Abstract
OBJECTIVES
To identify the cochlear segment in which spiral ganglion neuron (SGN) loss may more severely impact discrimination thresholds.
MATERIALS and METHODS
Thirteen temporal bones from 13 subjects between 55 and 77 years of age were analyzed. The organ of corti was analyzed to identify the loss of hair cells, and the number of SGNs in each cochlear segment were counted. The results of the speech perception test (SPT) and pure tone audiometry (PTA) tests were collected. PTA averages for low and high frequencies were calculated. One-way analysis of variance (ANOVA), Pearson, Spearman, and multilinear regression tests were performed.
RESULTS
No statistically significant correlation was identified between the patient’s age and number of SGNs. Statistically significant differences were observed between the number of SGNs in the different cochlear segments (one-way ANOVA: p<0.0001) and between poor PTA average and SPT scores (negative correlation) (p=0.03). A statistically significant correlation was identified between the overall number of cochlear SGNs and SPT scores (p=0.02) and between the number of SGNs in cochlear segments I (p=0.04) and II and the SPT score (p=0.03).
CONCLUSIONS
We identified that residual SGNs in the basal and middle turns of the cochlea might be determinants of speech perception.
Keywords: Speech perception, spiral ganglions, cochlea segmentation, word understanding
INTRODUCTION
The number of spiral ganglion neurons (SGNs) is a fundamental element for predicting cochlear implant (CI) outcomes after surgery [1], although controversial results have been reported [2]. SGNs progressively die with increase in age and the phenomenon affect all segments of the cochlea [3]. Each cochlear segment contains cells deputed to discrimination of specific frequencies [4] and the correct stimulation of each segment is fundamental to correctly identifying sounds and words [4–6].
The importance of preserving low-frequency hearing for the role that these frequencies have on speech perception and understanding is still controversial [7,8], as it is the relationship between residual SGNs and speech perception score, and the impact of electrode penetration on the discrimination ability [1,2].
To date, the effect of spiral ganglion neuron (SGN) distribution in the different cochlea segments on speech perception are still unclear. In this study we analyzed the temporal bones (TB) of elderly subjects with normal hearing function and compared the SGNs using their pure tone audiometry (PTA) and speech perception test (SPT) results to evaluate the potential correlation between the number of SGNs and SPT score and to identify which of the four cochlear segments contain SGNs that have a predominant role in the word recognition process.
MATERIALS AND METHODS
Temporal bones available in a university temporal bone permanent laboratory were analyzed to identify a sample of subjects over 50 years of age for whom the results of auditory and word recognition tests were available. Sample collection and human specimen storage were done in accordance with the Declaration of Helsinki. The TBs studied were prepared using the same standardized protocol [9].
The study was approved as a retrospective study on preexisting specimens by the local Internal Review Board.
The inclusion criteria were age over 50 years, good condition of the specimen, presence of normal inner hair cells (IHC) and outer hair cells (OHC) for the age, availability of PTA and SPT results performed within a year before death, no previous ear surgery.
The following data were collected for each subject: side of temporal bone studied, PTA results in the 250–8000 Hz frequency range, SPT (recorded as a percentage of correctly repeated words after being read a word list in a silent cabin), and the cause of death. Because results of PTA and SPT were available for each subject, we collected details about the auditory threshold (identified by PTA) and the SPT scores, by including only the results of the ear that we analyzed; for example, if we study a right TB we only included auditory tests of this side.
Pure tone audiometry average was calculated for low (250, 500, and 1000 Hz) and high frequencies (2000, 4000, and 8000 Hz). The averages were calculated to compare them with the SG number in segments I, II, III, and IV.
