Abstract
OBJECTIVES
We retrospectively evaluated a chart review of 3,525 patients evaluated for any acute disturbance. A total of 1,504 patients with acute vestibular syndrome (AVS) received an instrumental vestibular assessment within 72 h from the onset of the symptoms evaluated using simultaneously a combination of ocular vestibular-evoked myogenic potential (oVEMPs), cervical vestibular-evoked myogenic potential (cVEMPs), video head-impulse test (vHIT), and subjective visual vertical (SVV) were included in this study.
MATERIALS and METHODS
A total of 41 patients with AVS that showed a normal horizontal canal function tested with vHIT, a normal cVEMP function, unilaterally reduced or absent oVEMP n10, and an altered SVV were enrolled.
RESULTS
We found that although these patients referred acute vertigo and presented spontaneous nystagmus, they showed physiological values of vHIT and a normal saccular function, as shown by symmetrical cVEMPs.
CONCLUSION
Our findings support the hypothesis that a percentage of patients evaluated during an AVS using an instrumental vestibular assessment could present selective utricular macula dysfunction.
Keywords: Tomography, optical coherence, cochlea, hair cells, auditory, inner, hair cells, auditory, outer
INTRODUCTION
An acute vertigo is a challenge for clinicians, who must differentiate a central from different peripheral problems in the acute clinical setting. These conditions cause vertigo and nystagmus, but the assessment, treatment, therapeutic progression, and prognosis could be very different. The bedside oculomotor exam (Head-impulse—nystagmus—test-of-skew (H.I.N.T.S.)) could help in differentiating peripheral from central causes of acute vestibular syndrome (AVS) in the emergency department; however, to establish the damage of vestibular organs and to determine their possible functional recovery, more exams are required.[1–3] Furthermore, it is crucial to have information at the time of the attack (<72 h from the onset of symptoms). Oculomotor findings and video head-impulse test[4,5] (vHIT) are commonly used in combination and are often complemented by neuroimaging [(magnetic resonance imaging (MRI)] to determine the classical signs and symptoms that depend on the vestibular function absence that can differentiate central from peripheral vestibular disorders. To date, every semicircular canal in both labyrinths can be evaluated using the video head-impulse technique[6–8] to test every otolithic sense organ by vestibular-evoked myogenic potentials (VEMPs).[9–13] The ocular VEMP (oVEMP) tests mainly dynamic utricular function[9,10] and the cervical vestibular-evoked myogenic potential (cVEMP) tests mainly dynamic saccular function[10,11,14,15]; therefore, the combination of vHIT and VEMPs provides a complete picture of peripheral vestibular function.[16,17] Several studies[18,19] reported single cases of selective utricular loss in an acute patient diagnosed with oVEMPs or with subjective visual vertical (SVV), while the remaining sense organs showed normal function on specific tests. In these clinical cases, the authors reported patients with an AVS, rotatory vertigo accompanied by nausea and vomiting, and with spontaneous nystagmus, but who, on testing, had normal horizontal canal function, as shown by normal caloric and normal vHIT testing. On tests of dynamic otolith function, these patients had symmetric cVEMPs indicating symmetrical saccular function, but asymmetric oVEMPs and altered SVV. It was concluded that in these patients, the AVS was probably due to peripheral unilateral utricular function loss.
This retrospective chart review study aims to confirm the possibility of diagnosing a selective utricular macula dysfunction in a large cohort of patients during an AVS.
MATERIALS AND METHODS
Study Design
This is a retrospective chart review study aimed to investigate the loss of the utricular macula function in patients with AVS in the first 72 h after the attack. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation and with the Helsinki Declaration of 1975. This study was carried out according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. All participants provided written consent to publish the results obtained from their clinical examinations and instrumental tests.
Setting
Medical records of patients with AVS evaluated in the first 72 h and with at least one follow-up at the MSA ENT Academy Center Clinic, a tertiary vestibular referral center, were reviewed.
Participants
All medical records of patients who were admitted between January 1, 2015 and July 31, 2020 with an AVS—vertigo, postural unsteadiness, nausea, vomiting, and having spontaneous nystagmus suppressed by vision, and so giving the appearance of a vestibular neuritis—were screened.
