Abstract
Background
Disturbance of networks was recently proposed to be associated with the occurrence of depression in Parkinson’s disease (PD). However, the neurobiological mechanism of depression underlying PD remains unclear.
Objective
This study was conducted to investigate whether intra-network and inter-network brain connectivity is differently changed in PD patients with and without depression (PDD and PDND patients, respectively).
Methods
Forty-one PDD patients, 64 PDND patients, and 55 healthy controls (HCs) underwent resting-state functional magnetic resonance imaging (fMRI). The default mode network (DMN), executive control network (ECN), salience network (SN), precuneus network (PCUN), and sensorimotor network (SMN) were extracted using independent component analysis (ICA), and then the functional connectivity (FC) values within and between these networks were measured.
Results
PDD patients exhibited abnormal FC values within the DMN, ECN, SN, PCUN, and SMN. In addition, PDD patients demonstrated decreased connectivity between anterior SN (aSN) and bilateral ECN, between posterior SN (pSN) and dorsal DMN (dDMN), and between PCUN and dDMN/SMN/bilateral ECN. Connectivity within the left hippocampus of dDMN and the right medial superior frontal gyrus of aSN was a significant predictor of depression level in PD patients.
Conclusions
Aberrant intra- and inter-network FC is involved in several important hubs in the large-scale networks, which can be a biomarker for distinguishing PDD from PDND.
Keywords: Parkinson’s disease, depression, independent component analysis, triple-network model, resting state
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease (Mason et al., 2014); it is characterized by a combination of cardinal motor features and non-motor symptoms (NMS), including different prominent psychiatric disturbances (McLaughlin et al., 2014). A progressive degeneration of the nigrostriatal dopaminergic projections leads to classical motor symptoms in PD (Davie, 2008). Depression is one of the most common NMSs in PD, and almost half of PD patients have mild to major depressive symptoms (Kalia and Lang, 2015). Unfortunately, rarely have studies analyzed the circuit-level degeneration in PD with depression (PDD).
The recently proposed “triple-network” model emphasized the cooperation among networks. The triple-network model includes the default mode network (DMN), executive control network (ECN), and salience network (SN) (Menon, 2011). They are the three most important intrinsic networks of the human brain activation. DMN controls internal thought and autobiographical memory (Buckner et al., 2008). ECN is involved in multiple cognitive processes, such as attention, language, and memory (Lerman et al., 2014). SN has both abundant structural connectivity and functional connectivity (FC) with many brain regions of the DMN and ECN (Averbeck and Seo, 2008; Craig, 2009), and it is well known for its functions in processing salience information (Craig, 2009) and regulating balance between different large-scale networks (Menon, 2011) such as DMN and ECN. Abnormalities in the triple-network model have been observed across several psychiatric disorders including PDD (Manoliu et al., 2014; Wang et al., 2014; Fan et al., 2017; Weng et al., 2019). However, these network abnormalities were found in PD patients from a small cohort. The precuneus network (PCUN) hub consists of the precuneus, posterior inferior parietal lobule, middle cingulate cortex, and dorsal angular gyri. PCUN responds to a wide range of cognitive processes, including self-related processing (Lou et al., 2004); reflective, awareness, and conscious information processing (Vogt and Laureys, 2005); episodic memory (Dörfel et al., 2009); and visuospatial processing (Wenderoth et al., 2005). The sensorimotor network (SMN) includes somatosensory (e.g., postcentral gyrus) and motor (e.g., precentral gyrus) regions and extends to the supplementary motor areas. However, the connectivity within these specific networks has not been documented in PDD patients.
Resting-state functional magnetic resonance imaging (RS-fMRI) is a novel, non-invasive approach without performing any specific task. Previous RS-fMRI studies in depressed PD patients using the amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) analysis have demonstrated alterations in regional brain activity in the prefrontal–limbic system, the basal ganglia (BGN), and the DMN (Wen et al., 2013; Luo et al., 2014; Sheng et al., 2014; Hu et al., 2015a). Studies with FC analysis have observed FC disruption in the emotional, limbic, motor, and DMNs in depressed PD patients (Hu et al., 2015b; Huang et al., 2015; Liang et al., 2016). However, the networks do not function independently, and investigation of the connectivity within one specific network may not be enough or may be somewhat unjustified. A study using independent component (IC) analysis (ICA) confirmed the involvement of BGN, DMN, left frontoparietal network (LFPN), and SN in depression in PD patients, facilitating the development of more detailed and integrative neural models of PDD (Wei et al., 2017). Based on the above studies, we hypothesized that PDD may be related to the damage of specific large-scale neural networks, rather than on the disruption of a single, random, discrete brain region; not only the connectivity within these networks but also the coordination between them can be a prominent feature of PDD.
Alternatively, the ICA is a seed-free approach for detecting connectivity of brain networks. ICA offers an effective means for identification of functional systems within the brain during rest, typically referred to as “resting-state networks” (RSNs) or “intrinsic connectivity networks” (ICNs) (Beckmann et al., 2005; Damoiseaux et al., 2006). The ICA components extracted by enforcing orthogonality spatially represent the brain networks, while the FC correlation values between each network pair reflect interaction. Thus, ICA not only can investigate the FC within one specific network but also can test the FC across the networks. Moreover, using ICA, the subnetworks can be independently extracted. A previous study with a small sample size suggested that ICA is effective to identify the abnormal intrinsic FC within and between large-scale neural networks in depressed PD patients (Wei et al., 2017). Therefore, we proposed that determining neural correlations with ICA could lead to the discovery of biomarkers to improve the diagnosis of PDD.
This study was proposed to use the RS-fMRI and ICA to systematically investigate not only the connectivity within but also the connectivity between DMN, ECN, SN, PCUN, and SMN in PDD patients and PD without depression (PDND) patients. Specifically, we first used the ICA to identify and decompose the networks into independent z-maps (reflecting the intrinsic FC of areas within each network, intra-FC), and then we computed the FC correlation values between each network pair representing the intrinsic FC between networks (inter-FC). Second, we would compare the intra-FC and inter-FC data among PDD patients, PDND patients, and healthy controls (HCs).