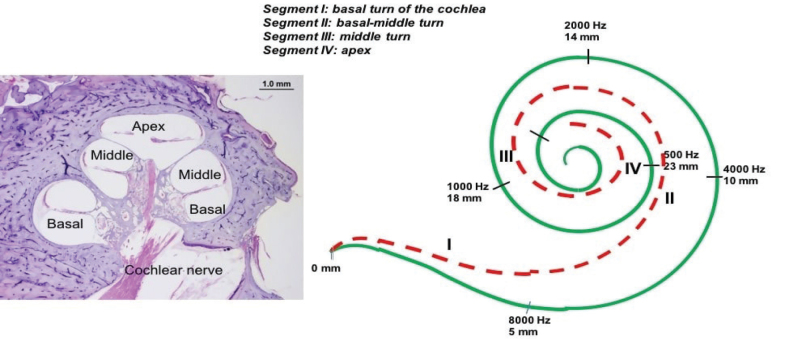
All TBs included in the study were studied with light microscopy (Olympus CH2; Olympus- lifescience, Shinjuku, Tokyo, Japan) to identify the organ of Corti, in which the IHC, OHC and SGNs were counted. An ocular grid and 20x magnification were used to facilitate counting [10]. The SGNs were counted manually in each section of the cochlea from the basal turn (segment I) to the apex (segment IV). The cochlea was divided into four segments as follows: a) segment I, from the hook region halfway to the basal turn; b) segment II, from the end of segment I until the beginning of the cochlear middle turn; c) segment III, corresponding to the middle turn of the cochlea; d) segment IV, corresponding to the cochlear apex. Total neuronal count per cochlea was then estimated by multiplying SGNs by 10 to account for intervening sections and by 0.91 to correct for dual-counting of nuclei spanning section boundaries based on the current recommendation.
Statistical Analysis
Stata® (StataCorp LLC, 4905 Lakeway Drive, College Station, Texas 77845-4512, USA) was used to analyze the data. Patient age and number of SGNs were correlated using the Pearson Correlation Coefficient. The total number of SGNs (segment I, II, III, and IV) were correlated with age, and the same correlation analysis (age and SGNs) was performed for each segment. The correlation between patient age and the SPT score was measured by the Pearson correlation. The low and the high-frequency PTA results were correlated separately with the SPT scores using the Pearson and Spearman correlations. A one-way analysis of variance (ANOVA) was performed to identify a statistically significant difference in the SGN number between each segment, and a post hoc Bonferroni-Holm (BH) test was used to identify the details of these differences. The Pearson test was used to identify a correlation between the loss of SGNs in segment I (basal turn) and segment IV (apex of cochlea). Multiple linear regression models were used to correlate the SGNs in segments I and II with the high-pure tone average, and the SGNs in segments III and IV with low-pure tone average. The same correlation was performed to evaluate the relationship between SGNs in each segment and the auditory threshold at a specific frequency; the number of SGNs in segment I was compared with the results of PTA at 8000 Hz and that in segment II with 4000 Hz. Finally, the Pearson correlation coefficient was used to correlate the total number of SNGs in all segments and the SPT scores, and the number of SNGs in the first segment were correlated with the SPT; this analysis was performed for each segment. The Spearman correlation was used to identify the correlation trend between the data and to confirm the results of the Pearson test when they were statistically significant. p<0.05 was considered to be significant.
RESULTS
Thirteen TBs from 13 adult patients (age range, 52–77 years; median age, 64.4 years) met the inclusion criteria and were included in the study; of these, seven were right TB and six left TB. All the TB included in the study were extracted within 24 hours of death (average postmortem time, 11 hours; range, 2.30–18 hours) and were prepared and processed as described above to guarantee homogeneity (Table 1).
Table 1.
SNGs, SPT, and the auditory threshold of each subject who was included in the study
| Patients | Age | Spiral ganglions in each segment | Speech (%) | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | Total | |||
| 1 | 69 | 2340 | 9572 | 5646 | 6043 | 23601 | 100 |
| 2 | 50 | 2574 | 7259 | 3793 | 5409 | 19035 | 100 |
| 3 | 50 | 2776 | 6743 | 3533 | 4446 | 17498 | 100 |
| 4 | 77 | 1965 | 5338 | 3545 | 2968 | 13816 | 92 |
| 5 | 77 | 1520 | 3927 | 2994 | 2309 | 10750 | 74 |
| 6 | 70 | 1935 | 6035 | 4279 | 4530 | 16779 | 90 |
| 7 | 74 | 1819 | 6481 | 3315 | 3778 | 15753 | 96 |
| 8 | 74 | 2407 | 8856 | 4387 | 5094 | 20744 | 80 |
| 9 | 60 | 1016 | 3695 | 2128 | 2815 | 9654 | 80 |
| 10 | 60 | 1904 | 5824 | 3386 | 4097 | 15211 | 80 |
| 11 | 63 | 758 | 5785 | 4103 | 4732 | 15378 | 88 |
| 12 | 52 | 2156 | 7687 | 4359 | 3641 | 17843 | 100 |
| 13 | 52 | 2448 | 7405 | 4862 | 4563 | 19278 | 100 |
SNGs: spiral ganglion neurons; SPT: speech perception test.