The inclusion criteria were: 1) unilaterally absent or markedly reduced oVEMP n10 potential in response to 500-Hz Fz bone-conducted vibration (BCV) stimulation recorded beneath the contralesional eye, indicating that there was reduced or absent utricular function in the affected ear; 2) normal bilateral semicircular canal function shown by normal Vestibular Ocular Reflex (VOR) gains on the vHIT tests for both directions of rotation, and/or the absence of systematic corrective saccades during the test; 3) cVEMPs on the ipsilesional side still present in response to 500-Hz Fz BCV stimulation, indicating that those saccular otolith afferents were functional in the inferior vestibular nerves; 4) absence of auditory signs to exclude possible cases of labyrinthitis; 5) absence of other alterations of the outer, middle, and inner ear; 6) history of sudden vertigo and/or postural unsteadiness and/or subjective lateral shift; 7) absence in the collected medical history of closed cranial trauma; 8) absence of symptoms and signs of central nervous system dysfunctions; 9) normal or near-normal vestibular and auditory function in the unaffected ear; and 10) presence of H.I.N.T.S peripheral pattern. We excluded the medical records of patients who showed one of the following exclusion criteria: 1) other vestibular diagnosis (e.g., unilateral vestibular neuritits (UVN), Ménière disease, bilateral vestibular loss, vestibular migraine, benign paroxysmal positional vertigo, etc.), somatic or psychiatric disorders; 2) presence of neurological diseases; and 3) presence of H.I.N.T.S central pattern.
Demographic and clinical characteristics at baseline are reported in Table 1.
Table 1.
Demographic and clinical characteristics
| Participants | Total |
|---|---|
| N | 41 |
| Age in years±SD | 45.78±15.96 |
| Gender (%) | |
| Female | 28 (68.3) |
| Male | 13 (32.7) |
| Side affected (%) | |
| Left | 9 (21.95) |
| Right | 32 (78.04) |
| oVEMPs AR ±SD | 63.69%±23.62 |
| cVEMPs AR ±SD | 12.99%±9.46 |
| vHIT horizontals (mean gain ±SD) | |
| Affected side | 1.11±0.13 |
| Healthy side | 1.14±0.15 |
| vHIT superiors (mean gain ±SD) | |
| Affected side | 0.87±0.04 |
| Healthy side | 0.90±0.05 |
| vHIT posteriors (mean gain ±SD) | |
| Affected side | 0.86±0.04 |
| Healthy side | 0.85±0.03 |
| SVV range | (26)>9.27±2.15 |
| (number of patients, N)±SD | (15)<–7.38±1.8 |
SD: standard deviation; oVEMPs: ocular vestibular-evoked myogenic potential; cVEMPs: cervical vestibular-evoked myogenic potential; AR: asymmetry ratio; vHIT: video head-impulse test; SVV: subjective visual vertical.
All patients with AVS were undergone a vestibular assessment that included horizontal and vertical semicircular canals with bedside head impulse test+vHIT, air-conducted sound, and BCV cervical and ocular VEMPs, and SVV. On the basis of tentative diagnosis of unilateral utricular loss (UUL), all of these patients were referred to a tertiary radiological center for MRI scans of the posterior cranial fossa using paramagnetic contrast enhancement to rule out other diseases.
On one occasion, a total of 20 normal healthy subjects (14 females and 6 males; mean age: 45.6±8.76 years) were tested. The remaining were patients’ partners. None of the healthy participants reported any (apart from standard refractive errors) auditory, vestibular, neurological, or visual issues.
Statistical Analysis
The asymmetry ratio (AR) between the affected and healthy sides was calculated using a version of the standard Jongkees formula for asymmetry calculations in vestibular testing, for both oVEMP n10 and cVEMP p13-n23 amplitudes: AR=100’(larger VEMP – smaller VEMP)/(larger VEMP + smaller VEMP). The AR was considered pathological with AR ≥40 for the oVEMPS and ≥30 for the cVEMPs. The average horizontal slow phase eye velocity VOR gain for each side was calculated at T0 and T1 as the sum of the VOR gains for each trial. Statistical analysis was performed with IBM SPSS Statistics software (v23, IBM Corp., Armonk, NY, USA). The values are expressed as mean±SD or ±two-tailed 95% confidence intervals. The significance level was set at 0.05. Each subject was tested in all of the conditions (oVEMP, cVEMP, vHIT, and VVS) for all experiments. Pearson’s correlation coefficient was calculated between SVV and oVEMP AR.
