Abstract
Patient: Female, 7-year-old
Final Diagnosis: Septic hip brucelosis
Symptoms: Hip pain
Medication: —
Clinical Procedure: —
Specialty: Orthopedics and Traumatology
Objective:
Rare disease
Background:
Brucellosis is a major health problem, especially in endemic countries. Although musculoskeletal system involvement is common with brucellosis, the development of septic arthritis in connection with brucellosis has been rarely reported.
Case Report:
A 7-year-old girl presented with high-grade fever and severe right hip pain. Based on her clinical picture, laboratory tests, and radiographic images, septic arthritis was diagnosed and subsequently managed with standard septic arthritis treatment. The severity of hip pain significantly decreased after hip drainage. However, a high-grade fever persisted, raising suspicion of brucellosis, which was confirmed by serological testing. Standard treatment of brucellosis was administered and the patient responded well.
Conclusions:
Brucella arthritis should be considered as a differential diagnosis of septic hip, especially in endemic areas. The nonpurulent nature of the hip aspirate should raise suspicion of brucella arthritis. Early diagnosis of brucella septic arthritis is important to avoid serious complications.
Keywords: Arthritis, Infectious; Brucellosis; Zoonoses
Background
Septic arthritis is a common and a harmful medical condition with significant morbidity and mortality. It has a bimodal distribution of incidence, with peaks in both children and the elderly. Its yearly incidence is estimated to be 4 to 37 cases per 100 000 people. The most common isolated microorganism associated with septic arthritis is Staphylococcus aureus. Higher rates of septic arthritis are reported in patients who are immunocompromised, participate in high-risk behavior, such as drug abuse, and have prosthetic joints. In endemic regions, some rare microorganisms should be considered, such as brucella and tuberculosis [1–3].
Brucellosis is a major health problem, especially in endemic countries, and is the most common zoonotic disease worldwide [4]. The incidence of brucellosis is underestimated because of misdiagnosis owing to its subtle nature and multi-systemic involvement. Early detection of brucellosis decreases infection relapse and potential debilitating complications [5,6]. Although the musculoskeletal system is commonly affected in brucellosis, brucella septic arthritis has been rarely reported [7–10]. Herein, we report a case of brucella septic arthritis.
Case Report
A 7-year-old girl without a previous medical history presented after 6 days of fever and right hip pain. The patient’s fever was continuous, partially relieved with antipyretic, and associated with chills. Her hip pain was a nonradiating groin pain, progressive in severity, continuous, and associated with limping and the inability to bear weight. The patient’s mother reported that the patient had a history of minor trauma that occurred 2 days before the symptoms started. The patient lives on a farm that has cows and goats. The patient’s family history and medical and surgical history were unremarkable. Three days after the symptoms started, the patient sought medical care and was misdiagnosed with transient synovitis and treated with bed rest. Three days later, she was referred to our center. The patient was hemodynamically stable with a 41°C fever. She was complaining of severe pain in the hip and refusing to put weight on it. When lying in bed, she kept her hip in a flexed, abducted, and externally rotated position. Her passive and active hip motion was restricted because of the pain. The examination of her remaining musculoskeletal system and other body organs was unremarkable. There were no palpable lymph nodes and no evidence of hepatosplenomegaly. Laboratory blood tests showed a normal leukocyte count of 4380×103/mm3. The differential count showed decreased neutrophils (32%) and increased lymphocytes (52%). An increased erythrocyte sedimentation rate (31 mmHg) and C-reactive protein level (27.02) were also noted. The hemoglobin level was 10.30 g/dL, and the blood culture was negative.
