Abstract
Understanding physiologic T-cell development from hematopoietic stem (HSCs) and progenitor cells (HPCs) is essential for development of improved hematopoietic cell transplantation (HCT) and emerging T-cell therapies. Factors in the thymic niche, including Notch 1 receptor ligand, guide HSCs and HPCs through T-cell development in vitro. We report that physiologically relevant oxygen concentration (5% O2,physioxia), an important environmental thymic factor, promotes differentiation of cord blood CD34+ cells into progenitor T (proT) cells in serum-free and feeder-free culture system. This effect is enhanced by a potent reducing and antioxidant agent, ascorbic acid. Human CD34+ cell-derived proT cells in suspension cultures maturate into CD3+ T cells in an artificial thymic organoid (ATO) culture system more efficiently when maintained under physioxia, compared to ambient air. Low oxygen tension acts as a positive regulator of HSC commitment and HPC differentiation toward proT cells in the feeder-free culture system and for further maturation into T cells in the ATO. Culturing HSCs/HPCs in physioxia is an enhanced method of effective progenitor T and mature T-cell production ex vivo and may be of future use for HCT and T-cell immunotherapies.
Keywords: cord blood, differentiation, hematopoietic stem and progenitor cells, hypoxia, physioxia, progenitor T cells, T cells
Graphical Abstract
1 |. INTRODUCTION
T cells are critical in defense against various microorganisms, and manifest antitumor activities. T cells are important for hematopoietic cell transplantation (HCT); they are protective against fatal cytomegalovirus infection and invasive aspergillosis in HCT.1,2 The capability of T cells to attack and kill residual leukemic stem cells by graft-vs-leukemia effect is considered for treatment of relapsed/refractory hematologic malignancies.3,4
Among various subsets of immune cells originating from hematopoietic stem cells (HSCs) in bone marrow (BM), T cells require a unique thymic niche for differentiation and maturation. The presence of Delta-like ligand 1 (DLL1) or DLL4, the ligand of Notch 1 receptor, is an essential component of the thymic niche during T-cell development.5–7
Lowered O2 tension is a significant but largely overlooked part of human physiology. The microenvironment of organs/tissues in vivo is more hypoxic than tissues/cells cultured in ambient air.8,9 BM where most HSC reside is of low oxygen concentration (<5%).9–11 Oxygenation of umbilical vein where cord blood (CB) is collected from is also low.12,13 Considering that the O2 concentration of the microenvironment in vivo is quite low, the appropriate term for low oxygen concentration (≤5% O2) in this situation is physioxia, a physiologically relevant oxygen concentration rather than the term hypoxia.
Oxygen concentrations in thymus can be lower than BM or spleen14; thus, physioxia might be important for T-cell commitment and development ex vivo. We hypothesized that culture of cells under physioxia with ascorbic acid, an antioxidant,15 would enhance HSC-commitment to progenitor T cells and HPC-differentiation toward mature T cells.
2 |. MATERIALS AND METHODS
2.1 |. Isolation of human CD34+ cells
Mononuclear fractions were separated from human CB (CordUse, Orlando, Florida, and Cleveland Cord Blood Center, Cleveland, Ohio) by Ficoll Paque Plus (GE Healthcare, Piscataway, New Jersey) density gradient centrifugation. CD34+ cell fractions were further isolated through a human CD34 MicroBead Kit (Miltenyi Biotec, San Diego, California). These CB CD34+ cells were purified further by fluorescence-activated cell sorting (FACS; up to 99% CD34+ cell purity) using FACS Aria or FACS Fusion (Becton Dickinson, Erembodegrem, Belgium), or passing through separation column once more.
2.2 |. Preparation of DLL4-coated plates and feeder-free cultures
A 10 μg/mL of recombinant (r) human (hu) delta-like ligand 4 protein labeled with Fc tag (DLL4-Fc) (Sinobiological, Beijing, China) and a 5 μg/mL of rhu fibronectin fragment CH-296 (RetroNectin, Takara Shuzo, Otsu, Japan) were diluted in prechilled phosphate-buffered saline (PBS), and 50 μL/well coated in nontissue culture treated 96-well plates and incubated overnight at 4°C.15 Wells were incubated further for at least 1.5 hours with 2% bovine serum albumin (BSA) diluted in PBS and washed once. Purified CD34+ cells (86.8% ~ 99.1% CD34+ purity) were seeded at a density of 4000 cells/well and cultured in serum-free Iscove-modified Dulbecco medium (IMDM) (Gibco) with 20% BSA, insulin, and transferrin serum substitute (BIT; Stemcell Technologies), 1% Glutamax (Gibco), and 1% penicillin/streptomycin (Lonza). Medium was supplemented with 100 ng/mL rhu stem cell factor, 100 ng/mL rhu Fms-like tyrosine kinase 3 ligand (FLT3L), 100 ng/mL rhu thrombopoietin (TPO) (R&D Systems, Minneapolis, Minnesota), and 100 ng/mL rhu interleukin-7 (IL-7) (PeproTech, Rocky Hill, New Jersey).16 Indicated concentrations of L-ascorbic acid sesquimagnesium salt-2 phosphate hydrate (AA) (Sigma Aldrich, St. Louis, Missouri) was added in media of AA treatment groups, since AA is more stable than L-ascorbic acid under typical culture conditions.17 AA was freshly made every week, as it was reported that only freshly reconstituted AA had effects on HSC differentiation into progenitor T cell.15 Feeder-free culture plates were refreshed by half-medium changes on day 4 and then three times/week. Culture plates were changed to new rhuDLL4-Fc-coated plates at day (D) 7. Cells were comparatively cultured for 2 weeks at 37°C with 5% CO2 and ~21% O2 (ambient air) or at 5% CO2 and 5% O2 (physioxia).
2.3 |. Artificial thymic organoid culture
MS5 cells expressing human DLL1 (MS5-hDLL1) were purchased from EMD Milipore (cat #SCC167) and expanded with high-glucose Dulbecco’s Modified Eagle Medium (DMEM) (Lonza), 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin. Artificial thymic organoid (ATO) cultures were as described.18 Briefly, MS5-hDLL1 cells at less than passage 5 were mixed with progenitor cell-enriched cells (up to 73.6% CD7+ cells) generated in feeder-free culture plates, placed in microcentrifuge tubes, and centrifuged at 300g for 5 minutes at 4°C. Cell pellets were loosened by light vortexing, and the cell slurry (about 5 μL) was gently placed on a 0.4-μm Costar Transwell insert (Corning, Kennebunk, Maine, Cat. 3450) in six-well plates. One insert with two ATOs was placed in a well containing 2 mL medium. Serum-free medium to maintain ATOs was composed of Roswell Park Memorial Institute (RPMI) 1640 (Gibco), 4% B27 supplement (ThermoFisher Scientific, Grand Island, New York), 1% Glutamax, 1% penicillin/streptomycin, 0.5% amphotericin B (Gibco), 5 ng/mL rhuFLT3L, 5 ng/mL rhuIL-7, with and without 100 μM AA. Medium for ATO was made fresh weekly and completely changed every 3 to 4 days. ATOs were maintained up to 6 weeks in an incubator at 37°C,5% CO2 and either ~21% O2, or 5% O2. ATOs were maintained for up to 10 weeks in some experiments. At the time of ATO cell harvest, ATOs were briefly disaggregated and aspirated using 1000 μL pipettes with flow cytometry staining buffer, and passed through a 70 μm nylon strainer (Corning, Cat. 431751) to filter MS5-hDLL1 cells out. Two ATOs placed onto a single well were harvested and pooled for cell counts and further analysis.