The Vestibular Assessment
Vestibular-Evoked Myogenic Potentials
The oVEMP n10 is a small (5–10 μV) negative (excitatory), crossed, VEMP of the stretched inferior oblique eye muscles [12,20] recorded by surface electromyography (EMG) electrodes on the skin beneath the eyes in response to stimulation by BCV delivered to the midline of the forehead at the hairline (Fz). Based on evidence of utriculo-ocular projections [21] and neural evidence of the preferential activation by 500-Hz BCV of irregular otolithic afferent neurons,[22–27] oVEMP (n10) is mainly indexed to utricular activity for these stimuli.
The cVEMP to these stimuli is a positive (inhibitory) uncrossed potential recorded by surface EMG electrodes over the tensed sternocleidomastoid (SCM) muscle in response to stimulation by BCV delivered to the midline of the forehead at the hairline (Fz), and the initial positive component (p13-n123) of the cVEMP is held to index mainly saccular function.[10,12,13]
SVV Methods
The SVV test was performed in a sitting position with the head of the subject erect. For the determination of SVV, we used a 40-cm-long 1-cm-wide dimly lit bar of light in a dark room at 1 m away from the subject, subtending a visual angle of 22.6°. The bar was displayed on a computer screen, and the movement was controlled by the subject using a joystick. The subject wore a mask, which occluded vision of screen edges, and was asked to set the bar to his true gravitational vertical. Setting of the upper tip of the bar to the subject’s left was scored negative. The angle in degrees was recorded with a resolution of 0.1°. A total of 6 measurements were made with the starting position of the bar alternating between ±45° from vertical. The determination of SVV was also carried out in 20 normal subjects who did not have a history of vertiginous episodes and/or balance disorder past or present. In normal subjects, estimation of vertical is when they are able to indicate the vertical very accurately.[28] In our clinic, we define the normal range of the SVV in the upright position (SVu) as −2° to +2°.
In the event of a sudden loss of unilateral vestibular function, the subject tilts the upper end of the bar toward the dysfunctional ear, shifting by several degrees with respect to the gravitational axis.[29] The error in perception could be due to an ipsilesional ocular torsion deviation, as an integral part of a postural static synkinesis known as ocular tilt reaction.[30]
Video Head-Impulse Test
The function of the semicircular canals was measured using vHIT (OtosuiteV®, GN Otometrics, Denmark) during Head Impulse. The gain value <0.68 identifies the affected side of UVN with 100% sensitivity and 100% specificity.[6]
RESULTS
A total of 3,525 medical records of patients who referred vertigo were reviewed; 1,504 patients with AVS who received an instrumental assessment within 72 h were included.
A total of 41 patients (2.7%) (28 females and 13 males, with the mean age of 45.78±15.96 years) met the inclusion criteria and were enrolled in this study. The averaged responses from patients and healthy participants show the main features of the oVEMP and the cVEMP responses to 500-Hz Fz BCV and vHIT. In the healthy participants, the stimulus produced a small (about 5–10 μV) negative potential (n10) at a latency of about 10 ms and of approximately equal amplitude beneath both eyes. However, the enrolled patients had an asymmetric oVEMP response to the same 500-Hz BCV stimulus, with the n10 component being markedly reduced beneath the eye opposite the affected side (Figure 1), so that the AR exceeded the limits of normal (40%). The cVEMP of the patient’s response was symmetrical, as well as the vHIT responses of horizontal canals.
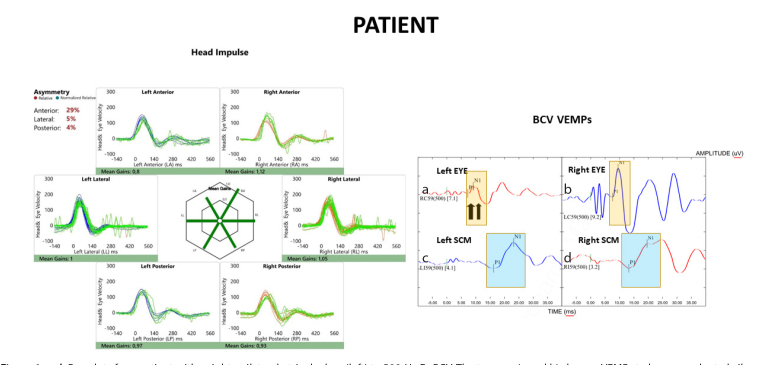
Figure 1. a–d.
