Abstract
Prevention of age-related disorders is increasingly in focus of health policies, and it is hoped that early intervention on processes of deterioration can promote healthier and longer lives. New opportunities to slow down the aging process are emerging with new fields such as personalized nutrition. Data-intensive research has the potential to improve the precision of existing risk factors, e.g., to replace coarse-grained markers such as blood cholesterol with more detailed multivariate biomarkers. In this paper, we follow an attempt to develop a new aging biomarker. The vision among the project consortium, comprising both research and industrial partners, is that the new biomarker will be predictive of a range of age-related conditions, which may be preventable through personalized nutrition. We combine philosophical analysis and ethnographic fieldwork to explore the possibilities and challenges of managing aging through bodily signs that are not straightforwardly linked to symptomatic disease. We document how the improvement of measurement brings about new conceptual challenges of demarcating healthy and unhealthy states. Moreover, we highlight that the reframing of aging as risk has social and ethical implications, as it is generative of normative notions of what constitutes successful aging and good citizenship.
Keywords: Philosophy of aging, Aging biomarker, Medicalization, Healthy aging, Personalized nutrition
Introduction
Scientific technologies are never produced in a cultural or political vacuum (Douglas 2009; Elliot and McKaughan 2014), and aging research illustrates well the intertwinement of scientific and political problems. Numerous health reports call for better scientific and technological solutions to address the socio-economic challenges resulting from population aging in Western societies (OECD 1998; European Commission 2018; Sundhedsstyrelsen 2010; WHO 2002, 2015). Science should, however, not be viewed solely as a mediator of political ambitions to support longer and more active lives. As sociologist Tiago Moreira reminds us, we should also attend to “how science, technology and medicine have been themselves implicated in the making of the ageing society” (Moreira 2017, p. 7). Science not only offers solutions to a predefined societal problem but also contributes to the framing of aging as a health problem. This paper explores the intertwined epistemic and social implications of the attempt to prevent disease by measuring and preventing aging. Towards this end, we follow an interdisciplinary project within the field of personalized nutrition that aims to develop a novel aging biomarker.
For decades, aging research has explored the potential of promoting healthier and longer lives by studying the factors that speed up or slow down the aging process. Aging is typically defined as progressive deterioration of body functions over time, and aging is hence associated with a higher prevalence of chronic diseases (Sprott 2010). Although aging is unavoidable, the notion of healthy aging highlights that the rate of deterioration differs among individuals and is modifiable through actions recommended in health policies (WHO 2002, 2015; Lamb 2017; Lassen and Moreira 2014). Health policy recommendations are dependent on scientific strategies for measuring and monitoring aging, e.g., via molecular signs called aging biomarkers (Butler and Sprott 2000). The quest for aging biomarkers has recently intensified with the emergence of omics fields, precision medicine, and personalized nutrition, thus opening the possibility of measuring aging more precisely through multivariate biomarkers (Levin 2014; Moskalev 2019).1
We focus in this paper on the recent reconceptualization of aging as risk, which is implied in the aforementioned policy reports and in scientific publications highlighting, e.g., how aging is “the biggest risk factor for a majority of chronic diseases driving both morbidity and mortality” (Kennedy et al. 2014, p. 709). Previous papers in philosophy of science discuss the epistemic and ethical problems associated with risk profiling and screening of cancer and other complex diseases (e.g., Biddle 2016; Green and Vogt 2016; Plutynski 2012, 2017). However, the analysis of the specific implications of prevention of age-related disease has so far received less attention in this field (see however Blasimme 2017, 2020). Following Blasimme (2020), we contend that the “geriatric style of thought”2 brings optimism about the potential of managing later-life pathologies, but also faces difficult challenges of scientifically demarcating what counts as healthy and unhealthy aging. Moreover, our case study adds to previous scholarship showing how attempts to naturalize aging are constitutive of societal norms for managing and disciplining aging bodies (Katz 1996; Oxlund and Whyte 2014).
Our analysis combines theoretical philosophical and anthropological analysis and ethnographic fieldwork within an ongoing research project aiming to improve strategies to counteract sarcopenia, understood as age-related loss of muscle mass. We approach science as performative, and look into how data and statistics are practiced, and negotiated in day-to-day science writings and settings (Law 2002; Mol 2002). Following the emergence of a new biomarker entails unfolding the techniques and forms of knowledge in sociomaterial practices. We aim to do so by describing the visions, strategies, and challenges involved in the examined project. The empirical material consists of semi-structured qualitative interviews with researchers involved in the project and food industry partners, as well as visits to the laboratories. Explicit reference to the project name and use of quotations are approved by the involved researchers, but we anonymize quotes from interviews used in this paper.
We begin with an analysis of the potentials and challenges of current research aiming to identify aging biomarkers (Sect. 2) and explore how these challenges play out in practice in the research project we have followed (Sect. 3). Our case illustrates how the identification of aging biomarkers not only requires more precise tools for measurement, but also conceptual clarification of where to draw the line in a vaguely defined spectrum between health and disease. Finally, we discuss how this epistemic problem has social implications for the reconceptualization of aging itself (Sects. 4 and 5).
Biomarkers to promote healthy aging
A biological marker, or biomarker, is a measurable indicator of health conditions that can inform disease diagnosis, treatment, or prevention. Biomarkers are typically defined according to their role in guiding medical decision making. Diagnostic biomarkers offer information that supports or leaves out some diagnostic possibilities. A simple example is a COVID-19 test for the presence or absence of virus RNA or anti-bodies. Predictive biomarkers are typically used to guide selection of treatment, as exemplified by tests for genetic variations to guide treatment selection for some types of cancer. Finally, prognostic biomarkers aim to predict future health outcomes, as exemplified in BRCA-testing for breast cancer. Variants (or biomarkers) that increase disease susceptibility are also referred to as risk factors. Aging biomarkers combine elements of all of these features by providing new tests that can diagnose age-related conditions or provide a risk profiling of such consideration, while also being suggestive of preventive measures to slow down the aging process.
The concept of aging biomarkers emerged in the literature on gerontology in the 1980s, in response to the growing recognition of limitations of using chronological age to predict morbidity and lifespan in humans and other species:
A biomarker of aging is a biological parameter of an organism that either alone or in some multivariate composite will, in the absence of disease, better predict functional capability at some late age than will chronological age (Baker and Sprott 1988, p. 223).
The cited early definition illustrates a central premise for aging research, namely that a “biological” or “functional age” can be distinguished from chronological age, where the former reflects an evaluation of a person’s physiological and mental health states (see also Nathan, this issue). The distinction between functional and chronological age is supported by observations of great variation in the speed of decline among individuals within the same (chronological) age group. Differences encountered between populations of “fast” and “slow agers” are hoped to point to modifiable molecular signs of aging. If biomedical technologies can detect these before a functional decline manifests at higher (phenotypic) levels of biological organization, it may be possible to take action to age in “healthier” ways.
