Abstract
This randomized clinical trial examines the superiority of a single dose of rituximab vs low-dose mycophenolate mofetil in preventing the recurrence of steroid-dependent nephrotic syndrome in children and young adults.
Children and young adults with steroid-dependent nephrotic syndrome (SDNS) are exposed to the toxic effects of both steroids and common steroid-sparing agents, such as calcineurin inhibitors, reporting an increased risk of kidney failure.1 Alternative low-toxicity, long-acting therapies are needed to treat SDNS.1
Methods
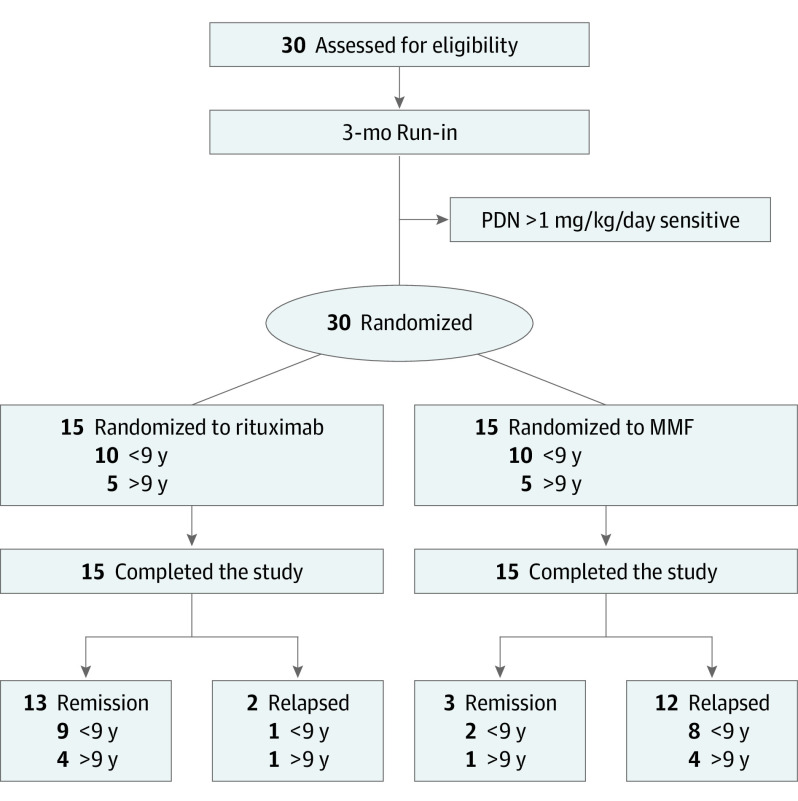
This randomized clinical trial was designed to test the superiority of a single dose of rituximab (375 mg/m2) vs low-dose mycophenolate mofetil (MMF) (350 mg/m2 twice daily) in preventing the recurrence of SDNS. Eligibility criteria were age 3 to 24 years and SDNS requiring prednisone 0.3 mg/kg/d to 1 mg/kg/d during at least 6 months before enrollment.
Eligible White patients were randomly assigned in a 1:1 ratio to receive either rituximab (intervention) or oral MMF (active comparator) (Figure 1). Sex and age were equally distributed. The trial protocol (Supplement 1) and study were approved by the regional review board (Liguria, IT) and Italian Drugs Agency (AIFA) and the trial protocol was registered at ClinicalTrials.gov (NCT04402580). Written consent was obtained by patients or by their parents.
Figure 1. Schematic View of Trial Design.
MMF indicates mycophenolate mofetil; PDN, prednisone.
The primary outcome was the occurrence of a relapse of SDNS within 12 months of randomization; the secondary outcome was the probability of remaining relapse-free up to 24 months from randomization. Relapse was defined by a urine protein/creatinine ratio (uPCR) of 2000 mg/g or more. Logistic regression was used to compare the risk of relapse at 1 year with a 2-sided P value of <.05 as statistical significance.
Results
The monitoring board recommended stopping the trial after the randomization of only 30 study participants owing to unexpectedly high rates of relapse in the MMF arm (Figure 1). Of note, no relapses occurred in the intervention arm in the first 6 months. After rituximab, CD19+ B cells immediately decreased to 0%, maintaining low values until 6 to 9 months after infusion, with no differences between relapse or not.
