Abstract
Purpose
Suboptimal glycemic control among individuals with diabetes is a leading cause of hospitalizations and emergency department utilization. Use of flash continuous glucose monitoring (flash CGM) improves glycemic control in type 1 and type 2 diabetes, which may result in lower risk for acute and chronic complications that require emergency services and/or hospitalizations.
Methods
In this retrospective, real-world study, we analyzed IBM MarketScan Commercial Claims and Medicare Supplemental databases to assess the impact of flash CGM on diabetes-related events and hospitalizations in a cohort of 2463 individuals with type 2 diabetes who were on short- or rapid-acting insulin therapy. Outcomes were changes in acute diabetes-related events (ADE) and all-cause inpatient hospitalizations (ACH), occurring during the first 6 months after acquiring the flash CGM system compared with event rates during the 6 months prior to system acquisition. ICD-10 codes were used to identify ADE for hypoglycemia, hypoglycemic coma, hyperglycemia, diabetic ketoacidosis, and hyperosmolarity.
Results
ADE rates decreased from 0.180 to 0.072 events/patient-year (hazard ratio [HR]: 0.39 [0.30, 0.51]; P < 0.001) and ACH rates decreased from 0.420 to 0.283 events/patient-year (HR: 0.68 [0.59 0.78]; P < 0.001). ADE reduction occurred regardless of age or gender.
Conclusions
Acquisition of the flash CGM system was associated with reductions in ADE and ACH. These findings provide support for the use of flash CGM in type 2 diabetes patients treated with short- or rapid-acting insulin therapy to improve clinical outcomes and potentially reduce costs.
Keywords: continuous glucose monitoring, type 2 diabetes, hospitalizations, hypoglycemia, hyperglycemia
The global prevalence of diabetes is estimated to increase from 9.3% (463 million people) in 2019 to 10.2% (578 million people) by 2030 [1], with the total cost of care rising to US $2.2 trillion [2]. Hospitalizations and emergency department utilization are primary contributors to these costs. In a recent American Diabetes Association report, the total direct cost of diabetes was estimated to be US $237.3 billion in 2017 [3]. Approximately 38% of these costs were attributed to hospital inpatient care ($69.7 billion), hospital outpatient treatment ($12.1 billion), emergency department utilization ($8.0 billion) and ambulance services ($332.0 million).
Hospitalizations are prevalent among individuals with type 2 diabetes, who account for 90% to 95% of all diabetes cases [4]. Adults with type 2 diabetes are admitted for emergency department treatment or hospitalized for numerous health conditions, including severe hyperglycemia and hypoglycemia [5-9]. These events are particularly common among patients with large fluctuations in glycated hemoglobin (HbA1c) and very high or very low average HbA1c levels [10].
Suboptimal glycemic control using traditional blood glucose monitoring persists among a substantial number of patients with type 2 disease [11, 12]. However, randomized controlled trials have demonstrated that use of continuous glucose monitoring (CGM) significantly lowers HbA1c [13], with reductions in hypoglycemia [14, 15] and improved treatment satisfaction [13, 14] in various type 2 diabetes populations.
Unlike traditional self-monitoring of blood glucose (SMBG), CGM systems provide a continuous stream of glucose data, indicating the current interstitial glucose level and the direction and velocity of changing glucose. This information allows users to quickly intervene to prevent or reduce acute hypoglycemia or hyperglycemia.
The FreeStyle Libre 14-day system, manufactured by Abbott Diabetes Care, Alameda, CA, is the only flash CGM system currently available in the US. An earlier version (10-day system) was available from 2017 to 2019. Unlike traditional blood glucose monitors, which provide only a single “point-in-time” glucose value, flash CGM systems utilize a single-use, factory-calibrated sensor that continuously measures interstitial glucose levels. By scanning the sensor with the reader or smartphone, the user can view the current glucose value as well as the glucose pattern over the past 8 hours with trend arrows, which indicate the direction and velocity of changing glucose levels.