2.4 |. Cells and organoids cultured under hypoxia
To minimize effects of ambient air, cells were cultured in a hypoxia incubator (5% O2, 5% CO2, and N2 balance), and media for feeder-free and organoid cultures changed inside a custom-configured, O2-, and CO2-controlled glove box (Hypoxic Chamber, Coy), maintained at 3% O2, 5% CO2, and N2 balance. For subsequent ATO culture, plates from the hypoxia incubator (5% O2) were quickly moved into a hypoxia chamber set at 3% O2 within 30 seconds. A 5% O2 was appropriate for cell incubation and the hypoxia chamber was at 3% O2.19 All supplies and media were acclimated for at least 18 hours in the hypoxia chamber. Liquids with less than 1 mL were acclimated in the hypoxia chamber for at least 3 hours, considering the time of equilibrium of a hypoxic environment.20 Transfer of cells under physioxia to ATO was performed in the 3% O2 hypoxia chamber to minimize ambient air O2 (~21%) effects.
2.5 |. Flow cytometry
Cells were harvested and stained with antibodies at 4°C for 30 minutes, by washing with 1 mL flow cytometry staining buffer, fixed with 1% formalin solution at 4°C for 30 minutes, and resuspended with staining buffer (PBS, 0.5% BSA, and 2 mM EDTA) before analysis. For flow cytometry analysis of ATO cell harvests, a Fc receptor blocking solution, Human TruStain FcX and a fixable viability dye, zombie yellow (BioLegend, San Diego, California) were added. Antibodies (BD Biosciences, San Jose, California) used for cell surface staining were: CD34-APC (581), CD38-PE (HIT2), CD45RA-PE-CF594 (HI100), CD90-BV421 (5E10), CD10-PE-Cy7 (HI10a), CD7-APC-H7 (M-T701), CD5-BV421 (UCHT2), CD1a-FITC (HI149), CD3-FITC or APC (UCHT1), CD4-PE-CF594 (RPA-T4), CD8-PerCP-Cy5.5 (RPA-T8), TCRαβ-FITC (IP26), TCRγδ-BV421 (B1), CD19-PE (HIB19), CD33-PE-Cy7 (WM53), CD56-FITC (B159). Samples analysis was on an LSR4 flow cytometer (BD Biosciences), and data analyzed using the FlowJo software (Tree Star, Washington).
2.6 |. ATO T-cell cytokine assays
Harvested cells were seeded at 1 × 105 cells/well with Minimum Essential Medium (MEM) alpha (Gibco) plus 10% FBS and 1% penicillin/streptomycin. Phorbol 12-myristate 13-acetate (PMA), ionomycin, Brefeldin A, and Monesin (eBioscience Cell Stimulation Cocktail, Cat #00-4975), and incubated overnight according to manufacturer’s protocol. Cells were washed with PBS and incubated with Hu TruStain FcX and zombie yellow (BioLegend) for 15 minutes and stained with surface antibodies. For intracellular staining, cells were permeabilized using a Cell Permeabilization Kit (BD Biosciences). The following antibodies from BioLegend antibodies were used for intracellular staining: TNFα-PE (Mab11), IL-2-PE-Cy7 (MQ1-17H12), and IFNγ-BV421 (B27) (BioLegend). Intracellular cytokine production was assessed by LSR4 flow cytometer (BD Biosciences).
2.7 |. Quantitative real-time polymerase chain reaction
CD34+ cells were plated at 5 × 104 cells/well onto rhuDLL4-Fc-coated 96-well plates and harvested at 48 hours. Total RNA was extracted using the PureLink RNA Micro Kit (Invitrogen) according to manufacturer’s protocol. For physioxia cell groups, the collection tube, syringe with needle, lysis buffer, and ethanol were acclimated in the hypoxia chamber at least 18 hours prior to RNA extraction. With regards to physioxia groups, harvesting cells, lysis, and homogenization of harvested cells were performed in the hypoxia chamber (3% O2) and the procedures after the homogenization step were performed in ambient air conditions, outside the hypoxia chamber, in order to minimize the effect of ambient air O2 levels on gene transcription of cells. With regard to the ambient air groups, every step of RNA extraction including harvesting cells, lysis, and homogenization steps were performed in ambient air conditions. Synthesis of cDNA from total RNA was performed using SuperScript III Reverse Transcriptase (Invitrogen) according to manufacturer’s protocol. cDNA was amplified together with respective primers in Fast SYBR Green Master Mix (Applied Biosystems). Thermocycling and quantification were performed using QuantStudio 6 Flex (Applied Biosystems). Relative expression levels of each gene were calculated by the delta cycle threshold (Δ-Ct) method with expression of β-actin as an internal reference. Polymerase chain reaction (PCR) primer sequences are shown in Supplementary Table 1.
2.8 |. Statistical analysis
“N” is described as the number of biological replicates, which means the number of cord units used in a certain experiment. Data points from each biological replicates were calculated as mean ± standard error of the mean (SEM) and is shown in bar or dot graphs with raw biological data points as dots. Two-way analysis of variance (ANOVA) determined effects of two independent variables of physioxia vs ambient air, and presence of AA on dependent variables. Post hoc Tukey comparisons were performed for P-values between multiple subgroups. P < .05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism program v8.0 (GraphPad Software, San Diego, California).
