Abstract
The clinical course of COVID-19 is very heterogeneous: most infected individuals can be managed in an outpatient setting, but a substantial proportion of patients requires intensive care, resulting in a high rate of fatalities. We performed a biomarker study to assess the impact of prior infections with seasonal coronaviruses on COVID-19 severity. Sixty patients with confirmed COVID-19 infections were included (age 30–82; 52 males, 8 females): 19 inpatients with critical disease, 16 inpatients with severe or moderate disease, and 25 outpatients. Patients with critical disease had significantly lower levels of anti-HCoV OC43-NP (P = 0.016) and HCoV HKU1-NP (P = 0.023) antibodies at the first encounter compared to other COVID-19 patients. Our results indicate that prior infections with seasonal coronaviruses might protect against a severe course of disease.
Keywords: COVID-19, OC43, HKU1, Seasonal coronaviruses
Introduction
At present, approximately 10–20% of COVID-19 patients need medical treatment in hospitals, and approximately 5% need long-term treatment in intensive care units (ICU). The majority of COVID-19 patients can be managed in an outpatient setting. Known important risk factors are age, male gender, high body mass index and pre-existing chronic conditions (Jordan et al., 2020). However, young and seemingly healthy individuals are also at risk to die from COVID-19 infections. At present, this heterogeneity of the disease course is not well understood. Partial immunity against SARS-CoV-2 might contribute to this phenomenon, as recently discussed in reports about cross-reactivity against SARS-CoV-2, where T-cell responses to SARS-CoV-2 in unexposed human individuals were described (Grifoni et al., 2020, Le Bert et al., 2020, Mateus et al., 2020). In a recent survey, patients with a mild course of COVID-19 reported frequent contact with small children (Dugas et al., 2020). Exposure to childhood-related infections might modify the disease severity of COVID-19, which corresponds to the low incidence of severe COVID-19 infections in small children (Ludvigsson, 2020). Seasonal coronaviruses mainly cause mild respiratory tract infections and are frequently found in children. Like SARS-CoV-2, those viruses belong to the subfamily of Orthocoronavirinae. The objective of this work is to assess if prior infections with a seasonal coronavirus–as indicated by antibody levels–modify the disease course of COVID-19.
Materials and methods
Serum samples from 60 patients with COVID-19 infections confirmed by RT-qPCR were analyzed in the context of the Coronaplasma Project (local ethics committee approval: AZ 2020-220-f-S) and COVID-19 biomarker study (local ethics committee approval: AZ 2020-210-f-S) at the University Hospital of Münster, Germany. The median age of patients was 58 years (range 30–82); 52 males and 8 females were included. Outpatients were manually selected to match the age and gender of inpatients. After informed consent, serum was collected at the first patient contact. The median age was 58 years for outpatients (22 male, 3 female), 58 years for inpatients with critical disease (ICU group: 17 male, 2 female) and 55 years for inpatients with severe or moderate disease (non-ICU group: 13 male, 3 female). Critical disease was defined by invasive ventilation or ECMO therapy; severe disease by oxygen insufflation; and moderate disease by hospitalization for other reasons without oxygen treatment. The median length of stay (LoS) for all inpatients was 10 days (range 2–55); 3 fatalities occurred.
Antibody measurement
Antibodies against seasonal coronaviruses and SARS-CoV-2 were measured with the immunostrip assay recomLine SARS-CoV-2 IgG from Mikrogen GmbH, Neuried, Germany. This assay measures immunoglobulin G (IgG) antibodies directed against the nucleocapsid protein (NP) of HCoV 229E, NL63, OC43 and HKU1. With respect to SARS-CoV-2, NP-specific and spike protein (S)-specific antibodies directed against the S1 subunit and the receptor-binding domain (RBD) were determined. Measurements were performed at the Institute of Virology/Department of Clinical Virology of the University Hospital Münster according to the manufacturer's guidelines. A dilution series and repetitive antibody measurements (2 replicates) were performed to test precision and reliability. Negative and positive controls were analyzed.