Suboptimal glycemic control among individuals with diabetes is a leading cause of hospitalizations and emergency department utilization. Use of flash continuous glucose monitoring (flash CGM) improves glycemic control in type 1 and type 2 diabetes, which may result in lower risk for acute and chronic complications that require emergency services and/or hospitalizations.
We analyzed the effects of system acquisition on inpatient and emergency outpatient acute diabetes-related event (ADE) and all-cause hospitalization (ACH) rates, in a large population of patients with type 2 diabetes who were treated with short- or rapid-acting insulin therapy. This represents the early real-world experience of patients using flash CGM systems.
Materials and Methods
Design and Study Sample
This retrospective database study assessed the effects of flash CGM system acquisition on occurrence rates of ADE and ACH within a large cohort of patients with type 2 diabetes treated with short- or rapid-acting insulin therapy. Both analysis structure and outcomes were prespecified. Patients were included if they had a diagnosis of type 2 diabetes, were ≥ 18 years of age, received a prescription for short- or rapid-acting insulin, were naïve to CGM, and acquired either the 10-day or 14-day sensor system between November 2017 and September 2018. In addition, patients were required to be continuously enrolled in the inpatient, outpatient, and pharmacy databases for at least 6 months prior to system acquisition.
Diabetes type was determined from the closest relevant diagnosis claim prior to flash CGM acquisition. In the rare case the closest claim had billing codes related to both type 1 and type 2 diabetes, the patient was not included. In addition, patients with a gestational diabetes diagnosis in the 6 months prior to flash CGM acquisition were excluded.
Data Sources
Patient data were obtained from the IBM Watson Health MarketScan Commercial Claims and Medicare Supplemental databases, which capture paid and adjudicated billing claims from inpatient hospital stays, outpatient encounters, and pharmacy prescriptions for privately insured and Medicare Supplemental patients throughout the United States. This nationally representative database has been used to support publications in the field of diabetes research [16, 17]. A monthly patient enrollment indicator shows whether a patient is under observation, which allows for longitudinal analysis. Patients can be lost to follow-up for a wide variety of reasons, including switching employers, losing their jobs or death. The dataset does not provide information on why a patient is no longer under observation.
International Classification of Diseases, 9th and 10th Revision (ICD-10) codes were used to identify patients with diagnosed type 2 diabetes.
Existence of a comorbidity was defined by the presence of a related diagnosis code in either inpatient or outpatient claims at any time from the beginning of each patient’s data availability through the day of flash CGM acquisition. Within the identified population, National Drug Code (NDC) data were used to identify patients who acquired a flash CGM system during the required observation period. We identified patients who were treated with short- or rapid-acting insulin therapy, as indicated by acquisition of short- or rapid-acting insulin in the NDC data within 6 months prior to system acquisition.
To ensure that patients were naïve to CGM, we excluded those with evidence of prior CGM purchase, including sensor, transmitter, or receiver, identified via either NDC codes or Healthcare Common Procedure Coding System (HCPCS) codes.
Outcome Measures
The primary outcome measure was change in ADE during the full 6 months following system acquisition compared with 6 months prior to use. Acute events included: hypoglycemia, hypoglycemic coma, clinical hyperglycemia, diabetic ketoacidosis, and hyperosmolarity. These were identified as either inpatient events with the associated ICD-10 code as the primary diagnosis code or emergency outpatient events, which included emergency department services, urgent care, or ambulance services with the associated ICD-10 code in any position. The ICD-10 codes for acute events were as follows: hypoglycemia (E16.1, E16.2, E10-11.649, E13.649), hypoglycemic coma (E10-11.641, E13.641), hyperglycemia (E10-11.65, E13.65), diabetic ketoacidosis (E10.1x, E13.1x), and hyperosmolarity (E11.00, E13.0x). For each patient, medical billing codes associated with the same service or admit date were counted as a single event. The change in ACH rates was assessed as a secondary outcome. Event rates are calculated by dividing the number of observed events by the total observation time. A patient that is lost to follow-up before 6 months results in a lower total observation time, leading to a higher estimate of the rate if the same number of events were still detected.