3 |. RESULTS
3.1 |. Physioxia (5% O2) enhances proliferation/differentiation into progenitor T cells in rhuDLL4-Fc-coated feeder- and serum-free cultures
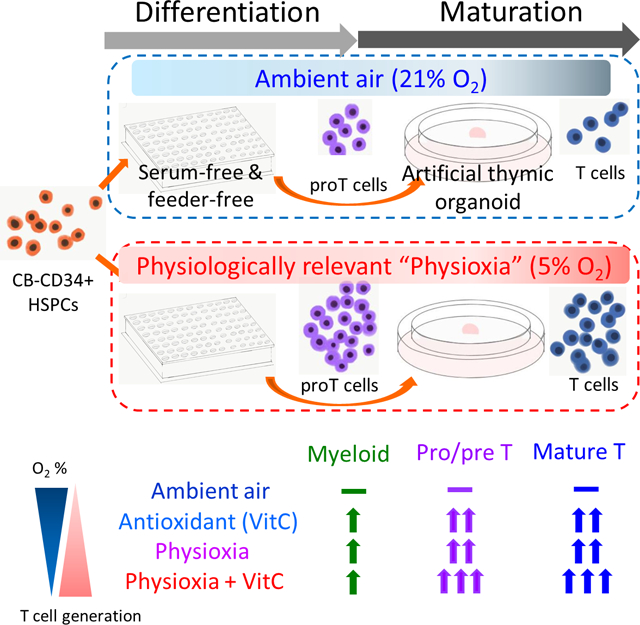
Human CD34+ cell differentiation towards progenitor T cells was descried.15 The concentration of rhuDLL4-Fc for coating culture plates was 10 μg/mL, based on previous reports.15,16 Four thousand CB CD34+ cells were plated per well in 96-well plates precoated with rhuDLL4-Fc (Figure 1A; diagrammatic representation). Mean CD34+ cell purity at day 0 was 94.4% and mean percent of CD3+ cells at day 0 was 0.15% (Supplementary Figure 1). After 2 weeks culture, total cell expansion was higher in the group cultured with AA under physioxia, compared to groups cultured without AA under ambient air (Supplementary Figure 2). Total cell expansion was decreased in cells cultured in rhuDLL4-Fc-coated wells compared to cells cultured in non-DLL4-Fc-coated wells (Supplementary Figure 3). T-cell development begins from CD34+ cells which enter the thymus from BM through post-capillary venules located in the corticomedullary junction area of thymus.21 CD34+CD45RA+ early T progenitors in the thymic cortex develop to double-negative (DN), double-positive (DP), and single positive (SP) stages.22 In the DN stage, CD7, CD5, and CD1a are sequentially expressed and cells categorized as progenitor T1 (proT1, previously described as DN1), progenitor T2 (proT2, previously described as DN2), and precursor T (preT and DN3).23–25 CD1a-expressed T-lineage-committed precursors are followed by immature single positive (iSP) T cells with CD4 expression, then an immature DP stage with coexpression of CD4 and CD8. Finally, CD3 is expressed on these DP T cells. DP T cells transit to the medulla of the thymus and develop into CD4 or CD8 SP T cells via positive and negative selection in the thymic microenvironment (diagrammed in Figure 1B).26,27 Progenitor T-cell marker, CD7 was expressed in more than 20% of cells by day 14. Absolute CD7+ cell (proT1) numbers were different between groups cultured without AA under ambient air (non-physioxia: N group, mean ± SEM, 17 588 ± 8396) and groups cultured with AA under physioxia (HA group, 89 049 ± 28 523) on day 14, and proT1 cell output was different between the ambient air and physioxia groups (Figure 1C,D). Absolute CD7+CD5+ progenitor T cell (proT2) numbers were significantly increased in physioxia and AA groups (Figure 1C,E). ProT2 cells on day 14 were lowest in the N group, followed by increases in groups without AA under physioxia (H group) and groups with AA under ambient air (NA group). Highest numbers of proT2 cells were in the HA group (mean ± SEM, 32 038 ± 10 476/4000 CD34+ cells), followed by 9978 ± 3950 (NA group), and 6536 ± 2165 (H group). ProT2 cells were lowest in the N group (922 ± 480). Two-way ANOVA analysis showed physioxia and presence of AA were independent factors for CD7+CD5+ cell output (Supplementary Table 2). CD7+CD5+ numbers were higher at 100 μM compared to 0 and 1 μM in the physioxia groups (Supplementary Figure 4). Thus, we used 100 μM AA in our subsequent experiments. Others used a concentration of AA similar to ours (95 μM).15
FIGURE 1.
Differentiation of CB-CD34+ cells in a feeder- and serum-free culture system. A, Schematic of culture system. B, Conceptual schema of human T-cell development. CD7, CD5, and CD1a are sequentially expressed on CD34+ cells entering into the thymus (DN cells), and develop into CD4- or CD8-expressing single positive (SP) cells through immature SP (iSP) and immature double-positive (iDP). Finally, mature SP T cells leave thymus and spread to periphery. C, Representative histograms and flow cytometry plots on day 14. Mean ± SEM values are presented for percent of CD7+ and CD7+CD5+ cells. D, The CD7+ (proT1) cell numbers on day 14 were different between groups without AA under ambient air and groups with AA under physioxia. CD7+ cell number showed differences between physioxia and ambient air groups (n = 9, two-way ANOVA, *P < .05, **P < .01). E, CD7+CD5+ (proT2) cell numbers showed differences between ambient air and physioxia groups (n = 9, two-way ANOVA, *P < .05). F, Folds of numbers of CD33+ (myeloid) cells on day 14 (n = 4, *P < .05). Cell numbers were normalized as cell numbers plated into one well (4000 CD34 + HSC/HPCs). Plots are presented as mean ± SEM. H, physioxia; HA, physioxia with AA; N, ambient air (non-physioxia); NA, ambient air with AA; NS, not significant
CD4+ iSP T cells, and B-lineage (CD19+), and natural killer cell (CD56+), were not different between physioxia and ambient air groups on day 14 (Supplementary Figure 5). Myeloid (CD33+) cells were higher in AA groups than non-AA groups under ambient air, but were not different between physioxia and ambient air groups (Figure 1F).
3.2 |. HSC/HPC populations in physioxia vs ambient air
To evaluate different stem and progenitor cell subpopulations among groups cultured under physioxia and ambient air, immunophenotyping for these cells was performed on day 7. Cells enriched for HSCs and HPCs (CD34+CD38−), HSC (CD34+CD38−CD45RA−CD90+), and multipotent progenitor (CD34+CD38−CD45RA−CD90−) cells were not different between ambient air and physioxia groups (Figure 2A–C). Combined populations of lymphoid-primed multipotent progenitor (CD34+CD38−CD45RA+CD90−/loCD10−)28 and multi-lymphoid progenitor (CD34+CD38−CD45RA+ CD90−/loCD10+)29 cells were increased in physioxia compared to ambient air (P = .056, Figure 2D). Flow cytometry data on day 14 in the differentiation phase in suspension cultures showed that CD34+CD45RA+ and CD34+CD7+ cell outputs were not different among groups cultured under physioxia and ambient air, whereas CD45RA+CD7+ cells were higher in physioxia groups (Supplementary Figure 6). Quantitative real-time PCR (qRT-PCR) was performed at 48 hours from D0 in the differentiation stage (Figure 1A) to check if Notch 1 signaling was altered. No statistically significant differences were noted in the Notch 1 signaling pathway (Notch1, DELTEX, HES1, GATA3, and TCF7) or for PU.1, a master regulator of myeloid differentiation between physioxia and ambient air groups (Figure 2E).
FIGURE 2.

HSC/HPC populations in physioxia vs ambient air groups. Cell numbers of (A) HSC/HPC (CD34+CD38−), (B) HSC (CD34+CD38−CD45RA−CD90+), and (C) multipotent progenitor (MPP, CD34+CD38−CD45RA−CD90−) were not different between physioxia and ambient air groups. D, Higher numbers of Lymphoid-primed multipotent progenitors (LMPP, CD34+CD38−CD45RA+CD90lo/−CD10−) and multi-lymphoid progenitors (MLP, CD34+CD38−CD45RA+CD90lo/−D10+) were observed in physioxia groups than in ambient air groups (P = .056) on day 7 (n = 3). E, Gene expression of the Notch1 signaling pathway (Notch1, HES1, DELTEX, TCF7, and GATA3) and PU.1 were not differently expressed between physioxia and ambient air groups at 48 hours (n = 4). Data are expressed as mean ± SEM. H, physioxia; HA, physioxia with AA; N, ambient air; NA, ambient air with AA; NS, not significant
AA is a cofactor of Fe2+ and α-ketoglutarate-dependent dioxygenases to facilitate enzymatic reactions of Ten-eleven translocation methylcytosine dioxygenase (TET; involving DNA methylation by catalyzing conversion of the 5-methylcytosine [5-mC] to 5-hydroxymethylcytosine [5-hmC]30). Ascorbic acid mimics TET2 restoration, and suppresses acute myeloid leukemia cell line progression.31 We tested expression of TET genes in cultured CB-CD34+ cells at early time points to see if differentiation towards proT cells might be associated with TET function in HPCs, but did not find differences in TET1, 2, and 3 expression (Supplementary Figure 7).
