Abstract
This study’s aim was to establish outcomes following cochlear implantation (CI) in patients with mitochondrial disorders associated with deafness. Systematic review and narrative synthesis. Databases searched: Medline, EMBASE, Web of Science, COCHRANE, and ClinicalTrials.gov. No limits on language or year of publication. Review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Searches identified 437 abstracts and 37 full text articles, of which 11 studies met the inclusion criteria reporting outcomes in a total of 17 patients. All implants achieved good hearing outcomes, and follow-up ranged between 1 week and 12 months. The methodological quality of the included studies was sufficient, scoring grades 3 to 4 using the Oxford Centre for Evidence Based Medicine grading system. All studies were retrospective and consisted of case reviews and case reports. All cases of CI showed positive outcomes in speech perception and detection. There is some qualitative evidence to suggest improvement in quality of life and satisfaction postoperatively. There was very limited information available on secondary outcomes such as surgical complications, quality of life, and method of cochlear implant insertion. The small sample size of our patient cohort and quality of studies suggests a need for large-scale studies with more robust methodology to assess the effectiveness of CI. There is a need for studies that assess other factors to be considered when counseling patients about cochlear implants, such as adverse events, surgical complications, and long-term benefits.
Keywords: Cochlear implantation, mitochondrial disorders, mitochondrial deafness
INTRODUCTION
Mitochondrial diseases are a genetically and phenotypically heterogenous group of disorders caused by dysfunctional mitochondria. Mitochondria are the organelles that are primarily responsible for the generation of cellular adenosine triphosphate (ATP). Mitochondrial diseases can be caused by mutations in mitochondrial DNA (mtDNA) or in the nuclear genes that encode the mitochondrial proteins. Common mutations include deletions and point mutations, which can manifest through maternal inheritance or de novo during embryonic development [1]. However, the way these mutations manifest is phenotypically variable. For example, the m.3243A>G mutation can be responsible for multiple syndromes such as mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) or Kearns-Sayre syndrome (KSS) at the same time [2]. The phenotypes of mitochondrial mutations may present as a disease in a single organ or as systemic disease with myopathic and neurological manifestations, including sensorineural hearing loss. They can also present as different syndromes including KSS, neurogenic weakness with ataxia and retinitis pigmentosa (NARP), MELAS, maternally inherited diabetes and deafness (MIDD), and myoclonic epilepsy with ragged-red fibers (MERRF) [3]. The symptoms of a disorder vary greatly from person to person. For example, KSS can present as bilateral deafness, diabetes, dementia, or as a heart block [4]. A more comprehensive list of genetic mutations responsible for mitochondrial disorders and mitochondrial sensorineural hearing loss can be found in our search methods.
It is important to note the difficulty in ascertaining the current prevalence of mitochondrial diseases, considering the many ways in which they manifest, their rarity, and the challenges of identifying the mitochondrial mutations in multifactorial conditions such as diabetes and Alzheimer’s disease [5]. For example, studies have reported prevalence rates of MIDD ranging from 0% to 60% in those with diabetes [6].
Diagnosis of mitochondrial disease is often clinical, which includes the use of blood and cerebrospinal fluid lactate levels, neuroimaging, and cardiac investigations. Mitochondrial DNA is inherited maternally, which warrants the need for genetic counseling in patients presenting with mitochondrial disease. The diagnosis can be confirmed by molecular genetic testing, using blood and urine from children, and skeletal muscle biopsy in adults [4].
Currently, the treatment of mitochondrial disease is challenging owing to the multitude of ways the patients present and the manifold disease manifestations. Previous literature has highlighted the need for further research to provide better therapeutic options for this cohort of individuals [7]. Subsequently, current management options are symptom focused and focus on alleviating the symptoms that ensue from mitochondrial diseases. For example, in MELAS, there is an emphasis placed on treating the acute stroke-like episodes that arise from the condition [8].
Hearing loss is a common feature of mitochondrial disease and mitochondrial dysfunction. Hearing loss can occur in isolation (non-syndromic), and as a feature of systemic mitochondrial disease (syndromic) [9]. Sound amplification using hearing aids with or without hearing assistive technology systems are currently the mainstay of treatment [10]. With severe to profound hearing loss, a retrospective case review found that cochlear implantation (CI) significantly improved speech perception and showed long-term improvements in hearing performance in selected patients [11]. Small cohort studies have deemed cochlear implants are successful in treating mitochondrial deafness, according to the improvement in patients’ speech perception scores [12]. However, large-scale studies focusing on the efficacy of cochlear implants are limited. Therefore, this systematic review and narrative synthesis aimed to review the current evidence/literature surrounding the use of CI in patients with mitochondrial deafness.