Materials and Methods
Participants
Forty-one PDD, 64 PDND patients, and 55 HCs were recruited with a written informed consent from the Second Xiangya Hospital between December 2015 and February 2020. The study was approved by the Ethical Committee of Medical Research of the Second Xiangya Hospital (2015-533). The criteria for recruited patients were as follows: (1) they met the United Kingdom Parkinson’s Disease Society Brain Bank Criteria (Daniel and Lees, 1993); (2) they were right-handed Han Chinese; (3) they stopped anti-PD or anti-depressant medications for 12 h before imaging and neuropsychological examination; (4) their motor symptoms were evaluated by the Unified Parkinson’s Disease Rating Scale (UPDRS-III) and Hoehn and Yahr (H-Y) scale; (5) they had an H-Y staging score of between 1 and 3; (6) depression was diagnosed with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria by an experienced psychiatrist, and patients had a 17-item Hamilton Depression Rating Scale (HDRS-17) score higher than seven (Wang et al., 2018). Afterward, the severity of depression was quantified with the HDRS-17 and the 21-item Beck’s Depression Inventory (BDI); and (7) they had no apparent cognitive impairments, which were determined by the Mini-Mental State Examination (MMSE) score not falling lower than the corresponding education level (Li et al., 2016). An MMSE score >17 for illiterate participants, >20 for grade-school literate participants, and >23 for junior high school and higher education literate participants were defined as normal MMSE scores in our patients. Patients were excluded if they had a history of head injury, stroke, or other neurologic or psychiatric disease; had an abnormal MMSE score; or had any disorders that interfere assessment of the manifestations of PD (Wang et al., 2020). The same exclusion criteria listed above were applied to the control group. Neuropsychological examinations were used to excluded dementia and depression in controls. Five PDD patients, four PDND patients, and three HCs were excluded from the present analyses due to the stringent inclusion and exclusion criteria. The demographic and clinical information is presented in Table 1.
TABLE 1.
Demographic and clinical characteristics of the participants (mean ± SD).
| Variable | HC (N = 52) | PDD (N = 36) | PDND (N = 60) | P |
| Gender (M/F) | 23/29 | 15/21 | 35/25 | 0.189a |
| Age (years) | 56.212 ± 8.465 | 56.361 ± 8.319 | 56.950 ± 9.967 | 0.903b |
| Education (years) | 8.087 ± 3.656 | 6.694 ± 3.069 | 7.367 ± 3.361 | 0.168b |
| Disease duration (years) | NA | 2.667 ± 2.588 | 2.304 ± 1.733 | 0.415c |
| MMSE | 26.673 ± 3.294 | 26.250 ± 2.882 | 27.067 ± 2.469 | 0.401b |
| HDRS-17 | 2.289 ± 2.615 | 14.639 ± 4.859 | 3.283 ± 2.116 | <0.001b |
| BDI-21 | 9.804 ± 9.353 | 23.000 ± 10.570 | 8.733 ± 5.862 | <0.001b |
| UPDRS-III | NA | 19.056 ± 11.810 | 14.800 ± 9.896 | 0.061c |
| H-Y | NA | 1.639 ± 0.743 | 1.550 ± 0.642 | 0.538c |
| REL-GMV | 0.408 ± 0.028 | 0.405 ± 0.026 | 0.403 ± 0.028 | 0.686b |
| REL-WMV | 0.338 ± 0.021 | 0.337 ± 0.02 | 0.335 ± 0.024 | 0.727b |
| Mean Power_FD | 0.096 ± 0.05 | 0.074 ± 0.037 | 0.09 ± 0.04 | 0.078b |
Significant post hoc two-sample t-tests (p < 0.05, Bonferroni corrected): DRS-17: PDD vs. PDND; PDD vs. HC; BDI-21: PDD vs. PDND; PDD vs. HC. PDD, Parkinson’s disease with depression; PDND, PD patients without depression; HC, healthy control; HDRS-17,17-item Hamilton Depression Rating Scale; BDI-21, 21-item Beck’s Depression Inventory; UPDRS-III, Unified Parkinson’s Disease Rating Scale-motor part III; H-Y, Hoehn and Yahr staging scale; MMSE, Mini-Mental State Examination; REL-GMV, relative gray matter volume; REL-WMV, relative white matter volume; FD, framewise displacement; NA, not applicable. For comparisons of demographics: ap value for the gender difference was obtained by chi-square test. bp values were obtained by one-way analysis of variance (ANOVA) tests. cp values were obtained by two-sample t-test.
MR Image Acquisition and Data Preprocessing
The procedure and parameters to obtain resting-state fMRI images were previously described (Liao et al., 2020). During the image processing, the first 10 volumes were removed from each participants’ series, slice timing was aligned, and the head motion was corrected. Data from nine participants (five PDD and four PDND) who displayed excessive head motion were excluded from further analyses. The images were then spatially normalized to a standard Montreal Neurological Institute (MNI) template, resampled to the voxel size of 3 mm × 3 mm × 3 mm, and spatially smoothed with a Gaussian kernel (8 mm × 8 mm × 8 mm full width at half maximum).
Independent Component Analysis and Selection of Networks of Interest
The ICs were analyzed by the Group ICA (the GIFT software1). The procedures based on this toolbox included three steps: (1) data reduction, (2) applying ICA algorithm, and (3) back-reconstruction for individual-level components. In this study, 38 ICs were auto-estimated through the minimum description length (MDL) criteria, and Group ICA was performed 20 times. Nine meaningful components were identified as anatomically and functionally classical RSNs via visual inspection. They were extracted from all participants, including the ventral DMN (vDMN), dorsal DMN (dDMN), PCUN, two SMNs, right ECN (RECN), left ECN (LECN), anterior SN (aSN), and two posterior SNs (pSN). Seven were related to the “triple-network” model, with three components reflecting the SN (one for the aSN and two for the pSN), two reflecting the DMN (one each for the vDMN and dDMN), and two reflecting the ECN (one each for the LECN and RECN) subnetworks.