Statistical Analysis
The analysis was structured as patient-as-own-control. Rates for all primary and secondary measures were calculated in the 6-month windows pre- and post-system purchase but are reported in units of events per patient-year (ev/pt-yr). Rates were adjusted for variable follow-up after system purchase. Cumulative events figures are based on Nelson-Aalen estimator. All hazard ratios, 95% confidence bounds, and P values are based on weighted Cox regression with Andersen-Gill extension for repeated events, adjusted for comorbidities listed in Table 1. Weighted Cox regression was used to account for nonproportionality of hazards, as tested via Schoenfeld residuals. Interactions were tested in the same model. All P values are reported without correction for multiple comparisons. RStudio version 1.0.153 (Boston, MA, USA) with R version 3.4.0 was used for statistical analysis.
Table 1.
Patient Characteristics
| Mean age, years (mean ± SD [10%, 90% deciles]) | 54.2 ± 9.6 (41, 64) |
| Male gender, % (n) | 52.9% (1304) |
| Follow-up days [10%, 90% deciles] | 173 [163, 182] |
| Comorbidities, % (n) | |
| Lipid disorder | 89.7% (2210) |
| Hypertension | 87.5% (2155) |
| Obesity | 60.0% (1479) |
| Neuropathy | 48.1% (1185) |
| Retinopathy | 31.2% (768) |
| Depression | 29.4% (724) |
| Pulmonary disease | 28.8% (709) |
| Hypothyroidism | 26.2% (646) |
| Anemia | 25.8% (635) |
| Myocardial infarction or coronary artery disease | 24.6% (606) |
| Liver disease | 20.8% (513) |
| Renal disease | 19.5% (480) |
| Peripheral vascular disease | 17.4% (429) |
| Heart failure | 13.1% (323) |
| Insurance type, % (n) | |
| Commercial insurance | 93.5% (2302) |
| Medicare supplemental insurance | 6.5% (161) |
Results
Patient Characteristics
We identified a cohort of 2463 patients with type 2 diabetes for assessment. Most patients were over the age of 50. The majority of patients had hypertension and dyslipidemia, and more than half were obese. Patient characteristics are presented in Table 1.
Outcomes
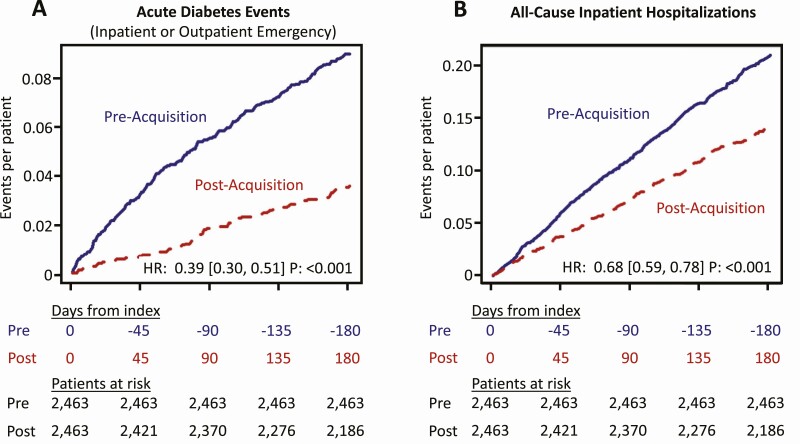
During the 6-month assessment period, we observed a reduction in ADE from 0.180 to 0.072 events/patient-year (hazard ratio [HR] 0.39 [0.30, 0.51]; P < 0.001) (Fig. 1A). Reductions in ACH were also observed, from 0.420 to 0.283 events/patient-year (HR 0.68 [0.59, 0.78]; P < 0.001). (Fig. 1B) The number of ADE, ACH, and patients experiencing these events dropped markedly during the 6-month post-acquisition period. (Table 2)
Figure 1.