Objective
Our systematic review analyzes the current evidence available and assesses and synthesizes the outcomes for CI as a means for treatment for profound sensorineural hearing loss resulting from mitochondrial dysfunction.
Population
Children or adults with mitochondrial sensorineural hearing loss
Intervention
CI
Comparison
Control group with no cochlear implant or conservative treatment only
Outcomes
Primary outcomes
Audiological outcomes and/or sound detection using an objective measure, speech perception, and production.
Secondary outcomes
Efficacy of different cochlear implant choices, adverse events, and quality of life following CI using satisfaction scales.
MATERIALS AND Methods
Our study protocol was registered in the PROSPERO database of systematic reviews, (CRD42020192989) and has been created according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Study inclusion criteria
All primary sources such as case reports, case reviews, cross-sectional studies, and randomized control trials were included in our search. Studies were excluded if they described outcomes of CI in patients with deafness yet did not specify the effects in the subgroup of mitochondrial disorders. We excluded studies based on animal or pharmacological models, and studies where CI was not the mainstay of treatment. If a study did not explicitly record CI outcomes on a measurable scale, it was excluded. Studies whereby the patients acquired a mitochondrial disorder owing to aminoglycoside exposure were also excluded.
Search Strategy
The following databases were searched: PubMed MEDLINE, Ovid EMBASE, ISI Web of Science, CENTRAL, ClinicalTrials.gov, and Cochrane register of Controlled Trials. Two independent reviewers (NZ/YN) screened all titles and abstracts according to the population, intervention, comparison, outcomes (PICO), and disagreements were resolved by discussion between them. Remaining studies were then screened according to the full text available by independent reviewers, and disagreements were resolved in the same way. No discrepancies required the adjudication of a third reviewer.
Search terms included (example from PubMed):
Cochlear implantation.mp OR cochlear implant OR cochlear implantation
Mitochondrial disorder OR mitochondrial sensorineural hearing loss OR Kearns-Sayre OR Chronic progressive external ophthalmoplegia OR MELAS OR MERRF OR MIDD OR MNGIE OR mitochondrial OR POLG OR TFAM OR OPA1 OR AGK OR MPV17 OR MFN2 OR FBXL4 OR TYMP OR ABAT OR DNA2 OR TWNK OR POLG2 OR SUCLA2 OR SUCLG1 OR SLC25A4 OR RRM2B OR TK2 OR RNASEH1 OR KSS OR DGUOK
1 AND 2
Preliminary searches were performed in order to refine the search terms above. Modified versions of this search strategy were used for other electronic databases. No language or date limitations were applied.
Data Extraction
Data were extracted into an Excel spreadsheet by the first reviewer (NZ) and checked by the second reviewer (YN). Our datasheet consisted of study characteristics, patient characteristics, audiological (primary) outcomes, satisfaction scales, quality assessment, and risk of bias. Missing data were sought, where possible, by email contact with the study authors. Summary data for each study is included in Table 1.
Table 1.
Study Characteristics
| References | Year | Country | Study type | Number of patients | Number of implants |
|---|---|---|---|---|---|
| Yamamoto et al. | 2015 | Japan | Case series | 5 | 5 (insertion not mentioned) |
| Sampaio de Oliveira et al. | 2017 | Brazil | Case series | 1 | 1 (unilateral left) |
| Li et al. | 2011 | China | Case report | 1 | 1 (insertion not mentioned) |
| Sudo et al. | 2011 | Japan | Case report | 1 | 1 (left) |
| Howes et al. | 2008 | UK | Case report | 1 | 1 (left) |
| Ulubil et al. | 2005 | UK | Case report | 1 | 1 (left) |
| Karkos et al. | 2004 | UK | Case series | 2 | 2 (1 left, 1 bilateral) |
| Manuso et al. | 2004 | Italy | Case report | 1 | 1 (left) |
| Counter et al. | 2001 | UK | Case report | 1 | 1 (left) |
| Raut et al. | 2002 | UK | Case report | 1 | 1 (left) |
| Yasumura et al. | 2003 | Japan | Case report | 1 | 1 (right) |
Risk of Bias Quality Scoring
The quality of the included studies was assessed independently by 2 reviewers (NZ and YN) using the 2012 risk-of-bias checklist for non-randomized studies by Brazzelli et al. [13] and the Oxford Centre for Evidence Based Medicine (OCEBM) 2011 Levels of Evidence 2.1 Grading system [14]. Two reviewers (NZ/YN) independently carried out the scoring using separate checklists, and discussion ensued to resolve disagreements. Any disagreements were resolved through discussion between the 2 review authors, and, where necessary, consultation with a wider author team (JM, PK, PM, MB).