Outcome Measures
The 10 ICs corresponding to the triple networks, PCUN, and SMN were then extracted from all participants. For each subject, each component’s z-map and its corresponding temporal cortex (TC) reflected the measures of intra-FC. Linear detrending, despiking, and temporal filtering were conducted for all TCs of the networks of interest before evaluating the inter-FC (Buckner et al., 2008; Luo et al., 2014). We calculated Pearson’s correlation coefficients of the TCs for each network pair among the SN, DMN, central executive network (CEN) subsystems; PCUN; and SMN and then transformed the coefficients into z-scores by Fisher’s z-transformation. These transformed z-scores indexed the inter-FC of each network pair.
Statistical Analysis
SPSS v19 software was used for data analysis (IBM, United States). The differential brain regions were identified among the three groups using one-way analyses of variance (ANOVAs) and post hoc two-sample t-test in a pair-wise manner.
To statistically analyze the FC within each selected ICN, it was calculated voxel-wise with participants’ reconstructed spatial maps using one-sample t-test SPM12 [p < 0.001, false discovery rate (FDR) corrected]. Comparisons of connectivity within each ICN among PDD, PDND, and HC groups were performed using a design model of one-way ANOVA in SPM12, followed by post hoc two-sample t-test (voxel level, p < 0.001, cluster size >10 voxels, corresponding to a corrected p < 0.05 as determined by AlphaSim correction). The between-groups differences of connectivity among selected ICNs were assessed using ANOVA, and post hoc two-sample t-test was used to determine connectivity changes between each pair of the three groups (p < 0.05, AlphaSim corrected).
The correlations between the detected connectivity abnormalities and the HDRS as well as the UPDRS-III scores were assessed using Pearson correlation coefficient for the PDD and PDND patients. A p < 0.05 was considered significant.
To test whether the intra-connectivity can predict depression level in the PD patients, hierarchical regression analyses were performed on the depression level as indicated by HDRS scores. Five regression models were run in total. Age, gender, educational years, MMSE scores, UPDRS-III scores, and illness duration were entered as predictors in step 1. To evaluate which connectivity could significantly predict depression level and beyond the predictors included in step 1, regression model 1 included connectivity of all four brain regions, which were different between PDD and PDND as predictors in step 2, and a stepwise method was used to determine which predictors to retain in the final model. To evaluate the predictive value of connectivity of each of the four regions, the connectivity of one of the four regions was entered as the only predictor in step 2 for each of the other four regression models.
Results
Demographic Information
No significant differences in H-Y staging, UPDRS-III, and PD duration were observed between the PDD and PDND patients, while no significant differences in gender, age, education level, relative gray matter volume (REL-GMV), MMSE scores, relative white matter volume (REL-WMV), and mean FD were observed between the three groups (Table 1). In contrast, significant differences in the HDRS-17 and BDI scores were observed among the three groups (p < 0.001).
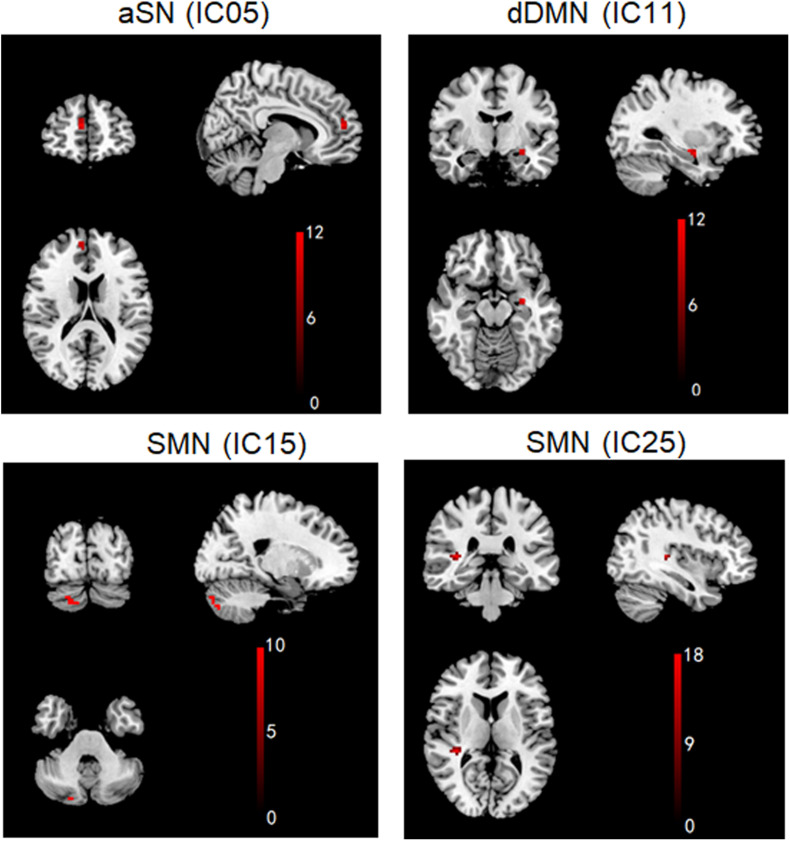
Identification of Network of Interests
The respective spatial patterns of 10 networks of interests for the three groups (PDD, PDND, and HCs) were revealed by the one-sample t-test (Figure 1, FDR corrected, p < 0.05), including the LECN (IC20), RECN (IC06), dDMN (IC11), pSN (IC09/12), vDMN (IC04), aSN (IC05), SMN (IC15/IC25), and PCUN (IC26).
FIGURE 1.
Large-scale networks including the 10 resting-state networks (RSNs) identified by independent component analysis. Left executive control network, LECN (IC20); right executive control network, RECN (IC06); dorsal default mode network, dDMN (IC11); posterior salience network, pSN (IC09/IC12); ventral default mode network, vDMN (IC04); anterior salience network, aSN (IC05); sensorimotor network, SMN (IC15/IC25); precuneus network, PCUN (IC26).
