for the proportion with at least 30% reduction from baseline to follow-up comparing CBT-I with EOC at 2 months and at 12 months. Odds ratios are adjusted for baseline values of relevant outcome, age, race, educational level, Charlson Comorbidity Index score, and drug (antidepressant, opioid, and sedative/hypnotic) use.
Pairwise P values for CBT-I minus EOC. P values correspond to a 2-sided test of the null hypothesis that the difference between the 2 means is zero.
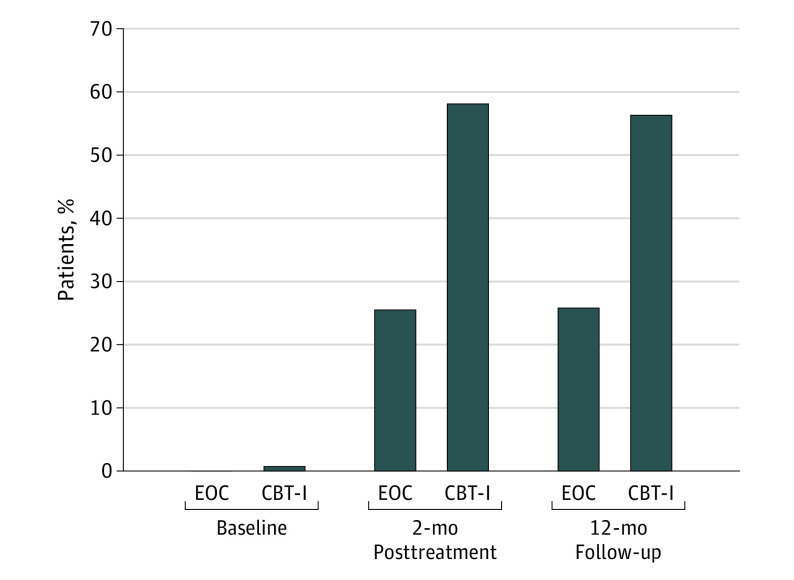
Post hoc analyses examined the proportion of individuals classified as insomnia remitters. At baseline, there was 1 participant in the CBT-I group and none in the EOC group with total ISI scores in the range of no insomnia (0-7 points). At 2 months posttreatment, 79 of 136 (58.1%) in the CBT-I group vs 37 of 145 (25.5%) in the EOC group, and at 12-month follow-up 67 of 119 (56.3%) in the CBT-I group vs 33 of 128 (25.8%) in the EOC group, were classified as having no insomnia (Figure 2). The OR comparing the proportion of remitters from baseline in the CBT-I group vs the EOC group was significant at both 2 months posttreatment (OR, 5.9 [95% CI, 3.3-10.7]) and 12-month follow-up (OR, 6.0 [95% CI, 3.1-11.7]).
Figure 2. Insomnia Severity Index Remission Over Time.
Scores in the range of no insomnia (0-7 points) are shown. CBT-I indicates cognitive behavioral therapy for insomnia; EOC, education-only control.
Severe Subgroup Analyses
Post hoc subgroup analyses performed on participants (n = 160) with severe baseline pain and insomnia (BPI-sf score, ≥5.0 and ISI score, ≥15) showed a pattern of results comparable with the overall analyses (eTable 4 in Supplement 2).
Adverse Events
During the course of the study, there were a total of 99 adverse events; 37 vs 31 events occurred baseline to 2 months posttreatment, and 18 vs 13 events occurred 2 months posttreatment to 12-month follow-up in the CBT-I group and EOC group, respectively. There were 4 serious adverse events (SAEs) baseline to 2 months posttreatment and 23 SAEs 2 months posttreatment to 12-month follow-up. No SAEs were determined to be related to treatment.
Discussion
Statewide provision of telephone CBT-I improved insomnia among older persons with chronic insomnia and comorbid OA pain. Treatment effects for insomnia were large, robust, and sustained at 12-month follow-up, with comparable benefits among persons with more severe insomnia and pain symptoms at baseline. The CBT-I intervention also reduced fatigue, indicating improved daytime function. Less robust CBT-I benefits were observed for pain. Differences between the CBT-I and EOC groups for depression were modest and not consistently statistically significant, perhaps because relatively few patients had elevated baseline depression scores.
Strengths
To our knowledge, the OATS trial is the largest controlled evaluation of telephone CBT-I to date. Participants were recruited statewide, with two-thirds residing in MU/HPSAs. Health plan participants were selected with few eligibility restrictions, increasing generalizability of study findings to primary care patients with OA who had limited or no access to CBT-I services. Two-stage screening ensured participants had chronic insomnia and pain. A final strength was the use of telephone EOC to control for nonspecific treatment effects.