Synthesis of Results
Study findings have been grouped based on the outcome scales used and the measures of CI efficacy. On assessment of the clinical and methodological heterogeneity of the studies, it was not deemed appropriate to conduct a meta-analysis.
RESULTS
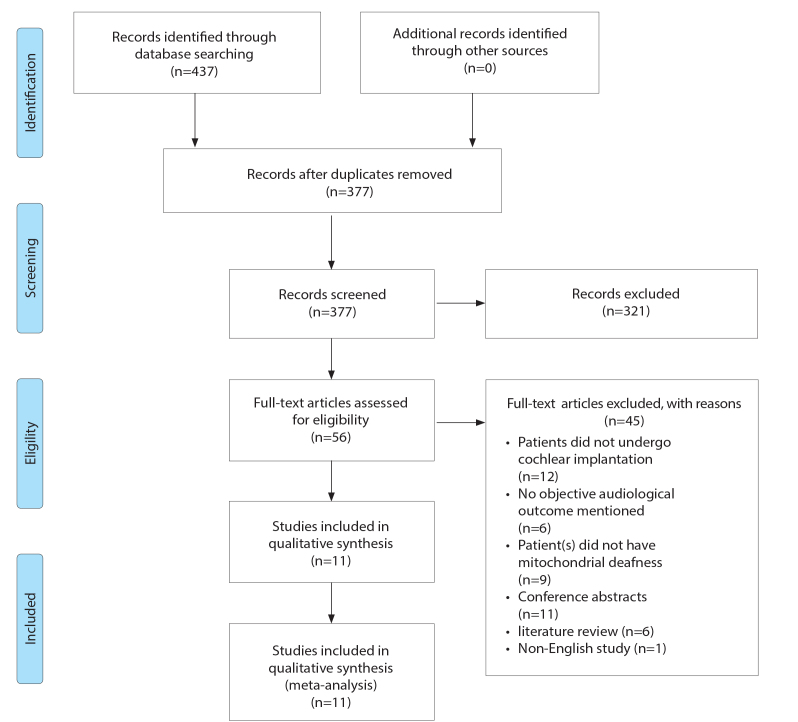
Searches were run on June 7, 2020. Figure 1 displays a flowchart detailing the study selection according to the PRISMA guidelines. The initial search yielded a total of 437 papers, and after deletion of duplicates 377 papers remained. Titles and abstracts were screened for their eligibility against our objectives (PICO), and this yielded 56 papers. We then screened the full papers according to our PICO, which resulted in 11 papers.
Figure 1.
Mitochondrial deafness PRISMA flowsheet.
Description of Studies
A total of 11 studies met the inclusion criteria with a total of 16 patients and 16 implants. There were 3 case series and 8 case reports. All studies were published between 2001 and 2017. Only 1 of the studies included pediatric patients exclusively, 9 included adult patients only, and 1 included both. No studies reported the average age of patients on the date of diagnosis of mitochondrial deafness or at CI. Of the studies, 2 described MELAS, 3 analyzed MIDD, and 1 analyzed A1555G, A3242G, OPA-1, and KSS.
Study characteristics are summarized in Table 1.
Quality of Studies
The methodological quality of the included studies was limited, predominantly consisting of case reports (n=11). A total of 10 studies were OCEBM grade 4, and 1 study was OCEBM grade 3. All studies were retrospective, and 8 studies included 1 patient only. There were limitations in the reporting of the surgical techniques, surgical complications, and outcomes relating to quality of life. Heterogeneity of reporting of audiological outcomes precluded a meta-analysis. Quality assessment of the studies is summarized in Table 2, using the Brazzelli risk of bias assessment.
Table 2.