Intra-Network Connectivity Analysis
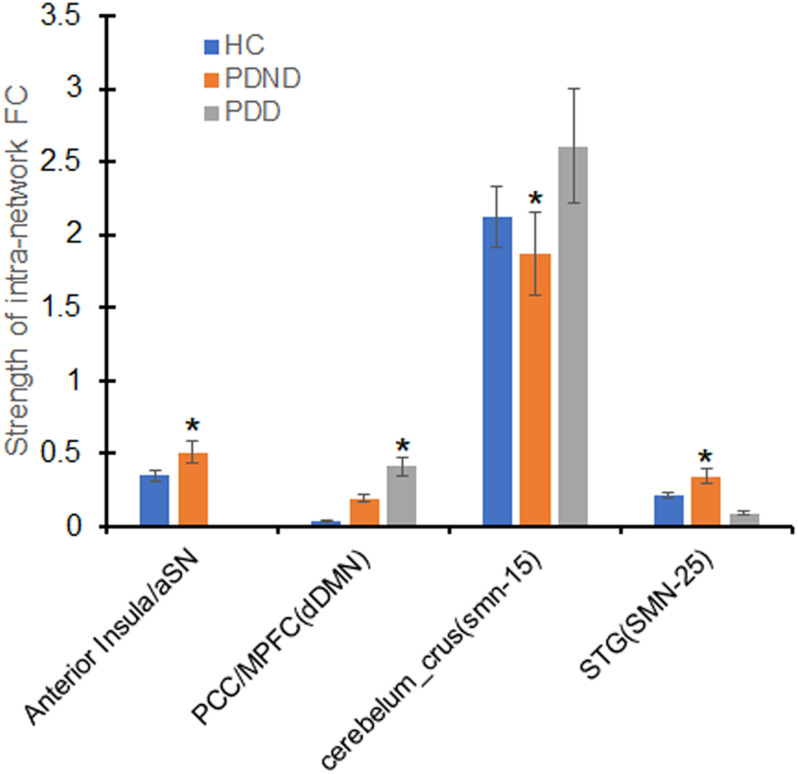
aSN, dDMN, and SMN exhibited significant changes in FC among the PDD, PDND, and HC groups (ANOVA, p < 0.05, Figure 2). Compared with the HC group, the PDD and PDND patients showed increased connectivity in the left hippocampus of the dDMN (Hippocampus_L). The PDD patients exhibited an increased connectivity in the left hippocampus of the dDMN (Hippocampus_L) and the right Cerebelum_Crus2 of the SMN (Cerebelum_Crus2_R) and a decreased connectivity in the right medial superior frontal gyrus (mSFG) of the aSN (Frontal_Sup_Medial_R) and the right superior temporal gyrus (STG) of the SMN (Temporal_Sup_R) than did the PDND group (p < 0.05, Figure 3 and Table 2). No group differences in intra-network connectivity of the vDMN, PCUN, left CEN (LCEN), and right CEN (RCEN) were observed.
FIGURE 2.
Differences of in the intra-network functional connectivity (FC) analysis. The x-axis represents the brain regions of the resting-state networks (RSNs) (parentheses), and the y-axis represents the mean value of intra-network FC strength. Error bars indicate the standard errors of the means. ∗p < 0.05, uncorrected, PDD vs. PDND. aSN, anterior salience network; SMN (IC15/IC25), sensorimotor network; dDMN, dorsal default mode network; STG, superior temporal gyrus; PDD, Parkinson’s disease with depression; PDND, PD patients without depression; HC, healthy control.
FIGURE 3.
Brain regions with significant differences in intra-network functional connectivity (FC) between PDD and PDND patients. (Post hoc two-sample t-tests, p < 0.05, uncorrected, PDD vs. PDND). aSN (IC05), anterior salience network; IC, independent component; dDMN (IC11), dorsal default mode network; SMN (IC15/IC25), sensorimotor network.
TABLE 2.
Brain regions with significant differences in intra-network functional connectivity (FC) between PDD and PDND patients.
| RSN | Brain region (AAL) | Number of voxels (cluster size) | MNI coordinates of local maxima |
Peak Z/T value | ||
| X | Y | Z | ||||
| aSN (IC05) | Frontal_Sup_Medial_R | 14 | 9 | 51 | 15 | 11.1233 |
| dDMN (IC11) | Hippocampus_L | 12 | −03 | −6 | −51 | 11.2688 |
| SMN (IC15) | Cerebelum_Crus2_R | 18 | 24 | −48 | −33 | 9.4682 |
| SMN (IC25) | Temporal_Sup_R | 13 | 36 | −33 | 9 | 16.6457 |
Post hoc two-sample t-tests, p < 0.05, uncorrected. PDD vs. PDND. aSN (IC05), anterior salience network; IC, independent component; dDMN (IC11), dorsal default mode network; SMN (IC15/IC25), sensorimotor network.
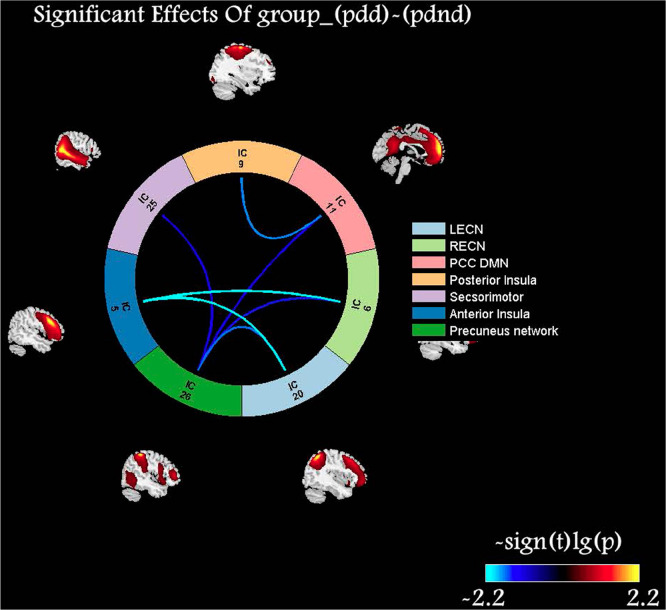
Inter-Network Connectivity Analysis
The PDD patients exhibited a decreased connectivity between aSN and bilateral ECN, between pSN and dDMN, and between PCUN and dDMN/SMN/bilateral ECN than did PDND patients (Figure 4). The PDD patients exhibited decreased connectivity between aSN/pSN and dDMN and between PCUN and dDMN/vDMN/SMN/RECN than did the HCs. The PDND patients exhibited a decreased connectivity between aSN and pSN, and PCUN and vDMN, as well as an increased connectivity between aSN and RECN than did the HCs. These alterations indicated an impaired FC between SN/PCUN and the other networks in the PDD and PDND patients.