Changes in ADE (A) and ACH (B). Figure shows reduction in acute diabetes-related events (ADE) and all-cause inpatient hospitalizations (ACH) from the 6-month period pre-flash CGM acquisition (solid line, blue) to the 6-month period post-flash CGM acquisition (dotted line, red). Cumulative event rate is based on a Nelson-Aalen estimator. ADE include diabetes-related inpatient hospitalizations and diabetes-related outpatient emergency visits including ambulance, urgent care, and emergency room visits. Results of weighted Cox regression with Andersen-Gill extension are presented as a hazard ratio (HR) with 95% confidence bounds and P values.
Table 2.
Number of Events and Number of Patients Affected
| Event type | 6-months pre-acquisition # events (# affected) | 6-months post-acquisition # events (# affected) |
|---|---|---|
| All-cause inpatient hospitalizations (ACH) | 516 (357) | 331 (239) |
| Acute diabetes events (ADE)a | 221 (181) | 84 (73) |
| Hypoglycemic ADE | 24 (21) | 17 (16) |
| Hyperglycemic ADE | 199 (166) | 69 (62) |
Each event type shows number of events (number of patients with event).
a In rare cases, when a hypoglycemic and hyperglycemic billing code appear on the same day, a single ADE is counted. So, totals on this row will be slightly lower than the sum of bottom 2 rows.
Subgroup Analysis
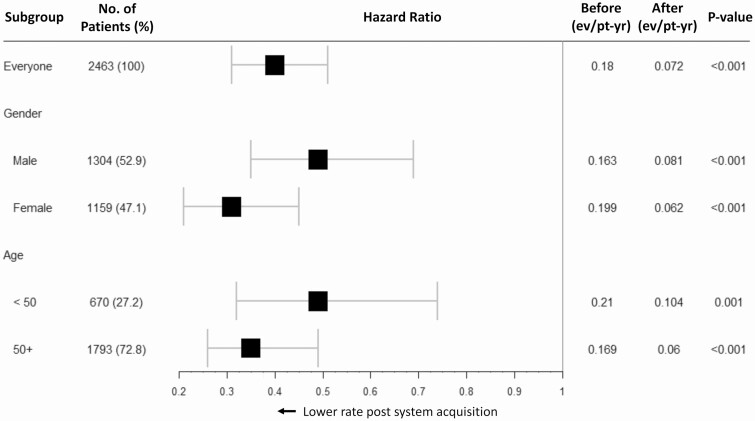
Risk reductions were significant regardless of gender or age, with trends toward greater risk reductions among female patients (HR 0.30 [0.20 0.44]; P < 0.001) and patients age ≥50 years (HR 0.35 [0.25 0.48]; P < 0.001); however, neither interaction term P value, 0.054 and 0.183 respectively, achieved statistical significance (Fig. 2).
Figure 2.
ADE by gender and age. Acute diabetes-related events (ADE) are analyzed for men and women and patients of different ages. Results of weighted Cox regression with Andersen-Gill extension for each patient group are presented as a hazard ratio with 95% confidence bounds and P values. Event rates per patient-year for the 6-month period before flash CGM acquisition and the 6-month period after flash CGM acquisition are illustrated.
Causes of ACH
Circulatory system disorders continued to be the primary cause of ACH after flash CGM acquisition (Table 3). However, Endocrine, Nutritional, and Metabolic system disorders, which is the category most related to diabetes, fell from the second to fifth most common major diagnostic category. Substantial decreases in infectious and parasitic diseases, respiratory system events, and kidney and urinary tract conditions were also observed.
Table 3.