All data were normally distributed. There was no statistically significant correlation between age and the total number SGNs (Pearson: p=0.07), and between the subjects’ ages and spiral ganglions in segment I (Pearson: p=0.28), segment II (Pearson: p=0.57), segment III (Pearson: p=0.81), or segment IV (Pearson: p=0.35).
Statistically significant differences in the SGN number between the four cochlear segments were identified (one-way ANOVA: p<0.0001); differences were particularly significant between segments I and II (BH: p<0.0001), segments I and III (BH: p<0.0001), segment I and IV (BH: p<0.0001), segment II and III (BH: p<0.0001), and segments II and IV (BH: p<0.0001). No statistically significant differences were observed between SGNs in cochlear segments III and IV (BH: p=2.9). Despite the differences in the number of cells between the four segments, no significant correlation was identified between the number of SGNs present in segment I (basal turn of the cochlea) and those in segment IV (apex of the cochlea) (Pearson: p=0.09).
High-frequency PTA average and SPT scores were negatively and significantly correlated (Pearson: p=0.03; rs: −0.615, p=0.02), while no correlation was found between the low-frequency PTA average and the SPT scores (Pearson: p=0.7). Multiple linear regression analysis showed that the number of SGNs in segments I and II were not predictive of the results of high-frequency PTA average (p=0.07; r=0.50; F=3.81), and the SGNs in III and IV were not predictive of the results of low-frequency PTA average (p=0.2; r=0.03; F=1.42)
To confirm the PTA average results, we separately correlated each specific cochlear segment with the corresponding tonotopic frequency threshold (Figure 1) to investigate the correlation between the SGN number and the auditory threshold. There was no statistically significant correlation between SGNs in segment I and the auditory threshold at 8000 Hz (Pearson: p=0.22), between those in segment II and 4000 Hz (Pearson: p=0.15) or between those in segment II and 2000 Hz (Pearson: p=0.11). No statistically significant differences were found by comparing the SGNs in segment III and auditory thresholds at 2000 Hz (Pearson: p=0.29) or between those in segment III and 1000 Hz (Pearson: p=0.34). There was also no statistically significant correlation between the SGNs in segment IV and the auditory threshold at 500 Hz (Pearson: p=0.054) or between those in segment IV and 250 Hz (Pearson: p=0.15).
Figure 1.
The drawing (image on the right) illustrates the tonotopic distribution of the hearing frequencies in a human cochlea (figure on the left).
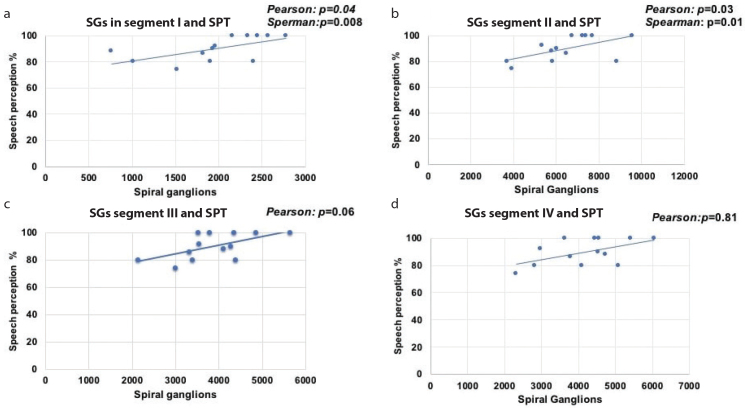
We observed a statistically significant positive correlation between the total number of SGNs (segment I to IV) and the SPT scores (Pearson: p=0.02; Spearman: rs: 0.642, p=0.01; Figure 2). By analyzing each segment and the SPT, we observed a positive significant correlation between the SGNs in segment I and SPT scores (Pearson: p=0.04; Spearman: rs: 0.693, p=0.008) (Figure 3a) and SGNs in segment II and SPT scores (Pearson: p=0.03; Spearman: rs: 0.642, p=0.01) (Figure 3b), but there was no correlation between the SGNs in segment III and SPT scores (Pearson: p=0.06; Figure 3c) or SGNs in segment IV and SPT scores (Pearson: p=0.81; Figure 3d).