FIGURE 4.
Comparisons of inter-network functional connectivity (FC) alterations in the resting-state networks (RSNs) between the Parkinson’s disease with depression (PDD) and PD without depression (PDND) groups. Color scale denotes the t value. Compared with PDND patients, PDD patients exhibited a decreased connectivity between anterior salience network (SN) (aSN) and bilateral executive control network (ECN), between posterior SN (pSN) and posterior default mode network (pDMN), and between precuneus network (PCUN) and pDMN/SMN/bilateral ECN.
Correlation Analysis of Functional Connectivity With Neuropsychological Test Scores and Clinical Characteristics
The Pearson correlation analysis demonstrated no significant correlations between altered FC values and any clinical variables (UPDRS-III/HDRS-17/BDI-21) in the patients with PD.
Intra-Network Connectivity Predicts Depression Levels in Parkinson’s Disease Patients
After demographical and clinical variables were controlled for, including age, gender, educational years, illness duration, MMSE scores, and UPDRS-III scores, connectivity within the left hippocampus of dDMN and the right mSFG of aSN was a significant predictor of depression level in the PD patients, when these FCs were considered either simultaneously or separately in the regression model after correction (p < 0.05/4). Connectivity within the right Cerebelum_Crus2 of the SMN reached a level of p < 0.05, while it could not survive the correction (Table 3).
TABLE 3.
Intra-network connectivity predicting depression levels in PD patients (N = 96).
| Predictors | Variable statistics |
Model statistics |
||||
| β (s.e.) | t | P | ΔR2 | F | p | |
| Step 1 (for all models) | ||||||
| Age | 0.09 (0.06) | 1.61 | 0.111 | 0.20 | 3.51 | 0.004 |
| Gender | 1.28 (1.14) | 1.12 | 0.26 | |||
| Educational years | 0.05 (0.17) | 0.27 | 0.788 | |||
| UPDRS-III | 0.21 (0.05) | 4.39 | <0.001 | |||
| MMSE | −0.04 (0.22) | −0.20 | 0.846 | |||
| Duration | −0.11 (0.28) | 0.42 | 0.679 | |||
| Step 2 | ||||||
| Model 1a | ||||||
| dDMN (Hippocampus_L) | 5.04 (1.43) | 3.53 | 0.001 | 0.23 | 6.91 | <0.001 |
| aSN (Frontal_Sup_Medial_R) | −2.57 (0.71) | −3.58 | 0.001 | |||
| SMN_15 (Cerebelum_Crus2_R) | 1.16 (0.57) | 2.02 | 0.047 | |||
| SMN_25 (Temporal_Sup_R) | – | – | – | |||
| Model 2 | ||||||
| dDMN (Hippocampus_L) | 5.58 (1.55) | 3.61 | 0.001 | 0.11 | 3.51 | 0.004 |
| Model 3 | ||||||
| aSN (Frontal_Sup_Medial_R) | −2.78 (0.78) | −3.56 | 0.001 | 0.10 | 5.22 | <0.001 |
| Model 4 | ||||||
| SMN_15 (Cerebelum_Crus2_R) | 1.58 (0.64) | 2.45 | 0.016 | 0.05 | 4.04 | 0.001 |
| Model 5 | ||||||
| SMN_25 (Temporal_Sup_R) | −2.48 (1.37) | −1.81 | 0.073 | 0.03 | 3.51 | 0.002 |
aPredictors included intra-connectivity of dDMN, aSN, SMN_15, and SMN_25. Using the stepwise method, connectivity of dDMN, aSN, and SMN_15 was significant and retained in the final model. SMN_15 could not survive the Bonferroni correction (p < 0.05/4). UPDRS-III, Unified Parkinson’s Disease Rating Scale-motor part III; MMSE, Mini-Mental State Examination; aSN (Frontal_Sup_Medial_R), anterior salience network (the right medial superior frontal gyrus); dDMN (Hippocampus_L), dorsal default mode network (the left hippocampus); SMN_15 (Cerebelum_Crus2_R), sensorimotor network_15 (the right Cerebelum_Crus2); SMN_25 (Temporal_Sup_R), sensorimotor network_25 (the right superior temporal gyrus).
Discussion
The present study systematically investigated the connectivity changes within and between PCUN, SMN, and the triple-network in the PDD and PDND patients. Compared with the PDND patients, the PDD patients showed a decreased intra-connectivity in the aSN and SMN; increased intra-connectivity in the dDMN and SMN; and decreased inter-connectivity between aSN and bilateral ECN, between pSN and dDMN, and between PCUN and dDMN/vDMN/SMN/RECN. Our study provided evidence of aberrant connectivity patterns across broad-scale networks in PDD.
In this study, we found that the PDD patients showed decreased inter-connectivity between aSN and bilateral ECN, and between pSN and dDMN. The triple-network model supports cognitive, affective, perceptual, and social functions (Menon, 2011). Thus, the disconnected communication from SN to ECN/DMN may cause disrupted top-down cognitive modulation over the limbic and subcortical structures, which may be involved in the onset and maintenance of depression. In addition, the occurrence of depression might arise from disturbance in more distributed neural networks, such as functional disruption of the prefrontal–limbic network (Luo et al., 2014). Consistently, previous studies also demonstrated that the SN is involved in PDD. For instance, Huang et al. (2020) reported that the disrupted connection between the SN and ECN might contribute to depression in PD. Wei et al. (2017) reported that the involvement of BGN, DMN, LFPN, and SN in PD facilitates the development of depression. Therefore, the impaired interactions between the triple network are critical for the occurrence and maintenance of depression in PD.