Quality Assessment of Studies
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yamamoto et al. | ||||||||||||||||||
| Sampaio de Oliveira et al. | ||||||||||||||||||
| Li et al. | ||||||||||||||||||
| Sudo et al. | ||||||||||||||||||
| Howes et al. | ||||||||||||||||||
| Ulubil et al. | ||||||||||||||||||
| Karkos et al. | ||||||||||||||||||
| Manuso et al. | ||||||||||||||||||
| Counter et al. | ||||||||||||||||||
| Raut et al. | ||||||||||||||||||
| Yasumura et al. |
Green = yes (low risk of bias), red = no (high risk of bias), yellow = unclear (unclear risk of bias)
1. Were participants a representative sample selected from a relevant patient population?
2. Were the inclusion/exclusion criteria of participants clearly described?
3. Were participants entering the study at a similar point in their disease progression?
4. Was selection of patients consecutive?
5. Was data collection undertaken prospectively?
6. Were the groups comparable on demographics? *
7. Was the intervention (and comparison) clearly defined?
8. Was the intervention undertaken by someone experienced at performing the procedure?
9. Were the staff, place, and facilities where the patients were treated appropriate for performing the procedure (for example, access to back-up facilities in hospital or special clinic?)
10. Were any of the important outcomes considered (that is, clinical effectiveness, cost-effectiveness, or learning curves)?
11. Were objective (valid and reliable) outcome measures used, including satisfaction scale?
12. Was the assessment of main outcomes blind? *
13. Was follow-up long enough (≥1 year) to detect important effects on outcomes of interest?
14. Was information provided on non-respondents, dropouts?
15. Were the characteristics of withdrawals/dropouts similar to those who completed the study and therefore unlikely to cause bias?
16. Was length of follow-up similar between comparison groups? *
17. Were the important prognostic factors identified (for example, age, duration of disease, and disease severity)?
18. Were the analyses adjusted for confounding factors? *
questions 6, 12, 16, 18 are not applicable to case series and case reports.
Audiological Outcomes
Audiological outcomes are summarized in Table 3. The follow-up time ranged from 1 week to 12 months. A reliable average follow-up time could not be calculated as one study did not specify the date of preoperative assessment. A total of 7 different audiological outcome measures were used, excluding crude assessment. This included 1 outcome scale measuring speech perception – Ling test in the study by Sampiao de Oliviere et al. [15] In terms of sound detection, 8 studies measured pure tone audiometry pre- and postoperatively. Sound perception was additionally measured via the Hearing In Noise Test (HINT) in 3 studies [15–17], the Bamford-Kowal-Bench (BKB) sentence testing in 3 studies [18–20], the City University of New York (CUNY) sentences in 3 studies [16, 19, 21], and the Arthur Boothroyd words test in one study [19]. Speech perception was also measured using unnamed scales in 2 studies [11, 22]. Communication mode was mentioned in only 2 studies and was assessed using non-quantitative measures, whereby patients in both studies were mentioned to be able to lip-read before the operation [15, 21].
Table 3.
Audiological outcomes
| References | Patient demographics | Cause of mitochondrial deafness | Preoperative measures and results | Implanted device | Postoperative measures and results | Complications | Follow-up time |
|---|---|---|---|---|---|---|---|
| Yamamoto et al. | 1 male, 4 females | 3 patients with MELAS, 1 patient with MIDD, 1 unclassified patient | None recorded | Not mentioned | 4/5 patients: good performance in speech perception performance test after CI | None | Not recorded |
| Sampaio de Oliveira et al. | 25-year-old female | Kearns-Sayre syndrome | Communication mode: good vocabulary and oral language Audiometry: moderate hearing loss, moderately severe hearing loss above a frequency of 2000. Unable to conduct audiometry with hearing aid as patient did not give consent Speech perception: Ling test: detection of Ling test sounds for /a/, /u/, /i/ and /m/= 100% and for /s/ and /∫/ = 0%; Name discrimination = 100%; Discrimination of the question affirmation = 0%; Vocabulary extension = 44%; Identification of sentence length = 60% and sentence identification = 0% the left ear (LE), In the scores found were: detection of Ling test sounds for /a/, /i/, /u/ and /m/ = 100% and for /s/ and /∫/ = 0%; Name discrimination 90%; Discrimination = of the question /affirmation = 0%; Vocabulary extension = 28%; Identification of sentence length = 30% and sentence identification = 0%. |
Not mentioned | Audiometry: mild hearing loss Speech perception: patient detected all the sounds of the Ling test. SPT test: open set: 45% for the list of sentences Patient could not perform other speech tests as was not yet accustomed |