Aging biomarkers are thus intended not only as diagnostic tools for age-related disease but also as prognostic tools for health screening. Preventive interventions associated with aging biomarkers include so-called geroprotective drugs and personalized nutrition, that aim to extend the general lifespan of an individual by lowering the risk of several age-related diseases (Moskalev 2019, ed.).3 Blasimme uses the notion of ground-state prevention to highlight how prevention of age-related decline aims to boost the baseline vitality as a way to counteract several age-related conditions (Blasimme 2020 this issue). But ground-state prevention also reawakens debates on the extent to which aging and age-related disease can and should be separated (Sprott 2010), and about where to draw the line between the normal and the pathological (Canguilhem 1966/1991; Blasimme forthcoming). In his book entitled The Metric Society, macro-sociologist Steffen Mau states: “While a diagnosis works on the binary principle of ill versus healthy, health scores are based on scales or points. Their purpose is to motivate us not ‘merely’ to be healthy, but to keep striving for improvement” (Mau 2019, p. 68). While going beyond the dichotomy between disease and health, ground-state prevention raises new question about how to define the spectrum of health states. Moreover, it raises the intertwined epistemic and ethical question of whether there is (or should be) an upper limit to health improvement. As we shall show, our case illustrates how researchers are confronted with these philosophical questions when aiming to develop a new aging biomarker.
Biomarker research is currently advanced through technologies that allow for quantitative measurement of finer-grained bodily signs. However, the improvement of measurement does not automatically lead to improved prognosis or diagnosis. The introduction of the most iconic symbol of medicine, the stethoscope, illustrates well the persistent challenge. The invention of the stethoscope by Laënnec in 1816 provided a more objective and distanced measurement of what is “inside the body” and marked the beginning of technology-driven developments in medicine (Reiser 1978). Nevertheless, the extension of human senses also came with new challenges of interpretation. While the stethoscope allowed for more precise detection and classification of heart murmurs among hospitalized patients, the status of heart murmurs as a diagnostic criterion for heart failure remained controversial (Binney 2016). Particularly problematic was the introduction of the stethoscope as a vehicle for early diagnosis of heart disease. The Scottish cardiologist James Mackenzie criticized how such examinations impacted the lives of individuals with detected heart murmurs, as they were advised against doing physical labor and had to pay more for medical insurance, without having established firm knowledge about the relation between such signs and symptomatic heart problems (Mackenzie 1919; Binney 2016, p. 89). Since most of these never developed heart problems, the example illustrates how introduction of prognostic technologies can unintentionally lead to harm if bodily signs are overinterpreted as indications of future disease.4
We use the example of the stethoscope to illustrate how uncertainty about a diagnostic or prognostic test can arise when there is a lack of knowledge about the spectrum of variation of the physiological condition measured, or what is sometimes called spectrum bias or spectrum effect (Mulherin et al. 2002). Different methodological designs have been explored to overcome this problem in biomarker research. A standard procedure in case–control and cohort studies compares the prevalence of a candidate risk factor in a patient population versus a healthy control group. The need for such methods underscores how biomarkers are inherently comparative and relational. They are statistically identified and defined as marking a measurable difference between reference populations, rather than representing a fixed biological entity or well-defined causal factor (Russo and Vineis 2016). A common approach to identify aging biomarkers is to compare “fast-agers,” characterized by a given type of functional deterioration, to “slow agers.” Here, the control group represents healthy aging, which can be individuals with above-average health or centenarians as an extreme example of “slow aging” (Franceschi and Bonafé 2003; Moreira 2017; Moskalev 2019, ed.). Yet, the selection of the relevant reference populations can be challenging in aging research, as difficult choices must be taken when determining which individuals are sufficiently healthy to represent the control group.5 Moreover, reference values for “normal” aging (functional performance or blood metabolites) change over a person’s lifetime, and studies must be designed to account for the limitations of comparing and extrapolating test results across age groups.6
Biomarkers can be validated by testing their predictive capacity on new populations (or on data from similar studies). If successful, the next step is to demonstrate the effects of an intervention on the risk factor associated with adverse health outcomes. This is typically attempted through randomized controlled trials, comparing the effects of intervening on risk factors in one group to a control group. Positive effects are typically most significant where there is a relatively simple and strong relationship between risk factor and disease, such as between smoking and lung capacity or lung cancer. But it has often proven difficult to change the development of complex diseases via interventions on risk factors. For instance, a large randomized controlled study recently documented a successful reduction in risk factors of ischemic heart disease (blood cholesterol, blood pressure, obesity) through lifestyle intervention. However, after ten years, the researchers observed no long-term effect on morbidity and mortality of the intervention group compared to the control group (Jørgensen et al. 2014). The challenge of prevention via intervention on classical risk factors such as blood cholesterol is also reflected in discussions about the benefit-to-harm ratio of statin treatment, especially among individuals with relatively low risk of heart disease (Abrahamson et al. 2013; Collins et al. 2016; Dumit 2012; Greene 2007; Ruscica et al. 2018). As we shall see below, this challenge is also motivating the project we have followed.
A possible explanation for the difficulties of preventing common diseases is that traditional biomarkers, such as blood cholesterol, only give a coarse-grained picture of risk that is not predictive at the individual level. It is hoped that improvement of measurement, via multivariate statistics on large datasets, can better account for interactions between multiple causal factors, thus giving way for more precise profiling of aging and risk (Levin 2014; Putin et al. 2016). The aim to account for patient-specific differences that impact disease development is pursued in the fields of personalized medicine and personalized nutrition. The latter is based on the idea that molecular profiling (via blood or stool samples) can help suggest personalized diets of benefit for the individual (Scrinis 2015). In the following, we explore how opportunities and challenges play out in this field through insights from ethnographic fieldwork in a data-intensive research project that aims to develop a rapid and robust screening tool for age-related changes in lipoprotein metabolism. The researchers we followed teamed up with a large food industry consortium that wants to explore the possibilities of delivering new functional food products for individualized prevention based on screening for aging biomarkers.
The COUNTERSTRIKE project
The COUNTERSTRIKE project will develop a novel analytical methodology for LPD determination, sufficiently rapid to allow for nation-wide screening efforts, which facilitate early diagnostics of not only sarcopenia, but also metabolic syndrome and related conditions, thus paving the way for development of personalised nutritional recommendations.7
The aim of the COUNTERSTRIKE project
The COUNTERSTRIKE project (COUNTERacting Sarcopenia with proTeins and exercise—screening the CALM cohort for lIpoprotein biomarKErs) is a collaboration between the University of Copenhagen and the University of Amsterdam, along with three industrial partners: Arla Amba group, Bruker, and Unilever. It is a highly interdisciplinary project with researchers from metabolomics, chemometrics, health statistics, physiology, and medicine. The participating researchers and industry partners describe the project as highly exploratory, as an experiment in how far it is possible—and desirable—to realize the vision stated in the above quote from the project’s webpage.