3.3 |. Effects of physioxia on ATO T-cell development
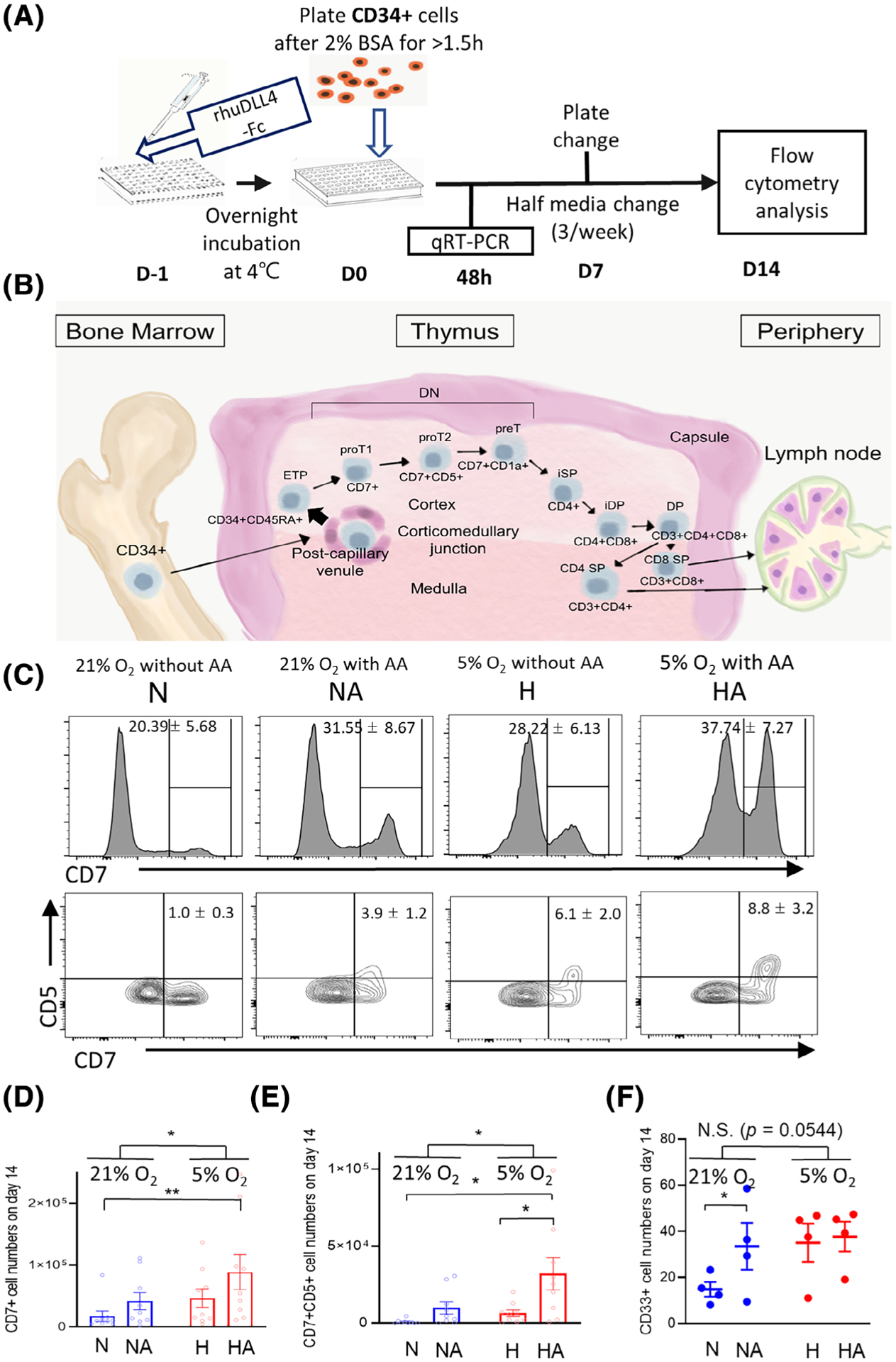
Cells cultured in serum and feeder-free medium and rhuDLL4-Fc-coated culture plates were transferred to ATO at week 2. Cells (7500) generated in culture plates were mixed with 1.5 × 105 MS5-hDLL1 cells.18 ATO cultures were maintained for 4 (6 weeks since CD34+ cell seeding on day 0) or more weeks in the same conditions for each group (Figure 3A; diagrammatic representation). Total cell expansion was highest in the HA group, followed by the H and NA groups, and lowest in the N group (Supplementary Figure 8). Gross shapes of ATO cultures were not different among groups, but the N group did not grow well compared to the other groups (Figure 3B).
FIGURE 3.
Maturation of progenitor T cells on artificial thymic organoid cultures (ATO). A, Experimental schema. B, ATO at week 6. Images of ATOs divided by four or nine pieces at ×10 magnification were taken using inverted microscope (Eclipse Ts2R, Nikon, Tokyo, Japan), then merged those into one images using a large imaging stitching tool with NIS-elements Basic Research software (Nikon). C, Representative flow cytometry plot showing progenitor T cells and T-cell development on ATOs. Week 2 is the timing of placing progenitor cells onto ATOs, and week 6 is the timing for harvesting ATO cells for further analysis. More abundant expression of progenitor T and mature T-cell markers on cells were noted at week 6 than at week 2, with a comparison of cells maintained under ambient air (21% O2) vs under physioxia (5% O2) (n = 3)
When cell subsets at week 6 from CD34+ cell seeding (4 weeks after the transfer to ATO) were compared to those at week 2 from CD34+ cell seeding (day 0 of ATO), output of CD7+CD1a+ cells (preT) were higher in physioxia vs ambient air (Figures 3C and 4A). At week 6, CD34+CD45RA+ (early T progenitor) cells and proT1 (Supplementary Figure 9) were higher in physioxia vs ambient air (Figures 3C and 4A). There was a trend toward higher CD34+CD7+ numbers in the physioxia groups (Supplementary Figure 10A). However, there was no effect of physioxia on CD45RA+CD7+ and CD7 +CD5+ cell output at week 6 (Supplementary Figures 10B and 11). Numbers of CD3+ cells per one ATO (Supplementary Figure 12A) and per 4000 CD34+ cells (Supplementary Figure 12B) were different among physioxia and ambient air groups. Significantly increased numbers of CD3+CD4+CD8+ (DP) T cells, CD3+CD4+ (CD4 SP) T, CD3 +CD8+ (CD8 SP) T cells, CD3+TCRαβ+ cells, and CD3+TCRγδ+ cells were noted in groups cultured under physioxia (H and HA) compared to groups cultured under ambient air (N and NA) (Figures 3C and 4B–D; Supplementary Figure 13).
FIGURE 4.