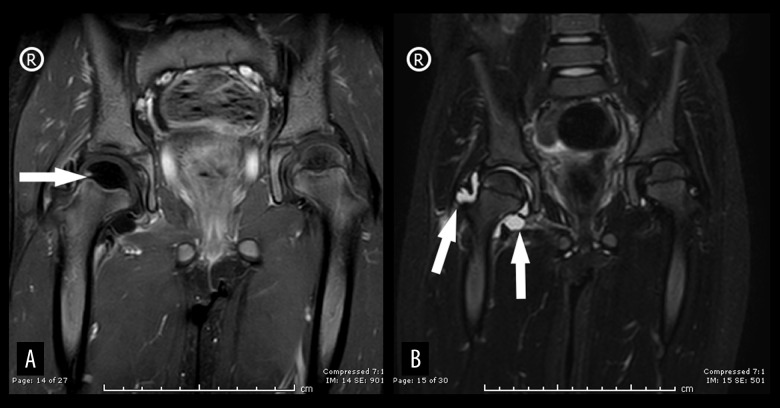
A pelvic X-ray, ultrasound, and magnetic resonance imaging (MRI) were ordered. The X-ray showed no changes (Figure 1), and the ultrasound showed joint effusion. The MRI revealed a moderate amount of joint effusion with enhanced synovium. The synovium appeared of low signal intensity on T2 and STIR, which could have been related to blood products. It also showed a small enhanced wall collection anterolateral to the neck of the right femur, measuring about 0.4 cm × 0.5 cm and associated with surrounding enhanced intramuscular edema. The right femoral head appeared diffusely hypo-enhanced compared to the left, raising concern of impending avascular necrosis (Figures 2, 3). Septic arthritis was the first differential diagnosis made. On admission, the patient was treated with vancomycin. Owing to her worsening clinical condition (fever continued with worsening hip pain), and impending avascular necrosis, which was revealed on the MRI, we decided to perform right hip joint debridement. The hip was approached anteriorly using the Smith-Peterson approach. A gush of serous yellow fluid, as shown in Figure 4, was aspirated and sent for Gram staining and culture. Copious irrigation was done by 6 liters of 0.9% normal saline, and the wound was closed layer by layer after drain insertion. Cytological analysis results of the synovial fluid revealed 20 000 cells/mm3 with a neutrophilic predominance (90%). The Gram stain results of the synovial fluid showed no organisms, and the culture showed no growth.
Figure 1.
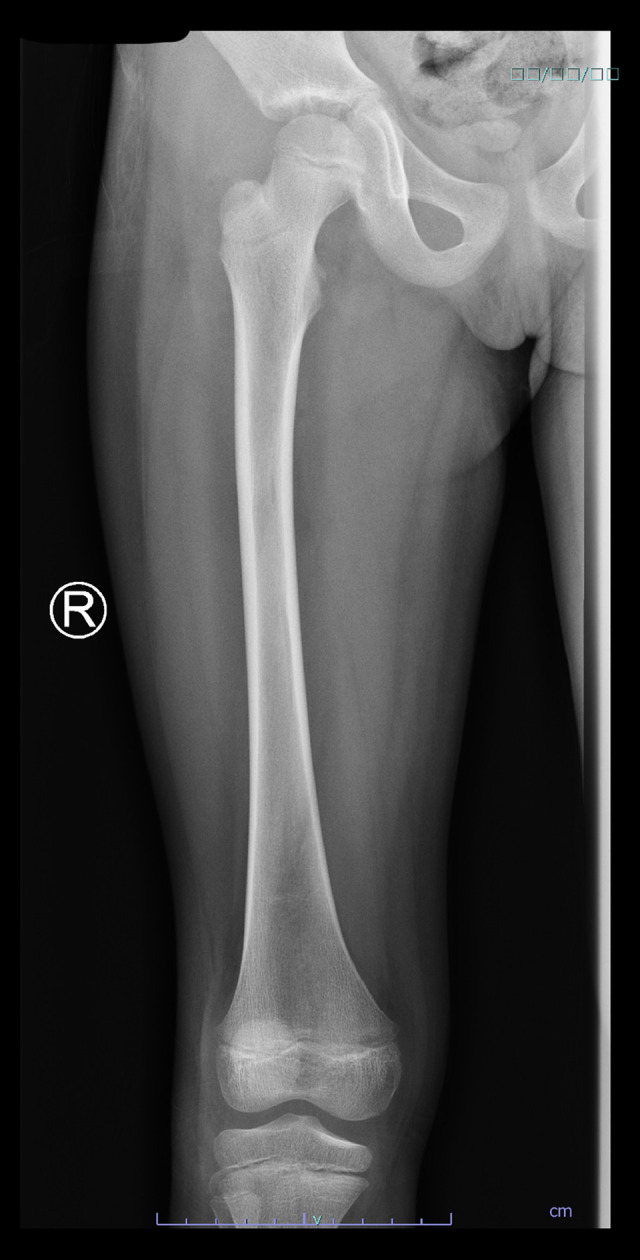
X-ray of the right femur; finding was unremarkable.