The project is focused on developing a valid biomarker of lipoprotein particle distribution by creating a protocol for clinical screening based on a comparison of lipoprotein profiles of research participants with diet, physiological parameters, physical function, gut microbiome, and health perception. Among the methods is a full metabolomic investigation of 3000 biological samples collected from healthy persons above the age of 65.8 A key goal is to precisely determine the lipoprotein particle distribution (LPD) in blood samples by developing a method that can provide rapid and reliable results. Secondly, to link the test results to metabolic measures and physiological parameters predictive of age-related deterioration. The project was created as an extension of another research project that focused on the prevention of loss of muscle strength within the elderly population (called Counteracting Age-related Loss of Skeletal Muscle Mass—abbreviated as CALM), and the samples from this study as well as an additional enrollment of participants to diversify the sample comprise the COUNTERSTRIKE study cohort.
The CALM and COUNTERSTRIKE projects are hoped to collect data and develop methods that can improve classical biomarkers. A medical doctor, who has been involved in both projects as part of his PhD project, expressed the problem with risk profiling via blood cholesterol and blood glucose as follows:
I have some concerns about both biomarkers, because we often assume that we understand the connections. The concern is justified by the very poor effect of lowering cholesterol medically, through statins, on prevention of cardiovascular events, including blood clots. […] You need to treat 138 people to prevent one cardiovascular event. To me, this indicates that we haven’t understood the real problem.
A possible explanation for the low effect of the intervention on blood cholesterol relates to the many other factors that influence how aging and disease develop in specific individuals. Therefore, the researchers hope to improve the predictability of testing procedures through identification of multivariate biomarkers (Levin 2014). The researcher in the quote clarified that their project is valuable because it offers potential for a complete description of each participant through data from questionnaires to macroscopic functional tests and a battery of blood tests. The latter makes possible an analysis of the plasma metabolome, the skeletal muscular metabolism, and the plasma amino acid profile. The task for the multivariate analysis is then to integrate various types of data to provide a more nuanced view of the relation between lipoproteins and age-related diseases.
Lipoproteins, i.e., macromolecules consisting of fat and protein, are often classified based on their tendency to increase the risk of coronary artery disease. For instance, low-density lipoprotein (LDL) cholesterol is commonly described as the “bad cholesterol” because it has a strong tendency to build up plaques in the arteries, which blocks blood flow, whereas high-density lipoprotein (HDL) cholesterol is seen as “good cholesterol” because it can help remove LDL from the bloodstream. There are further subclasses, such as intermediate and very-low-density lipoproteins (IDL and VLDL), as well as subclasses of each which differ in their molecular composition (Monsonis-Centelles et al. 2019). However, a central hypothesis in the COUNTERSTRIKE project is that not only the total concentration, but also the relative distribution of these and other lipoproteins, matters. As stressed by a co-PI:
Instead of just having a single-value biomarker, it's a spectrum of lipoprotein distribution. And this is what doctors have shown earlier: you get a different distribution of some of the lipoproteins, indicating for example obesity and some cardiovascular diseases. So yes, we work holistically, it sounds nice, but multivariate. Doctors have one thing, be it blood pressure or cholesterol or glucose levels in the blood, and if it is above some limit, you are sick, and if it is below, you are not sick. That doesn't make much sense to us. In all the things we investigate, it will rarely look like this. It is typically a multivariate picture, because nature is multivariate.
Rather than merely classifying lipoproteins into good or bad types, or interpreting test results according to a binary measure of above or below a standard threshold, the hope is that lipoprotein distribution profiling (LPD) will provide a more adequate picture of how a person’s health state relates to the combinations of several variables. LPD profiles are associated with obesity and insulin resistance and may serve as a predictor of cardiovascular risk. In the COUNTERSTRIKE project, LPD is further hypothesized to be a more generic aging biomarker that provides a “snapshot of the lipid metabolism, making it possible to assess how lipid metabolism differs in health and disease states” (Monsonis-Centelles et al. 2019). LPD profiling is in the COUNTERSTRIKE project explored as a facilitator of ground-state prevention, by pointing to the need for nutritional changes that could delay the onset of several age-related conditions. One of these is sarcopenia, defined as age-related loss of skeletal muscle mass, which can result in frequent falls, broken bones, or loss of autonomy due to physical disability (Blasimme 2017).9
In the following, we analyze the key tasks involved in the project and their associated challenges. Different tasks were often associated with different disciplines. A large part of the project has focused on the development of experimental testing procedures that allow for rapid and reproducible measurement of lipoprotein distributions. We describe this task in Sect. 3.2 as a step towards precision measurement. Another task focused on clinical applications and involved the collection and modeling of data from an intervention study. Examining this task, we highlight how putting precision measurement to use is dependent on the ability to address the theoretical challenge of defining a reference scale to represent the spectrum of more or less healthy states. In Sects. 3.3. and 4, we analyze how this persistent challenge of the conceptual demarcation of health forces stakeholders to reflect on the methodological and theoretical foundations of the experimental analyses.
Towards precision measurement
Any successful biomarker is preconditioned upon precise and reproducible testing procedures. It is relatively cheap and fast to measure total cholesterol, LDL, and HDL-cholesterol, as is widely done in routine medical health checks. However, the measurement of lipoprotein distribution is much more complicated and labor-intensive, as it involves ultracentrifugation procedures to separate the different lipoproteins. Therefore, an important aim of the COUNTERSTRIKE project has been to develop a faster method relying on nuclear magnetic resonance (NMR) spectroscopy.
Deriving information on LPDs from NMR-spectra requires a calibration model that establishes a relation between NMR-readings to existing references based on ultracentrifugation. An important outcome of COUNTERSTRIKE was to develop such a calibration model and demonstrate the reliability and reproducibility of the NMR-based prediction method in a cohort of fasting individuals (Monsonis-Centelles et al. 2019). To further establish whether the approach is reproducible in different settings, they conducted a so-called “ring test” where collaborators in different countries analyzed the same blood sample results in their laboratories for comparison. After successfully validating NMR-measurement in the ring test on the same cohort, the next step is to validate the method across cohorts and explore the prognostic utility of the multivariate biomarker in practice. A co-PI summarizes the status of the project at this point as follows:
We didn’t know much about [the relation between LPD profiling and sarcopenia] when we started the project. It was a hypothesis. And what do we know now? We now have the method and can use it to see if it works in practice. […] A company that makes NMR-instruments is very interested in this, because it’s the only way you can make these measurements fast, within an hour or half an hour, while an LPD with ultracentrifugation will take about a week and involve a lot of work and multiple samples. So there are lots of benefits, and that is what we aimed for. The method can be used for other purposes such as [tests for] obesity and cardiovascular disease.
The researcher further highlighted the importance of showing what he terms “biological replicates” instead of mere “replication of measurement.” In other words, the group has to show that the calibration method can travel across laboratory contexts and test populations. Commercial companies has previously developed such a calibration method. But an important aim in COUNTERSTRIKE is to develop openly accessible standard operating procedures that will make rapid NMR-based prediction possible as part of routine clinical procedures.
However, the strategy of meeting biological complexity through the upscaled complexity of measurements is not without challenges. It is generally more challenging to relate markers with multidimensional characteristics, such as the LPD and gut microbiome, to clinically relevant phenotypic features across cohorts. It is often assumed that increasing the number of variables in a risk analysis will automatically provide a more accurate picture of the multiple factors influencing disease risk or aging. However, the advantage of access to data on an increasing number of variables often comes with an increased risk of overfitting the model. This challenge is also present in the COUNTERSTRIKE project, as illustrated in the following quote by a statistical modeler:
[I]f you want to have a biomarker that is really useful, [it should] come up in different datasets and not only in your own. And you should also be quite clever about finding these biomarkers, because of what we in the field call overfitting. Maybe you have 100, 200, or 300 patients, which is a lot, but when you measure so much on these patients, you can always find something to discriminate between them.
Researchers often have too much and too little data at the same time. When the number of potentially relevant variables and their combinations (the dimensionality) becomes too large, compared to the sample size (N), it becomes tremendously challenging to identify the most relevant measures. If one compares two population groups to a high number of variables, then it is almost always possible to identify statistically significant differences among these groups. The results of such a “brute force” method are often not predictable when transferred to other populations, or may not be causally relevant for the phenomenon studied.10 This is a general problem for research on aging biomarkers, as also expressed in the following by aging researchers Veytsman et al. who work on genetic biomarkers:
[T]o generate a reliable biomarker test we need to initially profile many more patients than genes… In studies of aging, we essentially predict a “continuous” outcome, as rate of descending along the curve of ageing may change non-linearly. This requires either enrolling multiple cohorts of individuals, each cohort being within certain age bracket, or longitudinal profiling of individuals, with multiple sets of measurements collected long their ageing journey. (Veytsman et al. 2019, p. 15).
The quotes illustrate how improvement of measurement technologies is not only dependent on the accuracy of testing procedures, but also on how the number of variables relate to the size and variation of the reference populations. A strategy used in the COUNTERSTRIKE project to deal with this problem was to test the model based on data from the study that had not been used to develop the model.11 However, the researchers were confronted with the problem, well known in classical epidemiology, that their sample contained too little variation.
In the COUNTERSTRIKE project, the study cohort (volunteers above 65 years) turned out to be a relatively homogenous cohort of very healthy and fit people. This homogeneity provided a challenge for the statistical analysis of the relationship between molecular markers and physical deterioration, and for the effects of the intervention study itself. A positive effect on the increase in muscle mass and overall health was expected to be established in the intervention group via training and dietary changes. However, the intervention group’s sub-cohort were all above 83 and were already relatively healthy (some of them revealed themselves as former marathon runners during the intervention). As a result, they observed no statistically significant differences between the intervention group and a control group that did not undergo training intervention (Bechshøft et al. 2017). The homogeneity of the cohort also presented a problem for the validation of the NMR model, as clarified by a co-PI in the project:
Unfortunately, our cohort consists of old people with little variation in age. And it turns out that they are relatively healthy. […] When we develop methods, we would like to be able to include extremes. In part to see if [the relation] is linear, and if the findings fit in the model. And we haven’t gained that.
The researchers had hoped to use their developed NMR model to define a health spectrum, corresponding to a scale of health scores discussed previously, but the cohort only allowed them to map a small spectrum of possible health states. We shall return to this point in Sect. 4. First, we document how the aim of measuring aging often led to discussions about how to conceptually distinguish between healthy and unhealthy states.
Measuring aging and (re)defining health
An essential vision for the COUNTERSTRIKE project is to develop a so-called health landscape. A health landscape, or “health space,” is a concept increasingly used in metabolomics and preventive systems medicine (Levin 2014). Disease development or aging are represented as trajectories from healthy to unhealthy states (understood as compositions of some parameters). Possible interventions and their effects can be represented as vectors that can alter the speed or direction of an aging trajectory. In the context of the COUNTERSTRIKE project, the health landscape offers a conceptual framework for understanding LPD profiles as points in a multidimensional state space, and the development of these as trajectories of healthy or unhealthy aging. As clarified by a statistical modeler in COUNTERSTRIKE:
The meaningful connection should be described not as causal but as correlative. The task is not to identify an entity or pattern, but more like drawing the map of a health-landscape, in order to establish the relationship between lipoprotein distribution and phenotype.
In other words, rather than aiming to identify specific causal factors, the LPD profile and other measures are envisioned to provide information about how the co-occurrence and distribution of multiple factors contribute to the health status of an individual, via a statistical correlation between LPD values and phenotypic measures. This perspective aligns with reasoning styles in systems biology, where focus is shifted from the identification of specific disease-related causes or mechanisms to an exploration of the spectrum of factors that influence dynamic states, from resilience to deterioration (Sholl and Rattan 2019; see also Sholl this issue). Some areas in the statistically defined landscape are envisioned to be associated with increased risk of disease, and aging can accordingly be viewed as a trajectory towards diseased states that may be counteracted through interventions that push the system into a healthier state or trajectory.
The vision of a health landscape thus entails a view of aging processes as modifiable, similar to how reports on aging by WHO often feature a functional capacity model showing possible outcomes on intervention on aging trajectories (WHO 2002; Lassen and Moreira 2014). However, health landscapes in personalized nutrition differ from general functional capacity models by aiming to account for more individualized factors. In the context of COUNTERSTRIKE, the interventions are envisioned to be recommendations that target a personal profile developed from finer-grained measures, such as LPD profiling or gut microbiome sequencing. In the words of a co-PI:
It’s down to the personal level – what is good for you, and what is not good for you. And it may be that the good nutrition for you is bad for others. If we can, in a relatively simple way, via a blood test or a stool sample, measure something that adequately predicts your response to nutrition, or a physical activity intervention, then we would be much closer to be able to help some. Or help the right people at least, and we could give much more targeted diet recommendations, for instance, that would actually work.
The co-PI further pointed out that many general dietary recommendations lack proper evidence of benefits, and personalized nutrition is hoped to change this situation. Making health landscapes actionable for personalized disease prevention is, however, still a vision to be realized. It requires that it is possible to draw boundaries around what should count as more or less healthy states or trajectories, i.e., it requires a scale on which health scores can be related to phenotypic measures. As seen above, this task was complicated by the homogeneity of the COUNTERSTRIKE cohort. The more fundamental problem is that such scales may be defined in multiple ways, depending on the disciplinary perspective and the means of measurement.
At project meetings between academic collaborators and industry partners, Hillersdal observed how the researchers struggled to find a useful health definition for this purpose. While industry partners were asking for test results that allow them to develop and market protein products to specific LPD-profiles, statisticians expressed difficulties in delivering quantitative results without prior agreement on what health is. In one of the meetings, a statistician drew a circle in a coordinate system (Fig. 1) to represent the requirement of boundaries that quantitatively demarcate data points representing healthy and unhealthy states. Pointing to the circle representing such a boundary, he said: “Define health for me, and I will find it for you,” To that, a metabolomics industry partner replied: “No, give me a pattern, and I will give you health!”.
Fig. 1.
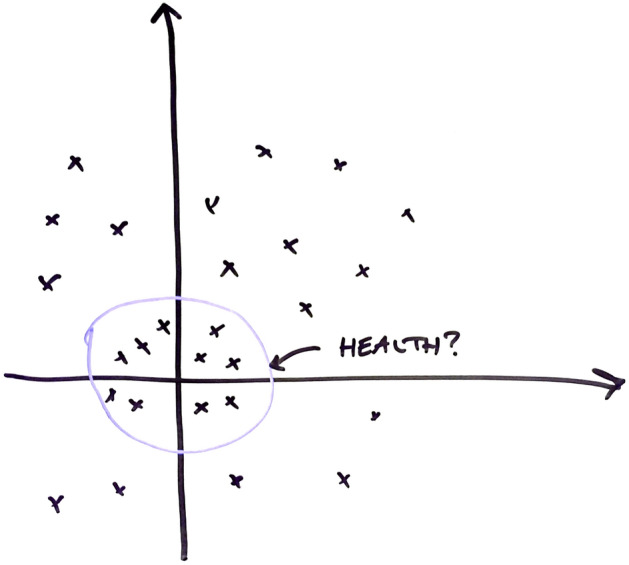
A reconstruction of the illustration drawn by the statistician in a meeting to illustrate the need to draw boundaries between healthy and unhealthy states, represented as test results in a health landscape
The discussion raises interesting questions about who has the capacity and right to define health, and in which scientific field this conceptual demarcation should be made. While the statistician expects the health sciences to provide the starting criteria for his analysis, the metabolomics industry partner’s response illustrates a common expectation that a quantitative definition of health will emerge from a statistical analysis. However, the statistician insists that his analysis cannot get off the ground without a predefined clinical notion of health that he can label the data-populations according to.
The challenges experienced in the COUNTERSTRIKE project is reminiscent of broader discussions in personalized medicine, where pioneers such as Leroy Hood have called for the development of wellness landscapes comparing “the expression level of a sample of molecules in a specimen from the individual with a health-associated reference expression region of the sample of molecules” (Hood and Siegal 2011, §2). However, as documented in an analysis by Vogt, Hoffman, and Getz (2016a, b), identifying a “reference expression” presupposes a health reference population that cannot itself be identified via data-intensive methods. Hence, the requirement for a reference population, makes the effort of biomarker precision inherently dependent on preexisting notions of health that the same field is trying to update and go beyond. The circularity in the task to improve definitions of health or aging need not be vicious, as ongoing scientific analyses can iteratively improve existing biomarkers and sharpen medical definitions (cf. Chang 2004). But it illustrates how translation of precision measurements into prognostic tools requires critical reflection on what is measured.
The difficulty of defining healthy aging was in the COUNTERSTRIKE project further complicated by disciplinary differences, as highlighted in the following quote by a statistician:
Well, I think that is … [the disciplines] have a very different views on what health actually is, and how we actually… With these tools, are we measuring health? Or what aspects of health are we measuring? To what extent are we measuring which aspects of health, and how are they connected? This is super interesting in connection to methodologies, because what has NMR to do with health? What has big data got to do with health?
Following their discussions, Hillersdal observed how the researchers struggled to find a way to integrate various disciplinary perspectives in a joint measurement of healthy aging. Similar challenges of interdisciplinary integration have been reported in the literature (Green and Andersen 2019; MacLeod 2018; Nersessian 2017), including the difficulty of translating dynamic notions of health and disease into quantitative measures (Levin 2014). The challenge to evaluate the utility of the multivariate biomarker is particularly hard when the targets of intervention are not symptomatic people but healthy elderly citizens. Instead of labeling data populations according to clinically validated disease categories, and evaluating effects of intervention according to hard endpoints such as morbidity, the points of comparison are here degrees of health and risk. The challenge to document short-term effects of personalized risk analysis and dietary changes is illustrated in an interview with a metabolomics industry partner:
[T]hese data are not good enough to really say, this product will reduce your cardiovascular risk, but can it say, for example that it reduces LDL cholesterol, and this may be related to a reduced risk. So, it helps to understand the health benefits of our products. And if we understand the mechanisms of actions, we can also find ingredients which target these mechanisms of actions, and then we have much more control on what our products do to the metabolism.
While the quote signals optimism for future benefits, it also highlights how the vision may still be in the distant future. The industry partners further envisioned that supermarkets would begin to cater to the individual consumer and offer specific functional foods suitable for their phenotype. However, the quote also underscores how the relation between the risk factor and disease risk remains uncertain, and it is generally difficult to document the health benefits of nutritional changes that are solid enough to market (Scrinis 2015). The critical challenge for the food industry is to get evidence that documents the health benefits among a group of individuals who are already relatively healthy. In her own words:
[It] is very difficult to show beneficial effects. We have put a lot of money to demonstrate that for our ingredients, but humans are so diverse, and we are not a pharmaceutical company, so we don’t look at diseased people. We look at healthy people. And how do you then say that somebody becomes healthier? How do we define that? That is very difficult.
Reframing the task of biomarkers, from a reduction in morbidity or specific disease risk to make the healthy healthier, raises interesting questions about the scope of personalized nutrition and management of aging bodies in general. It also illustrates how the quest for aging biomarkers presupposes and potentially coproduces norms of what constitutes healthy aging. The quotes seem to suggest that any aging trajectory, no matter how slow it may be, could become a potential target for further risk reduction. Hence, research on aging biomarkers brings new light to discussions about how risk management is historically constructed through a complex interplay of epistemic and non-epistemic values (Douglas 2009; Hilgartner 1992). But whereas scholarship on medicalization has focused on the role of pharmaceutical companies (Dumit 2012; Greene 2007; Oxlund and Whyte 2014), personalized nutrition exemplifies how investments from the food industry also take part in the shaping of health concepts.
Aging as risk
Section 3 described the difficulties of developing a scale or health landscape to compare LPD test results to. A researcher involved in COUNTERSTRIKE commented on this challenge by emphasizing that the identification of a biomarker for sarcopenia requires a study design that divides population groups into different degrees of sarcopenia. He referred to the expression that “it is not physiology, but world records, that define how far man can jump”. This saying highlights how biomarker research needs a material referent of (extreme) variation to get off the ground. The researcher’s critical reflection on the limitations of their study design resonates with George Canguilhem’s criticism of Auguste Comte, who insisted that the normal and its “true limits of variation” could and should be defined before investigating pathological cases (Canguilhem 1966/1996, p. 53). In contrast, Canguilhem argued that the normal is always defined in relation to the pathological, and that neither are free of normative connotations. The discussion on sarcopenia illustrates this point well, as it is not clear where an age-related loss of muscle mass falls on the spectrum between the normal and the pathological.
Aging involves a gradual loss of muscle mass, but at what point does sarcopenia become a risk factor or a health issue that calls for intervention? Sarcopenia was introduced in 1989 as a medical term for age-related loss of muscle mass, but, in 2016, it was assigned a disease code in the International Classification of Disease (ICD-10) system (Bülow et al. 2019). Bülow et al. problematize the new consensus definition because it conflates loss of skeletal muscle mass with loss of physical function. Aside from the risk of making the definition tautological or circular, the definition conceals that loss of physical function can have many other causes. Moreover, loss of physical function is a complex measure that is not straightforwardly related to health problems, which raises ethical dilemmas. As Blasimme (2017) highlights, if sarcopenia is defined as functional decline, and hence as a potential reduction in a person’s freedom to maintain autonomy in daily life, then the health care system would be ethically committed to doing something about it (e.g., to implement a screening and treatment program). On the other hand, if a person could have an age-related loss of muscle mass without suffering from functional decline, the medicalization of sarcopenia becomes more controversial. Hence, debates on sarcopenia illustrate how scientific categories and social values are intertwined and may mutually constrain each other (Longino 2002; Plutynski 2012, 2017).
A recurring theme observed in the project meetings and interviews with participants in the COUNTERSTRIKE project was the question of what happens when the health system increasingly treats risk (e.g., hypertension or sarcopenia), and not only symptomatic disease. If specific profiles of lipoprotein distributions can be shown to be associated with sarcopenia, what is then measured by a test for the risk of sarcopenia? Are we testing for a risk factor of a disease? Or a risk factor of a risk factor? Some participants expressed concerns about the increasing focus on risk factors, about where to draw the line, and whether the benefits of interventions outweigh the potential harms and costs of medical testing. One researcher viewed the future visions for disease prevention as completely unrealistic, as what he described as a “prediction-risk-screening-mania”:
[I]n my view, loss of muscle mass is not a pathological phenomenon, but a symptom. […] It’s this whole idea of treating risk factors… As I said earlier, a disease is ontologically different from a risk factor. A disease is manifest, it exists at the present point in time. It has an ontology, whereas the risk factor, it doesn’t exist as such.
The quote nicely illustrates the awareness that risk categories are plastic and highly dependent on how we draw the lines between health and risk. Since medically or socially constructed kinds can become reinforced through scientific programs (Hacking 1995), the intensified focus on risk factors potentially entails an extension of conditions that need to be treated (see also Greene 2007; Dumit 2012). As illustrated in the example with the stethoscope and measurement of heart murmurs, it is difficult to evaluate the clinical significance of the loss of muscle mass or altered metabolism. As expressed by a statistician in the COUNTERSTRIKE project:
What are we measuring? Aging is not a disease, and it has not been proven that a 20-year-old always has a better metabolism.
The quote indicates a concern that the reframing of aging as risk may promote a vision of aging itself as a disease. Moreover, since the focus is shifted from intervening on symptomatic disease to improving metabolic conditions, the potential becomes open-ended. As Blasimme (forthcoming) points out, ground-state prevention does not necessarily confine itself to a process of normalization of the unhealthy. It can also be seen as a way of enhancing human capacities more generally, through the attempt to control and postpone aging.
It could be argued that reconceptualizing aging as risk is relatively harmless, as long as screening for aging biomarkers is primarily used to evaluate the need for lifestyle changes, such as recommendations for exercise or personalized diet. Such arguments have been advanced in the context of precision medicine, where proponents distinguish their strategies for health promotion from traditional screening (Magis et al. 2018). However, the negative impact of medicalization is not necessarily tied to increased medication. Instead, medicalization refers to the implications of defining more and more aspects of life in relation to aims defined within the medical domain (Conrad 2007; Vogt et al. 2016b). If there is no upper limit to what constitutes healthy aging, any sign of deterioration becomes a potential target for age-preventive strategies (Estes and Binney 1989; Oxford and Whyte 2014). As a result, attempts to slow down the aging process may become a life-long preoccupation, or what Blasimme (2020) calls the agificication of life itself.
In the context of personalized medicine and personalized nutrition, concerns also relate to the increasing organization of health care around individualized risk (Green and Vogt 2016; Hogle 2019). While the notions of active or healthy aging come with new possibilities for “disciplining aging,” they also involve increasing responsibilization of individuals and potential stigmatization of “passive” lifestyles as a failure to age successfully (Katz 1996; Katz and Marshall 2003; Lassen 2014; Lassen and Jespersen 2017). In other words, the emphasis on healthy aging and public health policies implies an imperative to implement strategies towards this aim. As a researcher in the COUNTERSTRIKE project problematized:
It again returns to the question of sarcopenia. Before we have even figured out what it is, we are supposed to move on to how we can make things better. But what does “better” mean? Isn’t that something that the individual in a free, liberal society has to decide for himself? To decide what is good or bad for him, as long as it doesn’t hurt anyone else?
The researcher here points to how the aim to avoid sarcopenia is not self-evident, as it is unclear to what extent sarcopenia presents a health problem, and for whom. The quote highlights how an ideal of healthy aging always promotes certain norms of socially and clinically acceptable forms of living and aging. By pointing to physiological problems and possible interventions to solve these, prevention programs implicitly assume that staying healthy is the most important priority. In practice, however, the norms that underlie health programs sometimes clash with the situated values of elderly individuals (Wareham 2018).
An ethnographic study associated with the CALM project documented how several project participants viewed the active aging policies in the European Union as an intrusion on how they should live their lives (Lassen and Jespersen 2017). Several participants viewed chronic diseases and functional decline as a natural part of life, of growing older, and not as something that necessarily calls for preventive action. As the researchers highlight:
While active aging provides the answer that independence and activity are good for all, it does not answer what happens to those who cannot live up to the expectations of an active and healthy old age, and who might envision other ways of aging well. (Lassen and Jespersen 2017, p. 153).
Alternative visions of aging well can also be observed among the resourceful. Even individuals who were very active, i.e., well above average for their age group, criticized the health policies as part of a “health regime” taken too far. They joked about how drinking schnapps and playing billiard were part of what they understood as “active aging” and that they had earned a right to live as they please. Similarly, Hillersdal observed in a focus group interview with elderly research participants in COUNTERSTRIKE how the suggestion of protein supplements to help prevent sarcopenia conflicted with their concept of healthy food. These observations resonate with another Danish study documenting how users of blood pressure meters and glucometers expressed resistance towards the idea of always being reminded of potential risks and responsibilities to control these (Andersen and Whyte 2014; Oxlund and Whyte 2014). Such observations should caution against overenthusiasm about the effects of techniques that can “yield data independent of the opinions and appearance of the patient” (Reiser 1978, p. 43). For healthy aging strategies to have the intended effect, they have to align with the target audience’s values.
The norms of healthy or active aging in health policies may be prone to what philosopher Nancy Jecker calls midlife bias, i.e., the tendency to assume that values important to individuals at mid-life also transfer to older age (Jecker 2020, 2021).12 Health policies may presuppose that values such as independence, physical activity, and autonomy are good for all and of equal importance at all times. Jecker highlights the extreme example of how we often try to respect autonomy long after it is beyond the capacities of a person with severe dementia (see also Gjødsbøl et al. 2017). Similarly, it is important to keep in mind that preventing sarcopenia to maintain physical functionality may not always be the primary goal, as it has to compete with other values of a good life, such as freedom from dietary restrictions or stigmatization of lifestyle choices. From the perspective of elderly citizens, it may also be relevant to consider how maintenance of autonomy, e.g., capacity to perform daily tasks despite sarcopenia, can often be achieved in other ways, such as via the use of assistive devices (Blasimme 2017). Our aim is not to problematize current health policies per se, but rather to expose the normative assumptions in the discourse on individualized strategies for healthy aging and open these for further scrutiny.13
Interestingly, these deep philosophical problems were also present in meetings in the COUNTERSTRIKE project. In one meeting, one of the researchers asked, “why don’t we talk about the good death?” The open question was posed as a reaction to the focus on the physiological aging body as something that needs to be shaped and intervened on, thus excluding vulnerability and decay as natural processes of old bodies (cf. Kaufman 2015). It highlights how the very notions of “aging biomarkers,” “healthy aging,” and “active aging” are simultaneously scientific concepts and policy tools that influence the very way aging is viewed and enacted (Lassen and Moreira 2014; Douglas 2009).
Conclusion
Advanced technologies of measurement and big data analysis are opening new venues for population-wide screening-efforts to determine disease risk and potential individual actions on entire age-segments. This paper has described an attempt to develop a multivariate aging biomarker, understood as a sign of functional deterioration that leads to increased risk of sarcopenia and other age-related conditions. The approach exemplifies what Blasimme (this issue) calls ground-state prevention, which aims to delay the onset of several life-limiting pathologies. In this case, the researchers developed a data-intensive tool for precise measurement of lipoprotein distribution. However, they were confronted with difficulties in interpreting the measures without a stable referent for what counts as healthy and unhealthy states.
It has been exciting to follow how the challenges encountered in the exploratory COUNTERSTRIKE project led participants to critically reflect on their own project’s implicit assumptions. Participants raised concerns about the potential increase in the medicalization of aging, which may follow the intensified focus on risk factors, to which their own project also contributes. However, the fieldwork also documented a joint commitment to reduce overmedicalization through imprecise testing. In this sense, precision measurement points to the potential of risk-stratified strategies in response to growing insights into how people age differently. We find that the development of the COUNTERSTRIKE project exemplifies an unusual space for scientific exploration, philosophical reflection, and at times expression of different viewpoints among the different participants. These aspects of scientific practices are not visible in scientific publications but can be documented through ethnographic fieldwork.
The COUNTERSTRIKE project illustrates how epistemic and ethical issues intersect when health policies and scientific efforts increasingly focus on “risk reduction”. The intensified focus on prevention of aging let us believe that we can “grow older without aging” (Katz and Marshall 2003), by providing possibilities of action to counteract the aging process. Yet, as this ideal seems achievable only to a limited extent, it raises the question of what it means to age “healthy” or “naturally,” whether there is an upper limit to healthy aging, and how such limits are shaped by science policy. The intensified focus on aging biomarkers and the management of age-related disease raise intriguing questions about the implications of medicalization and agification of life itself, and about what the promotion of better and longer lives entails. While science can inform us about the relations between phenotypic functions and resilience to perturbations, we cannot escape the difficult ethical discussion of where it is meaningful to draw the line.
Acknowledgements
We are grateful for our interlocutors’ time and confidence in letting us follow their discussions and research practice in the COUNTERSTRIKE project. We would like to thank Giovanni Boniolo, Marco Nathan, and Alessandro Blasimme for organizing the workshop “Rethinking Ageing: Philosophy of Science and Ethics”, held at the Civitas Vitae Reserch Centre, Fondazione OIC Onlus, Padova, Italy, on December 13, 2019, and for editing this topical collection. We received valuable feedback on an earlier version of this paper from the audience of the workshop, the MeInWe-group in Copenhagen, Robert Batterman, Sabina Leonelli, and two anonymous reviewers. Our research is supported by The Carlsberg Foundation (Semper Ardens grant CF17-0016 for the project Personalized Medicine in the Welfare State, MeInWe) and the Innovation Fund Denmark.
Footnotes
Multivariate biomarkers integrate many variables simultaneously, drawing on datamining strategies and multivariate statistics.
Geriatrics, in contrast to gerontology, does not aim at understanding aging per se, but at assessing aging in order to improve the management of age-related disease (Gilleard and Higgs 2016).
For an overview of candidates for human aging biomarkers, see the online database called Digital Aging Atlas (http/ageing-map.org, and Craig et al. 2014).
Similar concerns about the risk of overdiagnosis have been expressed in the historical introduction of other technologies of measurement, such as blood pressure measurement, electrocardiography, and X-ray technology (Mackenzie 1919, pp.194–195), cancer screening via imaging technologies (Plutynski 2012, 2017), and “big data screening” via omics technologies (Green and Vogt. 2016; Vogt et al. 2019).