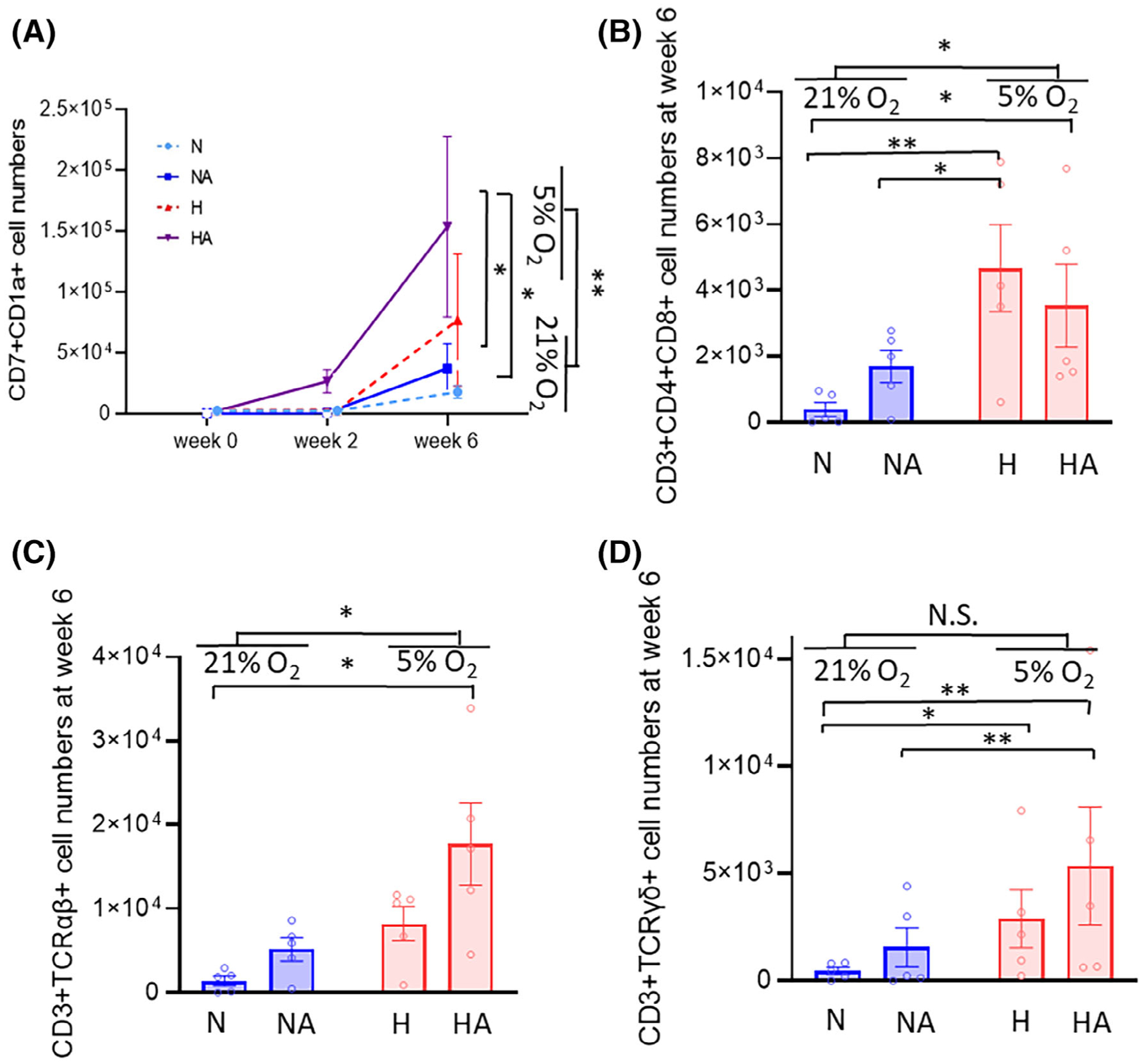
Progenitor T cell and T-cell output on ATO. H, physioxia; HA, physioxia with AA; N, ambient air; NA, ambient air with AA. A, Output of precursor T cell (CD7+CD1a+) subpopulations at week 0, week 2, and week 6 (n = 5) (two-way ANOVA, *P < .05, **P < .01). B, Output of CD3+CD4+CD8+ (DP) T-cell populations at week 6 (n = 5, two-way ANOVA, *P < .05, **P < .01). Output of (C) CD3+TCRαβ+ and (D) CD3+TCRγδ+ cells are shown. CD3+TCRαβ+ and CD3+TCRγδ+ cells were increased more in the HA than in the N group. More CD3+TCRαβ+ cells were generated after 6 weeks of culture in physioxia than in ambient air (n = 5, two-way ANOVA, *P < .05, **P < .01). Data are presented as mean ± SEM. NS, not significant
Some ATO’s cell cultures were maintained for up to 10 weeks; ATOs cultured without AA (N and H groups) could not be maintained up to 10 weeks (Supplementary Figure 14), with cells from these ATOs being punctuated, shrunken, and detached. MS5-hDLL1 cells might need AA supplementation for long-term survival. Medium for MS5-hDLL1 cultures in the datasheet provided by the manufacturer is an FBS-containing one, which may include AA in the FBS. Medium we used for maintenance of ATO was serum-free, we performed T-cell cytokine assays only in the NA and HA groups on week 10. Intracellular expression of IL-2, TNFα, and IFNγ after PMA and ionomycin stimulation was noted in CD3+ T cells in cells cultured under ambient air and physioxia with AA (Figure 5). CD3+ T cells32 isolated from CD34-negative CB mononuclear cells served as positive controls.
FIGURE 5.
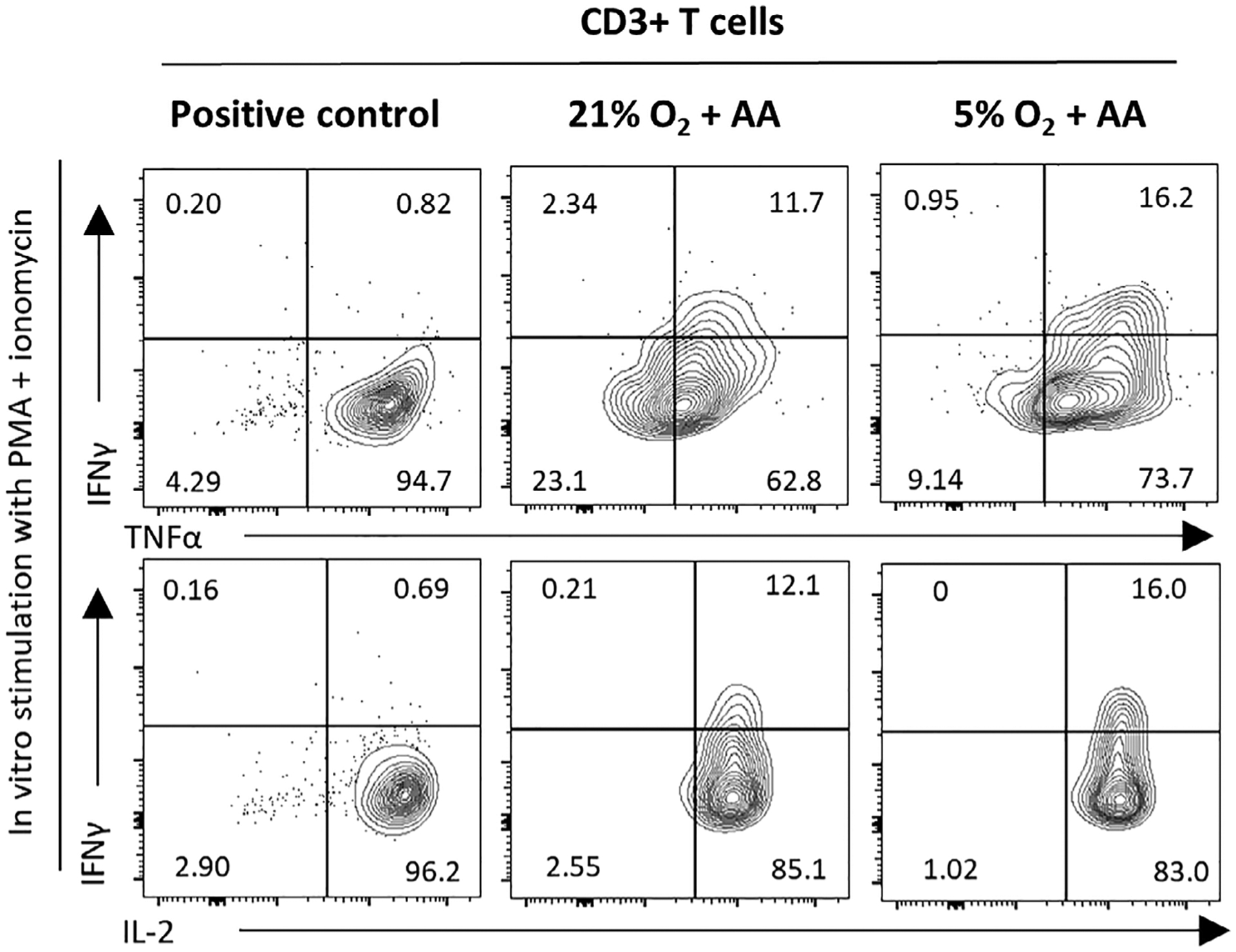
Representative flow cytometry plot (1 of 2 experiments) of cytokine production by ATO-derived CD3+ T cells at week 10. Cells were treated overnight with PMA and ionomycin, and intracellular flow cytometry analysis performed. FACS-sorted CD3+ T cells, abundant in cord blood, were used as positive controls
3.4 |. Effect of physioxia on ATO T-cell maturation
To determine if exposure to an environment with physioxia in vitro during maturation in ATOs would make a difference in terms of proT and T-cell output irrespective of exposure to physioxia during the differentiation phase in the feeder-free culture plate, we set up four groups (N, NA, H, and HA) cultured in a feeder- and serum-free culture for 2 weeks (differentiation phase) into two ATO groups. One was cultured under ambient air with AA (NA) and the other under physioxia with AA (HA) (ATO maturation phase) for up to 6 weeks (Figure 6A; diagrammed procedures). Expansion of cells was greater in ATOs maintained under physioxia, than ATOs maintained under ambient air (Figure 6B). PreT output showed significant differences at week 6 (Figure 6C), with more CD4 SP T and CD3+TCRαβ+ cells in ATO cells cultured under physioxia (Figure 6D–F).
FIGURE 6.
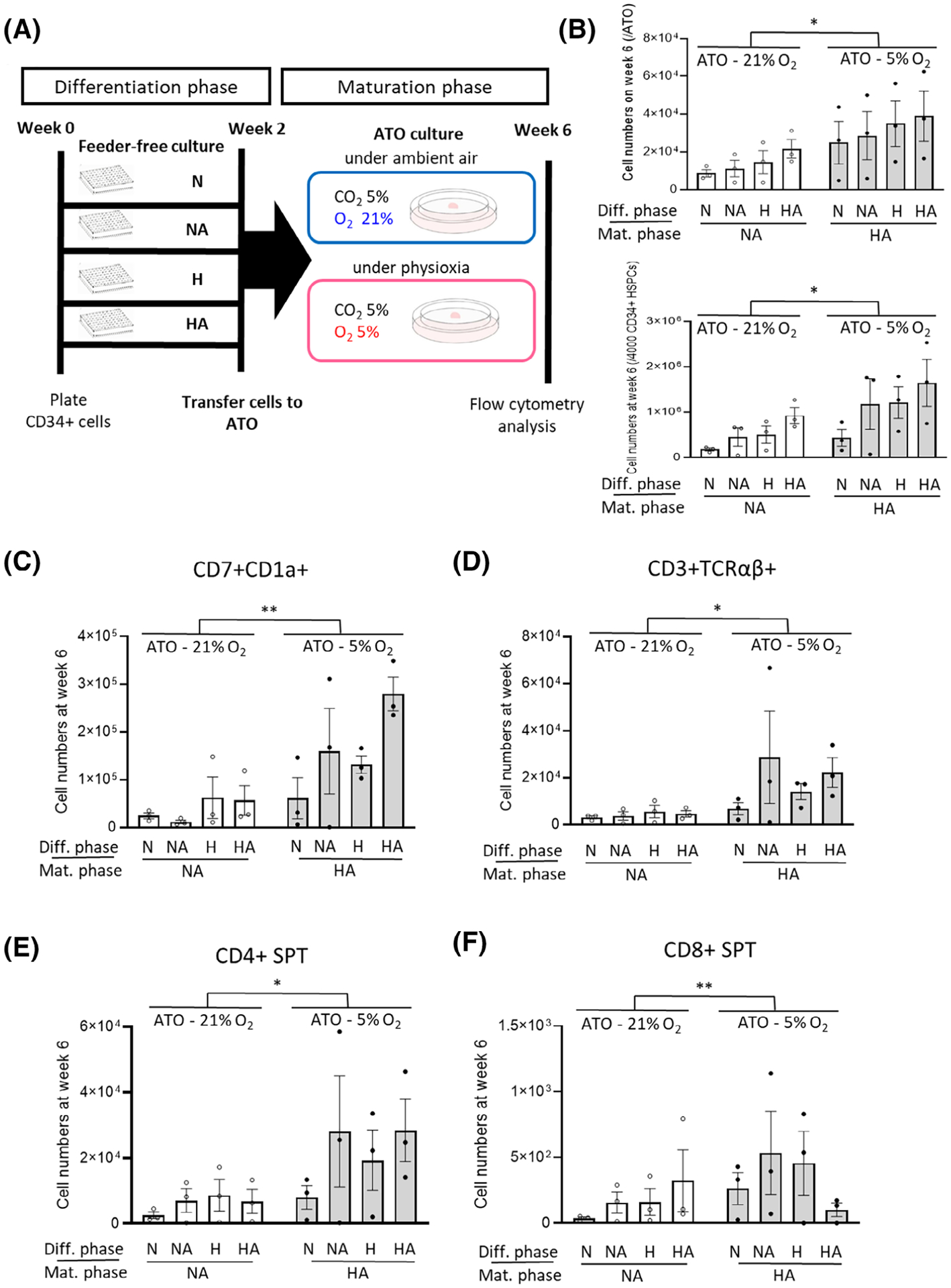
Effect of physioxia vs ambient air during the maturation phase in ATOs. A, Experimental schema. B, ATO cell numbers under physioxia (5% O2) were increased more than those under ambient air (21% O2). Cell numbers were normalized by cell numbers placed onto one ATO (7500 progenitors/ATO) and cell numbers plated into one well (4000 CD34+ cells/well) (n = 3, two-way ANOVA, *P < .05). C, Cell numbers of CD7+CD1a+ precursor T cells per 4000 CD34+ cells between physioxia vs ambient air groups in ATOs (n = 3, two-way ANOVA, **P < .01). D-F, Numbers of T-cell subsets per 4000 CD34+ cells between physioxia vs ambient air groups in ATOs on week 6 (n = 3, two-way ANOVA, *P < .05). Data are presented as mean ± SEM. Diff., Differentiation; Mat., Maturation; NS, not significant
4 |. DISCUSSION
Efficient generation of functional T cells from human HSC/HPCs ex vivo is important for cellular therapies, including engineered T cells. Approaches to produce progenitor T cell-primed cells and to expand progenitor T cells efficiently ex vivo are important, since rapid and robust T-cell reconstitution enables reduced risk of mortality and morbidity after HCT.33,34
We now report that CB CD34+ cells efficiently differentiate and mature into naïve CD4 and CD8+ SP T cells through 2 weeks of a differentiation phase in serum-free, feeder-free, and DLL4-coated plates, and after subsequent culture for 4 weeks during a maturation phase in a serum-free organoid culture system with human DLL1-expressing mouse feeder cells, effects greatest under physioxia, and enhanced in the presence of AA.
