Abstract
Background
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) are an established treatment for patients with type 2 diabetes (T2D). Differences between GLP‐1RAs in pharmacokinetics, dosing regimens and clinical effects, including cardiovascular (CV) outcomes, mean there may be benefits to switching from one to another. However, clinical guidance on switching is lacking and data from clinical trials are limited. This article provides a clinical perspective and consensus on the benefits of switching between GLP‐1RAs, the triggers for switching and how best to manage this in clinical practice. Once weekly (OW) semaglutide is used as an example to illustrate how the authors might switch to a different GLP‐1RA in clinical practice.
Methods
Literature was searched and perspectives from 10 healthcare professionals with experience in switching patients with T2D to OW semaglutide from another GLP‐1RA were collated.
Results
Medical triggers for switching to another GLP‐1RA included HbA1c targets not being met, a desire for additional weight loss, poor adherence, patients moving to increased CV risk status and adverse effects with the current GLP‐1RA. Non‐medical triggers for switching included patient preference, cost, formulary changes and insurance mandates. Once the decision to switch is made, an individualised approach is recommended, based on considerations that include reimbursement requirements, treatment duration with (and dose of) previous GLP‐1RA, the patient's experience initiating the prior GLP‐1RA, any concomitant treatment and clinical characteristics. When switching, it is important to emphasise that treatment burden will not increase and that if gastrointestinal adverse effects occur, they are typically transient. Any transient gastrointestinal adverse effects that may occur (or recur) when switching to another GLP‐1RA can be reduced by slow up‐titration and advising patients to reduce food portion sizes and fat intake.
Conclusion
Switching from one GLP‐1RA to another, such as OW semaglutide, can provide clinical benefits and may delay the need for treatment intensification.

Review criteria
Scientific literature was searched for publications reporting switching from one GLP‐1RA to once‐weekly (OW) semaglutide and identified publications were reviewed for relevance; no relevant publications were identified.
Therefore, publications were suggested by the authors (10 healthcare professionals with clinical experience switching patients with T2D to OW semaglutide from another GLP‐1RA) to support their recommendations.
Perspectives of the authors were collated, discussed and consensus sought, with the aim of providing guidance.
Message for the clinic
Switching from one GLP‐1RA to another can provide substantial clinical benefits and may delay the need for treatment intensification.
Triggers for initiating a discussion on switching may include HbA1c targets not being met, the need for additional weight loss, patients moving to an increased cardiovascular risk status and poor adherence.
Switching between GLP‐1RAs should be individualised to mitigate known adverse effects, in particular, those gastrointestinal in nature.
1. INTRODUCTION
Glucagon‐like peptide‐1 (GLP‐1) receptor agonists (GLP‐1RAs) are an established treatment for type 2 diabetes (T2D). Following failure of initial treatment with metformin and comprehensive lifestyle interventions, the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) consensus report and ADA Standards of Care for Diabetes recommend GLP‐1RAs for use throughout the treatment pathway for T2D, in particular if there is concern about hypoglycaemia or weight gain. 1 , 2 The ADA/EASD consensus report also recommends the use of GLP‐1RAs as first‐line pharmacotherapy for patients with a contraindication to metformin 2 and the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology guidelines include use of GLP‐1RAs as monotherapy for all patients with T2D. 3
There are several GLP‐1RAs available (Table 1) 4 , 5 , 6 , 7 , 8 , 9 , 10 and they vary in terms of their structure, pharmacokinetics, dosing regimen and clinical effects. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Exenatide and lixisenatide are analogues of exendin‐4 (a peptide agonist of the GLP‐1 receptor, obtained from the Gila monster) 13 ; albiglutide (no longer on the market, having been withdrawn for economic reasons 14 ), dulaglutide, liraglutide and semaglutide are analogues of human GLP‐1. 13 Some GLP‐1RAs are dosed daily (exenatide twice daily, lixisenatide, liraglutide and oral semaglutide), whereas others are taken once weekly (dulaglutide, exenatide extended‐release [exenatide‐ER] and semaglutide). 13 , 15 This review focuses on injectable GLP‐1RAs.
TABLE 1.
Available injectable GLP‐1RAs
| Drug | Exendin‐4 or human backbone (homology to native human GLP‐1) | Dosing frequency | Maintenance doses | Half‐life | How to initiate | Expanded CV indication? |
|---|---|---|---|---|---|---|
| Exenatide 4 | Exendin‐4 (53%) 41 | Twice daily |
5 µg 10 µg |
2.4 hours 71 |
Initiate at 5 µg twice daily May be increased to 10 µg twice daily after 1 month |
No |
| Exenatide ER 6 | Exendin‐4 (53%) 41 | Once weekly | 2 mg | 6‒7 days 72 | No | |
| Lixisenatide 5 | Exendin‐4 (~50%) 27 | Once daily |
10 µg 20 µg |
~3 hours | Initiate on 10 µg once daily for 14 days. Increase to 20 µg once daily starting on day 15 | No |
| Liraglutide 10 | Human GLP‐1 (97%) | Once daily |
1.2 mg 1.8 mg |
13 hours |
Initiate at 0.6 mg daily for 1 week then increase to 1.2 mg daily If additional glycaemic control is required, increase to 1.8 mg daily after 1 week of treatment with 1.2 mg daily |
Yes 10 |
| Dulaglutide 9 | Human GLP‐1 (90%) | Once weekly |
0.75 mg 1.5 mg |
~4 days 73 |
US PI: Initiate at 0.75 mg once weekly. May be increased to 1.5 mg once weekly for additional glycaemic control 9 EU SPC: For monotherapy, the dose is 0.75 mg; for potentially vulnerable populations, initiate at 0.75 mg and increase if appropriate to do so; for add‐on therapy, the dose is 1.5 mg and no up‐titration is required 64 |
Yes 9 |
| Semaglutide 7 | Human GLP‐1 (94%) | Once weekly |
0.5 mg 1.0 mg |
~7 days | Start at 0.25 mg once weekly. After 4 weeks, increase the dose to 0.5 mg once weekly. If after at least 4 weeks additional glycaemic control is needed, increase to 1.0 mg once weekly | Yes 7 |
Abbreviations: CV, cardiovascular; exenatide ER, exenatide extended release; GLP‐1, glucagon‐like peptide‐1; GLP‐1RA glucagon‐like peptide‐1 receptor agonist; PI, prescribing information; SPC, summary of product characteristics.
Differences in clinical efficacy have been observed in head‐to‐head comparisons between GLP‐1RAs in randomised controlled trials. 16 , 17 , 18 In addition, although no drug in this class has been observed to have a negative impact on cardiovascular (CV) outcomes, not all GLP‐1RAs have demonstrated CV benefits in CV outcomes trials. 19 Of the agents currently available, dulaglutide, liraglutide and once weekly (OW) semaglutide have demonstrated CV benefits and have expanded indications from the US Food and Drug Administration (FDA) for use in people with T2D at high CV risk, based on the results of these trials. 7 , 9 , 10 Liraglutide and OW semaglutide have indications for patients with T2D and established CV disease, whereas the indication for dulaglutide is for patients with T2D and established CV disease or multiple CV risk factors. 7 , 9 , 10 The differences in the indications reflect differences in CV risk of the populations enrolled in the CV outcomes trials. 20 , 21 , 22 The ADA Standards of Care for Diabetes recommend that GLP‐1RAs with demonstrated CV benefits are considered for patients with established atherosclerotic CV disease; the AACE guidelines and the European Society of Cardiology (ESC)/EASD guidelines also recommend GLP‐1RAs for these patients irrespective of glycaemic control. 1 , 3 , 23 In patients without established atherosclerotic CV disease but with indicators of high CV risk, the ADA Standards of Care for Diabetes GLP‐1RA indicate that GLP‐1RAs should be prescribed if sodium–glucose cotransporter‐2 (SGLT‐2) inhibitors are not tolerated or are contraindicated, or if the patient has less than adequate estimated glomerular filtration rate (eGFR) levels. 1
Given the differences within the drug class, switching from one GLP‐1RA to another may be beneficial and may delay the need to intensify therapy, thus avoiding an increase in treatment burden. Indeed, switching may even reduce the treatment burden by enabling the dose of concomitant oral glucose‐lowering drugs and/or insulin to be reduced and potentially improving adherence and persistence, if less frequently administered regimens are used. A review by Almandoz et al. provided advice on switching between GLP‐1RAs in clinical practice; 24 however, we are not aware of any other guidance on switching between GLP‐1RAs.