In this study, the PDD patients exhibited decreased inter-network connectivity between precuneus and DMN/SMN/ECN. Previous studies have demonstrated that the abnormal function of the striatum and the associated limbic–BGN circuitry is proposed to play a role in the emotional processing system in patients with depression (Jiao et al., 2011; Marchand et al., 2012; Andreescu et al., 2013). Studies have also shown that SMN is activated during bimanual motor tasks, suggesting the involvement of SMN in a pre-mediated state that prompts the brain in performing/coordinating a motor task (Beckmann et al., 2005). Thus, the vulnerability of the distinct PCUN and other large-scale networks plays a major role in the pathophysiology of neurodegenerative processes in the PDD patients. Our results suggest that the functional integrity of prefrontal–limbic–striatal–cortex circuitry function is involved in the pathogenesis of PDD.
In this study, the PDD patients had decreased connectivity in the right STG of the aSN and the right mSFG of the SMN and increased connectivity in the left hippocampus of the dDMN and the right Cerebelum_Crus2 of the SMN. Previous studies have shown hypoactivity, decreased metabolism, reduced density, and FC of the STG in PDD patients (Feldmann et al., 2008; Ballanger et al., 2012; Lou et al., 2015). The prefrontal cortex containing the mSFG serves an important function as a top-down modulator of emotional tasks (Phillips et al., 2003). The Sheng et al. (2014) study with ReHo and FC methods found that PDD patients had increased regional activity in the left frontal and medial frontal gyri than had non-depressed PD patients. Our previous study found abnormal ReHo values in both the cingulate cortex and the orbitofrontal area of depressed PD patients (Wang et al., 2020). Zhi et al. (2019) found that the right medial frontal gyrus activation could be a biomarker for the occurrence and the severity of depression in PD. The hippocampus is a major culprit of emotional dysregulation, contributing to the pathophysiology of depression in PD (van Mierlo et al., 2015). However, a previous study reported that PDD patients reduced connectivity of putamen with mesolimbic regions, particularly in the hippocampus (Luo et al., 2014). This discrepancy might be due to the neural network remodeling caused by anti-PD or anti-depressant medications. A meta-analysis study demonstrated that the Cerebelum_Crusl and Cerebelum_Crus2 areas were involved in executive functions and complex cognitive functions (including language, working memory, etc.) (Stoodley and Schmahmann, 2009). Both structural (Stoessl et al., 2011) and functional (Mentis et al., 2002) abnormalities of the cerebellum are present in movement and emotional disorders in depressed PD patients. Song et al. (2015) observed the interactive effects of motor and depressive symptoms on the bilateral posterior cerebellum.
Although some previous authors associated hyper-connectivity with functional compensation (Wang et al., 2006; Hillary et al., 2015), many others have considered increases in connectivity as a reflection of functional disruption (Esposito et al., 2010) and altered neuronal communication (Licata et al., 2013). It is therefore considered that “functional disconnection” implies both functional hypo-connectivity and hyper-connectivity. Recently, growing evidence (Küper et al., 2011; Song et al., 2015) has shown that aside from its traditional integration of motor functions, the cerebellum takes part in the regulation of non-motor functions, such as language, cognition, and emotion. Wen et al. (2013) observed an increased ALFF in the right cerebellum posterior lobe in depressed PD patients when compared with non-depressed PD patients. The increases in connectivity in the right cerebellum might be not only a compensatory mechanism for the defective motor system but also a reflection of functional disruption and altered neuronal communication. Thus, the STG, mSFG, hippocampus, and Cerebelum_Crus2 are likely the critical hubs in the neural circuits related with depression in PD patients.
Several limitations should be considered when interpreting the results of the present study. First, although the adoption of a 38-component ICA model was auto-estimated, there was no clear computational criterion for the number. Second, no significant correlations were found between the variables (intra-FC and inter-FC) and motor or non-motor scale scores (UPDRS-III/HDRS-17/BDI-21). This may be biased due to the small sample size in PDD group. Further studies in a larger sample size will be necessary to explore the relationship between connectivity abnormalities and the severity of depression in PDD. Third, it should be noted that none of our results could survive at the strict AlphaSim correction level of p < 0.001. However, in this study, the combination of the AlphaSim correction of p < 0.05 with individual voxel threshold at p < 0.001 and the required cluster size adopted was acceptable based on the previous studies (Kong et al., 2013; Fan et al., 2017). Our power analysis also indicated reliable effect sizes for our results. We were cautious in generalizing our results, and further studies are needed. Fourth, the patients in the current study were not drug naïve. Although the anti-PD or anti-depressant medications were stopped for 12 h before imaging and neuropsychological testing, the potentially confounding effects of chronic medications could not be avoided. Fifth, the lack of a primary depression group left the question of whether PDD shares a common neurobiological substrate with primary depression unanswered. Finally, this is a cross-sectional study. It remains unsolved whether the found alterations represent state or trait characteristics of our patient cohort.
In conclusion, abnormal FC between RSNs may reflect the neuroimaging characteristics of the prefrontal–limbic–striatal–cortex circuitry-related networks. The RSNs in PDD or PDND patients are not restricted to the triple-network model but rather spread to other neural networks such as PCUN and SMN. Specifically, we found that both the aberrant intra- and inter-network FC is involved in several important hubs in the large-scale networks. This study highlighted that aberrant intra-network FC and inter-network FC can be biomarkers for distinguishing PDD from PDND, and our findings can help understand the neural foundation of the depression in PD.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Research Ethical Committee of the Second Xiangya Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CT and XZ contributed to the conception and design of the study. HL, SC, QS, TW, YZ, ZM, TZ, and WS contributed to the data collection. HL and JF contributed to the data analysis. HL contributed to writing the manuscript. JL and JY contributed to the English-language revision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This study was supported by the National Natural Science Foundation of China (No. 81471646) and the Natural Science Foundation of Hunan Province (Nos. 14JJ2027 and 2019JJ40450).
https://www.nitrc.org/projects/gift, version 2.0a.
