Abstract
Background.
For patients at risk for multidrug-resistant organisms, IDSA/ATS guidelines recommend empiric therapy against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas. Following negative cultures, the guidelines recommend antimicrobial de-escalation. We assessed antibiotic de-escalation practices across hospitals and their associations with outcomes in hospitalized patients with pneumonia with negative cultures.
Methods.
We included adults admitted with pneumonia in 2010–2015 to 164 US hospitals if they had negative blood and/or respiratory cultures and received both anti-MRSA and antipseudomonal agents other than quinolones. De-escalation was defined as stopping both empiric drugs on day 4 while continuing another antibiotic. Patients were propensity adjusted for de-escalation and compared on in-hospital 14-day mortality, late deterioration (ICU transfer), length-of-stay (LOS), and costs. We also compared adjusted outcomes across hospital de-escalation rate quartiles.
Results.
Of 14 170 patients, 1924 (13%) had both initial empiric drugs stopped by hospital day 4. Hospital de-escalation rates ranged from 2–35% and hospital de-escalation rate quartile was not significantly associated with outcomes. At hospitals in the top quartile of de-escalation, even among patients at lowest risk for mortality, the de-escalation rates were <50%. In propensity-adjusted analysis, patients with de-escalation had lower odds of subsequent transfer to ICU (adjusted odds ratio, .38; 95% CI, .18–.79), LOS (adjusted ratio of means, .76; .75–.78), and costs (.74; .72–.76).
Conclusions.
A minority of eligible patients with pneumonia had antibiotics de-escalated by hospital day 4 following negative cultures and de-escalation rates varied widely between hospitals. To adhere to recent guidelines will require substantial changes in practice.
Keywords: community-acquired pneumonia, de-escalation, antimicrobial stewardship, negative cultures
For patients with risk factors for multidrug-resistant (MDR) pneumonia, the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines recommend empirical coverage for methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa with extended-spectrum antibiotics [1]. While most de-escalation of antimicrobials takes place following positive cultures, recently released IDSA/ATS guidelines also recommend de-escalation of empiric therapy to a narrower spectrum at 48 hours if microbial cultures do not reveal an MDR pathogen and the patient is clinically improving [1]. This recommendation represents a change from previous guidelines, which did not offer specific guidance regarding de-escalation of antimicrobials following negative cultures [2, 3].
Several observational studies have examined the outcomes associated with de-escalating antibiotics following negative cultures [4–6]. Although these were either small or limited to special populations, they have all reported that antimicrobial de-escalation following negative cultures was safe, reduces the length of hospital stay and duration of antimicrobial treatment, and is not associated with worse clinical outcomes. However, no studies have evaluated de-escalation practices following negative cultures across a representative sample of US hospitals [5, 7]. In particular, it is not known how much hospitals will have to change their practices to adhere to this new recommendation. In a large cohort of patients hospitalized with pneumonia receiving empiric broad-spectrum antibiotics in 164 acute-care hospitals, we aimed to assess de-escalation practices following negative cultures and to explore the association of de-escalation with hospital outcomes.
METHODS
The study protocol was deemed exempt by the Institutional Review Board of Cleveland Clinic as all data are de-identified and contain no protected health information.
Data Source
We conducted a retrospective cohort study including 164 US hospitals that participate in the Premier Healthcare Database (Premier Inc, Charlotte, NC). Further information about the database is provided in the Supplementary Information.
Cohort Identification
We included patients aged 18 years or older who were admitted with pneumonia to one of the participating Premier hospitals from July 2010 to June 2015. Patients were identified by an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) principal diagnosis of pneumonia, or a secondary diagnosis of pneumonia and a principal diagnosis of sepsis or respiratory failure as described previously [8]. In addition, to improve the specificity of our definition, all patients had to have a chest radiograph or computed tomography scan and blood or respiratory cultures performed by hospital day 1. All patients in the cohort received an empirical anti-MRSA drug together with at least 1 antipseudomonal drug other than a quinolone by hospital day 1. We did not include patients where quinolone was the only antipseudomonal agent, because quinolones are considered first-line therapy for community-acquired pneumonia, even for patients not at risk of resistant infections. We excluded hospital transfers, because we could not assess their initial severity and outcomes. Because we were interested in physicians’ responses to negative cultures, we excluded patients who had any positive bacterial cultures (blood, sputum, respiratory) and those with positive urine antigen tests for Streptococcus pneumoniae and Legionella pneumophila or positive polymerase chain reaction assays for Mycoplasma pneumoniae and Chlamydia pneumoniae. We also excluded patients for whom it would likely be inappropriate to de-escalate therapy: patients with cystic fibrosis and those on immunosuppressive medications (due to high risk of neutropenic fever). We excluded patients admitted directly to the intensive care unit (ICU) as decisions relating to antimicrobial de-escalation may be different in those patients. For the descriptive portion of the study, there were no additional exclusions.