Data processing and analysis
Antibody levels were visually determined according to the manufacturer's guidelines as ordinal values using the cutoff band of immunostrips as the internal reference. Results of individual coronavirus-specific bands were rated on an ordinal scale as non-detectable (−), below the cutoff (+/-), with cutoff intensity (+), above the cutoff (++), and very strong intensity (+++). Relative antibody levels were quantitatively determined with ImageJ (version 153, 64bit-Version for windows) using the signal intensity of the cutoff band of individual immunostrips as the internal reference. Results were expressed as the ratio of signal intensity HCoV-specific band to signal intensity cutoff band. Standardized photographs from immunostrip assays were used for this analysis. Demographical data, type of treatment and length of stay were extracted by the Medical Data Integration Center (MeDIC) from the hospital information system of the University Hospital Münster (ORBIS from Dedalus Healthcare Group). Descriptive statistics and statistical tests were performed with R (version 3.6.1). Ordinal and numerical values were analyzed with Wilcoxon tests. Spearman’s correlation was used to assess the association of numerical values. A two-sided P-value of 0.05 was considered significant.
Laboratory results
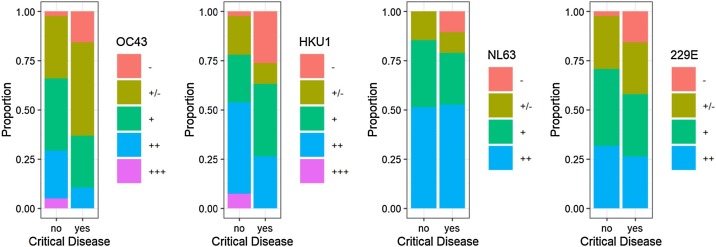
According to the visual determination of band intensities, levels of HCoV OC43- and HKU1-specific IgG antibodies were significantly lower for COVID-19 inpatients with critical disease than all other patients (P = 0.016 for OC43; P = 0.023 for HKU1; Wilcoxon test with ordinal measurement data). Figure 1 presents ordinal IgG antibody levels against HCoV for COVID-19 patients with critical disease compared to other COVID-19 patients. Serum samples at several time points per patient (0, 3, 8, 14 days after hospitalization) were available for 14 patients. There was no evidence for a change over time in antibody levels of HCoV OC43 and HKU1 (Supplemental Figure 1); therefore, these laboratory parameters are not biased by back boosting (Shrock et al., 2020). Similar results were obtained by densitometric antibody levels (Supplemental Figure 2).
Figure 1.
Proportion of ordinal HCoV antibody levels from COVID-19 patients with and without critical disease. (a) OC43 (P = 0.016) (b) HKU1 (P = 0.023) (c) NL63 (P = 0.82) (d) 229E (P = 0.30). COVID-19 patients with critical disease present low antibody levels more frequently than patients without critical disease. This difference is significant for OC43 and HKU1.
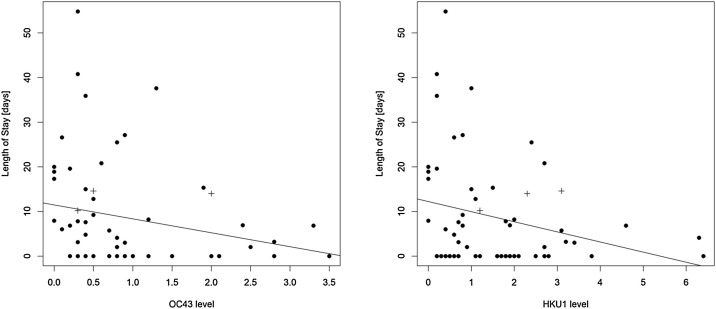
To further assess the potential clinical implications of antibodies against endemic coronaviruses for COVID-19 patients, the correlation of LoS and antibody levels against HCoVs OC43 and HKU1 was analyzed (Figure 2 ). Long hospitalization periods were predominantly seen in patients with low antibody levels.
Figure 2.
Correlation of length of stay with OC43 (a) and HKU1 (b) antibody levels. A trend is visible, but not significant (OC43: r = −0.237, P = 0.068; HKU1: r = −0.225, P = 0.083). Crosses denote fatal cases. Higher antibody levels are associated with reduced duration of hospitalization.
Supplemental Figure 3 presents results from SARS-CoV-2 IgG antibody measurements at first encounter. In general, patients with critical disease had higher SARS-CoV-2 IgG antibody levels than moderate/severe inpatients and outpatients.