References
- Andreescu C., Tudorascu D. L., Butters M. A., Tamburo E., Patel M., Price J., et al. (2013). Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 214 313–321. 10.1016/j.pscychresns.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck B. B., Seo M. (2008). The statistical neuroanatomy of frontal networks in the macaque. PLoS Comput. Biol. 4:e1000050. 10.1371/journal.pcbi.1000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanger B., Klinger H., Eche J., Lerond J., Vallet A. E., Le Bars D., et al. (2012). Role of serotonergic 1A receptor dysfunction in depression associated with Parkinson’s disease. Mov. Disord. 27 84–89. 10.1002/mds.23895 [DOI] [PubMed] [Google Scholar]
- Beckmann C. F., DeLuca M., Devlin J. T., Smith S. M. (2005). Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Andrews-Hanna J. R., Schacter D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Craig A. D. (2009). How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10 59–70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. S., Rombouts S. A., Barkhof F., Scheltens P., Stam C. J., Smith S. M., et al. (2006). Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U.S.A. 103 13848–13853. 10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S. E., Lees A. J. (1993). Parkinson’s disease society brain bank, london: overviewand research. J. Neural Transm. Suppl. 39 165–172. [PubMed] [Google Scholar]
- Davie C. A. (2008). A review of Parkinson’s disease. Br. Med. Bull. 86 109–127. [DOI] [PubMed] [Google Scholar]
- Dörfel D., Werner A., Schaefer M., von Kummer R., Karl A. (2009). Distinct brain networks in recognition memory share a defined region in the precuneus. Eur. J. Neurosci. 30 1947–1959. 10.1111/j.1460-9568.2009.06973.x [DOI] [PubMed] [Google Scholar]
- Esposito F., Pignataro G., Di Renzo G., Spinali A., Paccone A., Tedeschi G., et al. (2010). Alcohol increases spontaneous BOLD signal fluctuations in the visual network. NeuroImage 53 534–543. 10.1016/j.neuroimage.2010.06.061 [DOI] [PubMed] [Google Scholar]
- Fan J., Zhong M., Gan J., Liu W., Niu C., Liao H., et al. (2017). Altered connectivity within and between the default mode, central executive, and salience networks in obsessive-compulsive disorder. J. Affect. Disord. 223 106–114. 10.1016/j.jad.2017.07.041 [DOI] [PubMed] [Google Scholar]
- Feldmann A., Illes Z., Kosztolanyi P., Illes E., Mike A., Kover F., et al. (2008). Morphometric changes of gray matter in Parkinson’s disease with depression: a voxel-based morphometry study. Mov. Disord. 23 42–46. 10.1002/mds.21765 [DOI] [PubMed] [Google Scholar]
- Hillary F. G., Roman C. A., Venkatesan U., Rajtmajer S. M., Bajo R., Castellanos N. D. (2015). Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology 29 59–75. 10.1037/neu0000110 [DOI] [PubMed] [Google Scholar]
- Hu X., Song X., Li E., Liu J., Yuan Y., Liu W., et al. (2015a). Altered resting-state brain activity and connectivity in depressed Parkinson’s disease. PLoS One 10:e0131133. 10.1371/journal.pone.0131133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Song X., Yuan Y., Li E., Liu J., Liu W., et al. (2015b). Abnormal functional connectivity of the amygdala is associated with depression in Parkinson’s disease. Mov. Disord. 30 238–244. 10.1002/mds.26087 [DOI] [PubMed] [Google Scholar]
- Huang P., Guan X., Guo T., Zeng Q., Xuan M., Gu Q., et al. (2020). Damaged insula network contributes to depression in Parkinson’s disease. Front. Psychiatry 11:119. 10.3389/fpsyt.2020.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Xuan M., Gu Q., Yu X., Xu X., Luo W., et al. (2015). Abnormal amygdala function in Parkinson’s disease patients and its relationship to depression. J. Affect. Disord. 183 263–268. 10.1016/j.jad.2015.05.029 [DOI] [PubMed] [Google Scholar]
- Jiao Q., Ding J., Lu G., Su L., Zhang Z., Wang Z., et al. (2011). Increased activity imbalance in fronto-subcortical circuits in adolescents with major depression. PLoS One 6:e25159. 10.1371/journal.pone.0025159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia L. V., Lang A. E. (2015). Parkinson’s disease. Lancet 386 896–912. [DOI] [PubMed] [Google Scholar]
- Kong L., Chen K., Tang Y., Wu F., Driesen N., Womer F., et al. (2013). Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J. Psychiatry Neurosci. 38 417–422. 10.1503/jpn.120117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küper M., Dimitrova A., Thürling M., Maderwald S., Roths J., Elles H. G., et al. (2011). Evidence for a motor and a non-motor domain in the human dentate nucleus–an fMRI study. NeuroImage 54 2612–2622. 10.1016/j.neuroimage.2010.11.028 [DOI] [PubMed] [Google Scholar]
- Lerman C., Gu H., Loughead J., Ruparel K., Yang Y., Stein E. A. (2014). Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71 523–530. 10.1001/jamapsychiatry.2013.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Jia J., Yang Z. (2016). Mini-mental state examination in elderly Chinese: a population-based normative study. J. Alzheimers Dis. 53 487–496. 10.3233/jad-160119 [DOI] [PubMed] [Google Scholar]
- Liang P., Deshpande G., Zhao S., Liu J., Hu X., Li K. (2016). Altered directional connectivity between emotion network and motor network in Parkinson’s disease with depression. Medicine 95:e4222. 10.1097/md.0000000000004222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Fan J., Shen Q., Cai S., Wang M., Wang C., et al. (2020). Alterations of interhemispheric functional connectivity in Parkinson’s disease with depression: a resting-state functional MRI study. Front. Hum. Neurosci. 14:193. 10.3389/fnhum.2020.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata S. C., Nickerson L. D., Lowen S. B., Trksak G. H., Maclean R. R., Lukas S. E. (2013). The hypnotic zolpidem increases the synchrony of BOLD signal fluctuations in widespread brain networks during a resting paradigm. NeuroImage 70 211–222. 10.1016/j.neuroimage.2012.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H. C., Luber B., Crupain M., Keenan J. P., Nowak M., Kjaer T. W., et al. (2004). Parietal cortex and representation of the mental self. Proc. Natl. Acad. Sci. U.S.A. 101 6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y., Huang P., Li D., Cen Z., Wang B., Gao J., et al. (2015). Altered brain network centrality in depressed Parkinson’s disease patients. Mov. Disord. 30 1777–1784. 10.1002/mds.26321 [DOI] [PubMed] [Google Scholar]