Most Common Causes of All-Cause Hospitalization by Major Diagnostic Category
| Before flash CGM | After flash CGM | ||
|---|---|---|---|
| Hospitalizations (events/100 pt-yr) | Major diagnostic category | Hospitalizations (events/100 pt-yr) | Major diagnostic category |
| 7.8 | Circulatory system | 6.9 | Circulatory system |
| 6.4 | Endocrine, nutritional, and metabolic system | 2.8 | Infectious and parasitic DDs (systemic or unspecified sites) |
| 4.8 | Infectious and parasitic DDs (systemic or unspecified sites) | 2.8 | Musculoskeletal system and connective tissue |
| 3.5 | Respiratory system | 2.7 | Digestive system |
| 3.3 | Kidney and urinary tract | 2.6 | Endocrine, nutritional, and metabolic system |
| 3.2 | Musculoskeletal system and connective tissue | 2.1 | Respiratory system |
| 3.1 | Digestive system | 1.7 | Kidney and urinary tract |
| 2.9 | Nervous system | 1.6 | Nervous system |
| 2.4 | Hepatobiliary system and pancreas | 1.4 | Hepatobiliary system and pancreas |
Event rates per 100 patient years in the 6-month period pre-flash CGM acquisition and the 6-month period post-flash CGM acquisition are illustrated for major diagnostic categories. Each side of the table is sorted from highest to lowest event rate.
Discussion
We hypothesized that the availability of glucose data provided by the flash CGM system would be associated with a reduction in diabetes-related complications and resultant hospitalizations in individuals with type 2 diabetes who were treated with short- or rapid-acting insulin therapy. Results from the current analysis showed a significant association between acquisition of flash CGM and reductions in ADE requiring emergency outpatient/inpatient hospital services and all-cause events requiring inpatient hospitalization. The change in number of events per patient, particularly in ADE (Table 2), suggests a corresponding reduction in readmissions. Moreover, although the rate of hypoglycemic ADE was low prior to the flash CGM acquisition, the significant reduction in hyperglycemic ADE with slight reductions in hypoglycemia is a strong indicator of overall improved glycemic control. Both of these findings hold important clinical and financial implications. For example, hyperglycemia at hospital admission is a strong predictor of poor clinical outcomes for coronary artery bypass graft [18] and ischemic stroke [19-21]. As reported by Yun et al, each severe hypoglycemic event is significantly (P < 0.001) associated with increased risk for poor cardiovascular outcomes and all-cause mortality [22].
Because surveillance of hypoglycemia in the United States relies primarily on data from electronic health records (EHR) or administrative claims from hospital admissions and emergency department utilization, the actual incidence of severe hypoglycemia may be substantially underreported. In a recent survey of 13 359 individuals with diabetes who were treated with glucose-lowering medications, 11.7% reported having one or more severe hypoglycemic events requiring third-party assistance in the previous 12 months; however, 0.8% had a documented hypoglycemia-related emergency department or hospital utilization during the same time period [23].
Apart from its acute clinical outcomes, episodes of severe hypoglycemia can impact patient adherence to therapy, which can lead to poor glycemic control and increased risk of long-term complications [24, 25]. An international survey of 27 585 diabetes patients found that 25.8% to 46.7% of people with type 2 diabetes reduced their insulin dosages in response to hypoglycemia [26].
What makes our findings unique is that we saw a notable reduction in ADE and ACH within the first 45 days of the flash CGM post-acquisition period. Additionally, our findings from a real-world large patient cohort are consistent with results from prospective, observational studies involving both type 1 and type 2 diabetes [27, 28]. The FLARE-NL4 study, a prospective, nationwide registry in the Netherlands, assessed the impact of flash CGM use over 1 year in 1365 patients with type 1 diabetes (n = 1054), type 2 diabetes (n = 223), and others (n = 88) [27]. During the first year of use, the percentage of diabetes-related hospital admissions decreased 66% from 13.7% to 4.7% (P < 0.05). A similar study in Belgium assessed the impact of flash CGM use in 1913 adults with type 1 diabetes [28]. Investigators reported significant reductions in hospital admissions for severe hypoglycemia and diabetic ketoacidosis (from 3.2% to 2.2%; P = 0.031), the number of patients reporting severe hypoglycemic events (from 14.6% to 7.8%; P < 0.0001), and rates of hypoglycemic comas (from 2.7% to 1.1%; P = 0.001) following one year of flash CGM use. While these studies highlighted benefits in primarily the type 1 diabetes population, we wanted to explore the potential benefits of flash CGM in the type 2 diabetes population.