Physioxia, mimicking lowered O2 tension in the thymic niche, enhanced generation of progenitor T cells from CD34+ cells under xenogeneic feeder-free and serum-free culture conditions. A previous study in NSG mice showed that coinfusion of expanded progenitor T cells in DLL4-coated feeder-free plates and nonmanipulated CB sample led to accelerated T-cell reconstitution compared to nonmanipulated CB samples.33 Coinfusion of cells from two different donors may be feasible considering that double CB unit HCT is a strategy to treat adult patients requiring allogeneic CB HCT35 and so is coinfusion of cells from a haploidentical family donor plus a single CB.36 Our findings may be useful to improve outcomes of adult CB HCT with high treatment-related mortality (TRM) associated with delayed immune reconstitution after CB HCT.
HIF-1α induced by low oxygen tension potentiates Notch1 signaling in Jurkat and Sup-T1, T-acute lymphoblastic leukemia cell lines.37 Cell growth of Jurkat and Sup-T1 cells was increased and the G0/G1 cell cycle phase decreased under low oxygen concentration, consistent with our findings of increased cell numbers in the physioxia group on day 14 (Supplementary Figure 2). A recent study showed that short-duration (1 week) exposure to low oxygen tension increased the potential for lymphoid cell production from LMPP and pro-T/NKs.38 In contrast to our study, they cultured cells with feeder-cells in a serum containing media for 1 week, and did not investigate the role of sustained physioxia thereafter. They used a conventional coculture using MS5, a mouse feeder cell without DLL1 expression, and fetal calf serum-containing medium. Their culture system, although appropriate to see lymphoid development potential in BM, was not set up to understand the role of physioxia on T-cell development in a thymic microenvironment because of absence of essential thymic niche factors (Notch1 signal and IL-7). Our study demonstrates the role of physioxia on HSC commitment and HPC differentiation from huCB CD34+ cells into progenitor and mature T cells in context of the thymus. Cultures in physioxia are more likely to closely mimic microenvironmental effects.
Colony forming assay (CFA) in semi-solid agar plates or transplants into immunocompromised mice has been used as standards to determine functionality of various kinds of stem and progenitor cells. A CFA method for mouse B-lymphoid progenitors was established long ago,39 whereas a CFA method for human progenitor T cells is not yet reported. We chose an ATO system of 3D culture with MS5-hDLL1 cells to observe maturation of progenitor T cells under physioxia vs ambient air and to check the functionality of these T cells. Other studies have used mouse transplant assays to test the functionality of myeloid and B-lymphoid progenitor cells. However, generation of mature CD3+ T cells in immunodeficient mice after injection of CD34+ cells results in minimal or severely delayed engraftment because of the atrophied thymus.40,41 Due to this limitation, alternative transplant methods using intrahepatic injection of cells to neonate mice,42 intrathymic injection of cells,43 and cotransplantation with fetal thymic tissue44 have been used to assess T-cell development in vivo. However, it may be complicated to determine a role for physioxia on thymic maturation of T cells because the in vivo environment is already at low oxygen tension.
AA is an antioxidant reducing oxygen stress,45 which may in part mimic physioxia. When BM cells were harvested under physioxia, yields of HSC were increased.19 AA also induced increased yield of HSC, and further increased this yield under physioxia for mouse BM cells.46 Additive effects of AA on T-cell development may be through non-hypoxia-mimicking mechanisms. Mean plasma concentrations of ascorbic acid reported in healthy populations are 7.98 mg/L (range,0.50–17.4).47 When we converted these to molar concentrations, using molecular weight of L-ascorbic acid (176.12 g/mol), the mean plasma concentration of ascorbic acid was 45.3 μM (range, 2.8–98.8). Concentrations of AA (100 μM) used in our experiment converged with the upper limit of the normal range of the plasma concentration of ascorbic acid in healthy people. Standard cell culture conditions are often deficient in AA. Culture methods in vitro using primary human cells might negatively impact cell growth and may not represent true phenomenon occurring in the microenvironment in vivo. Thus, ascorbic acid might be a nutritional niche factor acting in association with the lowered O2 tension in the thymus.
Scale up production as well as the exclusion of xenogeneic feeder-cells and serum are needed for clinical application of produced pro cells. Although there has not been clinical experience of proT cell infusion for human subjects, there is a phase I/II clinical trial of haploidentical HCT and delayed infusion of ex vivo generated progenitor T cell (CD7+) scheduled soon for pediatric patients with severe combined immunodeficiency (ClinicalTrials.gov Identifier: NCT03879876); investigators are planning to use cell doses of 0.1 ~ 1.5 × 106 CD7+ cells/kg.48
In our results, a mean of 0.9 × 105 CD7+ cells were obtained from 4000 CD34+ cells in the HA group. If we scale up starting CD34+ cells to 1–3 × 106, an obtained cell dose from one CB unit, the expected yield of CD7+ cells in the HA group on day 14 is 6.6–19.8 × 107 (2.2–6.6 × 106/kg in pediatric patients with 30 kg body weight), a yield of proT cells that might be enough for pediatric patients based on this trial scheme. Greater improvement of proT cell yield is required for adult patients with higher body weights. A previous study using “engineered thymic niche”16 to improve proT cell yield reported up to 1 × 105 CD7+ cells starting from 4 × 103 CD34+ cells, which is similar to our data.
In summary, our study demonstrates that physioxia plus AA promotes CB CD34+ cell commitment and differentiation into progenitor T cells, and subsequently into mature T cells, suggesting that low oxygen tension is one essential factor of the thymic niche for T-cell development. Furthermore, this effect of physioxia can be boosted through addition of AA, a mimicker of physioxia.
It is possible that our results may be able to be translated into GMP-scale manufacturing and clinical application for patients treated with CB HCT and for immunocompromised patients, studies that require future evaluation.
5 |. CONCLUSION
In summary, our report is the first to investigate the role of physioxia on the whole sequence ex vivo of T-cell differentiation and maturation from human HSC/HPC. Physioxia promotes HSC commitment and HPC differentiation into progenitor T cells in non-xenogeneic feeder- and serum-free suspension culture, effects enhanced with ascorbic acid. Progenitor cells produced in this feeder-free culture system subsequently maturate into T cells in ATOs. Physioxia also enhances maturation to T cells in ATOs. This provides new insight to human T-cell development in hypoxic thymic niche, which may be translated to clinical application.
Supplementary Material
Significance statement.
This study is the first to investigate the role of physiologically relevant oxygen concentration (5% O2, Physioxia) on the ex vivo sequence of T-cell differentiation and maturation from human hematopoietic stem and progenitor cells. Physioxia promotes hematopoietic stem cell commitment and hematopoietic progenitor cell differentiation into progenitor/precursor T cells in suspension culture, effects enhanced with a potent antioxidant ascorbic acid, and which further facilitates T-cell maturation in artificial thymic organoids. This provides new insight into human T-cell development and may be useful for future clinical translation for patients treated with cord blood hematopoietic cell transplantation and for immunocompromised patients.
ACKNOWLEDGMENTS
This work was supported by Public Health Service Grants from the National Institute of Health to Hal E. Broxmeyer: (HL R35 139599 and U54 DK 106846), and by the Training Abroad Fund from the Seoul National University Hospital to Dong-Yeop Shin. Arafat Aljoufi and James Ropa were respectively supported as pre- and post-doctoral trainees on T32 DK 007519 to Hal E. Broxmeyer. We want to thank the rest of the Broxmeyer laboratory personnel for helpful assistance and discussions, and the Flow Cytometry Facility of the Indiana University School of Medicine for assistance. We would like to thank to Minchan Shin and Gyurim Shin for help with drawing some of the diagrams shown in the figures.