- Luo C., Chen Q., Song W., Chen K., Guo X., Yang J., et al. (2014). Resting-state fMRI study on drug-naive patients with Parkinson’s disease and with depression. J. Neurol. Neurosurg. Psychiatry 85 675–683. 10.1136/jnnp-2013-306237 [DOI] [PubMed] [Google Scholar]
- Manoliu A., Riedl V., Zherdin A., Mühlau M., Schwerthöffer D., Scherr M., et al. (2014). Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 40 428–437. 10.1093/schbul/sbt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand W. R., Lee J. N., Johnson S., Thatcher J., Gale P., Wood N., et al. (2012). Striatal and cortical midline circuits in major depression: implications for suicide and symptom expression. Prog. Neuropsychopharmacol. Biol. Psychiatry 36 290–299. 10.1016/j.pnpbp.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Mason A. R., Ziemann A., Finkbeiner S. (2014). Targeting the low-hanging fruit of neurodegeneration. Neurology 83 1470–1473. 10.1212/wnl.0000000000000894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin N. C., Piryatinsky I., Epstein-Lubow G., Marino L., Friedman J. H. (2014). Neuropsychiatric symptoms in an inpatient Parkinson’s disease sample. Parkinsons Dis. 2014:420240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Mentis M. J., McIntosh A. R., Perrine K., Dhawan V., Berlin B., Feigin A., et al. (2002). Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson’s disease. Am. J. Psychiatry 159 746–754. 10.1176/appi.ajp.159.5.746 [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Drevets W. C., Rauch S. L., Lane R. (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry 54 504–514. 10.1016/s0006-3223(03)00168-9 [DOI] [PubMed] [Google Scholar]
- Sheng K., Fang W., Su M., Li R., Zou D., Han Y., et al. (2014). Altered spontaneous brain activity in patients with Parkinson’s disease accompanied by depressive symptoms, as revealed by regional homogeneity and functional connectivity in the prefrontal-limbic system. PLoS One 9:e84705. 10.1371/journal.pone.0084705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Hu X., Zhou S., Xu Y., Zhang Y., Yuan Y., et al. (2015). Association of specific frequency bands of functional MRI signal oscillations with motor symptoms and depression in Parkinson’s disease. Sci. Rep. 5:16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoessl A. J., Martin W. W., McKeown M. J., Sossi V. (2011). Advances in imaging in Parkinson’s disease. Lancet Neurol. 10 987–1001. [DOI] [PubMed] [Google Scholar]
- Stoodley C. J., Schmahmann J. D. (2009). Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage 44 489–501. 10.1016/j.neuroimage.2008.08.039 [DOI] [PubMed] [Google Scholar]
- van Mierlo T. J., Chung C., Foncke E. M., Berendse H. W., van den Heuvel O. A. (2015). Depressive symptoms in Parkinson’s disease are related to decreased hippocampus and amygdala volume. Mov. Disord. 30 245–252. 10.1002/mds.26112 [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Laureys S. (2005). Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog. Brain Res. 150 205–217. 10.1016/s0079-6123(05)50015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen H., Wu J., Tao L., Pang Y., Gu M., et al. (2018). Altered resting-state voxel-level whole-brain functional connectivity in depressed Parkinson’s disease. Parkinsonism Relat. Disord. 50 74–80. 10.1016/j.parkreldis.2018.02.019 [DOI] [PubMed] [Google Scholar]
- Wang L., Dai Z., Peng H., Tan L., Ding Y., He Z., et al. (2014). Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum. Brain Mapp. 35 1154–1166. 10.1002/hbm.22241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zang Y., He Y., Liang M., Zhang X., Tian L., et al. (2006). Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. NeuroImage 31 496–504. 10.1016/j.neuroimage.2005.12.033 [DOI] [PubMed] [Google Scholar]
- Wang M., Liao H., Shen Q., Cai S., Zhang H., Xiang Y., et al. (2020). Changed resting-state brain signal in Parkinson’s patients with mild depression. Front. Neurol. 11:28. 10.3389/fneur.2020.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Hu X., Zhu Y., Yuan Y., Liu W., Chen H. (2017). Aberrant Intra- and internetwork functional connectivity in depressed Parkinson’s disease. Sci. Rep. 7:2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Wu X., Liu J., Li K., Yao L. (2013). Abnormal baseline brain activity in non-depressed Parkinson’s disease and depressed Parkinson’s disease: a resting-state functional magnetic resonance imaging study. PLoS One 8:e63691. 10.1371/journal.pone.0063691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N., Debaere F., Sunaert S., Swinnen S. P. (2005). The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur. J. Neurosci. 22 235–246. 10.1111/j.1460-9568.2005.04176.x [DOI] [PubMed] [Google Scholar]
- Weng Y., Qi R., Zhang L., Luo Y., Ke J., Xu Q., et al. (2019). Disturbed effective connectivity patterns in an intrinsic triple network model are associated with posttraumatic stress disorder. Neurol. Sci. 40 339–349. 10.1007/s10072-018-3638-1 [DOI] [PubMed] [Google Scholar]
- Zhi Y., Yuan Y., Si Q., Wang M., Shen Y., Wang L., et al. (2019). The association between DRD3 Ser9Gly polymorphism and depression severity in Parkinson’s disease. Parkinsons Dis. 2019: 1642087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.