Results from our study also highlight the need for reducing hyperglycemia without increasing the incidence and severity of hypoglycemia. Although recent data show similar rates for hypoglycemic and hyperglycemic ADE in the general diabetes population (8.8 vs 9.7 per/1000 patients, respectively) [29], the substantially larger number of hyperglycemic vs hypoglycemic ADE prior to flash CGM acquisition suggests that many study patients historically maintained elevated glucose levels. As reported by Gregg et al, there was a notable increase in hyperglycemia-related hospitalizations among young adults and middle-aged patients (aged 18-44 years) between 2009 and 2015 [30]. Although several factors may explain persistent hyperglycemia, it has been suggested that this may, in part, be an “unintended consequence” of relaxed treatment target recommendations from medical organizations [30]. Additionally, although cardiovascular disease remains the leading cause of mortality in both type 1 and type 2 diabetes, we are seeing an emergence in infectious, respiratory, renal, and liver diseases, which are likely attributable to persistent hyperglycemia [31]. Hospitalizations for all of these conditions were notably reduced after flash CGM acquisition. As shown in Table 3, we found notable decreases in hospitalizations for infections (41.7%), renal disease (48.5%), and liver disease (41.7%). Although the IBM Watson Health MarketScan datasets did not provide HbA1c values or other information regarding glycemic status, we believe that reductions in these comorbidities are likely due to improved glycemic control, as reported by McCoy et al [32].
An essential component of all available CGM systems is the ability to automatically transfer data to healthcare professionals via cloud-based software for interpretation and more informed decision making [33]. Although previously considered to be futuristic, the importance of telemedicine and digital medical device technologies has been demonstrated to be the best (or the only) option in delivering essential healthcare to patients as the COVID-19 pandemic progresses [34]. We expect use of telemedicine and cloud-based CGM data to expand and become a standard of diabetes care moving forward.
A key strength of our analysis was use of claims data from a large dataset, which provided reliable information about acquisition of the flash CGM system over time in 2463 patients with insulin-treated type 2 diabetes. Similarly, assessments of complications and utilization of healthcare resources (eg, emergency room visits, inpatient hospitalizations) based on ICD-10 codes allowed us to accurately quantify actual events and utilization without reliance on patient-reported data.
A notable limitation was the inability to empirically assess patient behaviors relevant to using the system. Specifically, we cannot conclude whether or to what degree patients used their system. Did they use their glucose data to make therapy decisions? Did they use it appropriately? In addition, as discussed earlier, because the IBM Watson datasets provided no information regarding HbA1c values, we could not assess changes in overall glycemic control. We also could not assess the socioeconomic, educational characteristics, or participation in a formal diabetes self-management education program, all of which could have affected outcomes. This is a limitation inherent to all claims data studies. Additionally, because our analysis only included data from commercial claims and Medicare Supplemental databases, our findings cannot be generalized to lower socioeconomic populations. Furthermore, although it was assumed that all of the study patients were using some form of short- or rapid-acting therapy we could not confirm through our database query the exact composition of the insulin regimen used by each patient. Importantly, since they comprise a small fraction of our study cohort, our findings cannot be generalized to older diabetes patients (≥65 years), who account for approximately 27% of diabetes in the United States [29], and in whom the risk for hypoglycemia is substantially greater [35-37] due to higher rates of hypoglycemia unawareness [38] and cognitive impairment [39]. However, an earlier randomized controlled trial showed significant reductions in time spent with glucose levels <70 mg/dL (<3.9 mmol/L) among flash CGM users compared with controls [15]. Nor did our population include Medicaid patients, who often develop diabetes at an earlier age with an increased level of severity, and who have different patterns of technology use compared with Medicare beneficiaries and privately insured patients [40]. Lack of a comparison group is an important limitation but is inherent to the retrospective design of the study. Although we were able to show associations between system acquisition and clinical outcomes, a causal relationship cannot be established.