Figure 2.
Bilateral hip magnetic resonance imaging (MRI). (A) Post-contrast fat-saturated bilateral hip T1W MRI, coronal view; arrow points to impeding avascular necrosis. (B) Bilateral hip MRI; T2W MRI, coronal view; arrows point to hyperintense area, indicating joint effusion.
Figure 3.
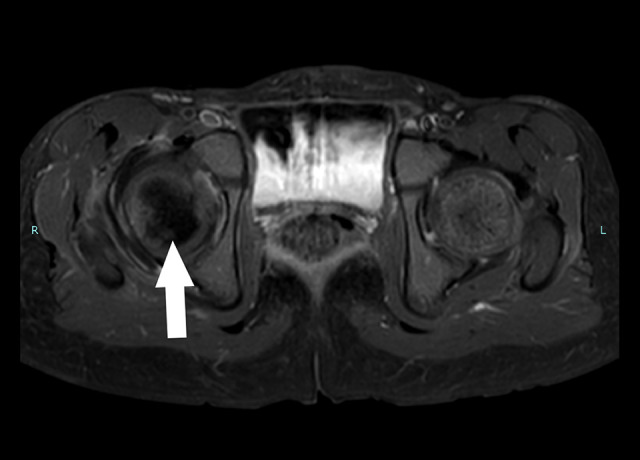
Bilateral hip magnetic resonance imaging (MRI); T2W MRI, axial view, arrow points to focal area of reduced marrow signal intensity, indicating impeding avascular necrosis.
Figure 4.
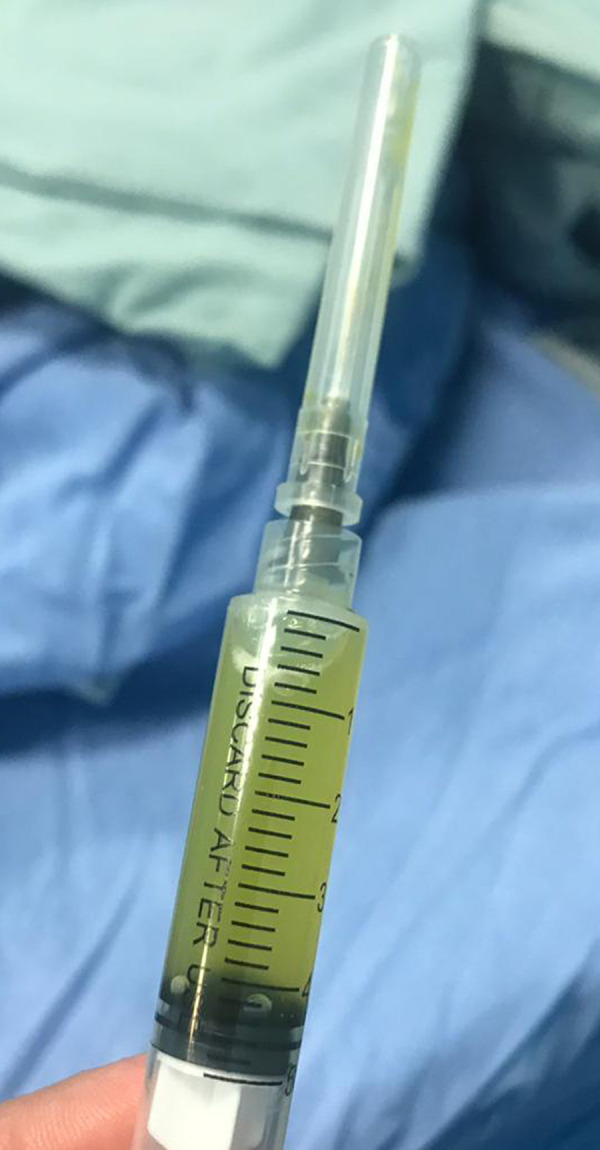
Synovial fluid; serous yellow in color.