As individuals with clinical experience with GLP‐1RAs, including switching from one GLP‐1RA to another, we have therefore developed this review article to provide a clinical perspective and consensus on the benefits of switching between GLP‐1RAs, the triggers for initiating switching and how best to manage this in clinical practice. Owing to the number of GLP‐1RAs available, and because of its clinical efficacy compared with other GLP‐1RAs, this review will focus on switching to OW semaglutide because this will highlight the main clinical considerations for switching.
2. LITERATURE SEARCH
The scientific literature was searched for publications reporting clinical experience switching from one GLP‐1RA to OW semaglutide. Publications were searched from the date OW semaglutide entered the market (international birth date [date of first global approval; approval by the FDA]: 5 December 2017) up to 3 October 2019. Identified publications were reviewed for relevant data and were included in the review if appropriate. No relevant publications were identified by this search; therefore, publications were suggested by the authors to support their recommendations.
3. BENEFITS OF SWITCHING FROM ONE GLP‐1RA TO ANOTHER
3.1. Improved glycaemic control
Glucose‐lowering efficacy differs between GLP‐1RAs. This has been observed in both clinical trials and analyses of real‐world data of GLP‐1RA‐naïve patients. In the DURATION 6, HARMONY 7 and LIRA–LIXI studies, liraglutide 1.8 mg was observed to reduce glycated haemoglobin (HbA1c) to a greater extent than exenatide ER 2.0 mg (1.5% vs 1.3%‐point), albiglutide 50 mg (1.0% vs 0.8%‐point) and lixisenatide 20 µg (1.8% vs 1.2%‐point), respectively. 25 , 26 , 27 In AWARD 6, similar reductions in HbA1c were observed with liraglutide 1.8 mg and dulaglutide 1.5 mg. 28 These differences have also been observed in clinical practice; for example, an analysis of real‐world data from the UK showed that liraglutide reduced HbA1c to a greater extent than lixisenatide (mean treatment difference [95% confidence interval (CI)] −0.3%‐point [−0.56; −0.04]). 29 In the SUSTAIN 3, SUSTAIN 7 and SUSTAIN 10 trials, OW semaglutide was observed to reduce HbA1c to a greater extent than exenatide ER 2.0 mg (semaglutide 1.0 mg; 1.5% vs 0.9%‐point), dulaglutide (semaglutide 0.5 mg vs dulaglutide 0.75 mg, 1.5% vs 1.1%‐point; semaglutide 1.0 mg vs dulaglutide 1.5 mg, 1.8% vs 1.4%‐point) and liraglutide 1.2 mg (semaglutide 1.0 mg; 1.7% vs 1.0%‐point), respectively. 16 , 17 , 18
Furthermore, improvements in glycaemic control have been observed in both randomised controlled trials and retrospective observational studies where patients were switched from one GLP‐1RA to another. In the DURATION 1 trial, after week 30, 130 patients with T2D who had been randomised to exenatide twice daily 10 µg were switched to exenatide ER 2.0 mg. 30 These patients experienced further decreases in HbA1c levels of 0.2%‐point in the 22 weeks following the switch. In this trial there was a short period of approximately 4 weeks after switching during which glycaemic control worsened but, subsequent to this, HbA1c levels improved. 30 In the LEAD 6 trial, following the main 26‐week, randomised, double‐blind phase, 187 patients who had been receiving exenatide twice daily 10 µg were switched to liraglutide 1.8 mg. 31 In the 14 weeks following switching to liraglutide, mean HbA1c decreased by 0.3%‐point and weight by 0.9 kg. 31
A retrospective analysis of 148 patients with T2D who initiated exenatide ER in Spanish tertiary care (CIBELES Project) included 30 (20.3%) patients who were switched from another GLP‐1RA. 32 In these patients, a mean [95% CI] HbA1c reduction of 0.5%‐point [0.17; 0.81] was observed after 6 months. 32 An analysis of US claims data from 107 patients with T2D, 56 of whom were already receiving a GLP‐1RA, who initiated OW semaglutide showed a 1.3%‐point reduction in HbA1c in the overall population. 33 A retrospective analysis of 164 patients with T2D who switched to OW semaglutide from either liraglutide or dulaglutide (REALiSe‐DM), having been on the initial GLP‐1RA for at least 3 months, also showed a reduction in HbA1c following the switch. 34 The patients were generally poorly controlled (mean HbA1c was 8.1%; 82.3% had HbA1c > 7.0%) the mean initial and final doses of OW semaglutide were 0.81 mg and 0.87 mg, respectively. The mean [95% CI] reduction in HbA1c was 0.7%‐point [0.51; 0.83]. Of the patients who were switched, 11.1% experienced a gastrointestinal (GI) adverse effect (AE). 34 A prospective, single‐arm, multicentre study in Japan, evaluating 58 patients who switched from exenatide twice daily to exenatide ER, observed improved glycaemic control (HbA1c decrease of 0.2%‐point over 24 weeks). 35 In addition, decreased fasting plasma glucose levels and improved beta‐cell function (measured by homeostasis model assessment) and C‐peptide index were noted in this study. 35 The incidence of hypoglycaemia was also significantly reduced at week 24. 35 In a retrospective, single‐centre study by Goncalves and Bell, 40 patients who were switched from liraglutide 1.8 mg (used for an average of 210 weeks) to OW semaglutide (average dose 0.76 mg) in clinical practice in the USA were followed for an average of 34 weeks. 36 Following the switch, HbA1c decreased from 7.46% ± 1.36% to 6.68% ± 1.00% (P < .001) and the number of patients requiring insulin dropped from 16 to 13. In patients receiving insulin, the average dose of insulin decreased from 49.5 units/d to 40.4 units/d. 36
These studies, therefore, demonstrate that switching between GLP‐1RAs can provide additional benefits in terms of glycaemic control. The benefits of switching on glycaemic control are supported by a modelling study evaluating the switch from another GLP‐1RA to OW semaglutide. 37 This modelling study used data from five trials in the phase 3 OW semaglutide clinical development programme (SUSTAIN 1, SUSTAIN 2, SUSTAIN 3, SUSTAIN Japan and SUSTAIN 7) and a phase 2 dose‐finding trial to investigate the impact of switching to OW semaglutide from liraglutide, dulaglutide or exenatide ER. Time‐course models for HbA1c changes with the four GLP‐1RAs were developed using population pharmacokinetic data from the included trials. The models suggested that switching to OW semaglutide from liraglutide, dulaglutide or exenatide ER would result in further reductions in HbA1c. This modelling study also suggested that when switching to OW semaglutide 0.25 mg there would be a slight initial deterioration in HbA1c before further improvements in HbA1c were observed.