The relationships of de-escalation to outcomes were analyzed at 2 levels. At the patient level, we wanted to assess patients with similar clinical characteristics who either did or did not undergo de-escalation 48 hours after being cultured, which generally corresponds to hospital day 4. Although for some patients this could be hospital day 3, we needed to choose a single day on which to perform our propensity adjustment. Therefore, we excluded patients (1) who died on or before hospital day 4, (2) who stopped both drugs before hospital day 4, or (3) who stopped both drugs on day 4 with no further antibiotics, because the diagnosis of bacterial pneumonia may have been in doubt. For the hospital-level analysis, we also excluded patients of hospitals with fewer than 100 otherwise eligible patients in order to provide stable estimates. For this analysis we considered those who stopped both drugs on hospital day 3 or 4, because we did not adjust for severity and therefore did not need to anchor on a specific day. This provided us with a larger patient sample. We also excluded from all outcome analyses the small fractions of patients with missing costs, discharge status, point of origin, or sex. Missing data were not imputed.
Antimicrobial Treatment
We defined broad-spectrum empiric antimicrobial therapy as following: receiving 1 anti-MRSA and at least 1 antipseudomonal agent (not including quinolones). The definition of anti-MRSA antibiotics and antipseudomonal antibiotics is provided as Supplementary Information. De-escalation was defined as stopping both agents (anti-MRSA and antipseudomonal) on day 4, but continuing other antibiotics.
Covariates
For each hospitalization, we extracted demographic data including age, gender, race, marital status, insurance payer, and admitting physician specialty. We categorized comorbidities using software from the Agency for Healthcare Research and Quality, based on the work of Elixhauser et al [9]. The combined comorbidity scores were calculated using the method described by Gagne et al [10]. We also noted relevant medications, tests, and treatments charged on the first and fourth hospital days. Additional variables included hospital characteristics (geographical region, bed size, urban/rural setting, and teaching status). However, test results of many laboratory variables including inflammatory biomarkers such as C-reactive protein and procalcitonin were not available.
Outcomes
Since results of day 1 cultures would ordinarily become available on day 3, we measured the associations of de-escalation on hospital day 4 with all-cause in-hospital mortality from hospital day 5 through day 14, and with day 5 or later transfer to ICU, invasive mechanical ventilation (IMV), vasopressor use, Clostridioides difficile infection (CDI), defined by ICD-9 code (not present on admission) and/or a positive laboratory test for C. difficile, length of stay (LOS), and cost. We also measured antibiotic re-escalation (defined as resumption of antipseudomonal and/or anti-MRSA therapy by day 7 after de-escalation). Hospital costs included entire cost of hospitalization (bed charge, medications, and laboratory tests). Hospital readmission data were not available and therefore not evaluated.
Statistical Analyses
Categorical variables were summarized by frequencies and proportions. Continuous variables were summarized as mean and SD or median and interquartile range (IQR; 25th–75th percentile [quartile 1–quartile 3]) depending on distributional symmetry. We compared the characteristics of patients whose empiric antimicrobial treatments for pneumonia were de-escalated on day 4 with those for whom they were stopped later or continued to discharge, using Wilcoxon’s t test, Pearson’s chi-square test, or Fisher’s exact test. Kaplan-Meier curves were used to depict and compare times to de-escalation of antipseudomonal agents, anti-MRSA agents, at least 1 of the 2 agents, and de-escalation of both antipseudomonal and anti-MRSA agents.
The relationships of de-escalation to outcomes were analyzed at 2 levels. At the patient level, we first calculated the absolute standardized differences of variables between patients who were and were not de-escalated on the fourth day. The variables compared included hospital characteristics, patient demographics, comorbidities, and treatments administered early (day 0 or 1) and on the fourth day after culture collection, serving as proxies for clinical severity. We considered a 10% standardized difference to be a minimally acceptable balance. We then used a propensity analysis to adjust for potential indication bias. The propensity to de-escalate was estimated for each patient using boosted additive linear logistic regression. We used the derived propensity scores to estimate the Average Treatment effects on outcomes in the Treated (ATT)—that is, in the de-escalated—patients using Inverse Probability of Treatment Weighting (IPTW). Post-IPTW balance in covariates was evaluated by calculating the absolute standardized differences between the de-escalated and weighted control patients [11, 12]. Propensity modeling and analyses of balance were conducted using the “twang” (Toolkit for Weighting and Analysis of Nonequivalent Groups) and gbm (Generalized Boosted Regression Models) R packages in R 3.5.0 [13–15].
To compare clinical outcomes (subsequent inpatient mortality by day 14, clinical deterioration, LOS, CDI and cost) between those who did and did not undergo day 4 de-escalation, we used multivariable logistic regression models for the 3 dichotomous outcomes (mortality, deterioration, and CDI), and gamma-generalized linear models with log link function for the continuous outcomes (LOS and cost). These models were fit using the ATT weights above and included the de-escalation treatment indicator and de-escalation propensity score as a covariate.
To further guard against confounding by indication, we performed a hospital-level analysis. If de-escalation on days 3 and 4 was harmful, we might expect to see poorer aggregate outcomes for patients in hospitals with the highest de-escalation rates. To examine this possibility, we divided the hospitals into quartiles by de-escalation fraction and compared patient outcomes across these quartiles. We also examined the likelihood of de-escalation based on the patient’s hospital de-escalation quartile and predicted mortality risk using a previously validated model whose performance is similar to the Pneumonia Severity Index [8]. Outcome analyses were done using SAS 9.4 (SAS Institute Inc, Cary, NC) and R 3.5.0 [13]. All tests were 2-sided and P < .05 was considered statistically significant.