None | 1 month |
| Li et al. | 28-year old female | MNGIE | Audiometry: audiogram showed no threshold at all frequencies Speech perception: Chinese Hearing in Noise Test (HINT): 0% |
CI24RE freedom implant | Audiometry: mild hearing loss Speech perception: monosyllabic words discrimination test: 56%. Sentence discrimination test: 89% Non-qualitative measures: patient able to conduct simple telephone conversations with acquaintances, was “very satisfied” with results |
None | 3 months |
| Sudo et al. | 11-year-old female | m.625G>A mutation | Audiometry: audiogram showed profound hearing loss. Only minimally improved by hearing aids Speech perception: 0% (scale for outcome measure not mentioned) |
CI24RCS | Audiometry: significant improvement, hearing thresholds of almost 25–45 dB. Speech perception: score increased to “almost 100%” Non-qualitative measures: 20 months after surgery: patient and parents were impressed with improved communication |
Intraoperative complication: Grand-mal seizure |
1 month |
| Howes et al. | 33-year-old female | MIDD | Audiometry: progressive hearing loss Speech perception carried out by the Arthur Boothroyd test, bamford Kowal-bench (BKB) test, CUNY (City of New York) sentences test and lip reading: pre implant: 10% of phenomes and 0% for CUNY (lipreading) and 40% lipreading with left hearing aid |
Nucleus 24M cochlear implant | Audiometry: audiogram showed mild hearing loss. Speech perception: phonemes: 67%, words 47% BKB Non-qualitative measures: patient able to use telephone at 6 weeks and communicated well with her husband Crude speech perception: able to hear the television and could use an MP3 player |
None None |
1 week 1 month |
| Arif Ulubil. | 80-year-old female | A1555G mutation | Audiogram: bilateral profound SNHL, Speech perception: HINT score was 29% |
Nucleus 24M cochlear implant | Audiometry: no hearing loss (sound-field thresholds were 20–25 dB from 250 to 4,000 Hz). Speech perception: CUNY sentences test: 60%. HINT: 65% Non-qualitative measures: able to perceive sound even with background noise |
||
| Karkos et al. | 2 female patients: 31 and 45 years old | MELAS | Audiometry: Audiogram showed profound hearing loss. Sound perception: did not volunteer any responses to BKB sentences Non-qualitative measures: able to lip-read husband but not other people |
Clarion cochlear implant | Audiometry: audiogram showed mild hearing loss Speech perception: patient did not attend Non-qualitative measures: husband noticed considerable improvement in hearing. Patient able to follow speech more easily and recognized environmental sounds. Patient said that she was pleased with the results |
None | 12 months |
| Macuso et al. | 73-year-old male | A3243G Mutation | Audiometry: left ear near total deafness and profound right sensorineural hearing loss. Speech perception test: outcome scale not mentioned: no open set speech discrimination without use of lipreading and hearing aids |
Nucleus 24M cochlear implant | Audiometry: no hearing loss Speech perception test: 95% open-set words and phrases recognition, 100% phrases comprehension score |
None | 12 months |
| Counter et al. | 44-year-old male | Unknown mutation | CUNY sentences test: lip reading and aided: 5%, sentence tests - aided alone 0%, lip reading alone 4%, | Med-El Combi 401 | CUNY sentences test score: post implant 84%, sentence aided alone 57%, AB word test 61%, environmental sound recognition 83%, telephone interview 66% | None | 3 months |
| Raul et al. | 42-year-old woman | Audiometry: bilateral profound deafness Speech perception: BKB scores 0% auditory and lipreading |
Nucleus 24M cochlear implant | Audiometry: mild hearing loss at low frequencies Speech perception: post-implant BKB scores: auditory: 90%, auditory and lipreading 100% |
None | 9 months | |
| Yasumura et al. | 29-year-old female | MELAS | Audiometry: bilateral profound hearing loss Speech perception: measured using a Japanese word list. Speech recognition scores: 0% and 4% in open and closed set hearing respectively |
Nucleus 24M cochlear implant | Audiometry: mild hearing loss Speech perception: word recognition score: 72%, 95% in closed-set listening. Sentence recognition score: 34% in open set hearing |
None | 3 months |
All 11 studies reported beneficial outcomes on either objective or subjective measures. It is difficult to synthesize them into pooled outcomes as heterogeneous outcome measures are used. Most showed improvement, and details are presented in Table 2, most are single case reports. No preoperative data were reported by the study carried out by Yamamoto et al. [11] The long-term benefits of CI beyond 1 year could not be assessed because of the limited follow-up time.