Prior studies of CBT-I for pain, with smaller samples and less rigorous control groups, have yielded mixed results.41 The current study shows that CBT-I was associated with short-term reductions in pain and suggests the possibility of a small and transient reciprocal sleep-pain relationship. However, improvements were not sustained long-term, and further research is needed.
Limitations
The study has limitations. Only KPW enrollees participated, thus findings may not be representative of other health care systems. The study sample was highly educated and predominately White. However, we enrolled persons statewide and included persons in MU/HPSAs, which somewhat mitigates these limitations. We did not evaluate primary sleep disorders (eg, sleep apnea), so we were unable to examine whether treatment effects were consistent for participants with such undiagnosed conditions. However, in clinical practice, patients with insomnia symptoms and undiagnosed or untreated sleep disorders often seek CBT-I treatment. Interventionists were not blinded to treatment assignment; however, all outcomes were collected by blinded research staff. Finally, outcomes were based on self-report, but for purposes of clinical practice and potential treatment scalability, these are important measures of effectiveness.
Conclusions
In conclusion, the OATS trial demonstrated short-term and long-term effectiveness of telephone CBT-I vs a credible attention control to improve sleep, fatigue, and, to a lesser degree, pain in a large statewide population of older adults with chronic insomnia and OA pain. The OATS trial findings have broad implications given that two-thirds of the study sample resided in MU/HPSAs where access to individualized specialized treatment, such as CBT-I, is limited at best and often nonexistent.
The ongoing coronavirus disease 2019 pandemic highlights the importance of being able to deliver effective health care remotely through a modality as widely available as the telephone. The OATS trial telephone CBT-I protocol is readily scalable and provides a template for accessible, individualized, and effective treatment for chronic insomnia comorbid with OA in older adult populations. Given abundant evidence that CBT-I is efficacious for persons with other comorbid conditions, including older adults with chronic pain,10,12,42 we believe the OATS trial findings are likely to be generalizable beyond the present OA study population.
Trial Protocol
eMethods 1. Cognitive-behavioral therapy for insomnia in older adults with osteoarthritis pain: Session outlines
eMethods 2. Treatment of sleep problems in older persons with osteoarthritis pain: education only: Session outlines
eTable 1. Baseline characteristics by intervention group, completer analysis (n=282)
eTable 2. Primary and secondary outcome results by treatment condition, all participants (n=327), using imputed data
eTable 3. Percentage of CBT-I and EOC responders (> 30% improvement from baseline) for insomnia, pain, depression, and fatigue at 2 months (post-treatment) and 12 months (n=327), using imputed data
eTable 4. Primary and secondary outcome results by treatment condition for severe subgroup participants (BPI > 5, ISI scores > 15) (n=160)
Data Sharing Statement
References
- 1.Wennberg AM, Canham SL, Smith MT, Spira AP. Optimizing sleep in older adults: treating insomnia. Maturitas. 2013;76(3):247-252. doi: 10.1016/j.maturitas.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM. Osteoarthritis and sleep: the Johnston County Osteoarthritis Project. J Rheumatol. 2008;35(6):1102-1107. [PMC free article] [PubMed] [Google Scholar]
- 3.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502. doi: 10.1016/j.jpsychores.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Sarzi-Puttini P, Cimmino MA, Scarpa R, et al. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35(1)(suppl 1):1-10. doi: 10.1016/j.semarthrit.2005.01.013 [DOI] [PubMed] [Google Scholar]
- 5.Blay SL, Andreoli SB, Gastal FL. Chronic painful physical conditions, disturbed sleep and psychiatric morbidity: results from an elderly survey. Ann Clin Psychiatry. 2007;19(3):169-174. doi: 10.1080/10401230701468099 [DOI] [PubMed] [Google Scholar]
- 6.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539-1552. doi: 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145(1-2):136-141. doi: 10.1016/j.pain.2009.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13(6):447-454. doi: 10.1007/s11916-009-0073-2 [DOI] [PubMed] [Google Scholar]
- 9.Tiede W, Magerl W, Baumgärtner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148(1):36-42. doi: 10.1016/j.pain.2009.08.029 [DOI] [PubMed] [Google Scholar]
- 10.Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472. doi: 10.1001/jamainternmed.2015.3006 [DOI] [PubMed] [Google Scholar]
- 11.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians . Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133. doi: 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 12.Tang NK, Lereya ST, Boulton H, Miller MA, Wolke D, Cappuccio FP. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38(11):1751-1764. doi: 10.5665/sleep.5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koffel E, Bramoweth AD, Ulmer CS. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med. 2018;33(6):955-962. doi: 10.1007/s11606-018-4390-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds SA, Ebben MR. The cost of insomnia and the benefit of increased access to evidence-based treatment: cognitive behavioral therapy for insomnia. Sleep Med Clin. 2017;12(1):39-46. doi: 10.1016/j.jsmc.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 15.Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID-19 outbreak: practical recommendations from a task force of the European CBT-I Academy. J Sleep Res. 2020;29(4):e13052. doi: 10.1111/jsr.13052 [DOI] [PubMed] [Google Scholar]