3.2. Additional weight loss
Weight reduction also varies between GLP‐1RAs. Liraglutide 1.8 mg reduced weight to a greater extent than exenatide ER (3.6 vs 2.7 kg), dulaglutide (3.6 vs 2.9 kg), albiglutide (2.2 vs 0.6 kg) and lixisenatide (4.3 vs 3.7 kg) in the DURATION 6, AWARD 6, HARMONY 7 and LIRA–LIXI trials, respectively. 25 , 26 , 27 , 28 OW semaglutide reduced weight to a greater extent than exenatide ER (semaglutide 1.0 mg; 5.6 vs 1.9 kg), dulaglutide (semaglutide 0.5 mg vs dulaglutide 0.75 mg, 4.6 vs 2.3 kg; semaglutide 1.0 mg vs dulaglutide 1.5 mg, 6.5 vs 3.0 kg) and liraglutide 1.2 mg (semaglutide 1.0 mg; 5.8 vs 1.9 kg) in the SUSTAIN 3, SUSTAIN 7 and SUSTAIN 10 trials, respectively. 16 , 17 , 18
In real‐world studies that included switching, weight reductions have been observed. In the REALiSe‐DM study, where patients were switched to OW semaglutide from either liraglutide or dulaglutide, the mean reduction in weight was 1.6 kg following the switch. 34 In the real‐world study by Goncalves and Bell, of 40 patients switched from liraglutide to OW semaglutide, weight dropped from 110.6 ± 20 to 106 ± 27 kg (P < .001) and body mass index from 36.4 ± 6.2 to 33.8 ± 6.2 kg/m2 (P < .001). 36 The benefits of switching on weight loss are supported by a modelling study evaluating the switch from another GLP‐1RA to OW semaglutide. 37 However, additional weight loss is not consistently observed and is likely dependent on the GLP‐1RAs switched from and to. In the retrospective CIBELES Project in patients who switched to exenatide ER from another GLP‐1RA (previous GLP‐1RA: twice daily exenatide, 63.3%; liraglutide. 30%; lixisenatide, 3.3%; dulaglutide, 3.3%), overall, no significant changes in weight were observed after 6 months of treatment. 32 This was also observed in a 24‐week study evaluating 58 patients with T2D who switched from exenatide twice daily to exenatide ER. 35
3.3. Cardiovascular benefits
In some countries, OW semaglutide and liraglutide have expanded indications for use in reducing the risk of major adverse CV events in adults with T2D and established CV disease 7 , 10 ; dulaglutide has an expanded indication for use in reducing the risk of major adverse CV events in adults with T2D and established CV disease or multiple CV risk factors. 9 Liraglutide was shown to reduce the risk for the composite of CV death, non‐fatal myocardial infarction (MI) and non‐fatal stroke compared with placebo (hazard ratio [HR] 0.87 [95% CI: 0.78; 0.97]) in patients with T2D and either CV risk factors or established CV disease over a median follow‐up of 3.8 years (LEADER trial). 20 OW semaglutide was shown to reduce the risk for the composite of CV death, non‐fatal MI and non‐fatal stroke compared with placebo (HR 0.74 [95% CI: 0.58; 0.95]) in patients with T2D and either multiple CV risk factors or established CV disease over a median follow‐up of 2.1 years (SUSTAIN 6 trial). 22 Dulaglutide was evaluated in the REWIND trial, which included a higher proportion of patients with multiple CV risk factors but without established CV disease compared with the LEADER and SUSTAIN 6 trials. 20 , 21 , 22 In the REWIND trial, dulaglutide was shown to reduce the risk for the composite of CV death, non‐fatal MI and non‐fatal stroke compared with placebo (HR 0.88 [95% CI: 0.79; 0.99]) in patients with T2D and either CV risk factors or established CV disease over a median follow‐up of 5.4 years. 21
Not all GLP‐1RAs have shown a beneficial effect on CV outcomes. 38 , 39 Lixisenatide was shown to be non‐inferior to placebo for reduction in risk of the composite of CV death, non‐fatal MI, non‐fatal stroke and hospitalisation for unstable angina over a median follow‐up of 25 months (HR 1.02 [95% CI: 0.89; 1.17]) in patients diagnosed with acute coronary syndrome within 6 months of screening (ELIXA trial). 38 Exenatide ER was shown not to be superior to placebo (HR 0.91 [95% CI: 0.83; 1.00]) for reducing the risk for the composite of CV death, non‐fatal MI and non‐fatal stroke in patients with T2D and either CV risk factors or established CV disease over a median follow‐up of 3.2 years (EXSCEL trial). 39
3.4. Better tolerance
Owing to the compositions and structures of the available GLP‐1RAs, a patient may react differently to different agents. The adverse effect (AE) profile, generally, differs between short‐ and long‐acting GLP‐1RAs, with short‐acting GLP‐1RA treatment more likely to cause nausea and/or vomiting and long‐acting GLP‐1RA treatment more likely to cause diarrhoea. 40 , 41 If a patient has intolerable GI AEs with one GLP‐1RA, but they wish to remain on a GLP‐1RA owing to the benefits of the drug class, switching to another drug in the class may ameliorate these AEs. 42
3.5. Improved adherence
Many factors are considered to influence adherence, one of which is the frequency of dosing, with some studies showing increased adherence to medication as dosing frequency decreases. 43 , 44 , 45 Patient preference studies indicate that frequency of injection is one of the most important considerations for both injection‐naïve and ‐experienced patients when selecting a GLP‐1RA. 46 , 47 , 48 , 49 , 50 An analysis of a nationwide, longitudinal prescription database in Germany observed that OW treatment with exenatide was associated with significantly higher adherence (evaluated as proportion of days covered [PDC] by prescriptions filled) compared with liraglutide, which is a once‐daily therapy (median PDC: 0.88 and 0.77, respectively; P < .05). 51 An evaluation of a cohort of Medicare patients with T2D ≥ 65 years old, initiated on exenatide ER, once‐daily liraglutide or twice‐daily exenatide, observed greater adherence with weekly versus daily dosing schedules. 52 An evaluation of Truven Health's MarketScan Research Databases of patients newly initiated on a GLP‐1RA between November 2014 and April 2015 observed that adherence was significantly higher for OW dulaglutide than for once‐daily liraglutide (mean PDC: 0.71 vs 0.67; P < .0001). 53 However, in this analysis, adherence to dulaglutide was also significantly higher compared with exenatide ER (mean PDC: 0.72 vs 0.61; P < .0001), despite both being OW, indicating that factors other than dosing schedule are also important when considering adherence. 53 This has also been observed in other real‐world studies. A retrospective observational claims study comparing adherence, persistence and glycaemic control over 12 months with dulaglutide, liraglutide and exenatide ER observed that a significantly higher proportion of patients were adherent (PDC ≥ 80%) with dulaglutide compared with liraglutide (51.2% vs 38.2%, respectively; P < .001) and exenatide ER (50.7% vs 31.9%; P < .001). 54 In this study a greater reduction in HbA1c was observed with dulaglutide compared with both liraglutide (34.24 vs 31.96 mmol/mol [3.13% vs 2.92%‐point]; P = .032) and exenatide ER (34.46 vs 31.84 mmol/mol [3.15% vs 2.92%‐point]; P = .056). 54
Poor adherence reduces the effectiveness of a therapy, and can be one of the causes of loss of glycaemic control or reduced effectiveness of a therapy compared with expectations. 55 , 56 In general, improved glycaemic control has been observed for injectable therapies, including GLP‐1RAs in patients with good adherence compared with patients with poor adherence. 57 Switching from a GLP‐1RA that is dosed either once or twice daily to a OW agent may, therefore, improve adherence, and in turn outcomes, in some patients.
3.6. More convenient device
The devices used to inject the different GLP‐1RAs vary. Patient preference studies have indicated that the delivery device and needle size are important aspects when selecting between GLP‐1RAs. 50 However, this is not observed in all studies and may be dependent on the therapy used by the patients involved when the study was conducted. 50 The needle size varies between devices; for example, the prefilled pen injector for exenatide OW requires use of a 23‐gauge disposable needle, 58 whereas the prefilled pen injectors for exenatide twice daily (29‐, 30‐ or 31‐gauge), dulaglutide (29‐gauge) and OW semaglutide (32‐gauge) use smaller diameter needles. 7 , 59 , 60 Use of a smaller diameter needle may reduce patient discomfort and the potential for injection‐site AEs. Furthermore, the needle is pre‐attached in the dulaglutide pen injector and is hidden, which may be beneficial for some patients. 9
In addition, the degree to which the dose can be selected varies between GLP‐1RA injection devices and use of a device that enables ‘microtitration’ (ie titration to intermediary doses) allowing slower up‐titration may help manage GI adverse effects. For example, the injection devices for exenatide ER, exenatide twice daily, lixisenatide and dulaglutide allow selection of a single dose, although multiple doses are available for dulaglutide. The multi‐dose pen injection device for OW semaglutide allows intermediary doses to be selected. This enables selection and use of the lower doses of OW semaglutide (0.25 and 0.5 mg) for dose escalation, in addition to the 1.0 mg dose; the 0.5 mg dose may also be used as a maintenance dose. It is also possible to ‘microtitrate’ a patient with OW semaglutide; microtitration is off‐label and refers to the use of doses selected based on the number of ‘clicks’ with the dose selection dial, with each click representing a proportion of the dose. In Europe, microtitration is specifically contraindicated in the SPC for OW semaglutide. 61 Although this is off‐label, selection of intermediary doses can enable ‘fine‐tuning’ of the dose, potentially enabling higher doses and the related benefits with reduced risk for GI AEs.
A decision to switch based on the delivery device should only be made if a patient indicates that they have had difficulty using the injection device of their current GLP‐1RA.
4. TRIGGERS FOR SWITCHING FROM ONE GLP‐1RA TO ANOTHER
Consideration of the potential benefits of switching from one GLP‐1RA to another suggests several medical triggers for initiating a discussion on switching (Figure 1). Triggers include HbA1c targets not being met, the need for additional weight loss, patients moving to an increased CV risk or more advanced chronic kidney disease (CKD) status, the presence of unwanted adverse effects and non‐medical causes, including patient preference, cost, formulary changes (including injection devices) and insurance mandates.
FIGURE 1.

Triggers for switching GLP‐1RAs, recommended GLP‐1RAs to switch to and the expected benefits of switching. Drugs are listed alphabetically when specific drugs are suggested, the order in the table is therefore not an order of preference. *Estimated glomerular filtration rate 15–30 mL/min/1.73 m2. AE, adverse effect; CKD, chronic kidney disease; CV, cardiovascular; exenatide ER, exenatide extended release; GI, gastrointestinal; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; OW, once‐weekly
4.1. HbA1c targets not being achieved
If HbA1c targets are not being achieved because of poor adherence, changing to a OW GLP‐1RA should be considered. If HbA1c targets are not being met owing to disease progression or if the current GLP‐1RA is not reducing HbA1c levels sufficiently, a switch to an agent with proven better glycaemic efficacy is recommended. Another reason why HbA1c‐lowering efficacy may be reduced with some GLP‐1RAs is the development of anti‐drug antibodies. An analysis of exenatide clinical trials showed that in patients who develop a high antibody titre (5% of patients treated with exenatide twice daily and 12% treated with exenatide ER) increasing antibody titre was associated with reduced average efficacy. 62 Switching patients who are suspected of developing anti‐drug antibodies to a different type of GLP‐1RA is recommended, and switching to a human GLP‐1 analogue may be beneficial.
4.2. The need for additional weight loss to improve outcomes
The majority of GLP‐1RAs, with the exception of liraglutide (at a different dose to that used for the treatment of T2D), 63 do not have approval for a weight‐loss indication. However, one of the beneficial effects observed with the drug class is weight loss, and this may be one reason for selecting a GLP‐1RA for the treatment of T2D. If weight loss is needed, we would recommend switching patients from their current GLP‐1RA to a GLP‐1RA with greater weight‐reducing capacity, as demonstrated in clinical trials; the ADA/EASD consensus report indicates that in people with T2D with a compelling need to minimise weight gain or promote weight loss, the most effective GLP‐1RA is OW semaglutide. 1 In addition, in our clinical experience, switching to a different GLP‐1RA may result in weight loss because of resumption of appetite suppression; however, this weight loss is dependent on switching to a GLP‐1RA with greater weight‐reducing capacity.
4.3. Increased CV risk
Patients who move from a low to a high risk of CV disease should be switched to a GLP‐1RA with an indication for use in patients with T2D and established CV disease (dulaglutide, liraglutide or OW semaglutide) or T2D and multiple CV risk factors (dulaglutide). 7 , 9 , 10
4.4. More advanced CKD status
Patients who progress to a more advance CKD status (ie to eGFR < 30 mL/min/1.73 m2) should have their medications reviewed. The ADA Standards of Care recommend that patients with CKD (eGFR 30–60 mL/min/1.73 m2 or UACR > 30 mg/g) should preferably be treated with an SGLT‐2 inhibitor with evidence of reducing CKD progression, if eGFR is adequate. 1 If an SGLT‐2 inhibitor is not tolerated, is contraindicated or if the patient has less than adequate eGFR then it is recommended that a GLP‐1RA with proven CV benefit should be used. 1 If the decision is made for the patient to remain on a GLP‐1RA, they should be switched to a GLP‐1RA that is not contraindicated at lower eGFR levels (dulaglutide, liraglutide or OW semaglutide). 7 , 9 , 10 , 61
4.5. The presence of unwanted adverse effects
It has been suggested that switching from one GLP‐1RA to another may result in resolution of GI adverse effects. 42 If a patient has persistent GI adverse effects with a GLP‐1RA, but it is felt that they are benefiting from the treatment, then an alternative GLP‐1RA could be tested.
5. HOW TO SWITCH FROM ONE GLP‐1RA TO ANOTHER
Once the decision has been made to switch and the GLP‐1RA to be switched to has been decided upon, the next step is to achieve this optimally; a proposed approach to this, based on our clinical experience, is overviewed below and shown in Figure 2. Reimbursement requirements may mean that the new GLP‐1RA must be initiated in a specific fashion and, if this is the case, the guidance from the entity providing reimbursement should be followed rather than the suggestions below. It is important to individualise the approach when switching from one GLP‐1RA to another, and the patient should be consulted before the switch is initiated.
FIGURE 2.
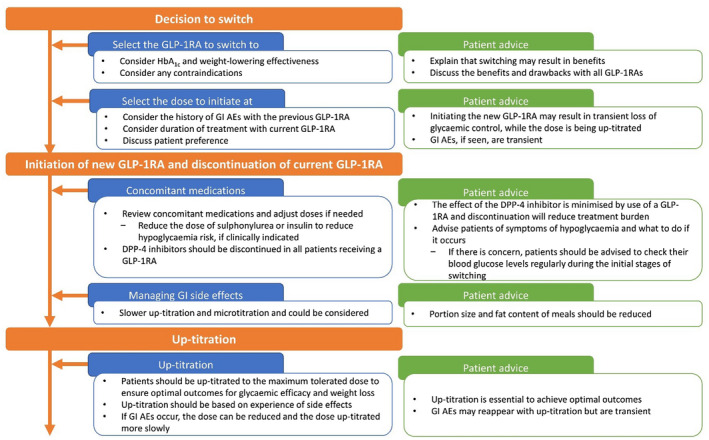
Overview of the proposed approach to switching to a new GLP‐1RA. AE, adverse effect; DPP‐4, dipeptidyl peptidase‐4; GI, gastrointestinal; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin
5.1. Consider any contraindications
Any contraindications or warnings should be considered when switching. The majority of the available GLP‐1RAs are not recommended for use in patients with end‐stage renal disease (estimated glomerular filtration rate [eGFR] <15 mL/min/1.73 m2) because of the limited available data in this population. 4 , 5 , 6 , 8 , 10 , 61 , 64 Exceptions to this are OW semaglutide and dulaglutide in the US, where the prescribing information does not contraindicate their use in patients with end‐stage renal disease. 7 , 9 Exenatide ER and exenatide twice daily should not be used in patients with severe renal impairment (creatinine clearance <30 mL/min), and should be used with caution in patients with moderate renal impairment (creatinine clearance 30–50 mL/min). 4 , 6 Lixisenatide is not recommended for use in patients with severe renal impairment (eGFR 15 to <30 mL/min/1.73 m2). 5 Caution should be exercised when escalating doses of liraglutide in patients with renal impairment (eGFR < 60 mL/min/1.73 m2), 10 and also if using dulaglutide in patients with severe renal impairment. 9
Patients with a history of diabetic retinopathy should be monitored when switching to OW semaglutide. 7 In dysregulated patients with a high HbA1c with long‐standing disease, there is the possibility of temporary worsening of the condition, most likely owing to rapid improvement in glycaemic control with OW semaglutide. 65 This is also observed with insulin treatment and should not be considered a barrier to switching patients to OW semaglutide, because the longer‐term benefits on complications outweigh the risk of temporary worsening of diabetic retinopathy. 65 , 66 In such patients, we recommend that OW semaglutide is up‐titrated more slowly (every 2–3 months) to provide a more gradual improvement in glycaemic control. Patients should have regular assessments for retinopathy, as per national standards. 67
5.2. Selecting the dose to initiate
First consider the patient's history of GI AEs with the previous GLP‐1RA. If there is a history, then it is recommended that the GLP‐1RA being switched to is one that enables slow up‐titration and that it is initiated at the lowest dose owing to the relationship between dose and risk of GI AEs with GLP‐1RAs; 68 with OW semaglutide this would be 0.25 mg and with dulaglutide it would be 0.75 mg. 7 , 9 Exenatide ER is only available as a single dose and can, therefore, not be initiated at a lower dose. 6 If the patient either had no or minimal GI AEs with the initial GLP‐1RA and/or the patient expresses a preference to initiate at a higher dose, for example because of the fear of losing glycaemic control, then, in our experience, this can be considered with a starting dose of OW semaglutide of 0.5 mg, although this is not in accordance with the label. 7 The authors do not recommend initiating OW semaglutide at 1.0 mg for most patients, owing to the risk of AEs when initiating on the highest dose; this is also not in accordance with the label. Depending on the presence and/or severity of GI AEs with the initial GLP‐1RA, the length of time before up‐titrating the new GLP‐1RA may also be adjusted. For example, if GI AEs were previously absent/minor, the dose of semaglutide might be increased every 2 weeks vs every 4 weeks, as stipulated in the label. However, it should be noted that OW semaglutide will have reached only 75% of steady state after 2 weekly doses, 69 and that up‐titration every 2 weeks will have cost implications, as the full dose might not be used from the pen injector. Conversely, if the patient had previously experienced substantial GI AEs, consideration should be given to up‐titrating more slowly, ie waiting longer than 4 weeks before increasing the dose.
Next, consider the details of the current GLP‐1RA treatment (how long the patient has been on it, the dose, the time of the next dose), as this will also help to guide the dose of the GLP‐1RA being switched to. If a patient has been receiving the initial GLP‐1RA for only a short duration (less than a month), then the approach to switching to the new GLP‐1RA should be the same as the approach taken when initiating that therapy in GLP‐1RA‐naïve patients. If a patient has been on the initial GLP‐1RA for a long duration (>1 month), then the dose of the current GLP‐1RA should be considered. If a maintenance dose lower than the maximum dose is being used, the new GLP‐1RA should be initiated at the lowest available dose. If the maximum dose of the initial GLP‐1RA is being used, then, in the our clinical experience, initiating at a higher dose could be considered; with OW semaglutide, for example, this could be 0.5 mg, although this is not in accordance with the label. 7
5.3. When to initiate the first dose of the new GLP‐1RA
When switching, the first dose of the new GLP‐1RA should be given at the time when the next dose of the previous GLP‐1RA would have been given.
5.4. Managing concomitant medications when initiating the new GLP‐1RA
The dose of concomitant therapies may need adjustment when switching, to reduce the risk of AEs. In patients who are receiving a sulphonylurea or insulin, the dose might need to be adjusted to reduce the risk for hypoglycaemia. An initial 50% dose reduction of a sulphonylurea is suggested for most patients, based on our collective clinical experience, depending on baseline HbA1c. According to clinical trial protocols, an initial dose reduction of insulin of 20% should be considered; however, this should also be based on baseline HbA1c. 70 Patients who proactively follow dietary changes may require greater dose reductions of sulphonylureas or insulin to avoid hypoglycaemia. All patients should regularly perform self‐monitoring of blood glucose in the short‐term following initiation of the new GLP‐1RA, to monitor for hypoglycaemia and inform any decisions concerning subsequent dose amends. In addition, it is important to remember to discontinue dipeptidyl peptidase‐4 inhibitors when initiating a GLP‐1RA, because the mechanism of action of these two drug classes is not synergistic. 2
5.5. Managing GI adverse effects and up‐titration following initiation
All GLP‐1RAs are associated with GI AEs. When switching between GLP‐1RAs, it is important to inform patients that some GI AEs are to be expected, but that these are usually transient. To minimise the risk of GI AEs, patients should be advised to reduce portion sizes and to reduce the fat content of their meals. Our clinical opinion is that reducing portion sizes is important because nausea may be partly related to delayed gastric emptying associated with GLP‐1RA use. Because a recommendation to reduce portion sizes may be interpreted differently by different patients, one approach is to advise patients to reduce the portion size to an amount that leads to satiation with no GI AEs or by a specific numerical figure (eg 50%). If a specific numerical figure is provided, it should be emphasised that this advice is for the meal immediately following injection to avoid the risk of undernourishment. This is effective because satiety is typically observed with a smaller meal when a GLP‐1RA is being used compared with when a patient is not on a GLP‐1RA.
Once the patient is initiated on a new GLP‐1RA, they should be monitored and, if needed, the dose up‐titrated to achieve greater effectiveness. If there are no GI AEs, then, in the authors’ clinical experience, up‐titration may be performed rapidly, for example, increasing to the next dose after 2‐4 weeks, although the lower end of this range may not be in accordance with the product label and cost may need to be considered when deciding on an up‐titration schedule. If GI AEs are present, then the authors recommend a more cautious approach, with slower up‐titration (every 4–8 weeks). If the GI AEs are intolerable to the patient after several weeks following up‐titration, the patient could be moved back to a lower dose. Anti‐emetics may be prescribed for a short period if the GI AEs are severe and the patient wishes to remain on a GLP‐1RA. If anti‐emetics are prescribed, the GI AEs should be monitored and, if they have not reduced once the course of anti‐emetics is complete, the patient should be switched from the GLP‐1RA to another glucose‐lowering therapy.
6. EXAMPLES OF SWITCHING TO OW SEMAGLUTIDE
Four case studies are provided in Figure 3, based on the authors’ clinical experience, to illustrate the considerations when switching from one GLP‐1RA to another, using OW semaglutide as an example. Reimbursement requirements may mean that the new GLP‐1RA must be initiated in a specific fashion, and if this is the case, the guidance provided by the entity providing reimbursement should be followed rather than the approaches suggested in the case studies.
FIGURE 3.
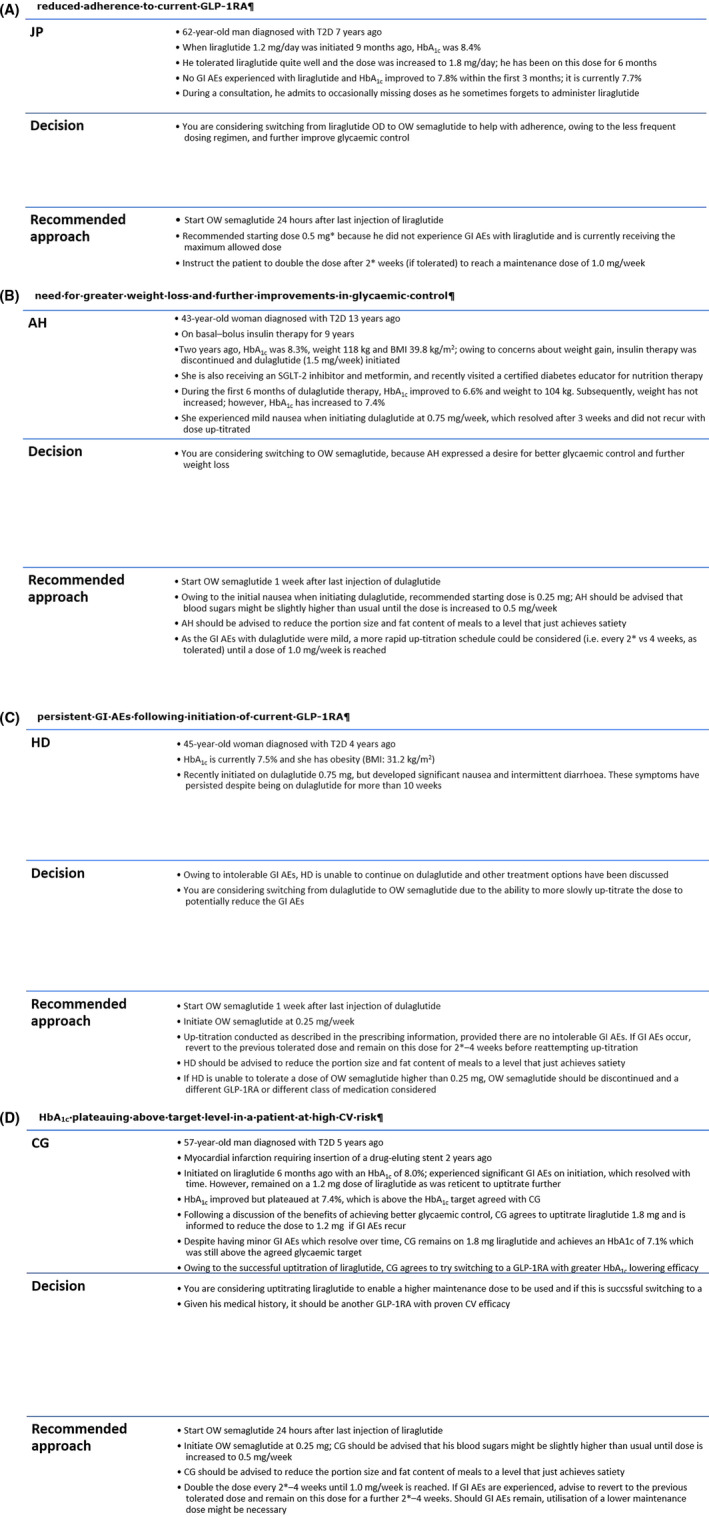
Switching from another GLP‐1RA to OW semaglutide: case studies. A, reduced adherence to current GLP‐1RA; B, need for greater weight loss and further improvements in glycaemic control; C, persistent GI AEs following initiation of current GLP‐1RA; D, HbA1c plateauing above target level in a patient at high CV risk. *A starting dose other than 0.25 mg and up‐titration after a time interval shorter than 4 weeks are off‐label and are recommendations from the authors, based on clinical experience. AE, adverse effect; BMI, body mass index; GI, gastrointestinal; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; OD, once‐daily; OW, once‐weekly; SGLT‐2, sodium–glucose cotransporter‐2; T2D, type 2 diabetes
7. BARRIERS TO SWITCHING FROM ONE GLP‐1RA TO ANOTHER
Patients may be reluctant to switch between GLP‐1RAs. This reluctance may have several causes, including:
The feeling that they are doing well‐enough in managing their diabetes and do not want to make a change even if it may improve outcomes
Concern that GI AEs may return
Not wanting to change a routine that they see as working for them
The change in device may be a barrier; for example, switching to a device that requires a greater amount of manipulation before an injection can be performed
Increased cost or lower reimbursement with an alternative GLP‐1RA.
To overcome these barriers, it can be helpful to discuss with the patient the benefits that may be obtained, together with providing reassurance that if GI AEs occur they are typically transient. 13 It is also important to emphasise that the treatment burden will not increase. Physicians may also hesitate to switch GLP‐1RAs, for the same reasons as patients, or because they incorrectly perceive that the HbA1c and/or weight‐lowering efficacy are consistent across the GLP‐1RA class. Another reason for hesitancy among physicians may be the lack of guidance on how to optimally achieve switching; we hope that this article, which summarises the clinical experience of the group on how to switch from one GLP‐1RA to another will help to address this gap.
8. CONCLUSIONS
In conclusion, we have observed in clinical practice that switching from one GLP‐1RA to another may provide substantial benefits and should be considered as an alternative to intensifying treatment. These benefits include improved glycaemic control, greater weight loss, increased adherence and use of a drug with proven CV benefits. The approach to switching should be individualised, based on considerations such as the duration of treatment with, and dose of, the previous GLP‐1RA and the patient's experience especially with GI symptoms when they initiated the prior therapy. When switching between GLP‐1RAs, transient GI AEs are expected and can be reduced by slow up‐titration, including use of intermediary doses, and advising patients to reduce portion sizes and fat intake. It is important to counsel patients that GI adverse effects are transient, and any loss of glycaemic control will be temporary, until the dose is up‐titrated.
DISCLOSURES
ABJ has received honoraria for speaking from Abbott, AstraZeneca, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Merck, Novo Nordisk and Sanofi, and has been involved in advisory boards for Abbott, AstraZeneca, Boehringer Ingelheim, Dexcom, Eli Lilly, Novo Nordisk and Sanofi. AA has received research funding from Amgen, Eli Lilly, Merck, Pfizer and Sanofi and has received personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, NAPP, Novo Nordisk, Sanofi and Takeda. JJGM has received personal fees from Abbott, AbbVie Inc, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esteve, Janssen, MSD, Mundipharma, Novo Nordisk, Pfizer, Roche and Sanofi. IH has received grants from AstraZeneca/Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Lexicon Pharmaceuticals, Medtronic, Merck & Co., Novo Nordisk, the Population Health Research Institute and Sanofi. She has received personal fees from AstraZeneca/Bristol Myers Squibb, Bausch Health, Boehringer Ingelheim/Eli Lilly, Dexcom, Eli Lilly, GlaxoSmithKline, Insulet Crop., Janssen, Medtronic, Merck & Co., Novo Nordisk and Sanofi. IH has received non‐financial support from AstraZeneca/Bristol Myers Squibb, Dexcom, GlaxoSmithKline, Insulet Corp., Janssen, Lexicon Pharmaceuticals, Medtronic, Merck & Co., Novo Nordisk, the Population Health Research Institute and Sanofi. KK has received personal fees for delivering educational talks from Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo UK, GlaxoSmithKline, Novo Nordisk and Pfizer, and has received personal fees for attending advisory boards from Amgen, Bristol Myers Squibb, Daiichi Sankyo UK and Pfizer. SM has received grants from Boehringer Ingelheim and Novo Nordisk, personal fees for advisory boards from Aventis, Boehringer Ingelheim, MSD and Novo Nordisk, and personal fees for lectures from Novo Nordisk. LP has received grants from Eli Lilly and Sanofi, personal fees from Eli Lilly, MSD, Novo Nordisk and Sanofi, and non‐financial support from Eli Lilly, Novo Nordisk and Sanofi. BDP has no conflicts of interest to disclose. JLS has been on the Speaker Bureau of Abbott, AstraZeneca, Boehringer Ingelheim/Eli Lilly, Janssen, Merck, Novo Nordisk, Salix and Sanofi, has been a Board Member/Advisory Panel for Ascensia Diabetes, Novo Nordisk, and Sanofi. She has acted as a consultant for the American Diabetes Association, Novo Nordisk and Sanofi, and has received editorial support from Novo Nordisk and Sanofi. TV has received grants from Boehringer Ingelheim, Eli Lilly and Novo Nordisk, has received personal fees for consulting from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Mundipharma, Sanofi, Sun Pharma and Novo Nordisk, and has received personal fees for attending advisory boards from Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sun Pharma.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception of the review, including discussion of proposals and guidance, critically reviewed for important intellectual content and approved the final version to be published.
ACKNOWLEDGEMENTS
We thank Alexander Jones, AXON Communications, for medical writing and editorial assistance (funded by Novo Nordisk A/S). Novo Nordisk was provided with the opportunity to perform a medical accuracy review.
Jain AB, Ali A, Gorgojo Martínez JJ, et al. Switching between GLP‐1 receptor agonists in clinical practice: Expert consensus and practical guidance. Int J Clin Pract. 2021;75:e13731 10.1111/ijcp.13731
REFERENCES
- 1. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes − 2020. Diabetes Care. 2020;43:S98‐S110. [DOI] [PubMed] [Google Scholar]
- 2. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm ‐ 2020 executive summary. Endocr Pract. 2020;26:107‐139. [DOI] [PubMed] [Google Scholar]
- 4. U.S. Food & Drug Administration . Prescibing information: BYETTA® (exenatide) injection; 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf. Accessed February 5, 2020.
- 5. U.S. Food & Drug Administration . Prescribing information: ADLYXIN® (lixisenatide) injection, for subcutaneous use; 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471orig1s000lbl.pdf. Accessed February 5, 2020.
- 6. U.S. Food & Drug Administration . Prescribing information: BYDUREON® (exenatide extended‐release) for injectable suspension, for subcutaneous use; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022200s026lbl.pdf. Accessed February 5, 2020.
- 7. U.S. Food & Drug Administration . Prescribing information: OZEMPIC® (semaglutide) injection, for subcutaneous use; 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf. Accessed April 28, 2020.
- 8. U.S. Food & Drug Administration . Prescribing information: TANZEUM® (albiglutide) for injection, for subcutaneous use; 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125431s000lbl.pdf. Accessed February 5, 2020.
- 9. U.S. Food & Drug Administration . Prescribing information: TRULICITY® (dulaglutide) injection, for subcutaneous use; 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125469s007s008lbl.pdf. Accessed April 28, 2020.
- 10. U.S. Food & Drug Administration . Prescribing information: VICTOZA® (liraglutide) injection, for subcutaneous use; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf. Accessed February 5, 2020.
- 11. Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon‐like peptide‐1 receptor agonists in type 2 diabetes: a systematic review and mixed‐treatment comparison analysis. Diabetes Obes Metab. 2017;19:524‐536. [DOI] [PubMed] [Google Scholar]
- 12. Witkowski M, Wilkinson L, Webb N, Weids A, Glah D, Vrazic H. A systematic literature review and network meta‐analysis comparing once‐weekly semaglutide with other GLP‐1 receptor agonists in patients with type 2 diabetes previously receiving 1–2 oral anti‐diabetic drugs. Diabetes Ther. 2018;9:1149‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gentilella R, Pechtner V, Corcos A, Consoli A. Glucagon‐like peptide‐1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019;35:e3070. [DOI] [PubMed] [Google Scholar]
- 14. GlaxoSmithKline UK Ltd . GlaxoSmithKline Safety Advisory: Reminder letter regarding the discontinuation of Eperzan▼(albiglutide) 1st July 2018; 2018. https://assets.publishing.service.gov.uk/media/5b4c89c5ed915d436ea7ea8b/Eperzan‐25062018.pdf. Accessed February 6, 2020.
- 15. Hughes S, Neumiller JJ. Oral semaglutide. Clin Diabetes. 2020;38:109‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41:258‐266. [DOI] [PubMed] [Google Scholar]
- 17. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275‐286. [DOI] [PubMed] [Google Scholar]
- 18. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once‐weekly semaglutide 1.0 mg vs once‐daily liraglutide 1.2 mg as add‐on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46:100‐109. [DOI] [PubMed] [Google Scholar]
- 19. Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP‐1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. [DOI] [PubMed] [Google Scholar]
- 22. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 23. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255‐323. [DOI] [PubMed] [Google Scholar]
- 24. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon‐like peptide‐1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020:cd190100. doi: 10.2337/cd19-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet. 2013;381:117‐124. [DOI] [PubMed] [Google Scholar]
- 26. Pratley RE, Nauck MA, Barnett AH, et al. Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289‐297. [DOI] [PubMed] [Google Scholar]
- 27. Nauck M, Rizzo M, Johnson A, Bosch‐Traberg H, Madsen J, Cariou B. Once‐daily liraglutide versus lixisenatide as add‐on to metformin in type 2 diabetes: a 26‐week randomized controlled clinical trial. Diabetes Care. 2016;39:1501‐1509. [DOI] [PubMed] [Google Scholar]
- 28. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384:1349‐1357. [DOI] [PubMed] [Google Scholar]
- 29. Feher M, Vega‐Hernandez G, Mocevic E, et al. Effectiveness of liraglutide and lixisenatide in the treatment of type 2 diabetes: real‐world evidence from The Health Improvement Network (THIN) Database in the United Kingdom. Diabetes Ther. 2017;8:417‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buse JB, Drucker DJ, Taylor KL, et al. DURATION‐1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33:1255‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buse JB, Sesti G, Schmidt WE, et al. Switching to once‐daily liraglutide from twice‐daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gorgojo‐Martínez JJ, Gargallo‐Fernandez MA, Brito‐Sanfiel M, Lisbona‐Catalan A. Real‐world clinical outcomes and predictors of glycaemic and weight response to exenatide once weekly in patients with type 2 diabetes: the CIBELES project. Int J Clin Pract. 2018;72:e13055. [DOI] [PubMed] [Google Scholar]
- 33. Visaria J, Dang‐Tan T, Petraro PV, Nepal BK, Willey V. Real‐world effectiveness of semaglutide in early users from a U.S. commercially insured (CI) and medicare advantage (MA) population. Diabetes. 2019;68:1006‐P. [Google Scholar]
- 34. Jain A, Kanters S, Khurana R, Kissock J, Severin N, Stafford S.Real world efficacy analysis of switch from liraglutide or dulaglutide to semaglutide in type 2 diabetes mellitus: REALiSe‐DM study. Presented at IDF 2019, 2‐6 December, Busan, Korea; 2019.