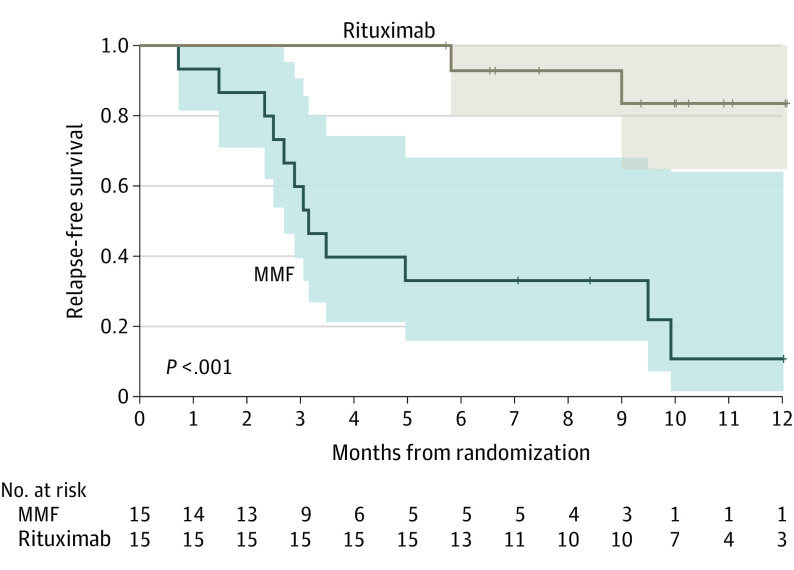
The risk of relapse was 0.8 (12/15) in the comparator arm and 0.13 (2/15; P = .008) in the intervention arm, with a significantly higher odds among children in the MMF arm (odds ratio [OR], 26; 95% CI, 2.9-311.0). The median time to the relapse in the active comparator arm was 3.15 months (95% CI, 2.5-9.9 months; Figure 2). No adverse effects were observed in either group and none related to rituximab infusion.
Figure 2. One-Year Relapse-Free Survival in the Rituximab and Mycophenolate Mofetil (MMF) Arms.
The risk of relapse was 0.8 (12/15) in the MMF and 0.13 (2/15; P = .008 in the rituximab arm, with higher odds in the MMF arm (odds ratio, 26; 95% CI, 2.9-311.0).
Discussion
Approximately 90% of children with idiopathic nephrotic syndrome develop steroid dependence.1 Considering the toxic effects of calcineurin inhibitors, alternative interventions or low-dose approaches are needed for long-term treatments.
The anti-CD20 monoclonal antibody rituximab emerged as a promising steroid-sparing drug in patients with SDNS.1,2 In the present study, single-dose rituximab, withdrawing steroids, resulted in effective control of SDNS. Given the limited adverse effects, rituximab should be considered as the main steroid-sparing drug in patients with SDNS.
Previous reports indicate that MMF can maintain steroid-free remission in children with SDNS.2 However, existing small and uncontrolled studies on MMF generated conflicting data2,3: the minimum dose of MMF sufficient to maintain remission of SDNS is unknown, and variable doses (ranging from 700 mg/m2-1200 mg/m2 daily) are reported.4
This study aimed to address the efficacy of low-dose MMF, and the inefficacy provides useful evidence for designing future trials. The adherence to MMF, monitored with monthly reports, was complete in all patients. The strategy of monitoring may represent a weakness and better strategies to estimate MMF exposure are to be defined.
Conclusions
Rituximab was effective in maintaining long-term remission of SDNS; MMF given at low dose (350 mg/m2 twice daily) was, instead, inadequate. The effect of higher doses (1200 mg/m2) deserve testing in randomized clinical trials. Lack of efficacy of low-dose MMF may have implications in clinical practice or research of other immune-mediated conditions.5,6
Trial Protocol
Data Sharing Statement
References
- 1.Ravani P, Rossi R, Bonanni A, et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol. 2015;26(9):2259-2266. doi: 10.1681/ASN.2014080799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravani P, Lugani F, Pisani I, et al. Rituximab for very low dose steroid-dependent nephrotic syndrome in children: a randomized controlled study. Pediatr Nephrol. 2020;35(8):1437-1444. doi: 10.1007/s00467-020-04540-4 [DOI] [PubMed] [Google Scholar]
- 3.Gellermann J, Weber L, Pape L, Tönshoff B, Hoyer P, Querfeld U; Gesellschaft für Pädiatrische Nephrologie (GPN) . Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol. 2013;24(10):1689-1697. doi: 10.1681/ASN.2012121200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novak I, Frank R, Vento S, Vergara M, Gauthier B, Trachtman H. Efficacy of mycophenolate mofetil in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2005;20(9):1265-1268. doi: 10.1007/s00467-005-1957-y [DOI] [PubMed] [Google Scholar]
- 5.Moudgil A, Bagga A, Jordan SC. Mycophenolate mofetil therapy in frequently relapsing steroid-dependent and steroid-resistant nephrotic syndrome of childhood: current status and future directions. Pediatr Nephrol. 2005;20(10):1376-1381. doi: 10.1007/s00467-005-1964-z [DOI] [PubMed] [Google Scholar]
- 6.Sinha A, Puraswani M, Kalaivani M, Goyal P, Hari P, Bagga A. Efficacy and safety of mycophenolate mofetil versus levamisole in frequently relapsing nephrotic syndrome: an open-label randomized controlled trial. Kidney Int. 2019;95(1):210-218. doi: 10.1016/j.kint.2018.08.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement