Abstract
A stable triarylmethyl spin probe whose Electron Paramagnetic Resonance (EPR) spectrum is highly sensitive to molecular tumbling is reported. The strong anisotropy of the hyperfine coupling tensor with the central carbon of a 13C1-labeled triarylmethyl radical enables the measurement of the probe rotational correlation time with applications to measure microviscosity and molecular dynamics.
Keywords: EPR spectroscopy, Microviscosity, Isotopic labeling, Triarylmethyl radical, Spin probe
Graphical Abstract
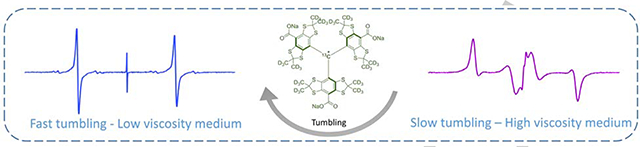
A stable triarylmethyl radical, whose EPR spectrum is highly sensitive to molecular tumbling, is presented.
Institute and/or researcher Twitter usernames: @IMMR_Chem, @WVUPharmacy, @WVUHealth
Electron paramagnetic resonance (EPR) spectroscopy with a molecular spin label or spin probe is a powerful technique used to study the topology, dynamics, and functions of proteins, nucleic acids or biological membranes in vitro[1] and to assess important biomarkers in vivo.[2] Two classes of stable radicals are widely used as spin probes or labels for biomedical EPR applications, namely nitroxide and triarylmethyl (TAM, trityl) radicals (Figure 1). Nitroxide radicals were developed as spin probes with spectral sensitivities to oxygen concentration, pH, thiols concentration, viscosity, polarity, etc.[3] Nitroxide spin labels allow for their conjugation to biomacromolecules to investigate their structure, conformational dynamics, and functions by taking advantage of the effect on the spectrum of restricted molecular motion of the label or by labeling a biomolecule with two nitroxides at selected sites and measuring the distance between the two spins using dipolar spectroscopy.[4] The most representative nitroxide spin label is the methanethiosulfonate MTSL label (Figure 1) that allows for easy conjugation to thiols. However, when used in vivo or in cells, nitroxide radicals are generally hampered by their fast reduction, leading to EPR-silent hydroxylamines. Recently, sterically shielded pyrrolidine nitroxides more resistant to reduction have been synthesized to address this issue.[5] Triarylmethyl radicals of type tetrathiatriarylmethyl bearing four sulfur atoms and one carboxylic acid per aromatic ring (see dFT and Ox071 in Figure 1) were synthesized by the late 90s to provide water solubility, high stability, and the absence of hyperfine splitting.[6] TAMs have extraordinary stability towards biological reducing and oxidizing agents. For example, the deuterated Finland trityl (dFT) has a half-life in human blood of more than 24h, and no significant decay of its EPR signal was observed upon incubation with an excess of GSH, ascorbic acid or hydrogen peroxide.[6–7] They also benefit from long relaxation times, leading to narrow EPR lines. TAMs typically exhibit a signal with a linewidth that is one order of magnitude smaller than nitroxides, leading to a high signal-to-noise ratio, which is critical for in vivo applications. TAM spin labels for distance measurements in DNA or protein [8] and spin probes with spectral sensitivities to oxygen concentration[9], pH[10], thiols concentration[11], inorganic phosphate[12], redox status[13] were reported.
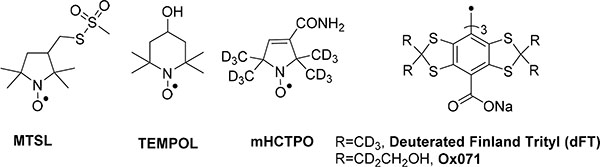
Figure 1.
Structures of molecular tumbling-sensitive nitroxides MTSL, TEMPOL and mHCTPO and non-sensitive to molecular tumbling dFT and Ox071 trityls
The EPR signals of nitroxide radicals such as TEMPOL or mHCTPO (Figure 1) are split by large hyperfine coupling (AN) to the nitrogen nucleus (l=1 for 14N and l=1/2 for 15N) of the nitroxide moiety. Both g and AN have significant anisotropies. In low-viscosity liquids, those anisotropies are averaged by the molecular tumbling, and only the average g (giso) and A (Aiso) are observed in the spectra. If the molecular tumbling is decreased because of higher viscosity of the solvent or by conjugation/interaction with a macromolecule or a membrane, the lines broaden, and features of the anisotropic spectrum are observed for sufficiently long tumbling correlation times.[3a] Spectral simulations permit determination of the tumbling correlation times (τR), which is defined as the time required for a molecule to rotate by one radian. This τR can be theoretically correlated to viscosity (η) through the Stokes-Einstein equation, τR= ηV/kT, where η is the viscosity, V is the particle volume, k is the Boltzman constant, and T is the temperature. Note that because of the molecular nature of the spin probe, the measurement reports the microscopic viscosity of the medium. Conversely, for tetrathiatriarylmethyl radicals reported to date, their EPR spectra are relatively insensitive to molecular tumbling, because of a very low anisotropy of the g-factors and no or small hyperfine splitting (A).[14]
In this communication, we report the synthesis of the first TAM radical whose EPR spectrum is highly sensitive to molecular tumbling and hence, to microviscosity. Analysis of the natural abundance 13C satellite lines in the EPR spectra for dFT, supported by quantum chemical calculations, revealed a strong anisotropy for the coupling with the central carbon C1.[15] An Az value of 160.1 MHz (57.13 G) was measured for the solid-state spectrum. Although the Ax=Ay could not be measured directly because of the overlap with the much more intense line from the natural abundance 12C1, values of Ax=Ay were estimated to be 20.6 MHz (7.35 G) based on the value of Az and the Aiso from the liquid-state spectrum.[15]
Because of the magnitude of the hyperfine coupling, the large anisotropy, and the high stability of TAMs, we thought that a dFT 13C-labeled at the central carbon would be an ideal EPR probe sensitive to motion.
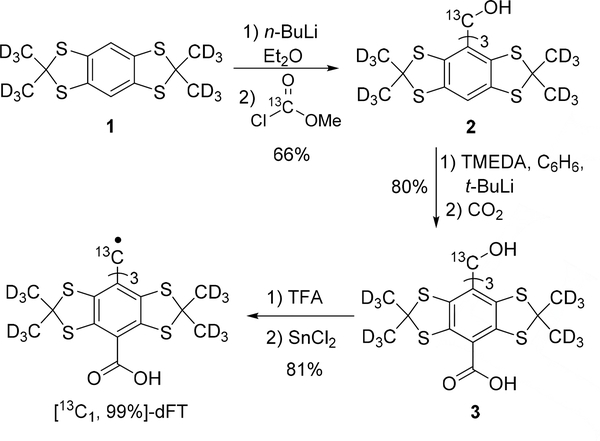
Our synthetic strategy is based on the well-optimized synthesis of dFT[16], with the use of a 99% enriched methyl chloroformate-(carbonyl-13C) for the formation of the trityl methanol 2 from the aryl 1 (Scheme 1). The subsequent carbonylation leading to 3 and the formation of the radical follow the strategy previously reported for dFT.
Scheme 1.
Synthesis of [13C1, 99%]-dFT.
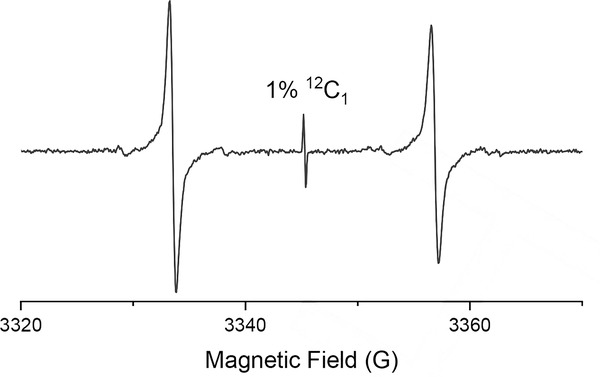
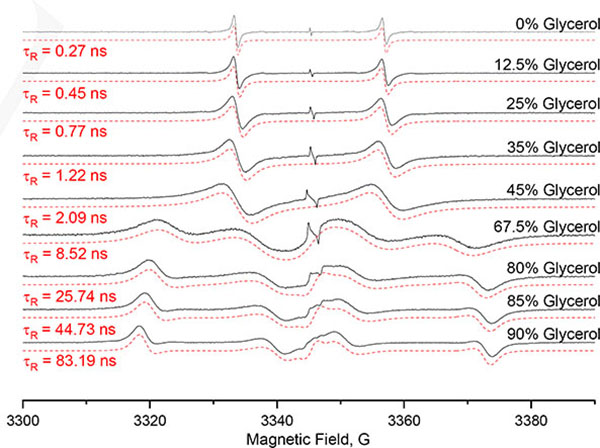
The EPR spectrum recorded at X-Band in deoxygenated PBS (10 mM, pH 7.4) at 22°C exhibits a large doublet as a result of coupling to the 13C1 (l=1/2), with a hyperfine splitting of 23.35±0.04 G (Figure 2). A single line corresponding to the 1% 12C1 dFT is also present. Interestingly, the EPR lines of the doublet are significantly broader than the single line peak corresponding to the non-labeled compound, ΔBpp= 0.57±0.01 G, compared to ΔBpp= 0.032±0.005 G§ for the non-labeled counterpart. The higher linewidth for the doublet is the result of an incomplete averaging of the A anisotropy at 22°C. To investigate the influence of tumbling on the EPR spectrum, samples of [13C1, 99%]-dFT were prepared with glycerol content varying from 0% to 90% at 22°C to increase viscosity and τR. The resulting EPR spectra (Figure 3) show a progressive transition from a doublet at 0% glycerol to an anisotropic immobilized spectrum at 90% glycerol. Values of Ax=Ay=17 MHz (6.07 G) and Az = 162 MHz (57.81 G) were determined by spectral simulation using EasySpin[17] for the spectrum at 90% glycerol.
Figure 2.
X-Band EPR spectrum of [13C1, 99%]-dFT (200 μM) in deoxygenated PBS (10 mM pH 7.4, NaCl 0.137M) at 22°C.
Figure 3.
X-Band EPR spectra (Black) of [13C1, 99%]-dFT (200 μM) in deoxygenated PBS with 0%, 12.5%, 25%, 35%, 45%, 67.5%, 80%, 85% and 90% (V/V) glycerol at 22°C. The spectra were simulated (red dashed lines) using the chili function of EasySpin, using gx=2.0031 gy=2.0031, gz=2.0027, and Ax=17 MHz, Ay=17 MHz, Az=162 MHz.
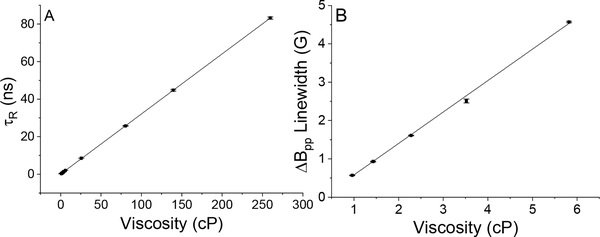
Computer simulations of the spectra permit determination of τR for each sample (see Table 1). Figure 4A shows the linear relationship between the determined τR and the viscosity, validating the simulation.
Table 1.
Influence of viscosity on τR determined using computer simulations and on the linewidths of the low field component of the doublet.
| Glycerol (%V/V)a | Viscosity (cP) | τR (ns)b | Measured Linewidth (G)c |
|---|---|---|---|
| 0 | 0.96 | 0.27±0.01 | 0.57±0.01 |
| 12.5 | 1.43 | 0.45±0.01 | 0.93±0.01 |
| 25 | 2.28 | 0.77±0.01 | 1.61±0.01 |
| 35 | 3.52 | 1.22±0.01 | 2.51±0.05 |
| 45 | 5.82 | 2.09±0.01 | 4.57±0.02 |
| 67.5 | 25.62 | 8.52±0.02 | - |
| 80 | 80.35 | 25.74±0.17 | -. |
| 85 | 139.52 | 44.73±0.47 | -. |
| 90 | 259.71 | 83.19±0.55 | -. |
%V/V Glycerol in deoxygenated PBS (10 mM, NaCl 0.137M) at 22°C.
Mean of τR calculated from three individual spectra.
Means of the peak-to-peak linewidths measured from three spectra for the low field peak.
Figure 4.
A) τR (ns) from spectral simulations versus viscosity (cP). Linear fit leads to the equation τR(ns)= 0.320*viscosity(cP)+0.095, R2=0.999. B) Measured ΔBpp linewidth for the low field component versus viscosity (cP). Linear fit leads to the equation ΔBpp (G)= 0.820*viscosity (cP)-0.256, R2=0.998.
By modulating the rate of bimolecular reactions, the microviscosity plays an important role in biochemistry. Abnormal microviscosity has been linked to diseases.[18] For example, a decreased average microviscosity of 1.8 cP in murine fibrosarcomas compared to 2.9 cP for the normal tissue was measured in vivo by EPR using the redox-sensitive mHCTPO probe.[18a] In order to further study microviscosity as a biomarker of cancer and other diseases directly in vivo, a probe that is resistant in biological media and highly sensitive to microviscosity is needed. Note that molecular rotors to measure viscosity using fluorescence techniques have been recently reported.[18b, 19] Unfortunately, light scattering within the tissue prevents the application of fluorescence-based techniques to deep tissues, whereas low-frequency magnetic resonance techniques (NMR, EPR) can penetrate more deeply.[20] In addition, EPR possesses the advantage over NMR to detect fast motions because of its intrinsic shorter timescale.
Below 45% glycerol (5.82 cP at 22 °C), the spectrum is essentially a doublet (Figure 3), and the effect of a longer τR is a line broadening. For some applications, such as in vivo, it may be more practical to measure the linewidth of one peak as an empirical parameter to extract the microviscosity without spectral simulation. Table 1 shows the measured linewidths for the low field component of the doublet for various viscosities, which allows calculation of the sensitivity of 0.820 G per cP (Figure 4B), which is roughly two orders of magnitude higher than the sensitivity of the linewidth to viscosity for TEMPOL at the same field.[3a] It is worth noting that a water-soluble perchlorinated trityl 50% labeled 13C1 at the central carbon with sensitivity to viscosity has been published. However, the anisotropy of the hyperfine splitting and the effect of viscosity on τR were not provided.[21]
Dissolved oxygen is also known to broaden the linewidth of TAM radicals through the Heisenberg spin exchange. However, the sensitivity of TAMs to oxygen is much smaller. The difference in linewidth measured for the low field peak between a solution in equilibrium with air (21% oxygen) and a deoxygenated solution reaches 110 mG (see SI). Note that in vivo values of viscosity in a range of 5 cP have been measured using a nitroxide probe [18a] this would correspond to a ≈4G linewidth range for [13C1, 99%]-dFT. Therefore, the effect of oxygen on the measurement of viscosity is minimal. Moreover, the utilization of the central line, corresponding to the non-labeled compound§§ (insensitive to viscosity), can be used to measure oxygen, which permits correction for oxygen concentration present in the milieu. Assuming an in vivo accuracy of measurement of 10% of the linewidth, it is possible to measure viscosity with an accuracy of ~0.1–0.5 cP for 1≤cP≤5. The small number of lines and the effect of viscosity limited to line broadening is an ideal feature for in vivo microviscosity imaging applications using the spectral-spatial EPR modality.
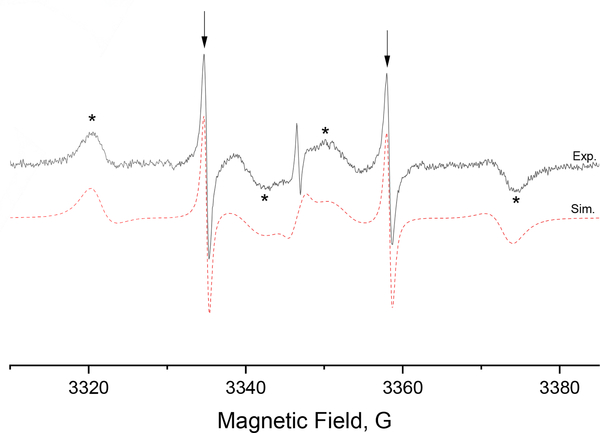
A motion-sensitive probe also enables the study of molecular interactions. The dFT radical is known to bind to bovine serum albumin (BSA) carrier protein.[22] The probe, when bound to BSA, is expected to have a slower tumbling rate because of the large volume of BSA. To study this interaction, [13C1, 99%]-dFT was mixed with BSA in water. The EPR spectrum in Figure 5 clearly shows the presence of two spectral components. A narrow doublet corresponding to the free probe (showed with arrows) with a fast tumbling rate and a second component with a slower tumbling rate corresponding to the probe bound to BSA (showed with asterisks). The spectral simulation allowed to determine τR = 36 ns for the probe bound to BSA, in good agreement with the correlation time of 30 ns for a nitroxide bond to BSA[23]. In comparison, the calculated τR for BSA with the Stokes-Einstein equation is 51 ns.[24]
Figure 5.
X-Band EPR spectra (Black) of [13C1, 99%]-dFT (400 μM) with BSA (500 μM) in deoxygenated water at 22°C. The arrows show the free probe in fast tumbling while the asterisks show the probes bound to BSA with a slower tumbling rate. The red dashed line shows the simulated spectra for two spectral components with τR =36 ns and 0.28 ns.
In conclusion, we demonstrated that the 13C1 isotope of a triarylmethyl spin probe used in vivo exhibits a sensitivity to molecular tumbling that can be used to measure microviscosity or molecular interaction. The measurement of viscosity is highly sensitive and can be carried out through spectral simulation or alternatively through the measurement of the EPR linewidth. The high stability of TAMs will enable measurements and imaging of viscosity in vivo, using EPR-based techniques. Moreover, further modifications of 13C1 dFT to target a specific biological compartment (e.g., intra-, extracellular space, blood pool) will allow studying the microviscosity of each compartment. A 13C1 Ox071[25], which does not bind albumin, will allow intravenous delivery. Also, a 13C1 TAM could be used to study molecular dynamics and interactions upon conjugation to a molecule of interest. Besides biological applications, 13C1 TAM and EPR could be used for other purposes, such as measuring viscosity in microfluidic devices, measuring glass transition temperature, macromolecular self-organization etc.
Supplementary Material
Acknowledgements
This work was partially supported by the NIH grant: EB023990. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. WVU HSC is acknowledged for start-up funds to B.D. The IMMR center and Valery. V. Khramtsov are acknowledged for access to the EPR facility. The authors gratefully acknowledge Sandra and Gareth Eaton (University of Denver) for the helpful discussion and advice on the experiments.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Supporting information for this article is given via a link at the end of the document.
The value of 0.032 G for the non-labeled dFT was measured using a modulation amplitude of 0.015 G and non-saturating power.
Using the 1% of the non-labeled compound or by adding dFT to the solution.
References
- [1].a)Sahu ID, Lorigan GA, Biomed Res. Int 2018, 2018, 3248289; [DOI] [PMC free article] [PubMed] [Google Scholar]; b)Fleissner MR, Bridges MD, Brooks EK, Cascio D, Kálai T, Hideg K, Hubbell WL, Proc. Natl. Acad. Sci. USA 2011, 108, 16241–16246; [DOI] [PMC free article] [PubMed] [Google Scholar]; c)Nguyen P, Qin PZ, WIREs 2012, 3, 62–72; [DOI] [PMC free article] [PubMed] [Google Scholar]; d)Qin PZ, Iseri J, Oki A, Biochem. Biophys. Res. Commun 2006, 343, 117–124; [DOI] [PMC free article] [PubMed] [Google Scholar]; e)Widomska J, Raguz M, Dillon J, Gaillard ER, Subczynski WK, BBA-Biomembranes 2007, 1768, 1454–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Khramtsov VV, Antiox. Red. Sign 2018, 28, 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a)Clark A, Sedhom J, Elajaili H, Eaton GR, Eaton SS, Concepts Magn. Reson. A 2016, 45A, e21423; [Google Scholar]; b)Bobko AA, Eubank TD, Voorhees JL, Efimova OV, Kirilyuk IA, Petryakov S, Trofimiov DG, Marsh CB, Zweier JL, Grigor’ev IA, Samouilov A, Khramtsov VV, Magn Reson Med 2012, 67, 1827–1836; [DOI] [PMC free article] [PubMed] [Google Scholar]; c)Weaver J, Burks SR, Liu KJ, Kao JPY, Rosen GM, J Magn Reson 2016, 271, 68–74; [DOI] [PMC free article] [PubMed] [Google Scholar]; d)Marsh D, Appl. Magn. Reson 2010, 37, 435–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jeschke G, Annu. Rev. Phys. Chem 2012, 63, 419–446. [DOI] [PubMed] [Google Scholar]
- [5].a)Paletta JT, Pink M, Foley B, Rajca S, Rajca A, Org. Lett 2012, 14, 5322–5325; [DOI] [PMC free article] [PubMed] [Google Scholar]; b)Karthikeyan G, Bonucci A, Casano G, Gerbaud G, Abel S, Thomé V, Kodjabachian L, Magalon A, Guigliarelli B, Belle V, Ouari O, Mileo E, Ang. Chem. Int. Ed 2018, 57, 1366–1370. [DOI] [PubMed] [Google Scholar]
- [6].Ardenkjær-Larsen JH, Laursen I, Leunbach I, Ehnholm G, Wistrand LG, Petersson JS, Golman K, J. Magn. Reson 1998, 133, 1–12. [DOI] [PubMed] [Google Scholar]
- [7].a)Song Y, Liu Y, Hemann C, Villamena FA, Zweier JL, J. Org. Chem 2013, 78, 1371–1376; [DOI] [PubMed] [Google Scholar]; b)Liu Y, Villamena FA, Zweier JL, Cehm. Commun 2008, 4336–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a)Giannoulis A, Yang Y, Gong Y-J, Tan X, Feintuch A, Carmieli R, Bahrenberg T, Liu Y, Su X-C, Goldfarb D, Phys. Chem. Chem. Phys 2019, 21, 10217–10227; [DOI] [PubMed] [Google Scholar]; b)Shevelev GY, Gulyak EL, Lomzov AA, Kuzhelev AA, Krumkacheva OA, Kupryushkin MS, Tormyshev VM, Fedin MV, Bagryanskaya EG, Pyshnyi DV, J. Phys. Chem. B 2018, 122, 137–143. [DOI] [PubMed] [Google Scholar]
- [9].Serda M, Wu Y-K, Barth ED, Halpern HJ, Rawal VH, Cehm. Res. Toxicol. 2016, 29, 2153–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marchand V, Levêque P, Driesschaert B, Marchand-Brynaert J, Gallez B, Magn Reson Med 2017, 77, 2438–2443. [DOI] [PubMed] [Google Scholar]
- [11].Tan X, Ji K, Wang X, Yao R, Han G, Villamena FA, Zweier JL, Song Y, Rockenbauer A, Liu Y, Angew. Chem. 2020, 132, 938–944. [DOI] [PubMed] [Google Scholar]
- [12].Bobko AA, Dhimitruka I, Zweier JL, Khramtsov VV, Ang. Chem. Int. Ed. 2014, 53, 2735–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu Y, Villamena FA, Rockenbauer A, Zweier JL, Cehm. Commun. 2010, 46, 628–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Owenius R, Eaton GR, Eaton SS, J. Magn. Reson. 2005, 172, 168–175. [DOI] [PubMed] [Google Scholar]
- [15].Bowman MK, Mailer C, Halpern HJ, J. Magn. Reson. 2005, 172, 254–267. [DOI] [PubMed] [Google Scholar]
- [16].Rogozhnikova OY, Vasiliev VG, Troitskaya TI, Trukhin DV, Mikhalina TV, Halpern HJ, Tormyshev VM, Eur. J. Org. Chem. 2013, 2013, 3347–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stoll S, Schweiger A, J. Magn. Reson. 2006, 178, 42–55. [DOI] [PubMed] [Google Scholar]
- [18].a)Halpern HJ, Chandramouli GVR, Barth ED, Yu C, Peric M, Grdina DJ, Teicher BA, Cancer Res. 1999, 59, 5836–5841; [PubMed] [Google Scholar]; b)Gupta N, Reja SI, Bhalla V, Gupta M, Kaur G, Kumar M, J. Mat. Chem. B. 2016, 4, 1968–1977; [DOI] [PubMed] [Google Scholar]; c)Aras S, Tek I, Varli M, Yalcin A, Cengiz OK, Atmis V, Atli T, Am. J. Alzheimer’s Dis. Other Dement. 2013, 28, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shimolina LE, Izquierdo MA, López-Duarte I, Bull JA, Shirmanova MV, Klapshina LG, Zagaynova EV, Kuimova MK, Sci. Reports 2017, 7, 41097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Röschmann P, Med. Phys. 1987, 14, 922–931. [DOI] [PubMed] [Google Scholar]
- [21].Elewa M, Maltar-Strmečki N, Said MM, El Shihawy HA, El-Sadek M, Frank J, Drescher S, Drescher M, Mäder K, Hinderberger D, Imming P, Phys. Chem. Chem. Phys. 2017, 19, 6688–6697. [DOI] [PubMed] [Google Scholar]
- [22].Song Y, Liu Y, Liu W, Villamena FA, Zweier JL, RSC Adv. 2014, 4, 47649–47656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cobb CE, Hustedt EJ, Beechem JM, Beth AH, Biophys. J. 1993, 64, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gelamo EL, Itri R, Alonso A, da Silva JV, Tabak M, Colloid J. Interface Sci. 2004, 277, 471–482. [DOI] [PubMed] [Google Scholar]
- [25].Poncelet M, Huffman JL, Khramtsov VV, Dhimitruka I, Driesschaert B, RSC Adv. 2019, 9, 35073–35076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.