Raw data for a patient with a right unilateral utricular loss (left) to 500-Hz Fz BCV. The top row (a and b) shows oVEMPs to bone-conducted vibration: the yellow rectangle shows the time of the n10 response, which is markedly reduced beneath the left eye of the patient (left column), indicating utricular macula hypofunction in the right ear. The patient’s left eye, corresponding to the right ear, shows a normal oVEMP response. The down row (c and d) shows the cVEMPs to 500-Hz Fz BCV: the blue rectangle shows the time of the p13 (p1) and n23 (n1) responses, marked with small vertical lines. These responses are normal in both the patient and the healthy subject (see Figure 2). The left side (for the viewer) of the figure shows results of vHIT testing of both left and right semicircular canals: eye velocity (green traces) and head velocity (blue traces for the left side and red traces for the right side) versus time. The signs of head velocity for rightward impulses and of eye velocity for leftward impulses have been inverted for easier comparison. Both the patient and the healthy subject (see Figure 2) show normal responses for head impulses to both sides—eye velocity matched head velocity closely.
The mean AR of the oVEMPs for patients was 63.69%±23.62, with a two-tailed 95% confidence interval of 70.76–55.97%. The mean AR for UUL patients here, 63.69%±23.62 SD, n=41, is similar to the mean AR of the 133 patients with superior vestibular neuritis,[31] mean AR=66.9% ±19.7, and n=133.
The SVV at the same testing occasion as the VEMP testing was altered in all tested patients; 26 patients showed values >9.27±2.15 and 15 patients showed values <–7.38±1.8. The patients consistently showed deviations of the SVV toward the affected ear. The mean of the horizontal VOR gain was 1.11±0.13 in the affected side and 1.14±0.13 in the healthy side; the superiors VOR gain was 0.87±0.04 in the affected side and 0.90±0.05 in the healthy side; and the posteriors VOR gain was 0.86±0.04 in the affected side and 0.85±0.03 in the healthy side. The mean AR of the cVEMPs for patients was 12.99%±9.46 (Figure 2).
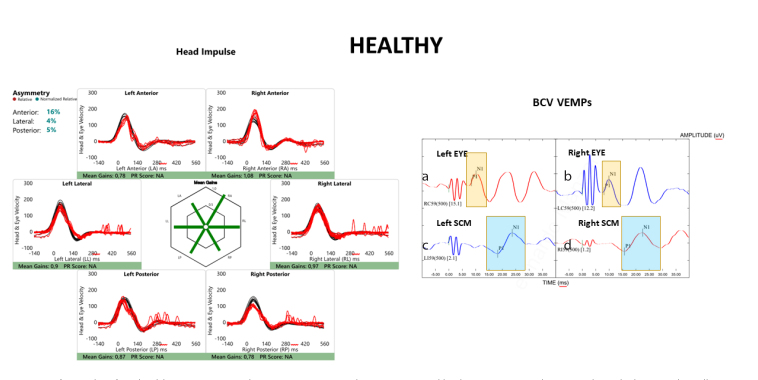
Figure 2. a–d.
Raw data for a healthy participant (right) to 500-Hz Fz BCV. The top row (a and b) shows oVEMPs to bone-conducted vibration: the yellow rectangle shows the time of the n10 response, which is symmetrical beneath the eyes of the subject indicating symmetrical utricular macula function. The patient’s eyes show a normal oVEMP response. The down row (c and d) shows the cVEMPs to 500-Hz Fz BCV: the blue rectangle shows the time of the p13 (p1) and n23 (n1) responses, marked with small vertical lines. These responses are normal and symmetrical. The left side (for the viewer) of the figure shows results of vHIT testing of both left and right semicircular canals: eye velocity (red traces) and head velocity (black traces) versus time. The signs of head velocity for rightward impulses and of eye velocity for leftward impulses have been inverted for easier comparison. Healthy subjects show normal responses for head impulses to both sides—eye velocity matched head velocity closely.
The correlation between the signed oVEMP n10 AR and the SVV setting was r=0.89, which is significant with p<0.001.