- 35. Watanabe Y, Saisho Y, Inaishi J, et al. Efficacy and safety of once‐weekly exenatide after switching from twice‐daily exenatide in patients with type 2 diabetes. J Diabetes Invest. 2020;11:382‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goncalves E, Bell DS. Efficacy of semaglutide versus liraglutide in clinical practice. Diabetes Metab. 2019:S1262‐3636(19)30155‐7. doi: 10.1016/j.diabet.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 37. Overgaard RV, Lindberg SØ, Thielke D. Impact on HbA1c and body weight of switching from other GLP‐1 receptor agonists to semaglutide: a model‐based approach. Diabetes Obes Metab. 2019;21:43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247‐2257. [DOI] [PubMed] [Google Scholar]
- 39. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon‐like peptide‐1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19:336‐347. [DOI] [PubMed] [Google Scholar]
- 41. Nauck MA, Meier JJ. MANAGEMENT OF ENDOCRINE DISEASE: are all GLP‐1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181:R211‐R234. [DOI] [PubMed] [Google Scholar]
- 42. Bain SC, McKenna J. Clinical experience with liraglutide. Int J Clin Pract Suppl. 2010;167:44‐48. [DOI] [PubMed] [Google Scholar]
- 43. Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18:527‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giorgino F, Penfornis A, Pechtner V, Gentilella R, Corcos A. Adherence to antihyperglycemic medications and glucagon‐like peptide 1‐receptor agonists in type 2 diabetes: clinical consequences and strategies for improvement. Patient Prefer Adherence. 2018;12:707‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kruk ME, Schwalbe N. The relation between intermittent dosing and adherence: preliminary insights. Clin Ther. 2006;28:1989‐1995. [DOI] [PubMed] [Google Scholar]
- 46. Qin L, Chen S, Flood E, et al. Glucagon‐like peptide‐1 receptor agonist treatment attributes important to injection‐experienced patients with type 2 diabetes mellitus: a preference study in Germany and the United Kingdom. Diabetes Ther. 2017;8:335‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qin L, Chen S, Flood E, et al. Glucagon‐like peptide‐1 receptor agonist treatment attributes important to injection‐naive patients with type 2 diabetes mellitus: a multinational preference study. Diabetes Ther. 2017;8:321‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gelhorn HL, Poon JL, Davies EW, Paczkowski R, Curtis SE, Boye KS. Evaluating preferences for profiles of GLP‐1 receptor agonists among injection‐naive type 2 diabetes patients in the UK. Patient Prefer Adherence. 2015;9:1611‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Polonsky WH, Fisher L, Hessler D, Bruhn D, Best JH. Patient perspectives on once‐weekly medications for diabetes. Diabetes Obes Metab. 2011;13:144‐149. [DOI] [PubMed] [Google Scholar]
- 50. Thieu VT, Robinson S, Kennedy‐Martin T, Boye KS, Garcia‐Perez LE. Patient preferences for glucagon‐like peptide 1 receptor‐agonist treatment attributes. Patient Prefer Adherence. 2019;13:561‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qiao Q, Ouwens MJ, Grandy S, Johnsson K, Kostev K. Adherence to GLP‐1 receptor agonist therapy administered by once‐daily or once‐weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes. 2016;9:201‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nguyen H, Dufour R, Caldwell‐Tarr A. Glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) therapy adherence for patients with type 2 diabetes in a medicare population. Adv Ther. 2017;34:658‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alatorre C, Fernández Landó L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon‐like peptide‐1 receptor agonists: Higher adherence and persistence with dulaglutide compared with once‐weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19:953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12‐month follow‐up in a real‐world setting in the United States. Diabetes Obes Metab. 2019;21:920‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lin LK, Sun Y, Heng BH, Chew DEK, Chong PN. Medication adherence and glycemic control among newly diagnosed diabetes patients. BMJ Open Diabetes Res Care. 2017;5:e000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aikens JE, Piette JD. Longitudinal association between medication adherence and glycaemic control in Type 2 diabetes. Diabet Med. 2013;30:338‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hamersky CM, Fridman M, Gamble CL, Iyer NN. Injectable antihyperglycemics: a systematic review and critical analysis of the literature on adherence, persistence, and health outcomes. Diabetes Ther. 2019;10:865‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. AstraZeneca . BYDUREON frequently asked questions; 2020. https://www.bydureon.com/pen/frequently‐asked‐questions.html. Accessed May 6, 2020.
- 59. Amylin Pharmaceuticals Inc. and Eli Lilly and Company . Byetta pen user manual; 2006. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021773s005lbl.pdf. Accessed February 5, 2020.
- 60. Matfin G, Van Brunt K, Zimmermann AG, Threlkeld R, Ignaut DA. Safe and effective use of the once weekly dulaglutide single‐dose pen in injection‐naïve patients with type 2 diabetes. J Diabetes Sci Technol. 2015;9:1071‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. European Medicines Agency .Summary of product characteristics: OZEMPIC® (semaglutide); 2019. https://www.ema.europa.eu/en/documents/product‐information/ozempic‐epar‐product‐information_en.pdf. Accessed December 10, 2019.
- 62. Fineman MS, Mace KF, Diamant M, et al. Clinical relevance of anti‐exenatide antibodies: safety, efficacy and cross‐reactivity with long‐term treatment. Diabetes Obes Metab. 2012;14:546‐554. [DOI] [PubMed] [Google Scholar]
- 63. U.S. Food & Drug Administration . SAXENDA® (liraglutide [rDNA origin] injection), solution for subcutaneous use; 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf. Accessed March 18, 2020.
- 64. European Medicines Agency .TRULICITY summary of product characteristics; 2019. https://www.ema.europa.eu/en/documents/product‐information/trulicity‐epar‐product‐information_en.pdf. Accessed May 11, 2020.
- 65. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Diabetes Control Complications Trial Research Group , Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. [DOI] [PubMed] [Google Scholar]
- 67. American Diabetes Association . 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes − 2020. Diabetes Care. 2020;43:S135‐S151. [DOI] [PubMed] [Google Scholar]
- 68. Sun F, Chai S, Yu K, et al. Gastrointestinal adverse events of glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Technol Ther. 2015;17:35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carlsson Petri KC, Ingwersen SH, Flint A, Zacho J, Overgaard RV. Semaglutide s.c. once‐weekly in type 2 diabetes: a population pharmacokinetic analysis. Diabetes Ther. 2018;9:1533‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cai Y, Wei L, Ma L, et al. Long‐acting preparations of exenatide. Drug Des Dev Ther. 2013;7:963‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Varin EM, McLean BA, Lovshin JA. Glucagon‐like peptide‐1 receptor agonists in adult patients with type 2 diabetes: review of cardiovascular outcome trials. Can J Diabetes. 2020;44:68‐77. [DOI] [PubMed] [Google Scholar]
- 73. Barrington P, Chien JY, Tibaldi F, Showalter HD, Schneck K, Ellis B. LY2189265, a long‐acting glucagon‐like peptide‐1 analogue, showed a dose‐dependent effect on insulin secretion in healthy subjects. Diabetes Obes Metab. 2011;13:434‐438. [DOI] [PubMed] [Google Scholar]


