Abstract
Aspergillus infection is a well-known complication of severe influenza and severe acute respiratory syndrome coronavirus (SARS-CoV), and these infections have been related with significant morbidity and mortality even when appropriately diagnosed and treated. Recent studies have indicated that SARS-CoV-2 might increase the risk of invasive pulmonary aspergillosis (IPA). Here, we report the first case of Aspergillus ochraceus in a SARS-CoV-2 positive immunocompetent patient, which is complicated by pulmonary and brain infections. Proven IPA is supported by the positive Galactomannan test, culture-positive, and histopathological evidence. The patient did not respond to voriconazole, and liposomal amphotericin B was added to his anti-fungal regimen. Further studies are needed to evaluate the prevalence of IPA in immunocompetent patients infected with SARS-CoV-2. Consequently, testing for the incidence of Aspergillus species in lower respiratory secretions and Galactomannan test of COVID-19 patients with appropriate therapy and targeted anti-fungal therapy based on the primary clinical suspicion of IPA are highly recommended.
Keywords: Disseminated aspergillosis, Aspergillus ochraceus, COVID-19, Immunocompetent patient
1. Introduction
Aspergillus species is considered one of the main causes of life-threatening infections in hospitalised patients with significant morbidity and mortality rates even when appropriately diagnosed and treated [1], [2], [3], [4]. The clinical spectrum of these fungi causes various infections, ranging from allergic and chronic infections to acute invasive aspergillosis (IA) [1], [2]. The incidence of this infection has increased in recent years, especially among hematopoietic stem cell recipients, solid organ transplant recipient, chronic pulmonary infections, and patients with severe influenza pneumonia [5], [6]. Previous studies indicated that influenza and coronavirus disease 2019 (COVID-19) were associated with an increased risk for invasive aspergillosis [7], [8], [9], [10]. They are also complicated by invasive pulmonary aspergillosis (IPA) in 6–24% of cases with a high mortality rate of over 45% [11]. Recent studies have demonstrated that COVID-19 due to SARS-CoV-2 may increase the risk of superinfections [7], [12] and particularly IPA co-infection [13], [14]. As recent studies have revealed, IPA emerges among SARS-CoV-2 infected patients in intensive care units (ICUs) with severe pulmonary abnormalities [15], [16]. Furthermore, COVID-19 associated pulmonary aspergillosis (CAPA) was reported in many countries [14], [17], [18]. Here, we present the first case of Aspergillus ochraceus in a SARS-CoV-2 positive immunocompetent patient in Isfahan, Iran.
2. Case presentation
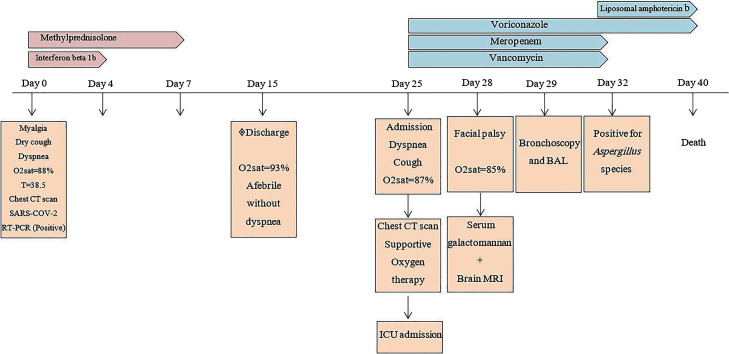
In June 2020, a 35-year-old immunocompetent man with a fever (38.5 °C) for six days, myalgia, dry cough, loss of appetite, and dyspnea with oxygen saturation (SpO2) of 88% on room air was admitted to the Emergency department, Isfahan University of medical sciences, Isfahan, Iran. Laboratory results showed leukopenia (900/mm3), elevated C-reactive protein (CRP) (95 mg/dL), and real-time reverse transcriptase–polymerase-chain-reaction (rRT-PCR) confirmed SARS-CoV-2 infection. Chest computed tomography (CT) scan demonstrated COVID-19 pneumonia. Supportive oxygen therapy with nasal cannula at 4 litres per minute and treatment with interferon Beta-1b 0.25 mg SQ every 48 hours for three dosages and methylprednisolone 125 mg for three days were started. After 8 days, SpO2 was 91 with improvement of symptoms. The patient was discharged on day 15 with SpO2 of 93% on room air with no dyspnea. Ten days after discharge, the patient was referred to the Emergency