DISCUSSION
This study aimed to verify the presence of a selective loss of utricular function in patients with AVS using the oVEMPs. For this purpose, we used simultaneously in the first 72 h from the onset of the symptoms different technologies for the study of the vestibular function (vHIT, BCV oVEMPs, BCV cVEMPs, and SVV). We believe that this type of evaluation approach is of fundamental importance in diagnosing selective inner ear dysfunctions, especially in view of understanding the symptomatic evolution of the clinical conditions. Patients who referred to acute vertigo and presented spontaneous nystagmus gave every indication of having a UVN, but objective tests of horizontal semicircular canal function using vHIT showed that the horizontal canals have normal function. These patients had a unilaterally reduced or absent oVEMP n10, supporting the clinical hypothesis of an isolated utricular dysfunction. According to pioneering studies, our results confirm that within 72 h from the AVS could have normal canal dynamic function associated with a systematic deviation of the SVV. We also found a significant correlation between the SVV and the oVEMP n10 in the acute phase. Indeed, we reported that when both SVV and oVEMP tests are conducted on the same patients in the acute phase, they indicate similar results confirming the UUL. Obviously, in these patients, the saccular function was normal, as shown by symmetrical cVEMPs results.
The above analysis is based on the argument that otolithic projections to the oculomotor system are predominantly from the utricular macula, and projections to SCM muscles are predominantly from saccular macula.[31] There are some sacculo-ocular projections, but they are weaker in comparison with the utriculo-ocular projections.[32,33]
Although most of these patients had spontaneous nystagmus with a clear horizontal component, there was no canal paresis in our sample. The neural mechanism that can explain the presence of spontaneous nystagmus could be the imbalance in average resting discharge rates of neurons in the two vestibular nuclei.[34–37]
From a clinical perspective, our results indicate that it should not be immediately concluded that when a patient has acute vertigo and spontaneous nystagmus, it is due to canal dysfunction. Indeed, they could have a transitory or permanent utricular loss.
To the best of our knowledge, this is the first study aimed to investigate the presence of a selective utricular macula dysfunction on this large cohort of patients. We believe that our data could be useful in the clinical routine to recognize this clinical entity and to reduce the risk of misdiagnose.
This study presents some limitations that should be mentioned. First, this is a retrospective study, with the inherent potential bias, and second, we do not use ocular cycloposition assessment with optical coherence tomography; we evaluate the test of skew with crossover test.[3] Another limitation could be the lack of a follow-up indeed the evaluation of the “dynamic”/“transient” vestibular-otolith sensory function if performed later in time (i.e., over 6 months) could remain substantially unchanged or could improve. Furthermore, we have to consider the sensitivity and specificity of these tests and also the frequency range tested in the vestibular system in the different stages of the disease. The evolution in the recovery of otolith function could affect the therapeutic destiny of these patients (i.e., rehabilitation of the otolithic function). Testing the static/sustained vestibular-otolith sensory function with SVV and the dynamic/transient vestibular-otolith sensory function with oVEMPs[38] simultaneously at the time of the attack and during the follow-up in patients with AVS led to highlight how the functions of the utricular macula are affected by the inflammatory process at very early stages of the disease.
CONCLUSION
Our findings showed that a percentage (2.7%) of patients evaluated during an AVS using an instrumental vestibular assessment could present a selective asymmetry of oVEMPs and normal canals and saccular function. Furthermore, our results support the hypothesis that combining vestibular instrumental assessment is a possible way to diagnose selective utricular macula dysfunction in patients with acute vertigo evaluated within 72 h from the onset of the symptoms.
MAIN POINTS.
In the first 72 h from the onset of the symptoms it could be useful to study the vestibular function with different technologies.
A Subjective Visual Vertical alteration and an Asymmetry Ratio of ocular vestibular-evoked myogenic potential in patients with acute vestibular syndrome can be a clinical sign of early stage of utricular loss.
Patients who referred to acute vertigo and presented spontaneous nystagmus can have normal semicircular canals function.
A percentage (2.7%) of patients evaluated during an acute vestibular syndrome using an instrumental vestibular assessment could present a selective asymmetry of ocular vestibular-evoked myogenic potential and normal canals and saccular function.
Footnotes
Ethics Committee Approval: All procedures performed were in accordance with the ethical standards of our institutional research committee, and in accordance with the tenets of the 1964 Helsinki Declaration and its later amendments, or with comparable ethical standards.