Surgical Operation and Implant Details
Only 2 studies elaborated on surgical outcomes [20, 21]. In 1 study, 1 subject experienced a grand-mal seizure as an intraoperative complication. Counter et al. mentioned that the patient in their case report surgically underwent CI via posterior tympanotomy and cochleostomy and was the only study to describe the operative procedure. No other study outlined rehabilitation details or whether residual hearing was retained after the CI procedure. Implants used included the Nucleus 24 cochlear implant system (Cochlear Ltd, Sydney, Australia), the Clarion cochlear implant (Advanced Bionics, Zürich, Switzerland), the Nucleus 22 implant (Cochlear Ltd, Sydney, Australia),, and the Med-El Combi 401 system (Med-El, Innsbruck, Austria). Of the studies, 2 did not specify the type of implant used [11, 15].
Quality of Life Outcomes
No studies recorded both pre- and postoperative quality of life outcomes. Howes et al [19]. used the Glasgow Health status inventory and the Glasgow Benefit Plot in order to assess quality of life after CI. This was the only study to use a scale to comment upon the satisfaction of patients. Yasumura et al. [23] mentioned that 9 months after the CI surgery, the patient was “satisfied” with the improvement in communication because of the implant, and 3 other studies also mentioned satisfaction but did not elaborate further [17, 21, 22, 23].
Statistical Analysis
Because of the heterogeneity in reporting the outcome measures, time points, and lack of objective measures, a meta-analysis was not performed.
DISCUSSION
This systematic review and narrative synthesis reports on outcomes of CI in patients with mitochondrial deafness. To the best of the authors’ knowledge, this is the first systematic review on the topic. Our systematic searches identified 11 studies for inclusion. All the studies reported favorable outcomes following CI, with an improvement in sound detection and speech perception. Only 4 studies reported quality of life outcomes in the form of “patient satisfaction,” and 1 study used a qualitative scale for this. There was no deterioration in hearing after CI; however, the length of follow-up was relatively limited with follow-up periods ranging from 1 week to 12 months.
The average age of CI in patients with mitochondrial deafness ranged from 11 to 80 years, and most patients were women. This constituted a total sample size of 16 patients. Previous reviews of CI in mitochondrial disorders did not elaborate on the characteristics of the study population [3, 7, 12]. No studies in this review detailed how their patients were chosen, which raises the risk of selection bias. It is important to note that the small total number of patients reported may not be representative of the entire population of patients with mitochondrial disease.
All studies, except the one by Sampaio de Oliveira et al. and Karkos et al. stated that genetic analysis was used in confirming the diagnosis of mitochondrial disorder in causing sensorineural hearing loss [15, 20]. Yamamoto et al. [11] mentions that the patients were diagnosed by neurologists based on genetic tests, biopsies, MRI, and clinical symptoms. Jia-nan et al. [17] conducted an MRI, genetic testing, and muscle biopsy. Clinical symptoms were also considered by a team of neurologists. In studies by Sudo et al. [22], Counter et al. [21], and Macuso et al. [23], genomic sequencing was used to diagnose mitochondrial disease. Howes et al. [19] confirmed diagnosis via MRI and leucocyte DNA analysis. Ulubil et al. [16] and Raut et al. [18] also confirmed the diagnosis via leucocyte DNA analysis. Although this range of diagnostic approaches represents the varied patterns of clinical practices across the world for these rare conditions, they do allow us to be confident that patients described in these studies did indeed have the disorders of interest.
Of the 11 studies, 9 reported favorable outcomes in sound detection via pure tone audiometry, with significant improvement from profound-moderate hearing loss to mild-no hearing loss. Karkos et al. [20] and Manuso et al. [23] conducted a follow-up period of review of the cohort of patients, which lasted 12 months, which implies that there is at least medium-term beneficial effect from CI. However, larger and longer scale studies are needed to draw any formal conclusions [18].
With regards to speech perception, all studies that measured it via a continuous scale measure found a significant improvement. Karkos et al. [20] were not able to elicit any responses to the speech perception test before the CI cochlear implantation operation; and therefore, a direct comparison could not be made. The tests used to measure speech perception, such as HINT, BKB, and CUNY are all standardized methods, therefore affirming the validity of the improvement in speech perception, but very difficult to compare with each other for a true meta-analysis.
Only 1 study used a satisfaction quality of outcomes scale, and this did not assess preoperative quality of life [22]. Therefore, although CI may be a viable option for improving hearing loss from an audiological point of view, other factors such as complications, discomfort, and day-to-day living are not considered.