Our findings provide evidence for the use of flash CGM in insulin-treated type 2 diabetes to improve clinical outcomes and potentially reduce the financial burden associated with hospitalizations and emergency department utilization due to ADE. Wider use of flash CGM may address the changing trends of increasing all-cause hospitalizations among younger and middle-age adults and the newly emerging trends of increased mortality due to infections, respiratory illness, and renal and hepatic complications. Further investigation of how patients utilize their glucose data in day-to-day diabetes management might provide additional insights that could guide the development of educational strategies and mechanisms for ongoing patient support systems that would encourage both persistent and appropriate use of the system.
Acknowledgments
The authors thank Chris Parkin of CGParkin Communications for providing medical writing support.
Financial Support: This research was funded by Abbott Diabetes Care.
Glossary
Abbreviations
- ACH
all-cause hospitalization
- ADE
acute diabetes-related event
- CGM
continuous glucose monitoring
- HbA1c
glycated hemoglobin
- ICD-10
International Classification of Diseases, 10th Revision
- NDC
National Drug Code
- SMBG
self-monitoring of blood glucose
Additional Information
Disclosures: R.M.B. has received research support, consulted, or has been on a scientific advisory board for Abbott Diabetes Care, Ascensia, CeCur Corporation, DexCom, Hygieia, Insulet, Johnson & Johnson, Lilly, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi, and United Healthcare. His technology research is funded in part by NIH/NIDDK. R.M.B.’s employer, nonprofit HealthPartners Institute, contracts for his services and no personal income goes to R.M.B. I.B.H. receives research funding from Medtronic Diabetes and Insulet, and has received consulting fees from Abbott Diabetes Care, Bigfoot, Roche, and Becton Dickinson. M.S.D.K., G.J.R., D.S., and Y.N. are employed by Abbott.
Data Availability
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels: International Diabetes Federation; 2019. Accessed February 8, 2020. www.diabetesatlas.org [Google Scholar]
- 2. Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41(5):963-970. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannon A, Handelsman Y, Heile M, Shannon M. Burden of illness in type 2 diabetes mellitus. J Manag Care Spec Pharm. 2018;24(9-a Suppl):S5-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang HJ, Stryer D, Friedman B, Andrews R. Multiple hospitalizations for patients with diabetes. Diabetes Care. 2003;26(5):1421-1426. [DOI] [PubMed] [Google Scholar]
- 6. Kim H, Ross JS, Melkus GD, Zhao Z, Boockvar K. Scheduled and unscheduled hospital readmissions among patients with diabetes. Am J Manag Care. 2010;16(10):760-767. [PMC free article] [PubMed] [Google Scholar]
- 7. Raval AD, Zhou S, Wei W, Bhattacharjee S, Miao R, Sambamoorthi U. 30-day readmission among elderly medicare beneficiaries with type 2 diabetes. Popul Health Manag. 2015;18(4):256-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eby E, Hardwick C, Yu M, et al. Predictors of 30 day hospital readmission in patients with type 2 diabetes: a retrospective, case-control, database study. Curr Med Res Opin. 2015;31(1):107-114. [DOI] [PubMed] [Google Scholar]
- 9. McCoy RG, Lipska KJ, Herrin J, Jeffery MM, Krumholz HM, Shah ND. Hospital readmissions among commercially insured and medicare advantage beneficiaries with diabetes and the impact of severe hypoglycemic and hyperglycemic events. J Gen Intern Med. 2017;32(10):1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Critchley JA, Carey IM, Harris T, DeWilde S, Cook DG. Variability in glycated hemoglobin and risk of poor outcomes among people with type 2 diabetes in a large primary care cohort study. Diabetes Care. 2019:dc190848. doi: 10.2337/dc19-0848. [DOI] [PubMed] [Google Scholar]
- 11. Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8(4):863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stone MA, Charpentier G, Doggen K, et al. ; GUIDANCE Study Group . Quality of care of people with type 2 diabetes in eight European countries: findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care. 2013;36(9):2628-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42(7):1178-1184. [DOI] [PubMed] [Google Scholar]