Discussion
Several groups have recently reported T-cell based immunological cross-reactivity against SARS-CoV-2 in unexposed individuals (Grifoni et al., 2020, Le Bert et al., 2020, Mateus et al., 2020). From a public health perspective, the relatively high proportion of COVID-19 patients with critical disease poses the key problem of this pandemic: overload of the healthcare system. If a prior infection with a known pathogen modified the course of COVID-19, i.e. decreased the proportion of intensive care patients, this could become a component to overcome the pandemic. Persons at risk for a severe course of disease could be identified before a SARS-CoV-2 infection and appropriate protective measures taken; this may also be relevant for vaccination strategies.
This biomarker study assessed a potential relationship between prior infections with seasonal coronaviruses–measured as antibody levels–and the severity of COVID-19 disease. It was shown that elevated antibody levels for HCoVs OC43 and HKU1 were associated with less need for intensive care therapy. A trend towards a reduced length of hospital stay was also observed. Our findings are concordant with a recent study based on data from electronic medical records (Sagar et al., 2020). One could argue that higher levels of antibodies against seasonal coronaviruses are merely a surrogate marker for a more active immune system. However, HCoVs OC43 and HKU1 are betacoronaviruses and, therefore, more closely related to SARS-CoV-2 than HCoVs 229E or NL63. Our study indicates that prior exposure to HCoVs OC43 and HKU1 has a stronger association with COVID-19 severity than past HCoV 229E or NL63 infections. A possible explanation might be that prior exposure to seasonal betacoronaviruses facilitates T-cell based immune response to SARS CoV-2. Further research is needed to assess the molecular mechanism behind our findings.
Regarding SARS-CoV-2 antibodies, an inverse pattern was detected: inpatients with critical disease demonstrated higher median antibody levels for NP, RBD and S1 of SARS CoV-2 than other patients. Similar results were reported recently (Kowitdamrong et al., 2020). Hence, there is no evidence for a general bias regarding antibody levels and the ability to mount a humoral immune response between the 3 groups (outpatient, non-ICU and ICU) in our cohort.
This study has limitations; it is a retrospective, single-site study with a limited number of cases, and association is not causation. However, it is notable that the effect of HCoV OC43 and HKU1-specific antibody levels reached statistical significance regarding the need for intensive care therapy with only 60 patients. Therefore, these findings should be validated in other sites with larger patient collectives. In a prospective setting (e.g. for risk groups with contacts to many persons like employees of hospitals or supermarkets), it should be tested if the absence of HCoV OC43 and HKU1-specific antibody levels can identify persons at risk for a severe course of COVID-19. Identification of vulnerable individuals is a key priority in the current stage of the pandemic to guide protective measures and design vaccination strategies.
Conclusion
Elevated levels of pre-existing antibodies against seasonal coronaviruses, specifically HCoV OC43 and HKU1, are associated with a less severe course of COVID-19. Further studies should validate this finding and explore the potential to identify persons at risk for severe disease before a SARS-CoV-2 infection.
Conflict of interest
None declared.
Author contributions
MD, PT, JK, RV and HS designed the study. TG, EL and JK developed the measurement procedure. TG performed lab measurements. PT and RV collected patient data. MD and TB analyzed data. MD wrote the manuscript.
Acknowledgement
Supported by a grant from BMBF (HiGHmed 01ZZ1802V, Use Case Infection Control).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.02.085.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Dugas M., Schrempf I.M., Ochs K., Frömmel C., Greulich L., Neuhaus P. Association of contact to small children with mild course of COVID-19. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.09.003. S1201-9712(20)30720-30727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7) doi: 10.1016/j.cell.2020.05.015. 1489-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368 doi: 10.1136/bmj.m1198. m1198. [DOI] [PubMed] [Google Scholar]
- Kowitdamrong E., Puthanakit T., Jantarabenjakul W., Prompetchara E., Suchartlikitwong P., Putcharoen O. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0240502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar M., Reifler K., Rossi M., Miller N.S., Sinha P., White L. Recent endemic coronavirus infection is associated with less severe COVID-19. J Clin Invest. 2020;(September):143380. doi: 10.1172/JCI143380. PMID: 32997649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrock E., Fujimura E., Kula T., Timms R.T., Lee I.H., Leng Y. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020;370(November (6520)) doi: 10.1126/science.abd4250. eabd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.