The sample was too small to conduct a subgroup analysis on whether CI was more effective in improving outcomes for 1 mitochondrial disorder than others. Furthermore, there was no analysis into which brand of cochlear implant gave better results. Owing to the lack of information in the studies summarizing the method of surgical implantation, rehabilitation details, and conduction of electro-acoustic stimulation; it was difficult to analyze whether these factors are important in improving outcomes of CI. Yamamoto et al. [11] mentions the risk of malignant hyperthermia when describing surgical findings during CI, although there was none with his cohort.
Like many rare diseases, it is recommended that centers collaborate to register and test patient outcomes in standardized testing formats so that more robust conclusions about cochlear outcome results can be drawn.
CONCLUSION
Hearing outcomes following CI in mitochondrial deafness are generally good, showing improvements in speech perception and sound detection. This is supported by our larger volume of studies and a more systematic approach but is in keeping with a previous (2010) review on their efficacy in these disorders [12].
The quality of evidence from which to draw conclusions is suboptimal, with only 11 studies found from 4 international databases ranging from OCEBM evidence levels 3 to 4. This highlights the need for studies which are of a higher methodological quality and also for longer follow-up outcomes. There was very limited information available on secondary outcomes such as surgical complications, quality of life, and neurological impairments that might complicate CI use. However, it appears that CI is a worthwhile and effective intervention for patients with profound sensorineural hearing loss because of mitochondrial dysfunction, in improving speech perception, detection, and possibly satisfaction. The rate of complications is low with only 1 patient undergoing a seizure during the perioperative period and no other described complications.
A significant opportunity for ongoing research in this area exists, for example exploring specific mutations and patient characteristics, to determine when CI should be recommended in these patient groups and when it is most cost-effective. Larger studies are needed to provide a higher level of evidence, and external factors should be considered when counseling patients regarding the benefits and risks of CI in mitochondrial disease. Given the rarity of these conditions, this would best be performed in an international approach underpinned by a common dataset.
MAIN POINTS.
This review identified 11 studies of cochlear implantation in mitochondrial deafness which met the inclusion criteria.
Studies described MELAS (2), MIDD (3), and 1 each analyzed A1555G, A3242G, OPA-1, and KSS.
The methodological quality of included studies was limited, consisting of case reports and small volume case series. Ten studies were OCEBM grade IV and one study was grade III.
Multicenter longitudinal studies with standardized comprehensive outcome measures including through health-related quality of life data will be key in establishing a better understanding of short and long-term post-CI outcomes.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – J.M., P.M., M.B.; Design – J.M., P.M., M.B.; Supervision – J.M., P.K., P.M., M.B.; Resource - J.M., P.K., P.M., M.B.; Materials - J.M., P.K, P.M., M.B.; Data Collection and/or Processing – N.Z., Y.N.; Analysis and/orInterpretation – N.Z., Y.N., P.K., J.M., P.M., M.B.; Literature Search – N.Z., Y.N.;Writing – N.Z., Y.N.; Critical Reviews – J.M., P.K., P.M., M.B.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Alston C, Rocha M, Lax N, Turnbull D, Taylor R. The genetics and pathology of mitochondrial disease. J Pathol. 2016;241:236–50. doi: 10.1002/path.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schapira A. Mitochondrial disease. The Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- 3.Kokotas H, Petersen M, Willems P. Mitochondrial deafness. Clin Genet. 2007;71:379–91. doi: 10.1111/j.1399-0004.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 4.Chinnery PF. Mitochondrial Disorders Overview. 2000 Jun 8 [Updated 2014 Aug 14] In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 1993–2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1224/ [Google Scholar]