- 14. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose-sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther. 2017;8(3):573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425-1432. [DOI] [PubMed] [Google Scholar]
- 17. Fitch K, Pyenson BS, Iwasaki K. Medical claim cost impact of improved diabetes control for medicare and commercially insured patients with type 2 diabetes. J Manag Care Pharm. 2013;19(8):609-20, 620a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109(12):1497-1502. [DOI] [PubMed] [Google Scholar]
- 19. Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD; Canadian Alteplase for Stroke Effectiveness Study Investigators . Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32(4):617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke. 2008;39(10):2749-2755. [DOI] [PubMed] [Google Scholar]
- 21. Bruno A, Biller J, Adams HP Jr, et al. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999;52(2):280-284. [DOI] [PubMed] [Google Scholar]
- 22. Yun JS, Park YM, Han K, Cha SA, Ahn YB, Ko SH. Severe hypoglycemia and the risk of cardiovascular disease and mortality in type 2 diabetes: a nationwide population-based cohort study. Cardiovasc Diabetol. 2019;18(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karter AJ, Moffet HH, Liu JY, Lipska KJ. Surveillance of hypoglycemia-limitations of emergency department and hospital utilization data. JAMA Intern Med. 2018;178(7):987-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr. 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haugstvedt A, Wentzel-Larsen T, Graue M, Søvik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with Type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study. Diabet Med. 2010;27(1):72-78. [DOI] [PubMed] [Google Scholar]
- 26. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079-1094. [DOI] [PubMed] [Google Scholar]
- 27. Fokkert M, van Dijk P, Edens M, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care. 2019;7(1):e000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43(2):389-397. [DOI] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report, 2020: estimates of diabetes and Its Burden in the United States. Accessed February 29, 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 30. Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. Jama. 2019;321(19):1867-1868. [DOI] [PubMed] [Google Scholar]
- 31. Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391(10138):2430-2440. [DOI] [PubMed] [Google Scholar]
- 32. McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Association of cumulative multimorbidity, glycemic control, and medication use with hypoglycemia-related emergency department visits and hospitalizations among adults with diabetes. JAMA Netw Open. 2020;3(1):e1919099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levine BJ, Close KL, Gabbay RA. Reviewing U.S. connected diabetes care: the newest member of the team. Diabetes Technol Ther. 2020;22(1):1-9. [DOI] [PubMed] [Google Scholar]
- 34. Hollander JE, Sites FD. The transition from reimagining to recreating health care is now. NEJM Catalyst 2020 April 8. Accessed May 13, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0093
- 35. Meneilly GS, Cheung E, Tuokko H. Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes. 1994;43(3):403-410. [DOI] [PubMed] [Google Scholar]
- 36. Meneilly GS, Tessier D. Diabetes in the elderly. Diabet Med. 1995;12(11):949-960. [DOI] [PubMed] [Google Scholar]
- 37. Schütt M, Fach EM, Seufert J, et al. ; DPV Initiative and the German BMBF Competence Network Diabetes Mellitus . Multiple complications and frequent severe hypoglycaemia in ‘elderly’ and ‘old’ patients with Type 1 diabetes. Diabet Med. 2012;29(8):e176-e179. [DOI] [PubMed] [Google Scholar]
- 38. Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32(8):1513-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Punthakee Z, Miller ME, Launer LJ, et al. ; ACCORD Group of Investigators; ACCORD-MIND Investigators . Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35(4):787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garfield SS, Xenakis JJ, Bastian A, McBride M. Experiences of people with diabetes by payer type: an analysis of the roper diabetes data set. Diabetes Ther. 2015;6(2):113-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.