Figure 2.
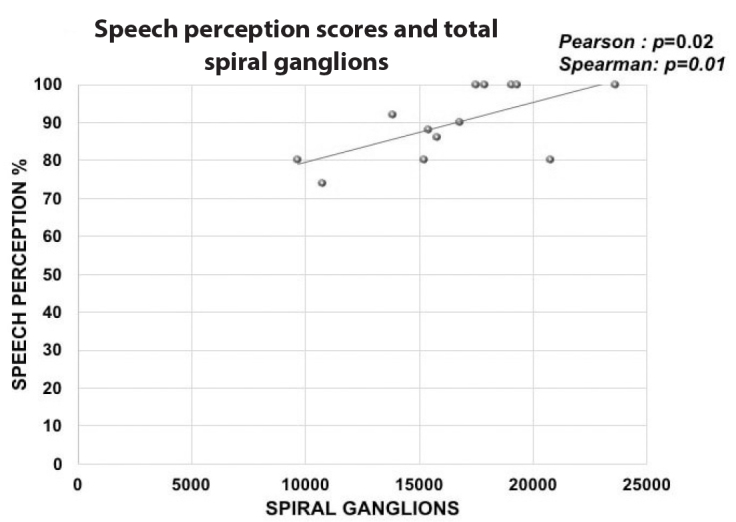
The graphic shows the linear correlation between the entire number of spiral ganglions identified in the cochlea and the results of the speech perception test.
Figure 3. a–d.
Four graphics illustrate the linear correlation between the spiral ganglions in segment I and II and the score of the speech perception test. In case of segments I and II, the correlation was statistically significant (a and b); in case of segments III and IV, despite the relationship between the number of spiral ganglion neuron and the scores of speech perception appears linear, the correlation was not statistically significant (c and d).
DISCUSSION
Based on the results of this study, we did not observe a direct correlation between subjects’ age and number of SGNs; this evidence is in contrast with the Schuknecht theory, supporting SGN death because of aging [3]. However, several subjects included in our study died of cancer and were treated with toxic drugs that could affect SGN survival [11–13] and account for the absence of correlation between the number of SGNs and the patients’ ages as observed in this study.
The distribution of SGNs among the four segments of the cochlea (I to IV) was significantly different. Although the basal turn (segments I and II) of the cochlea contained very few SGNs, the loss of these cells in these segments did not impact the residual number of SGNs in the apical turn of the cochlea (segments III and IV).
The number of residual SGNs in each segment was not correlated with the respective auditory threshold for each frequency. This confirmed the limitation of PTA as a single test to investigate the hearing capacity of patients during clinical investigations [14–17]; in fact, PTA investigates only hair cell function, and that has been shown to be not correlated with the number of residual SGNs in the cochlea [18,19].
Finally, the total number of residual SGNs in the cochlea (segments I to IV) was positively correlated with SPT scores. In particular, the SGNs in segments I and II [20] were correlated with SPT scores; this evidence showed that normal discrimination of frequency between 2000 and 8000 Hz is fundamental to the understanding of words [21].
The number of SGNs is a relevant point in CI surgery [20,22], as their preservation is fundamental to achieve the best hearing outcomes [21,23]. Currently, SGN preservation in the cochlea is considered by looking at the entire structure without focusing on a specific segment. Theoretically, preservation of the SGNs in the middle (<3000 Hz) and apical (500 Hz) cochlear turns [22, 23] should be fundamental to discriminating frequencies in the speech range [24]. On the contrary, we identified a correlation between SGNs in segments I and II and SPT scores, which suggests the importance of correct high-frequency discrimination in speech perception. This evidence is in accordance with Di Stadio et al [8] study, in which the authors have shown that children with cochlear malformation (in which all SGNs are located in the basal turn) undergoing CI could obtain the same results as children with normal cochlea as evidenced by the comparison of their auditory brainstem responses [8,25].