After surgery, the patient’s hip pain dramatically improved but she continued to have high-grade fever.
We suspected brucella infection and tested the patient for brucella serology using the Rose Bengal test, which was positive (titer level >160). The treatment regimen for brucellosis was initiated by gentamycin (115 mg IV every 24 h), doxycycline (50 mg orally every 12 h for 6 weeks), and rifampin (450 mg orally every 24 h for 6 weeks). The patient’s condition improved dramatically after the start of treatment.
Discussion
There are 4 species of brucella that cause most cases of diseases in humans, each with a different animal host reservoir. The most common is Brucella melitensis (goats, camels), followed by Brucella abortus (cattle), Brucella suis (pigs), and Brucella canis (dogs) [11].
There is more brucellosis with peripheral skeleton involvement than with vertebral features in the pediatric population, in contrast to that in adults [11]. It can present as arthralgia, enthesopathy, osteomyelitis, arthritis, bursitis, tendonitis, and tenosynovitis [12] in the most commonly affected peripheral joints, which are the knee, hip, sacroiliac, and ankle, respectively [12,13]. Chronic knee arthritis and osteomyelitis due to brucella infection have also been reported [10,14].
Septic arthritis caused by brucella progresses slowly. In the early stages, the arthritis begins with small pericapsular erosions. Blood culture is not sensitive for brucella; it is positive in only 20% to 70% of patients [11]. Also, synovial fluid Gram stain and culture results are frequently negative, and a low leukocyte count does not exclude brucella septic arthritis [15–17]. The diagnosis usually depends on the analysis of synovial fluid cytologically, epidemiological consideration, family history, and high suspicion [18].
Arthritis of the knee has a typical presentation and is easy to diagnose, while the diagnosis and treatment of arthritis of the hip are more difficult, and any delay in treatment can lead to serious complications, such as dislocation and avascular necrosis of the femoral head [14,15,19]. Brucellosis should be considered in the differential diagnosis of a patient presenting with symptoms of knee or hip arthritis in endemic regions to prevent misdiagnosis and serious complications.
Although Jordan is an endemic area of brucellosis, in the present case, the patient visited 2 hospitals and her condition was deteriorating due to misdiagnosis resulting from the absence of extra-articular manifestations, which increased the difficulty of diagnosing brucellosis. But after surgical intervention, brucellosis was suspected owing to the serous nature of the fluid, the Gram-negative results, and the absence of pus. In addition, the child had anemia, which often accompanies brucella infection of the musculoskeletal system.
The Center for Disease Control recommends not consuming unpasteurized dairy products and undercooked meats, and that those dealing with animal tissues wear gloves, goggles, and gowns to prevent brucellosis infection [20].
Conclusions
Brucella arthritis should be considered as a differential diagnosis of septic hip, especially in endemic regions. A nonpurulent hip aspirate should raise a suspicion of brucella arthritis. Early diagnosis of brucella septic arthritis is important to avoid serious complications.
Acknowledgments
We are grateful to Shahed Obeidat, Ahmad Salah Fares, Husam Aldean Abuhayyeh, and Mustafa Alwani for moderating this paper, thereby significantly improving the manuscript.
Footnotes
Conflict of Interest
None.