department of Amin hospital, Isfahan, Iran, with shortness of breath and cough. Chest X ray (Fig. 1 A and B) and chest CT scan (Fig. 2 A and B) showed parenchymal consolidation and soft tissue attenuated nodules with a random distribution. The patient was transferred to the intensive care unit (ICU) due to oxygen saturation of 87% on room air, dyspnea and severe bilateral pulmonary infiltrates (day 25). Laboratory analysis revealed white blood cell 3200 mm3, hemoglobin 13.5 g/dl, neutrophil 72.6%, lymphocytes 17%, platelets 242,000 mm3, blood urea nitrogen 11 mg/dL, and creatinine 0.8 mg/dL. Considering clinical manifestation, laboratory data and lung CT scan, meropenem (2 g/8 h) and vancomycin (1 g/12 h) were started. According to multiple nodules with a random distribution in the chest CT scan, intravenous voriconazole 6 mg/kg for the first day, and then 4 mg/kg every 12 hour were administered. On day 28, left facial nerve palsy occurred. Brain magnetic resonance imaging (MRI) was performed, and it revealed a focal mass with smooth ring enhancement in the left cerebellar peduncle. The mass showed diffusion restriction indicative of the brain abscess (Fig. 3 A–D). Human immunodeficiency virus (HIV) and nitroblue tetrazolium (NBT) results were negative. Blood and urine cultures were sterile. Serum Galactomannan antigen (GM) test using the platelia Aspergillus ELISA kit (Bio-Rad) was 2.1 on day 28. Bronchoscopy and bronchoalveolar lavage (BAL) were performed on day 29. Pulmonary cytopathology testing for malignancy was negative, but septate hyaline fungal hyphae with dichotomous branching were observed in histopathology findings (Fig. 4 A). The bronchoalveolar lavage (BAL) sample was cultured on both Sabouraud's dextrose agar (SDA; Merck) and Malt extract agar (MEA; Merck) supplemented with 0.5 mg/mL chloramphenicol at 35 °C and an amber-coloured granular texture, with reverse pale to brownish colonies grown within 4 days. Microscopy features with thick, slightly granular, conidiophores with globous vesicles, biseriate and hyaline, and globous pale yellow conidia were classified as Aspergillus species on day 32 (Fig. 4B and C). A voucher strain was deposited into the fungal diagnostic centre (FDC) culture collection Isfahan, Iran. DNA was extracted from 4-day-old culture and stored at −20 °C. The beta-tubulin gene was amplified using primers Bt2a (5’ GGTAACCAAATCGGTGCTGCTTTC 3’) and Bt2b (5’ ACCCTCAGTGTAGTGACCCTTGGC 3’) and sequenced as previously described [19]. The DNA sequence of the beta-tubulin gene was subjected to a BLAST search of the NCBI databases. The isolate was identified as Aspergillus ochraceus, having 99% sequence identity. The molecular results confirmed the mycological diagnosis of the disease as disseminated aspergillosis due to Aspergillus ochraceus. The beta-tubulin gene sequence of the strain from the current case has been deposited in the GenBank under the accession number MT799706. Antifungal susceptibility tests, using broth microdilution, were performed according to the Clinical and laboratory standard institute methods [20]. The MIC values were as follows: amphotericin B (1 μg/mL), itraconazole (0.5 μg/mL), voriconazole (0.5 μg/mL), caspofungin (0.016 μg/mL), anidulafungin (0.008 μg/mL) and micafungin (0.008 μg/mL). Liposomal amphotericin B (5 mg/kg/day) was added to his anti-fungal regimen, and meropenem and vancomycin were stopped. On day 40, despite anti-fungal therapy, the patient died 16 days after hospital admission due to respiratory distress and pulmonary failure (Fig. 5 ). This research was approved by the Ethics committee of Isfahan University of medical sciences (no. IR.MUI.MED.REC.1399.343), and written informed consent was obtained from the patient.
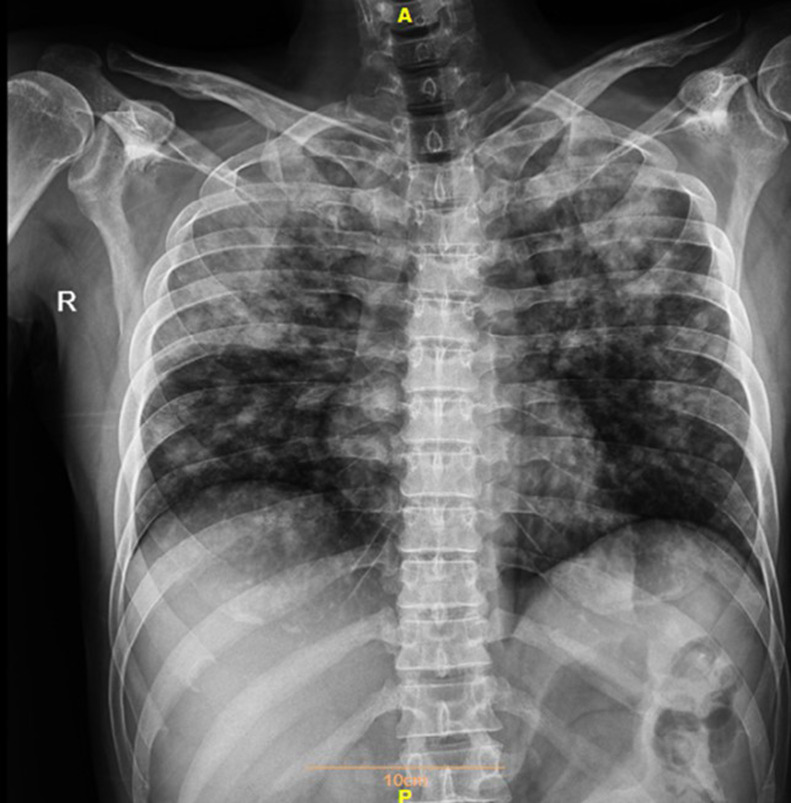
Fig. 1.
Chest X-ray revealed alveolar parenchymal opacification mainly in upper lobes.
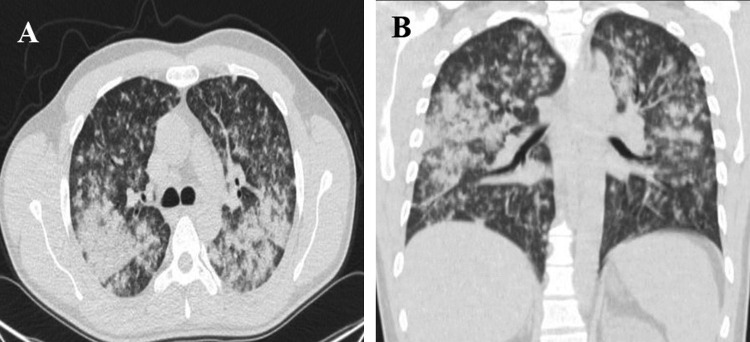
Fig. 2.
Chest CT scan revealed parenchymal consolidation and soft tissue attenuated nodules with a random distribution (A), in a coronal view, note the upper predominance of parenchymal consolidation (B).
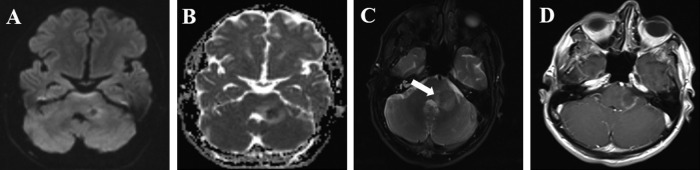
Fig. 3.
Axial diffusion-weighted MR image revealed hyperintensity in the mass (A), axial apparent diffusion coefficient MR image showed corresponding hypointensity confirming restricting diffusion (B), axial T2-weighted MR images showed a focal mass (white arrow) with intermediate signal intensity in the left brachium pontine surrounded by peripheral vasogenic edema (C), axial contrast-enhanced T1-weighted image showed smooth ring enhancement (D).
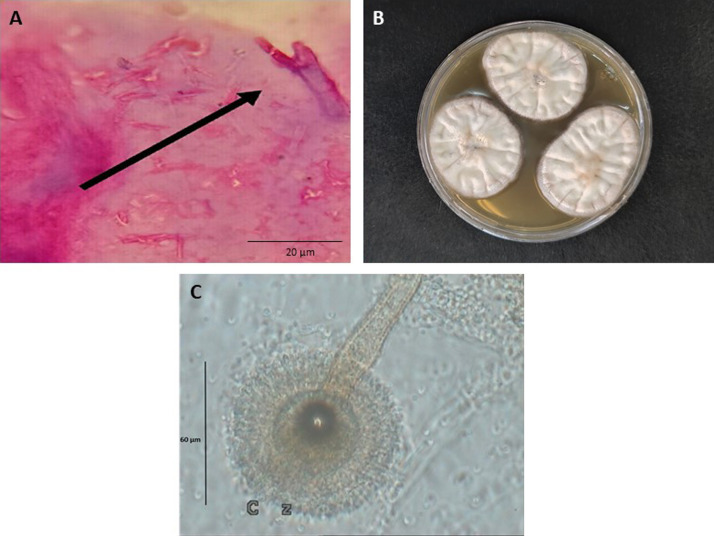
Fig. 4.
Dichotomous hyaline septate hyphae with acute-angle branching (black arrows) belonging to the Aspergillus species, periodic Acid–Schiff (PAS) stain, original magnification × 40 (A), Aspergillus ochraceus on the malt extract agar after 7 days (B), microscopic morphology of A. ochraceus showed thick, slightly granular, globous vesicles, biseriate and hyaline, and globous pale yellow conidia (C).
Fig. 5.
Timeline representing the course of COVID-19 pneumonia due to Aspergillus ochraceus.
3. Discussion
Since January 27, 2021, the COVID-19 pandemic due to SARS-CoV-2 has thus far killed over 57,651 people and infected over 1,392,314 individuals in Iran. Recent studies have shown that CAPA may occur in the immunocompetent host. We report the first case of Aspergillus ochraceus in a SARS-CoV-2 positive, which occurred in an immunocompetent host without an underlying condition. Most immunocompetent patients presenting severe types of COVID-19 have at least one underlying condition, such as diabetes mellitus, chronic obstructive pulmonary disease, chronic renal disease, and hypertension [21]; however, none of these risk factors is commonly associated with an increased risk for invasive fungal infections. In our patient, the WBC count was 900 per μL (with 90% neutrophil) at first admission with the diagnosis of COVID-19, which was 2100 per μL on day 3. On day 25, at second hospitalisation, the WBC count was 3200 per μL (with neutrophil 72.6%). In both times, the patient had leukopenia due to COVID-19 infection as a viral infection. Furthermore, the patient had not prolonged neutropenia as a risk factor for invasive aspergillosis, and it was transient on the first admission. At second hospitalisation, the patient was not neutropenic. Blaize et al. [22] reported a case of invasive aspergillosis in an immunocompetent male with severe COVID-19-associated pneumonia with several underlying chronic diseases, such as myelodysplastic syndrome and Hashimoto's thyroiditis. In the absence of host factors, as defined by the European Organisation for research and treatment of cancer/mycoses study group (EORTC/MSG) criteria for invasive aspergillosis, invasive or high-risk diagnostic methods, such as CT scan and biopsy, are essential to support the recognition of IPA [23]. The IPA in ICU patients is recognised as a valid algorithm to comparatively deal with the absence of host factors [24]. Bartoletti et al. recommended that CAPA criteria for diagnosis of invasive aspergillosis might provide earlier diagnosis than AspICU criteria and might prioritise prompt anti-fungal treatment [25]. The present case was diagnosed with proven CAPA, based on the positive Galactomannan test, culture-positive, and histopathological evidence [26]. Overall, cerebral aspergillosis has a high mortality rate, and survivors present various neurological deficiencies [27]. Aspergillus ochraceus has not been reported as a frequent causative agent of pulmonary infections, especially among asthmatic and immunocompromised patients. In this regard, Negri et al. showed that only one out of 133 patients was found to have A. ochraceus infection [28]. Similarly, Carpagnano et al. revealed colonisation with A. ochraceus in only three of 43 cancer patients [29]. Moazam et al. [30] reported a 39-year-old male case with chronic pulmonary aspergillosis (CPA) due to A. ochraceus with a history of chronic obstructive pulmonary disease (COPD). Contrary to the present case, he was healed and responded well to IV voriconazole. Overall, SARS-CoV-2 may be a risk factor for IA and other invasive fungal diseases [12]. Our case report highlights that clinical suspicion and screening are needed to evaluate the prevalence of IPA in immunocompetent patients infected with COVID-19. Consequently, testing for the presence of Aspergillus species in lower respiratory secretions, and the GM test in COVID-19 patients, as well as primary clinical suspicion of IPA with appropriate and targeted anti-fungal therapy according to national and international guidelines, are highly recommended.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
This study was financially supported by a grant from the School of medicine, Isfahan University of medical sciences, Isfahan, Iran (no. 199163), which we gratefully acknowledge.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.mycmed.2021.101124.