We speculate that our results might be helpful for CI surgery. Currently, we know that insertion of the electrode up to the apex of the cochlea may present some risks [26]; so, if the basal and the beginning of middle turn of the cochlea are more relevant than the apex to correctly discriminate speech, a shorter electrode could be a good option, especially in patients who present residual hearing. In fact, these patients generally have the low frequencies preserved; so the short electrode placed in the I and II turn of the cochlea might directly stimulate the areas where the hair cells are missing by combining bionic stimulation with the natural preserved functions [27,28].
Study Limitations
This study presents some limitations. The major limitation is the small sample size; this is a common limitation of these studies because of the limited availability of TB [1,3,27,28]. In addition, the condition of the central auditory pathways was not evaluated through magnetic resonance imaging, limiting the possibility of determining if central involvement had an impact on SPT scores. Another important limitation is that all subjects died of cancer or of cardiovascular diseases and were under multiple medical treatments, including specific ototoxic drugs that might have had an effect on the inner ear.
CONCLUSION
Our study showed that residual SGNs in the I and II turns of the cochlea might be relevant for speech perception and that these cells perform their discrimination function independent of the function of the organ of Corti. Additional studies on larger samples are necessary to confirm our preliminary results.
MAIN POINTS.
The preservation of a sufficient number of spiral ganglions is fundamental in cochlear implant surgery because these cells are necessary to correctly discriminate the sounds.
The results of speech perception test are generally related to the auditory thresholds, indicating that a good function of hair cells is determinant to correctly understand the speech.
Only high-frequency loss impacts the SPT scores, while low-frequency loss doesn’t affect the speech perception.
Loss of spiral ganglion neurons in each segment of the cochlea is independent from wellness of the hair cells in the same segment.
Only the reduction of SNG number in the basal turn of the cochlea affects the SPT scores, confirming that preservation of these cells in the I and II turn is fundamental for obtaining satisfactory auditory results after CI.
Acknowledgements
Thanks to Professor Nadol Director of Temporal Bone Bank of MEEI and to all staff members of temporal bone laboratory of Massachusetts Eye and Ear Infirmary for their support. Thanks to Dr Felipe Santos who introduced me in this interesting topic.
Footnotes
Ethics Committee Approval: The study was approved by IRB of the Temporal Bone Bank of MEEI in Boston.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.D.S.; Design - A.D.S.; Supervision - A.D.S., G.R.; Resource - A.G.; Materials - A.D.S., A.d.V., F.K.; Data Collection and/or Processing - A.D.S., A.d.V., M.R.; Analysis and/or Interpretation - A.D.S., M.R.; Literature Search - A.D.S., F.K., M.R.; Writing - A.D.S., A.d.V., M.R.; Critical Reviews - A.G.,. G.R.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Seyyedi M, Viana LM, Nadol JB., Jr Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol Neurotol. 2014;35:1446–50. doi: 10.1097/MAO.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB., Jr Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115:672–7. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- 3.Schuknecht HF. Presbycusis. Trans Am Laryngol Rhinol Otol Soc. 1955:401–18. doi: 10.1288/00005537-195506000-00002. discussion, 419–20. [DOI] [PubMed] [Google Scholar]
- 4.Di Stadio A. Which factors to induce hearing loss in professional musicians? Extensive literature review and histopathology findings can answer it. HBC. 2017;15:63–7.1. doi: 10.1080/21695717.2017.1311505. [DOI] [Google Scholar]
- 5.Di Stadio A, Messineo D, Ralli M, Roccamatisi D, Musacchio A, Ricci G, et al. The impact of white matter hyperintensities on speech perception. Neurol Sci. 2020 doi: 10.1007/s10072-020-04295-8. doi: 10.1007/s10072-020-04295-8. [DOI] [PubMed] [Google Scholar]