Informed Consent: Informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – L.M., M.T.; Design - L.M., M.T.; Supervision – G.K.; Resource – L.M.; Materials - L.M., M.T.; Data Collection and/or Processing - L.M., M.T.; Analysis and/or Interpretation L.M., M.T.; G.K..; Literature Search - L.M., M.T.; Writing - L.M., M.T.; Critical Reviews – G.K.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Newman-Toker DE, Kerber KA, Hsieh YH, Pula JH, Omron R, Saber Tehrani AS, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20:986–96. doi: 10.1111/acem.12223. [DOI] [PubMed] [Google Scholar]
- 2.Newman-Toker DE, Curthoys IS, Halmagyi GM. Diagnosing Stroke in Acute Vertigo: The HINTS Family of Eye Movement Tests and the Future of the “Eye ECG”. Semin Neurol. 2015;35:506–21. doi: 10.1055/s-0035-1564298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kattah JC. Use of HINTS in the acute vestibular syndrome. An Overview. Stroke Vasc Neurol. 2018;3:190–6. doi: 10.1136/svn-2018-000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manzari L, Tramontano M. Suppression Head Impulse Paradigm (SHIMP) in evaluating the vestibulo-saccadic interaction in patients with vestibular neuritis. Eur Arch Otorhinolaryngol. 2020;277:3205–12. doi: 10.1007/s00405-020-06085-6. [DOI] [PubMed] [Google Scholar]
- 5.Manzari L, Graziano D, Tramontano M. The Different Stages of Vestibular Neuritis from the Point of View of the Video Head Impulse Test. Audiol Res. 2020;10:31–8. doi: 10.4081/audiores.2020.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–41. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol Neurotol. 2013;34:974–9. doi: 10.1097/MAO.0b013e31828d676d. [DOI] [PubMed] [Google Scholar]
- 8.Macdougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS One. 2013;8:e61488. doi: 10.1371/journal.pone.0061488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. 2010;121:132–44. doi: 10.1016/j.clinph.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Curthoys IS. The interpretation of clinical tests of peripheral vestibular function. Laryngoscope. 2012;122:1342–52. doi: 10.1002/lary.23258. [DOI] [PubMed] [Google Scholar]
- 11.Curthoys IS, Vulovic V, Manzari L. Ocular vestibular-evoked myogenic potential (oVEMP) to test utricular function: neural and oculomotor evidence. Acta Otorhinolaryngol Ital. 2012;32:41–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Rosengren SM, Kingma H. New perspectives on vestibular evoked myogenic potentials. Curr Opin Neurol. 2013;26:74–80. doi: 10.1097/WCO.0b013e32835c5ef3. [DOI] [PubMed] [Google Scholar]
- 13.Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol. 2010;121:636–51. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson GP, McCaslin DL, Piker EG, Gruenwald J, Grantham SL, Tegel L. Patterns of abnormality in cVEMP, oVEMP, and caloric tests may provide topological information about vestibular impairment. J Am Acad Audiol. 2011;22:601–11. doi: 10.3766/jaaa.22.9.5. [DOI] [PubMed] [Google Scholar]
- 15.Oh SY, Kim JS, Yang TH, Shin BS, Jeong SK. Cervical and ocular vestibular-evoked myogenic potentials in vestibular neuritis: comparison between air- and bone-conducted stimulation. J Neurol. 2013;260:2102–9. doi: 10.1007/s00415-013-6953-8. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal Y, Bremova T, Kremmyda O, Strupp M. Semicircular canal, saccular and utricular function in patients with bilateral vestibulopathy: analysis based on etiology. J Neurol. 2013;260:876–83. doi: 10.1007/s00415-012-6724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiarovano E, Zamith F, Vidal PP, de Waele C. Ocular and cervical VEMPs: a study of 74 patients suffering from peripheral vestibular disorders. Clin Neurophysiol. 2011;122:1650–9. doi: 10.1016/j.clinph.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Manzari L, Burgess AM, Curthoys IS. Does unilateral utricular dysfunction cause horizontal spontaneous nystagmus? Eur Arch Otorhinolaryngol. 2012;269:2441–5. doi: 10.1007/s00405-012-2127-z. [DOI] [PubMed] [Google Scholar]