- 16.Hartley S, Colas des Francs C, Aussert F, et al. The effects of quarantine for SARS-CoV-2 on sleep: an online survey. Encephale. 2020;46(3S):S53-S59. doi: 10.1016/j.encep.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907. doi: 10.1016/j.bbi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Lan M, Li H, Yang J. Perceived stress and sleep quality among the non-diseased general public in China during the 2019 coronavirus disease: a moderated mediation model. Sleep Med. 2020;S1389-9457(20)30224-0. doi: 10.1016/j.sleep.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnedt JT, Cuddihy L, Swanson LM, Pickett S, Aikens J, Chervin RD. Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. Sleep. 2013;36(3):353-362. doi: 10.5665/sleep.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastien CH, Morin CM, Ouellet MC, Blais FC, Bouchard S. Cognitive-behavioral therapy for insomnia: comparison of individual therapy, group therapy, and telephone consultations. J Consult Clin Psychol. 2004;72(4):653-659. doi: 10.1037/0022-006X.72.4.653 [DOI] [PubMed] [Google Scholar]
- 21.Farrand P, Woodford J. Impact of support on the effectiveness of written cognitive behavioural self-help: a systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2013;33(1):182-195. doi: 10.1016/j.cpr.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 22.Laurel Franklin C, Walton JL, Raines AM, et al. Pilot study comparing telephone to in-person delivery of cognitive-behavioural therapy for trauma-related insomnia for rural veterans. J Telemed Telecare. 2018;24(9):629-635. doi: 10.1177/1357633X17732366 [DOI] [PubMed] [Google Scholar]
- 23.McCurry SM, Guthrie KA, Morin CM, et al. Telephone based cognitive-behavioral therapy for insomnia in peri- and postmenopausal women with vasomotor symptoms: a MsFLASH randomized trial. JAMA Internal Medicine. 2016;176(7):913-920. doi: 10.1001/jamainternmed.2016.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCurry SM, Von Korff M, Morin CM, et al. Telephone interventions for co-morbid insomnia and osteoarthritis pain: the OsteoArthritis and Therapy for Sleep (OATS) randomized trial design. Contemp Clin Trials. 2019;87:105851. doi: 10.1016/j.cct.2019.105851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 26.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734-739. doi: 10.1176/ajp.140.6.734 [DOI] [PubMed] [Google Scholar]
- 27.Morin CM, Beulieu-Bonneau S. Cognitive-behavioral therapy for insomnia in peri- and post-menopausal women. Universite Laval; 2013:32. [Google Scholar]
- 28.Balderson BH, McCurry SM, Vitiello MV, et al. Information without implementation: a practical example for developing a best practice education control group. Behav Sleep Med. 2016;14(5):514-527. doi: 10.1080/15402002.2015.1036271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morin CM. Insomnia: Psychological Assessment and Management. Guilford Press; 1993. [Google Scholar]
- 30.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601-608. doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487-2494. doi: 10.1185/03007990903167415 [DOI] [PubMed] [Google Scholar]
- 32.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129-138. [PubMed] [Google Scholar]
- 33.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163-173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184(3):E191-E196. doi: 10.1503/cmaj.110829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gradisar M, Lack L, Richards H, et al. The Flinders Fatigue Scale: preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med. 2007;3(7):722-728. doi: 10.5664/jcsm.27030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. Paper presented at: SAS Global Forum 2012; April 22-25, 2012; Orlando, FL. [Google Scholar]
- 38.Vitiello MV, McCurry SM, Shortreed SM, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. 2014;155(8):1547-1554. doi: 10.1016/j.pain.2014.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger PW. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27(12):85-96. [Google Scholar]
- 40.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 2004. [Google Scholar]
- 41.Ho KKN, Ferreira PH, Pinheiro MB, et al. Sleep interventions for osteoarthritis and spinal pain: a systematic review and meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2019;27(2):196-218. doi: 10.1016/j.joca.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 42.Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Cognitive-behavioral therapy for insomnia in older adults with osteoarthritis pain: Session outlines
eMethods 2. Treatment of sleep problems in older persons with osteoarthritis pain: education only: Session outlines
eTable 1. Baseline characteristics by intervention group, completer analysis (n=282)
eTable 2. Primary and secondary outcome results by treatment condition, all participants (n=327), using imputed data
eTable 3. Percentage of CBT-I and EOC responders (> 30% improvement from baseline) for insomnia, pain, depression, and fatigue at 2 months (post-treatment) and 12 months (n=327), using imputed data
eTable 4. Primary and secondary outcome results by treatment condition for severe subgroup participants (BPI > 5, ISI scores > 15) (n=160)
Data Sharing Statement