RESULTS
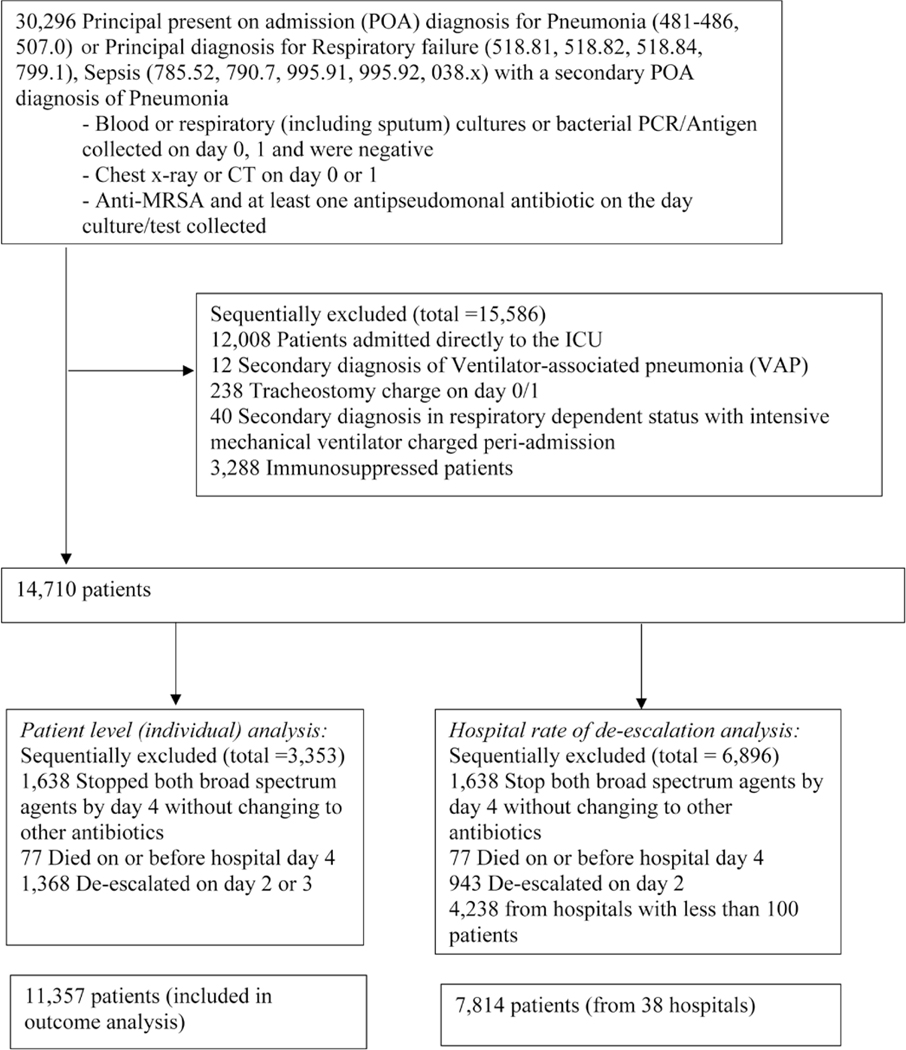
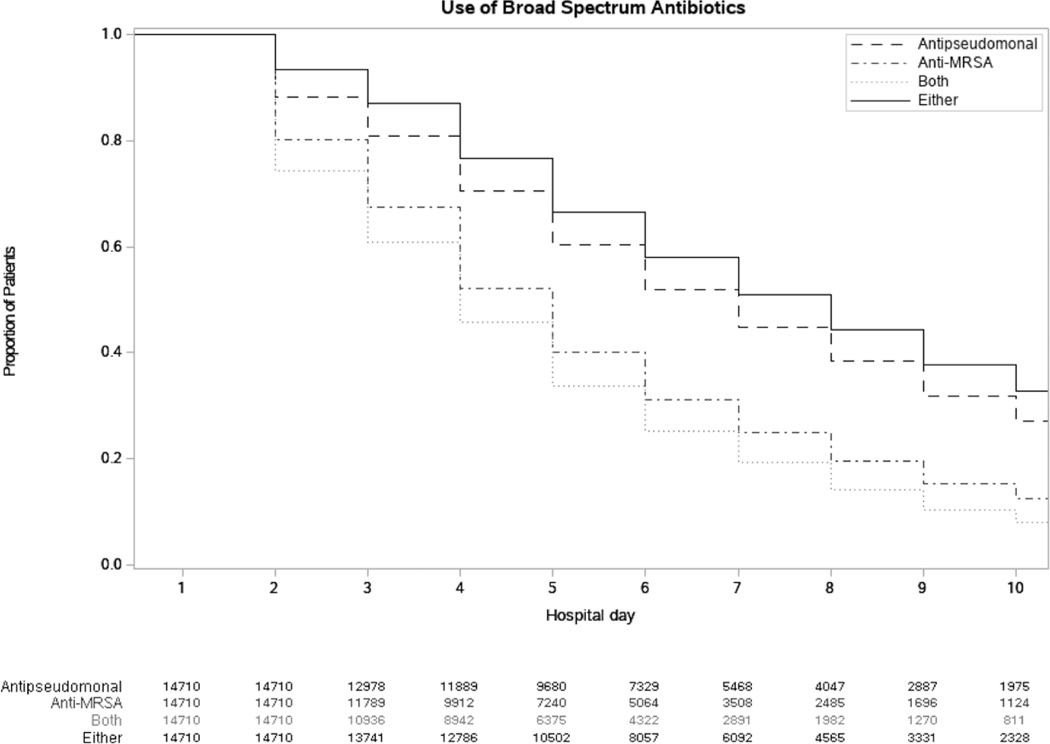
Overall, 14 710 patients from 164 hospitals met the initial eligibility criteria (Figure 1). Figure 2 describes the proportions of patients receiving an antipseudomonal agent, anti-MRSA agent, or both or either of the medications by hospital day. On hospital day 0, all patients received both medications. On each subsequent hospital day, fewer patients were taking both agents, as therapy was de-escalated. In general, anti-MRSA therapy was de-escalated faster than antipseudomonal therapy. By hospital day 4, 77 patients had died and 12 786 patients were on either of the medications. Therefore, by day 4, 1924 patients (13%) had both initial empiric drugs stopped, and by hospital day 10, 65% were de-escalated or discharged. Last, the de-escalation proportions (by hospital day) in patients with negative respiratory cultures were similar to those in patients with negative blood cultures.
Figure 1.
Patient selection flow chart to identify study eligible patients. Abbreviations: CT, computed tomography; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; PCR, polymerase chain reaction.
Figure 2.
Proportions of patients remaining on an antipseudomonal agent (dashed line), anti-MRSA agent (dot-dash line), both (dotted line), or either agent (solid line) by hospital day. Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Table 1 describes the characteristics of the entire cohort and the cohort stratified by de-escalation status on hospital day 4 after cultures were drawn. Patients with de-escalation were younger, had a lower comorbidity burden, and were less likely to be on dialysis than patients who were not de-escalated. Patients with negative MRSA nasal cultures were more likely than those with positive swabs to have anti-MRSA drugs stopped by day 4 (40.6% vs 26.6%; P = .005) but not appreciably more likely to have both drugs stopped on day 4 (10% vs 6.2%, P = .2).
Table 1.
Characteristics of Patients With Pneumonia With Negative Cultures Stratified by De-escalation Status on Hospital Day 4
| Factor | Total(N = 11 357) | De-escalated on Day 4 (n = 913) | Not De-escalated (n = 10 444) | P (2-sided) |
|---|---|---|---|---|
| Age, median [Q1, Q3], y | 76.0 [63.0, 85.0] | 74.0 [61.0, 84.0] | 76.0 [63.0, 85.0] | .013a |
| Gender, n (%) | .61b | |||
| Female | 5664 (49.9) | 448 (49.1) | 5216 (49.9) | |
| Male | 5693 (50.1) | 465 (50.9) | 5228 (50.1) | |
| Race, n (%) | .017b | |||
| White | 8602 (75.7) | 674 (73.8) | 7928 (75.9) | |
| Black | 1607 (14.1) | 123 (13.5) | 1484 (14.2) | |
| Hispanic | 65 (0.57) | 2 (0.22) | 63 (0.60) | |
| Other | 1083 (9.5) | 114 (12.5) | 969 (9.3) | |
| Marital status, n (%) | .003b | |||
| Married | 4183 (36.8) | 298 (32.6) | 3885 (37.2) | |
| Single | 6377 (56.2) | 528 (57.8) | 5849 (56.0) | |
| Other | 797 (7.0) | 87 (9.5) | 710 (6.8) | |
| Admission source, n (%) | .37b | |||
| Emergency room | 9481 (83.5) | 761 (83.4) | 8720 (83.5) | |
| SNF/ICF | 1458 (12.8) | 125 (13.7) | 1333 (12.8) | |
| Clinic | 404 (3.6) | 25 (2.7) | 379 (3.6) | |
| Others | 14 (0.12) | 2 (0.22) | 12 (0.11) | |
| Insurance payer, n (%) | .23b | |||
| Medicare | 8959 (78.9) | 694 (76.0) | 8265 (79.1) | |
| Medicaid | 846 (7.4) | 77 (8.4) | 769 (7.4) | |
| Managed care | 871 (7.7) | 83 (9.1) | 788 (7.5) | |
| Commercial indemnity | 312 (2.7) | 29 (3.2) | 283 (2.7) | |
| Others | 369 (3.2) | 30 (3.3) | 339 (3.2) | |
| Principal diagnosis, n (%) | .51b | |||
| Pneumonia | 5255 (46.3) | 439 (48.1) | 4816 (46.1) | |
| Aspiration pneumonia | 1614 (14.2) | 117 (12.8) | 1497 (14.3) | |
| Sepsis | 4147 (36.5) | 332 (36.4) | 3815 (36.5) | |
| Respiratory failure | 341 (3.0) | 25 (2.7) | 316 (3.0) | |
| Admission within last 6 months, n (%) | 2252 (19.8) | 172 (18.8) | 2080 (19.9) | .43b |
| Dialysis, n (%) | 783 (6.9) | 41 (4.5) | 742 (7.1) | .003b |
| Admitted from SNF/ICF, n (%) | 1458 (12.8) | 125 (13.7) | 1333 (12.8) | .42b |
| Comorbidities | ||||
| Combined comorbidity scores, median [Q1, Q3] | 4.0 [2.0, 6.0] | 3.0 [1.0, 5.0] | 4.0 [2.0, 6.0] | .001a |
| Hypertension, n (%) | 7870 (69.3) | 633 (69.3) | 7237 (69.3) | .98b |
| Chronic pulmonary disease, n (%) | 5081 (44.7) | 414 (45.3) | 4667 (44.7) | .70b |
| Anemia, n (%) | 4627 (40.7) | 288 (31.5) | 4339 (41.5) | <.001b |
| Diabetes, n (%) | 4053 (35.7) | 314 (34.4) | 3739 (35.8) | .39b |
| Early treatments (day 0/1), n (%) | ||||
| Blood cultures | 11 195 (98.6) | 903 (98.9) | 10 292 (98.5) | .38b |
| Respiratory cultures | 1236 (10.9) | 125 (13.7) | 1111 (10.6) | .004b |
| NIV | 923 (8.1) | 57 (6.2) | 866 (8.3) | .030b |
| Blood lactate | 6230 (54.9) | 519 (56.8) | 5711 (54.7) | .21b |
| Arterial and venous blood gas | 3807 (33.5) | 302 (33.1) | 3505 (33.6) | .77b |
| Influenza test | 1695 (14.9) | 163 (17.9) | 1532 (14.7) | .010b |
| Positive influenza test | 31 (0.27) | 2 (0.22) | 29 (0.28) | .99c |
| MRSA (PCR/nasal test), n (%) | .22b | |||
| Not tested | 10 830 (95.4) | 864 (94.6) | 9966 (95.4) | |
| Negative | 414 (3.6) | 42 (4.6) | 372 (3.6) | |
| Positive | 113 (0.99) | 7 (0.77) | 106 (1.0) | |
| Treatments received/billed on day 4, n (%) | ||||
| Arterial and venous blood gas | 861 (7.6) | 32 (3.5) | 829 (7.9) | <.001b |
| Brain natriuretic peptide | 575 (5.1) | 26 (2.8) | 549 (5.3) | <.001b |
| Blood lactate | 278 (2.4) | 10 (1.1) | 268 (2.6) | .006b |
| ICU | 685 (6.0) | 23 (2.5) | 662 (6.3) | <.001b |
| IMV | 431 (3.8) | 15 (1.6) | 416 (4.0) | <.001b |
| NIV | 658 (5.8) | 29 (3.2) | 629 (6.0) | <.001b |
| Vasopressor | 197 (1.7) | 5 (0.55) | 192 (1.8) | .004b |
| Chest x-ray | 2919 (25.7) | 159 (17.4) | 2760 (26.4) | <.001b |
| Blood culture | 311 (2.7) | 14 (1.5) | 297 (2.8) | .020b |
| Respiratory culture | 486 (4.3) | 17 (1.9) | 469 (4.5) | <.001b |
| Opiates | 2152 (18.9) | 133 (14.6) | 2019 (19.3) | .002b |
| Insulin | 1666 (14.7) | 98 (10.7) | 1568 (15.0) | .001b |
| Albuterol/ipratropium | 3190 (28.1) | 228 (25.0) | 262 (28.4) | .029b |
| Outcomes | ||||
| In-hospital 14-day mortality, n (%) | 667 (5.9) | 26 (2.8) | 641 (6.1) | <.001b |
| Late ICU (day 5+), n/total (%) | 367/10 450 (3.5) | 10/873 (1.2) | 357/9577 (3.7) | <.001b |
| Late IMV (day 5+), n/total (%) | 300/10 654 (2.8) | 5/874 (0.57) | 295/9780 (3.0) | <.001b |
| Late vasopressor (day 5+), n/total (%) | 322/10 822 (3.0) | 3/889 (0.34) | 319/9933 (3.2) | <.001b |
| CDI, n/total (%) | 77/9834 (0.78) | 6/790 (0.76) | 71/9044 (0.79) | .94b |
| Cost, median (Q1, Q3), USD$ | 10 539.8 [7158.7, 16 451.3] | 7855.1 [5546.3, 11 279.3] | 10 869.5 [7343.1, 17 020.6] | <.001a |
| Length of stay, median (Q1, Q3), d | 6.0 [4.0, 9.0] | 4.0 [3.0, 6.0] | 6.0 [5.0, 9.0] | <.001a |
| Duration of antibiotics treatments, median [Q1, Q3] | 7.0 [5.0, 9.0] | 5.0 [4.0, 7.0] | 7.0 [5.0, 9.0] | <.001a |
| Hospital characteristics (row percentage) | ||||
| Bed size, n (%) | <.001b | |||
| ≤200 beds | 1857 (16.4) | 110 (5.9) | 1747 (94.1) | |
| 201–400 beds | 4029 (35.5) | 275 (6.8) | 3754 (93.2) | |
| ≥401 beds | 5471 (48.2) | 528 (9.7) | 4943 (90.3) | |
| Region, n (%) | .26b | |||
| Midwest | 2682 (23.6) | 233 (8.7) | 2449 (91.3) | |
| Northeast | 2343 (20.6) | 187 (8.0) | 2156 (92.0) | |
| South | 5171 (45.5) | 391 (7.6) | 4780 (92.4) | |
| West | 1161 (10.2) | 102 (8.8) | 1059 (91.2) | |
| Urban/rural, n (%) | .10b | |||
| Rural | 1267 (11.2) | 87 (6.9) | 1180 (93.1) | |
| Urban | 10 090 (88.8) | 826 (8.2) | 9264 (91.8) | |
| Teaching hospital, % | <.001b | |||
| No | 5488 (48.3) | 343 (6.3) | 5145 (93.8) | |
| Yes | 5869 (51.7) | 570 (9.7) | 5299 (90.3) | |
Data in parentheses are percentages (%) of patients unless otherwise indicated.
Abbreviations: CDI, Clostridioides difficile infection; ICF, intermediate care facility; ICU, intensive care unit; IMV, invasive mechanical ventilation; MRSA, methicillin-resistant Staphylococcus aureus; NIV, noninvasive ventilation; PCR, polymerase chain reaction; Q, quartile; Q1, first quartile; Q3, third quartile; SNF, skilled nursing facility.
Wilcoxon test.
Pearson χ2 test.
Fisher exact test.
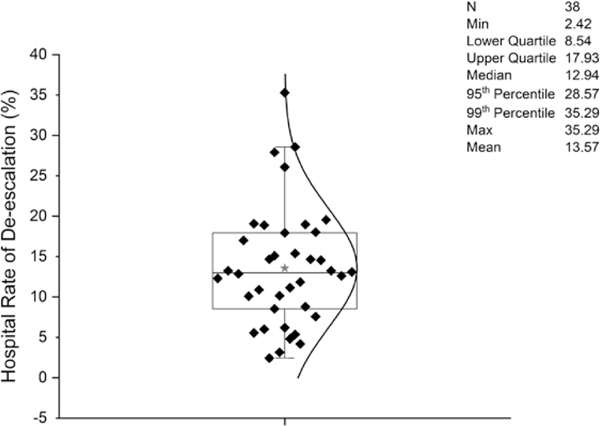
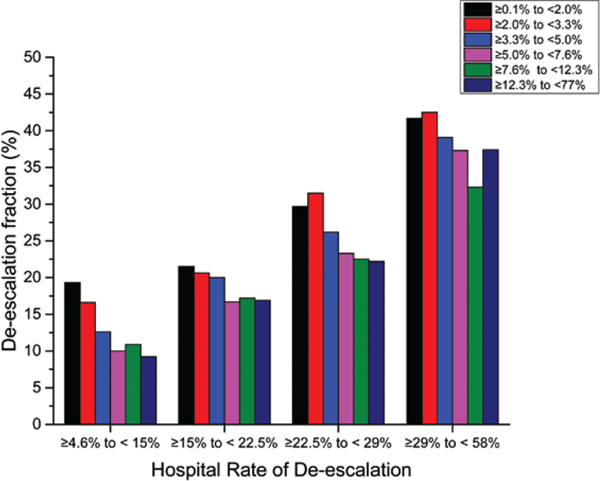
Hospital rates of de-escalation on day 4 ranged from 2% to 35% with a median of 13% (Figure 3). De-escalation was more common in larger hospitals (ie, with >401 beds) compared with smaller hospitals (ie, with <200 beds) (9.7% vs 5.9%), in teaching institutions compared with others (9.7% vs 6.3%), and in urban compared with rural hospitals (8.2% vs 6.9%). De-escalation also varied widely across regions in the United States. Figure 4 shows the hospitals divided into quartiles of de-escalation rate, and within each quartile patients are stratified by predicted mortality. Within each quartile of de-escalation rate, patients at higher predicted risk for mortality were less likely to be de-escalated. However, the trend was much stronger across hospital quartiles than within them, so that low-risk patients at hospitals with low rates of de-escalation were much less likely to be de-escalated than were high-risk patients at hospitals with high de-escalation rates. The de-escalation rate was less than 50% even among patients at lowest risk for mortality at hospitals in the top quartile of de-escalation.
Figure 3.
Distribution of de-escalation rates by hospital day 4 through hospital day 10 across US hospitals with at least 100 eligible pneumonia patients with negative cultures. *Represents mean. Abbreviations: Max, maximum; Min, minimum.
Figure 4.
Bar chart of hospital rates of day 4 de-escalation (y axis), grouped by hospital de-escalation quartile and further stratified by sextiles of predicted mortality (x axis).
Hospital-level Outcomes
The characteristics of patients with de-escalation on days 3 and 4 based on the hospital rates of de-escalation (quartiles of de-escalation) and unadjusted outcomes are described in Supplementary Table 1.
Patient-level Outcomes
The left-hand columns of Table 2 compare the outcomes for patients with day 4 de-escalation with those for patients not de-escalated on day 4 in models without propensity adjustment. De-escalation was associated with reductions in all negative outcomes with the exception of C. difficile incidence, which was similar in both groups. Post-IPTW adjustment, all standardized differences were less than 10%, as shown in the Love plot (Supplementary Figure 1). In the propensity-adjusted analyses using IPTW, de-escalation was not significantly associated with 14-day mortality (odds ratio [OR], .65; 95% confidence interval [CI], .39–1.08). De-escalation was significantly associated with less frequent late ICU admission (OR, .38; 95% CI, .18–.79]), late IMV (OR, .25; 95% CI, .09–.66), late vasopressor use (OR, .13; 95% CI, .04–.44), shorter LOS (risk-adjusted ratio of means, .76; 95% CI, .75–.78) and lower hospitalization cost (risk-adjusted ratio of means, .74; 95% CI, .72–.76). De-escalation was not significantly associated with developing CDI (OR, 1.33; 95% CI, .40–4.47). Last, among the patients with de-escalation, only 13 patients (1.4%) were ever re-escalated to broad-spectrum antibiotics (resumption of antipseudomonal and/or anti-MRSA therapy) during the hospitalization.
Table 2.
Patient Outcomes of Patients With Pneumonia With Negative Cultures Comparing Odds of Events for Those Who Were De-escalated With Those Who Were Not De-escalated on Hospital Day 4
| Outcome | Unadjusted OR (95% CI) | IPTW OR (95% CI) | P Value |
|---|---|---|---|
| In-hospital 14-day mortality | .45 (.30–.67) | .65 (.39–1.08) | .095 |
| Late ICU (day 5+)a | .30 (.16–.56) | .38 (.18–.79) | .01 |
| Late IMV (day 5+)a | .19 (.08–.45) | .25 (.09–.66) | .005 |
| Late vasopressor (day 5+)a | .10 (.03–.32) | .13 (.04–.44) | .001 |
| CDIb | .96 (.42–2.23) | 1.33 (.40–4.47) | .64 |
| Costc | .65 (.62–.68) | .74 (.72–.76) | <.001 |
| Length of stay | .70 (.68–.73) | .76 (.75–.78) | <.001 |
Data are presented as ORs (95% CIs) except for cost and length of stay (mean multipliers). Abbreviations: CDI, Clostridioides difficile infection; CI, confidence interval; ICU, intensive care unit; IMV, invasive mechanical ventilation; IPTW, Inverse Probability of Treatment Weighting; OR, odds ratio.
Among patients not previously in the ICU, on IMV, or on vasopressor, respectively.
Patients with Clostridium difficile diagnosis codes present on admission (008.45) or with a positive Clostridium difficile test by hospital day 4 were excluded.
Patients with no costs or from hospitals where most patients show no costs are excluded from this analysis.
DISCUSSION
The recent IDSA/ATS pneumonia guidelines conclude that de-escalation of broad-spectrum antibiotics is appropriate in stable patients 48 hours after negative cultures. In this large, multihospital, retrospective cohort of adult patients with pneumonia treated empirically with a combination of an anti-MRSA and an antipseudomonal agent, fewer than 1 in 7 such patients had their extended-spectrum coverage de-escalated by hospital day 4. De-escalation practices varied widely by hospital, with larger teaching hospitals having the highest rates of de-escalation. Although patients at hospitals with higher de-escalation rates did not suffer worse outcomes, no hospital de-escalated even 40% of its eligible patients. The overall de-escalation rates were less than 50% even among patients at lowest risk for mortality at hospitals in the top quartile of de-escalation, suggesting there is substantial opportunity for improvement.
The guidelines based their recommendations on a series of observational studies of antibiotic de-escalation. All of these studies found de-escalation to be safe, but the studies were generally small and not representative of US hospitals in general. The largest study examined 9319 patients (including both positive and negative cultures) admitted to 119 Veterans Administration (VA) facilities from 2008 to 2011. Overall, 28% of the patients who received initial broad-spectrum antibiotics were de-escalated by hospital day 4, although the rate varied 6-fold across facilities [5, 16]. De-escalation was uncommon even in culture-positive cases (36%) and only 27% of the culture-negative patients underwent de-escalation. In the propensity-matched cohort, de-escalation was associated with a shorter LOS but not with 30-day readmissions or CDI [16]. In a more recent single-center study, Cowley et al [17] examined de-escalation of anti-MRSA therapy among 279 patients with culture-negative nosocomial pneumonia, of whom only 33% were de-escalated by hospital day 4. There were no significant differences in 28-day mortality, but patients who were de-escalated had shorter LOS and lower incidence of acute kidney injury. Our study generally confirms these findings in a larger and more recent cohort. We also found that de-escalation was associated with a shorter LOS in our cohort, as well as with lower rates of inpatient mortality and late deterioration. Patients who de-escalated and hospitals with high de-escalation fractions did not have worse outcomes. Few patients had their antibiotics re-escalated, and re-escalation was not more common at hospitals with the highest de-escalation rates.
Our study is also novel in studying the variation within and across non-VA hospitals, which is important for 2 reasons. First, it is likely that our propensity-adjusted model included some residual confounding by indication. Within an institution, the patients who are de-escalated are the least sick and are therefore likely to have good outcomes. Inverse Probability of Treatment Weighting helps to overcome this bias, but may not be completely successful. Across hospitals, those with higher rates of de-escalation are likely not those with the least sick patients, since they tended to be larger, teaching hospitals. It is more likely that de-escalation in these hospitals is the result of intentional antimicrobial stewardship efforts. At the same time, looking at de-escalation rates across hospitals (2–35%) demonstrates that there is substantial room for improvement. In particular, the low rates of de-escalation among low-risk culture-negative patients highlight an easy target for antimicrobial stewardship, since close to 100% of such patients should be de-escalated.
In recent years, more hospitals have taken to performing MRSA swabs of the nares and de-escalating patients with negative swabs. Several studies support this practice [18, 19]. We found that only a small minority of patients had nasal testing, and among those who did, only about one-third had their anti-MRSA antibiotics tapered after a negative test. Moreover, nasal MRSA testing did not lead to overall antimicrobial de-escalation.