Footnotes
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Scheinberg P, Melenhorst JJ, Brenchley JM, et al. The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood. 2009;114:5071–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogonek J, Kralj Juric M, Ghimire S, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martelli MF, Di Ianni M, Ruggeri L, et al. “Designed” grafts for HLA-haploidentical stem cell transplantation. Blood. 2014;123:967–973. [DOI] [PubMed] [Google Scholar]
- 4.Handgretinger R, Schilbach K. The potential role of gammadelta T cells after allogeneic HCT for leukemia. Blood. 2018;131:1063–1072. [DOI] [PubMed] [Google Scholar]
- 5.Feyerabend TB, Terszowski G, Tietz A, et al. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67–79. [DOI] [PubMed] [Google Scholar]
- 6.Hozumi K, Mailhos C, Negishi N, et al. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med. 2008; 205:2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zúñiga-Pflücker JC. Direct comparison of Dll1- and Dll4-mediated notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol. 2010;185:867–876. [DOI] [PubMed] [Google Scholar]
- 8.Ivanovic Z Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol. 2009;219:271–275. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Trinh T, Aljoufi A, Broxmeyer HE. Hypoxia signaling pathway in stem cell regulation: good and evil. Curr Stem Cell Rep. 2018;4: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. [DOI] [PubMed] [Google Scholar]
- 11.Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arikan GM, Scholz HS, Petru E, Haeusler MCH, Haas J, Weiss PAM. Cord blood oxygen saturation in vigorous infants at birth: what is normal? BJOG. 2000;107:987–994. [DOI] [PubMed] [Google Scholar]
- 13.Victory R, Penava D, Da Silva O, et al. Umbilical cord pH and base excess values in relation to adverse outcome events for infants delivering at term. Am J Obstet Gynecol. 2004;191:2021–2028. [DOI] [PubMed] [Google Scholar]
- 14.Braun RD, Lanzen JL, Snyder SA, Dewhirst MW. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am J Physiol Heart Circ Physiol. 2001; 280:H2533–H2544. [DOI] [PubMed] [Google Scholar]
- 15.Huijskens MJ, Walczak M, Koller N, et al. Technical advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J Leukoc Biol. 2014;96:1165–1175. [DOI] [PubMed] [Google Scholar]
- 16.Shukla S, Langley MA, Singh J, et al. Progenitor T-cell differentiation from hematopoietic stem cells using Delta-like-4 and VCAM-1. Nat Methods. 2017;14:531–538. [DOI] [PubMed] [Google Scholar]
- 17.Hata R, Senoo H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissue like substance by skin fibroblasts. J Cell Physiol. 1989;138:8–16. [DOI] [PubMed] [Google Scholar]
- 18.Seet CS, He C, Bethune MT, et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat Methods. 2017;14:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantel CR, O’Leary HA, Chitteti BR, et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell. 2015;161:1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenger RH, Kurtcuoglu V, Scholz CC, Marti HH, Hoogewijs D. Frequently asked questions in hypoxia research. Hypoxia (Auckl). 2015;3:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. [DOI] [PubMed] [Google Scholar]
- 23.Haynes BF, Martin ME, Kay HH, Kurtzberg J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J Exp Med. 1988;168:1061–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awong G, Herer E, Surh CD, Dick JE, la Motte-Mohs RN, Zúñiga-Pflücker JC. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114:972–982. [DOI] [PubMed] [Google Scholar]
- 25.Meek B, Cloosen S, Borsotti C, et al. In vitro-differentiated T/natural killer-cell progenitors derived from human CD34+ cells mature in the thymus. Blood. 2010;115:261–264. [DOI] [PubMed] [Google Scholar]
- 26.Dik WA, Pike-Overzet K, Weerkamp F, et al. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med. 2005;201:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staal FJ, Wiekmeijer AS, Brugman MH, et al. The functional relationship between hematopoietic stem cells and developing T lymphocytes. Ann N Y Acad Sci. 2016;1370:36–44. [DOI] [PubMed] [Google Scholar]
- 28.Goardon N, Marchi E, Atzberger A, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell. 2011;19:138–152. [DOI] [PubMed] [Google Scholar]
- 29.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585–593. [DOI] [PubMed] [Google Scholar]
- 30.D’Aniello C, Habibi E, Cermola F, et al. Vitamin C and l-proline antagonistic effects capture alternative states in the pluripotency continuum. Stem Cell Rep. 2017;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cimmino L, Dolgalev I, Wang Y, et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell. 2017; 170:1079–95.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Arena G, Musto P, Cascavilla N, et al. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998;83:197–203. [PubMed] [Google Scholar]
- 33.Reimann C, Six E, Dal-Cortivo L, et al. Human T-lymphoid progenitors generated in a feeder-cell-free Delta-like-4 culture system promote T-cell reconstitution in NOD/SCID/gammac(−/−) mice. STEM CELLS. 2012;30:1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh J, Chen ELY, Xing Y, Stefanski HE, Blazar BR, Zúñiga-Pflücker JC. Generation and function of progenitor T cells from StemRegenin-1-expanded CD34+ human hematopoietic progenitor cells. Blood Adv. 2019;3:2934–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner JE Jr, Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371:1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Besien K, Hari P, Zhang MJ, et al. Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and graft-versus-host disease-free, relapse-free survival. Haematologica. 2016;101:634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou J, Li P, Lu F, et al. Notch1 is required for hypoxia-induced proliferation, invasion and chemoresistance of T-cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2013;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chabi S, Uzan B, Naguibneva I, et al. Hypoxia regulates lymphoid development of human hematopoietic progenitors. Cell Rep. 2019;29: 2307–20 e6. [DOI] [PubMed] [Google Scholar]
- 39.Kincade PW, Lee G, Scheid MP, et al. Characterization of murine colony-forming B cells. II. Limits to in vitro maturation, Lyb-2 expression, resolution of IgD+ subsets, and further population analysis. J Immunol. 1980;124:947–953. [PubMed] [Google Scholar]
- 40.Liu C, Chen BJ, Deoliveira D, et al. Progenitor cell dose determines the pace and completeness of engraftment in a xenograft model for cord blood transplantation. Blood. 2010;116:5518–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. [DOI] [PubMed] [Google Scholar]
- 42.Awong G, Singh J, Mohtashami M, et al. Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. Blood. 2013;122:4210–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuckett AZ, Thornton RH, O’Reilly RJ, van den Brink MRM, Zakrzewski JL. Intrathymic injection of hematopoietic progenitor cells establishes functional T cell development in a mouse model of severe combined immunodeficiency. J Hematol Oncol. 2017;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. [DOI] [PubMed] [Google Scholar]
- 45.Shaban S, El-Husseny MWA, Abushouk AI, et al. Effects of antioxidant supplements on the survival and differentiation of stem cells. Oxid Med Cell Longev. 2017;2017:5032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai Q, Capitano M, Huang X, Guo B, Cooper S, Broxmeyer HE. Combinations of antioxidants and/or of epigenetic enzyme inhibitors allow for enhanced collection of mouse bone marrow hematopoietic stem cells in ambient air. Blood Cells Mol Dis. 2018;71:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagel AF, Albrecht H, Dauth W, et al. Plasma concentrations of ascorbic acid in a cross section of the German population. J Int Med Res. 2018;46:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andre I, Simons L, Ma K, et al. Ex vivo generated human T-lymphoid progenitors as a tool to accelerate immune reconstitution after partially HLA compatible hematopoietic stem cell transplantation or after gene therapy. Bone Marrow Transplant. 2019;54:749–755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