References:
- 1.Wang YY, Zhao Z, Zhang JL, Huang F. [Clinical and imaging characteristics of 110 patients with infectious sacroiliitis] Zhonghua Nei Ke Za Zhi. 2020;59(2):134–39. doi: 10.3760/cma.j.issn.0578-1426.2020.02.008. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 2.Cohen E, Katz T, Rahamim E, et al. Septic arthritis in children: Updated epidemiologic, microbiologic, clinical and therapeutic correlations. Pediatr Neonatol. 2020;61(3):325–30. doi: 10.1016/j.pedneo.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Long B, Koyfman A, Gottlieb M. Evaluation and management of septic arthritis and its mimics in the emergency department. West J Emerg Med. 2019;20(2):331–41. doi: 10.5811/westjem.2018.10.40974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas G, Papadimitriou P, Akritidis N, et al. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 5.Shevtsov A, Syzdykov M, Kuznetsov A, et al. Antimicrobial susceptibility of Brucella melitensis in Kazakhstan. Antimicrob Resist Infect Control. 2017;6:130. doi: 10.1186/s13756-017-0293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanaei Dashti A, Karimi A. Skeletal Involvement of Brucella melitensis in Children: A systematic review. Iran J Med Sci. 2013;38(4):286–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Jalan D, Elhence A, Elhence P, Jain P. A case of acute septic arthritis hip caused by Brucella melitensis in an adolescent child. BMJ Case Rep. 2015;2015:bcr2015211678. doi: 10.1136/bcr-2015-211678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong TM, Lou N, Jin W, et al. Septic arthritis caused by Brucella melitensis in urban Shenzhen, China: A case report. J Med Case Rep. 2014;8:367. doi: 10.1186/1752-1947-8-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donoghue AF. Septic arthritis in the hip caused by Brucella melitensis. J Bone Joint Surg. 1933;2:506–8. [Google Scholar]
- 10.Wernaers P, Handelberg F. Brucellar arthritis of the knee: A case report with delayed diagnosis. Acta Orthop Belg. 2007;73(6):795–98. [PubMed] [Google Scholar]
- 11.Pappas G. The changing Brucella ecology: Novel reservoirs, new threats. Int J Antimicrob Agents. 2010;36(Suppl. 1):S8–11. doi: 10.1016/j.ijantimicag.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Esmaeilnejad-Ganji SM, Esmaeilnejad-Ganji SMR. Osteoarticular manifestations of human brucellosis: A review. World J Orthop. 2019;10(2):54–62. doi: 10.5312/wjo.v10.i2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg DL, Reed JI. Bacterial arthritis. N Engl J Med. 1985;312:764–71. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- 14.Ayaşlioğlu E, Ozlük O, Kiliç D, et al. A case of brucellar septic arthritis of the knee with a prolonged clinical course. Rheumatol Int. 2005;25(1):69–71. doi: 10.1007/s00296-004-0453-1. [DOI] [PubMed] [Google Scholar]
- 15.Cerit ET, Aydın M, Azap A. A case of brucellar monoarthritis and review of the literature. Rheumatol Int. 2012;32(5):1465–68. doi: 10.1007/s00296-011-1917-8. [DOI] [PubMed] [Google Scholar]
- 16.Fink CW, Nelson JD. Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–35. [PubMed] [Google Scholar]
- 17.Andonopoulos AP, Asimakopoulos G, Anastasiou E, Bassaris HP. Brucella arthritis. Scand J Rheumatol. 1986;15:377–80. doi: 10.3109/03009748609098207. [DOI] [PubMed] [Google Scholar]
- 18.Shmerling RH. Synovial fluid analysis: A critical reappraisal. Rheum Dis Clin North Am. 1994;20:503–12. [PubMed] [Google Scholar]
- 19.Sahu KK, Tsitsilianos N, Moselle L, Mishra AK. Septic arthritis of hip joint and its devastating complications. BMJ Case Rep. 2020;13(2):e233909. doi: 10.1136/bcr-2019-233909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Brucellosis prevention. 2020. http://www.cdc.gov/brucellosis/prevention/index.html.