- 19.Pelosi S, Schuster D, Jacobson GP, Carlson ML, Haynes DS, Bennett ML, et al. Clinical characteristics associated with isolated unilateral utricular dysfunction. Am J Otolaryngol. 2013;34:490–5. doi: 10.1016/j.amjoto.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Weber KP, Rosengren SM, Michels R, Sturm V, Straumann D, Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J Physiol. 2012;590:3091–101. doi: 10.1113/jphysiol.2011.226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol. 1969;68:350–62. doi: 10.3109/00016486909121573. [DOI] [PubMed] [Google Scholar]
- 22.McCue MP, Guinan JJ., Jr Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994;14:6058–70. doi: 10.1523/JNEUROSCI.14-10-06058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCue MP, Guinan JJ., Jr Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol. 1997;18:355–60. [PubMed] [Google Scholar]
- 24.Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175:256–67. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- 25.Manzari L, Tedesco AR, Burgess AM, Curthoys IS. Ocular and cervical vestibular-evoked myogenic potentials to bone conducted vibration in Ménière’s disease during quiescence vs during acute attacks. Clin Neurophysiol. 2010;121:1092–101. doi: 10.1016/j.clinph.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Curthoys IS, Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res. 2011;210:347–52. doi: 10.1007/s00221-010-2499-5. [DOI] [PubMed] [Google Scholar]
- 27.Curthoys IS, Vulovic V, Burgess AM, Cornell ED, Mezey LE, Macdougall HG, et al. The basis for using bone-conducted vibration or air-conducted sound to test otolithic function. Ann N Y Acad Sci. 2011;1233:231–41. doi: 10.1111/j.1749-6632.2011.06147.x. [DOI] [PubMed] [Google Scholar]
- 28.Böhmer A, Rickenmann J. The subjective visual vertical as a clinical parameter of vestibular function in peripheral vestibular diseases. J Vestib Res. 1995;5:35–45. doi: 10.1016/0957-4271(94)00021-S. [DOI] [PubMed] [Google Scholar]
- 29.Faralli M, Ricci G, Molini E, Longari F, Altissimi G, Frenguelli A. Determining subjective visual vertical: dynamic versus static testing. Otol Neurotol. 2007;28:1069–71. doi: 10.1097/MAO.0b013e31815aea1b. [DOI] [PubMed] [Google Scholar]
- 30.Brandt T, Strupp M. General vestibular testing. Clin Neurophysiol. 2005;116:406–26. doi: 10.1016/j.clinph.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Manzari L, Tedesco A, Burgess AM, Curthoys IS. Ocular vestibular-evoked myogenic potentials to bone-conducted vibration in superior vestibular neuritis show utricular function. Otolaryngol Head Neck Surg. 2010;143:274–80. doi: 10.1016/j.otohns.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Uchino Y, Kushiro K. Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci Res. 2011;71:315–27. doi: 10.1007/s002219900292. [DOI] [PubMed] [Google Scholar]
- 33.Isu N, Graf W, Sato H, Kushiro K, Zakir M, Imagawa M, Uchino Y. Sacculo-ocular reflex connectivity in cats. Exp Brain Res. 2000;131:262–8. doi: 10.1007/s002219900292. [DOI] [PubMed] [Google Scholar]
- 34.Curthoys IS, Manzari L, Smulders YE, Burgess AM. A review of the scientific basis and practical application of a new test of utricular function--ocular vestibular-evoked myogenic potentials to bone-conducted vibration. Acta Otorhinolaryngol Ital. 2009;29:179–86. [PMC free article] [PubMed] [Google Scholar]
- 35.Leigh RJ, Zee DS. The Neurology of Eye Movements. Philadelphia: F. A. Davis; 2006. [Google Scholar]
- 36.Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev. 1989;14:155–80. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel EA, Demetriades TD. Central compensation of labyrinth damage. Pflugers Archiv Fur Die Gesamte Physiologie des Menschen Und Der Tiere. 1925;210:215–22. doi: 10.1007/BF01722883. [DOI] [Google Scholar]
- 38.Halmagyi GM, Weber KP, Curthoys IS. Vestibular function after acute vestibular neuritis. Restor Neurol Neurosci. 2010;28:37–46. doi: 10.3233/RNN-2010-0533. [DOI] [PubMed] [Google Scholar]