- 5.Schaefer A, Taylor R, Turnbull D, Chinnery P. The epidemiology of mitochondrial disorders-past, present and future. Biochimica et Biophysica Acta. 2004;1659:115–20. doi: 10.1016/j.bbabio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Newkirk J, Taylor R, Howell N, Bindoff L, Chinnery P, Alberti K, et al. Maternally inherited diabetes and deafness: prevalence in a hospital diabetic population. Diabet Med. 1997;14:457–60. doi: 10.1002/(SICI)1096-9136(199706)14:6<457::AID-DIA372>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer G, Majamaa K, Turnbull D, Thorburn D, Chinnery P. Treatment for mitochondrial disorders. Cochrane Database Syst Rev. 2012;2012:CD004426. doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh S, Goldstein A, Koenig M, Scaglia F, Enns G, Saneto R, et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med. 2014;17:689–701. doi: 10.1038/gim.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurt B, Topal T. Mitochondrial Disease. Dis Mol Med. 2013;1:11. doi: 10.5455/dmm.20130107125901. [DOI] [Google Scholar]
- 10.Adigun O. Depression and Individuals with Hearing Loss: A Systematic Review. J Psychol Psychother. 2017;7:323. doi: 10.4172/2161-0487.1000323. [DOI] [Google Scholar]
- 11.Yamamoto N, Okuyama H, Hiraumi H, Sakamoto T, Matsuura H, Ito J. The Outcome of Cochlear Implantation for Mitochondrial Disease Patients with Syndromic Hearing Loss. Otol Neurotol. 2015;36:e129–33. doi: 10.1097/MAO.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 12.Vivero R, Fan K, Angeli S, Balkany T, Liu X. Cochlear implantation in common forms of genetic deafness. Int J Pediatr Otorhinolaryngol. 2010;74:1107–12. doi: 10.1016/j.ijporl.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazzelli MC. Collagenase clostridium histolyticum for the treatment of Dupuytren’s contracture. [Place of publication not identified]: NIHR Journals Library. 2015 [Google Scholar]
- 14.OCEBM Levels of Evidence - CEBM [Internet] CEBM; 2020. [cited 23 July 2020]. Available from: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/ [Google Scholar]
- 15.Oliveira L, Brosco K, Oliveira E, Alvarenga K. Cochlear implant in Kearns-Sayre syndrome: case study of twin sisters. Audiol Commun Res. 2017;22:e1841. doi: 10.1590/2317-6431-2017-1841. [DOI] [Google Scholar]
- 16.Ulubil S, Furze A, Angeli S. Cochlear implantation in a patient with profound hearing loss with the A1555G mitochondrial DNA mutation and no history of aminoglycoside exposure. J Laryngol Otol. 2005;120:230–2. doi: 10.1017/S002221510500318X. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Han D, Ji F, Chen A, Wu N, Xi X, et al. Successful cochlear implantation in a patient with MNGIE syndrome. Acta Oto-Laryngologica. 2011;131:1012–6. doi: 10.3109/00016489.2011.579623. [DOI] [PubMed] [Google Scholar]
- 18.Raut V, Sinnathuray A, Toner J. Cochlear implantation in maternal inherited diabetes and deafness syndrome. J Laryngol Otol. 2002;116:373–5. doi: 10.1258/0022215021910825. [DOI] [PubMed] [Google Scholar]
- 19.Howes T, Madden C, Dasgupta S, Saeed S, Das V. Role of mitochondrial variation in maternally inherited diabetes and deafness syndrome. J Laryngol Otol. 2008;122:1249–52. doi: 10.1017/S0022215108001503. [DOI] [PubMed] [Google Scholar]
- 20.Karkos P, Anari S, Johnson I. Cochlear implantation in patients with MELAS syndrome. Eur Arch Otorhinolaryngol. 2004;262:322–4. doi: 10.1007/s00405-004-0817-x. [DOI] [PubMed] [Google Scholar]
- 21.Counter P, Hilton M, Webster D, Wardell T, Taylor R, Besley G, et al. Cochlear implantation of a patient with a previously undescribed mitochondrial DNA defect. J Laryngol Otol. 2001;115:730–2. doi: 10.1258/0022215011908766. [DOI] [PubMed] [Google Scholar]
- 22.Sudo A, Takeichi N, Hosoki K, Saitoh S. Successful cochlear implantation in a patient with mitochondrial hearing loss and m.625G>A transition. J Laryngol Otol. 2011;125:1282–5. doi: 10.1017/S0022215111002453. [DOI] [PubMed] [Google Scholar]
- 23.Yasumura S, Aso S, Fujisaka M, Watanabe Y. Cochlear Implantation in a Patient with Mitochondrial Encephalopathy, Lactic Acidosis and Stroke-like Episodes Syndrome. Acta Otolaryngol. 2003;123:55–8. doi: 10.1080/0036554021000028081. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso M, Filosto M, Forli F, Rocchi A, Berrettini S, Siciliano G, et al. Anon-syndromic hearing loss caused by very low levels of the mtDNA A3243G mutation. Acta Neurol Scand. 2004;110:72–4. doi: 10.1111/j.1600-0404.2004.00254.x. [DOI] [PubMed] [Google Scholar]