- 6.Keithley EM. Pathology and mechanisms of cochlear aging. J Neurosci Res. 2019 doi: 10.1002/jnr.24439. doi: 10.1002/jnr.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dincer D’Alessandro H, Mancini P. Perception of lexical stress cued by low-frequency pitch and insights into speech perception in noise for cochlear implant users and normal hearing adults. Eur Arch Otorhinolaryngol. 2019;276:2673–80. doi: 10.1007/s00405-019-05502-9. [DOI] [PubMed] [Google Scholar]
- 8.Di Stadio A, Dipietro L, De Lucia A, Trabalzini F, Ricci G, Martines F, et al. E-ABR in patients with cochlear implant: a comparison between patients with malformed cochlea and normal cochlea. J Int Adv Otol. 2019;15:215–21. doi: 10.5152/iao.2019.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuknecht HF. Pathology of the Ear. 2nd ed. New York, NY: Lea and Febiger; 1993. [Google Scholar]
- 10.Di Stadio A, Ralli M, Ishai R, D’Ascanio L, Trabalzini F, Della Volpe A, et al. Nucleolus vs Nucleus Count for Identifying Spiral Ganglion in Human Temporal Bone. J Int Adv Otol. 2018;14:181–9. doi: 10.5152/iao.2018.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fetoni AR, De Bartolo P, Eramo SL, Rolesi R, Paciello F, Bergamini C, et al. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase inantioxidant defense. J Neurosci. 2013;33:4011–23. doi: 10.1523/JNEUROSCI.2282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baiduc RR, Poling GL, Hong O, Dhar S. Clinical measures of auditory function: the cochlea and beyond. Dis Mon. 2013;59:147–56. doi: 10.1016/j.disamonth.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer JS, Batts SA, Chu KK, Sahin MI, Leung HM, Tearney GJ, et al. Micro-optical coherence tomography of the mammalian cochlea. Sci Rep. 2016;6:33288. doi: 10.1038/srep33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyatepe-Coo EF, Park MA. Identifying the Role of the Spiral Ganglion in Speech Discrimination. Hear. 2015;19:1–2. doi: 10.1097/01.HJ.0000466874.97809.89. [DOI] [Google Scholar]
- 16.Ralli M, Greco A, De Vincentiis M, Sheppard A, Cappelli G, Neri I, Salvi R. Tone-in-noise detection deficits in elderly patients with clinically normal hearing. Am J Otolaryngol. 2019;40:1–9. doi: 10.1016/j.amjoto.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvi R, Ding D, Jiang H, Chen GD, Greco A, Manohar S, Sun W, Ralli M. Hidden Age-Related Hearing Loss and Hearing Disorders: Current Knowledge and Future Directions. Hearing Balance Commun. 2018;16:74–82. doi: 10.1080/21695717.2018.1442282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci. 2012;32:405–10. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linthicum FH, Jr, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–22. doi: 10.1097/MAO.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Euteneuer S, Hansen S, Ryan AF. The role of the spiral ganglion neurons in cochlear implants. Today and in future regenerative inner ear treatment. HNO. 2008;56:457–60. doi: 10.1007/s00106-008-1709-y. [DOI] [PubMed] [Google Scholar]
- 21.Rask-Andersen H, Liu W, Erixon E, Kinnefors A, Pfaller K, Schrott-Fischer A, et al. Human cochlea: anatomical characteristics and their relevance for cochlear implantation. Anat Rec (Hoboken) 2012;295:1791–811. doi: 10.1002/ar.22599. [DOI] [PubMed] [Google Scholar]
- 22.Frijns JH, Briaire JJ, Grote JJ. The importance of human cochlear anatomy for the results of modiolus-hugging multichannel cochlear implants. Otol Neurotol. 2001;22:340–9. doi: 10.1097/00129492-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 24.Dejonckere PH. Acoustic analysis of voice production. Production trial from a clinical perspective. Acta Otorhinolaryngol Belg. 1986;40:377–85. [PubMed] [Google Scholar]
- 25.Lee J, Nadol JB, Jr, Eddington DK. Depth of electrode insertion and postoperative performance in humans with cochlear implants: a histopathologic study. Audiol Neurootol. 2010;15:323–31. doi: 10.1159/000289571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quesnel AM, Nakajima HH, Rosowski JJ, Hansen MR, Gantz BJ, Nadol JB., Jr Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear Res. 2016;333:225–234. doi: 10.1016/j.heares.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seyyedi M, Nadol JB., Jr Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol. 2014;35:1545–51. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamakura T, O’Malley JT, Nadol JB., Jr Preservation of Cells of the Organ of Corti and Innervating Dendritic Processes Following Cochlear Implantation in the Human: An Immunohistochemical Study. Otol Neurotol. 2018;39:284–93. doi: 10.1097/MAO.0000000000001686. [DOI] [PMC free article] [PubMed] [Google Scholar]