Online Supplement. Supplementary data
References
- 1.Patterson T.F., Kirkpatrick W.R., White M., Hiemenz J.W., Wingard J.R., Dupont B., et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus study group. Medicine. 2000;79:250–260. doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kosmidis C., Denning D.W. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270–277. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 3.Taccone F.S., Van den Abeele A.-M., Bulpa P., Misset B., Meersseman W., Cardoso T., et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19(1):7. doi: 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meersseman W., Vandecasteele S.J., Wilmer A., Verbeken E., Peetermans W.E., Van Wijngaerdert E. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621–625. doi: 10.1164/rccm.200401-093OC. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis D.P., Marr K.A., Park B.J., Alexander B.D., Anaissie E.J., Walsh T.J., et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 6.Pappas P.G., Alexander B.D., Andes D.R., Hadley S., Kauffman C.A., Freifeld A., et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-associated infection surveillance network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 7.Nasri E., Shoaei P., Vakili B., Mirhendi H., Sadeghi S., Hajiahmadi S., et al. Fatal invasive pulmonary aspergillosis in COVID-19 patient with acute myeloid leukemia in Iran. Mycopathologia. 2020:1–8. doi: 10.1007/s11046-020-00493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H., Ding Y., Li X., Yang L., Zhang W., Kang W. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N Engl J Med. 2003;349:507–508. doi: 10.1056/NEJM200307313490519. [DOI] [PubMed] [Google Scholar]
- 9.Van de Veerdonk F.L., Kolwijck E., Lestrade P.P.A., Hodiamont C.J., Rijnders B.J.A., van Paassen J., et al. Influenza-associated aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2017;196(4):524–527. doi: 10.1164/rccm.201612-2540LE. [DOI] [PubMed] [Google Scholar]
- 10.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz I.S., Friedman D.Z., Zapernick L., Dingle T., Lee N., Sligl W., et al. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from Alberta, Canada. Clin Infect Dis. 2020;71(7):1760–1763. doi: 10.1093/cid/ciaa007. [DOI] [PubMed] [Google Scholar]
- 12.Gangneux J.-P., Bougnoux M.-E., Dannaoui E., Cornet M., Zahar J.R. Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med. 2020;30(2):100971. doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verweij P.E., Gangneux J.-P., Bassetti M., Brüggemann R., Cornely O., Koehler P., et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1(2):e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prattes J., Valentin T., Hoenigl M., Talakic E., Reisinger A.C., Eller P. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU – a case report. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasir N., Farooqi J., Mahmood S.F., Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63(8):766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19 associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alanio A., Delliere S., Fodil S., Bretagne S., Megarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill COVID-19 patients. Lancet Respir Med. 2020;8(6):e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubka V., Kolarik M. β-tubulin paralogue tubC is frequently misidentified as the benA gene in Aspergillus section Nigri taxonomy: primer specificity testing and taxonomic consequences. Persoonia. 2012;29:1–10. doi: 10.3767/003158512X658123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical laboratory standards institute. Clinical and laboratory standards institute; Wayne, PA, USA: 2008. Reference method for broth dilution anti-fungal susceptibility testing of filamentous fungi, 2nd ed; approved standard. CLSI document M38-A2. [Google Scholar]
- 21.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 22.Blaize M., Mayaux J., Nabet C., Lampros A., Marcelin A., Thellier M., et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26(7):1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European organisation for research and treatment of cancer and the Mycoses study group education and research consortium. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blot S., Rello J., Koulenti D. Diagnosing invasive pulmonary aspergillosis in ICU patients: putting the puzzle together. Curr Opin Crit Care. 2019;25(5):430–437. doi: 10.1097/MCC.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 25.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L., et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020:28. doi: 10.1093/cid/ciaa1065. [ciaa 1065] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koehler P., Bassetti M., Chakrabarti A., Chen S.C., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2020;14 doi: 10.1016/S1473-3099(20)30847-1. [S1473-3099(20)30847-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzolf G., Sabou M., Lannes B., et al. Magnetic resonance imaging of cerebral aspergillosis: imaging and pathological correlations. PLoS One. 2016;11(4):e0152475. doi: 10.1371/journal.pone.0152475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negri C., Gonçalves S., Xafranski H., et al. Cryptic and rare Aspergillus species in Brazil: prevalence in clinical samples and in vitro susceptibility to triazoles. J Clin Microbiol. 2014;52(10):3633–3640. doi: 10.1128/JCM.01582-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpagnano G.E., Lacedonia D., Palladino G.P., Crisetti E., Susca A., Logrieco A., et al. Aspergillus spp. colonisation in exhaled breath condensate of lung cancer patients from Puglia Region of Italy. BMC Pulm Med. 2014;14(1):22. doi: 10.1186/1471-2466-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moazam S., Denning D.W. Aspergillus nodules in chronic granulomatous disease attributable to Aspergillus ochraceus. Med Mycol Case Rep. 2017;17:31–33. doi: 10.1016/j.mmcr.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.