Our findings should be interpreted in light of several limitations. First, by relying on ICD-9 codes to define our study cohort, we may not have identified all patients with pneumonia, may have misclassified some patients, and also may have missed important confounders. Second, although we used propensity analysis to adjust for confounders, it is likely that there are residual unmeasured confounders. Nevertheless, our findings are in keeping with all other studies and support the conclusions of the new guidelines. Third, we lacked clinical data and therefore could not directly measure clinical severity. However, we utilized several indirect measures of severity, such as late ICU admission, vasopressor use, and mechanical ventilation, that we have shown previously to have excellent prognostic ability for inpatient mortality [8]. Last, we also lacked provider-level data and therefore could not account for de-escalation variations at the provider level.
Conclusions
Overall, fewer than 1 in 7 eligible patients with pneumonia had their empiric antibiotic coverage de-escalated following negative cultures. The de-escalation rates varied widely among hospitals and were low even among the lowest-risk patients. As expected, patients with de-escalation did not exhibit worse outcomes. These data suggest that physicians will need to substantially change their response to negative cultures for most non–critically ill patients if they are to adhere to the new guidelines. Since antibiotics are not benign and antimicrobial stewardship is an important priority, hospital antibiotic stewardship programs should emphasize de-escalation following negative cultures as an opportunity to reduce exposure to broad-spectrum antibiotics, improving both antimicrobial stewardship and medication safety by substitution of lower-toxicity agents.
Supplementary Material
Acknowledgments
Financial support. The work was supported by the Agency for Healthcare Research and Quality (grant number R01 HS024277-01A1; to M. B. R., S. S. R., S. H., P. K. L., P.-C. Y., M. D. Z, and P. B. I.). A. D. is supported by the Agency for Healthcare Research and Quality under award K08 HS025026. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Disclaimer. The funding agency had no role in the design or conduct of the study or in the drafting of the manuscript.
Potential conflicts of interest. A. D. has received research support from The Clorox Company and is on the advisory board of Ferring Pharmaceuticals. S. S. R. has received research support from bioMerieux, BD Diagnostics, Hologic, Diasorin, Affinity Lifescale, Accelerate, OpGen, and Roche. M. D. Z. is a consultant and has received research support from Astellas, Paratek, Cleveland Clinic, Lungpacer, Nabriva, Merck, The Medicines Company, Shionogi, Pfizer, Melinta, Tetraphase, and Spero. P. B. I. has consulted with Colgate Palmolive and G.E. Healthcare. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416. [DOI] [PubMed] [Google Scholar]
- 3.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckel WR, Stenehjem E, Sorensen J, Dean N, Webb B. Broad- versus narrow-spectrum oral antibiotic transition and outcomes in health care-associated pneumonia. Ann Am Thorac Soc 2017; 14:200–5. [DOI] [PubMed] [Google Scholar]
- 5.Madaras-Kelly K, Jones M, Remington R, et al. Antimicrobial de-escalation of treatment for healthcare-associated pneumonia within the Veterans Healthcare Administration. J Antimicrob Chemother 2016; 71:539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlueter M, James C, Dominguez A, Tsu L, Seymann G. Practice patterns for antibiotic de-escalation in culture-negative healthcare-associated pneumonia. Infection 2010; 38:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braykov NP, Morgan DJ, Schweizer ML, et al. Assessment of empirical antibiotic therapy optimisation in six hospitals: an observational cohort study. Lancet Infect Dis 2014; 14:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothberg MB, Pekow PS, Priya A, et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One 2014; 9:e87382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 10.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011; 64:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985; 39:33–8. [Google Scholar]
- 12.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. Available at: https://www.R-project.org/ [Google Scholar]
- 14.Greenwell B, Boehmke B, Cunningham J and GBM Developers. gbm:generalized boosted regression models. R package version 2.1.5. Available at: https://CRAN.R-project.org/package=gbm [Google Scholar]
- 15.Ridgeway G, McCaffrey D, Morral A, Burgette L, Griffin BA. Toolkit for weighting and analysis of nonequivalent Groups: a tutorial for the twang package. Santa Monica, CA: RAND Corporation, 2017. [Google Scholar]
- 16.Bohan JG, Remington R, Jones M, Samore M, Madaras-Kelly K. Outcomes associated with antimicrobial de-escalation of treatment for pneumonia within the Veterans Healthcare Administration. Open Forum Infect Dis 2017; 4:ofw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes associated with de-escalating therapy for methicillin-resistant Staphylococcus aureus in culture-negative nosocomial pneumonia. Chest 2019; 155:53–9. [DOI] [PubMed] [Google Scholar]
- 18.Mergenhagen KA, Starr KE, Wattengel BA, Lesse AJ, Sumon Z, Sellick JA. Determining the utility of methicillin-resistant Staphylococcus aureus nares screening in antimicrobial stewardship. Clin Infect Dis 2019; pii: ciz974. doi: 10.1093/cid/ciz974. [DOI] [PubMed] [Google Scholar]
- 19.Parente DM, Cunha CB, Mylonakis E, Timbrook TT. The clinical utility of methicillin-resistant Staphylococcus aureus (MRSA) nasal screening to rule out MRSA pneumonia: a diagnostic meta-analysis with antimicrobial stewardship implications. Clin Infect Dis 2018; 67:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.