This challenges is related to what is more generally discussed as the reference class problem, i.e., the problem of how the choice of references classes can affect the assignment of probabilities to the individual case (see also Reichenbach 1949; Hájek 2007).
Estimating how such changes occur over time (and thus how to classify age groups) is particularly challenging as one cannot assume that the speed of deterioration changes linearly with chronological age. A recent study suggests that blood metabolites change in wave-like dynamics with rapid shifts at certain points in time (Lehallier et al. 2019). Discussing the implications of such findings is, however, beyond the scope of this paper.
Project description at the official homepage of the COUNTERSTRIKE project: https://food.ku.dk/english/research_at_food/research-projects/2015/counterstrike-counteracting-sarcopenia-with-proteins-and-exercise/. Accessed Nov 26, 2020.
Among the exclusion criteria were health and elderly care dependency, serious chronic diseases, as well as rheumatic diseases affecting joints and muscles. For further information on exclusion criteria, see table 1 in (Bechshøft et al. 2016).
The specific definition of sarcopenia is currently subject to controversy. We discuss this further in Sect. 4.
Factors that look statistically predictive often turn out to be causally irrelevant, e.g., when Google Flu Trends predicted peaks in flu incidents based on the correlation of flu epidemics and the winter season (Lazer et al. 2014).
Another important strategy to deal with overfitting is feature selection, i.e., select for those known to be biologically relevant or to remove variables that are only weakly correlated with the age. This can for instance be done through a statistical procedure called Principal Component Analysis.
Similarly, Wareham (this issue) questions the the objectivity of midlife valuations of life at old age, considering both the aim to live longer and hoping to die before experiencing harms of aging.
Similarly, Sholl and Rattan (2019) emphasize that the most effective strategies to promote healthy aging may be to alter aspects of the environment, i.e., to shift the perspective towards prevention from the “outside-in”.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abramson JD, Rosenberg HG, Jewell N, Wright JM. Should people at low risk of cardiovascular disease take a statin? BMJ. 2013;347:f6123. doi: 10.1136/bmj.f6123. [DOI] [PubMed] [Google Scholar]
- Andersen JH, Whyte SR. Measuring risk, managing value: Health technology and subjectivity in Denmark. Anthropology & Medicine. 2014;21(3):265–276. doi: 10.1080/13648470.2014.907022. [DOI] [PubMed] [Google Scholar]
- Baker GT, Sprott RL. Biomarkers of aging. Experimental Gerontology. 1988;23(4–5):223–239. doi: 10.1016/0531-5565(88)90025-3. [DOI] [PubMed] [Google Scholar]
- Bechshøft RL, Reitelseder S, Højfeldt G, Castro-Mejía JL, Khakimov B, Ahmad HFB, Lassen AJ. Counteracting age-related loss of skeletal muscle mass: a clinical and ethnological trial on the role of protein supplementation and training load (CALM Intervention Study): Study protocol for a randomized controlled trial. Trials. 2016;17(1):397. doi: 10.1186/s13063-016-1512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechshøft RL, Malmgaard-Clausen NM, Gliese B, Beyer N, Mackey AL, Andersen JL, Holm L. Improved skeletal muscle mass and strength after heavy strength training in very old individuals. Experimental Gerontology. 2017;92:96–105. doi: 10.1016/j.exger.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Biddle JB. Inductive risk, epistemic risk, and overdiagnosis of disease. Perspectives on Science. 2016;24(2):192–205. [Google Scholar]
- Binney, N. (2016). On the Historical Contingency of Medical Knowledge: An integrated historical and philosophical investigation into the development and epistemic status of knowledge about heart failure. PhD dissertation, University of Exeter.
- Blasimme, A. (this issue). The plasticity of ageing and the rediscovery of ground-state prevention. History and Philosophy of the Life Sciences. [DOI] [PMC free article] [PubMed]
- Blasimme A. Physical frailty, sarcopenia, and the enablement of autonomy: philosophical issues in geriatric medicine. Aging Clinical and Experimental Research. 2017;29(1):59–63. doi: 10.1007/s40520-016-0714-3. [DOI] [PubMed] [Google Scholar]
- Blasimme A. Mind’s frailty: Elements of a geriatric logic in the clinical discourse about dementia prevention. In: Liebing A, Schicktanz S, editors. Preventing Dementia? Critical Perspectives on a New Paradigm of Preparing for Old Age. New York: Berghahn Books; 2020. pp. 110–130. [Google Scholar]
- Blasimme, A. (forthcoming). Producing health: Canguilhem and the contemporary clinical discourse about aging. In P.-O. Methot (ed.). The normal and the pathological: Writings on Georges Calguilhem. Paris, France: Herman Publisher.
- Bülow J, Ulijaszek SJ, Holm L. Rejuvenation of the term sarcopenia. Journal of Applied Physiology. 2019;126(1):255–256. doi: 10.1152/japplphysiol.00400.2018. [DOI] [PubMed] [Google Scholar]
- Butler RN, Sprott RL. Biomarkers of Aging: From Primitive Organisms to Man. New York, NY: International Longevity Center; 2000. [Google Scholar]
- Canguilhem G. The normal and the Pathological. New York: Zone Books; 1966. [Google Scholar]
- Chang H. Inventing Temperature: Measurement and Scientific Progress. New York: Oxford University Press; 2004. [Google Scholar]
- Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Evans S. Interpretation of the evidence for the efficacy and safety of statin therapy. The Lancet. 2016;388(10059):2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- Conrad P. The Medicalization of Society: On the Transformation of Human Conditions Into Treatable Disorders. Baltimore: John Hopkins University Press; 2007. [Google Scholar]
- Craig T, Smelick C, Tacutu R, Wuttke D, Wood SH, Stanley H, de Magalhaes JP. The Digital Ageing Atlas: Integrating the diversity of age-related changes into a unified resource. Nucleic Acids Research. 2014;43(D1):D873–D878. doi: 10.1093/nar/gku843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas H. Science, Policy, and the Value-Free Ideal. Pittsburgh: University of Pittsburgh Press; 2009. [Google Scholar]
- Dumit J. Drugs for Life. How Pharmaceutical Companies Define our Health. Durham and London: Duke University Press; 2012. [Google Scholar]
- Elliott KC, McKaughan DJ. Nonepistemic values and the multiple goals of science. Philosophy of Science. 2014;81(1):1–21. [Google Scholar]
- Estes CL, Binney EA. Thebiomedicalization of aging: dangers and dilemmas. The Gerontologist. 1989;29(5):587–596. doi: 10.1093/geront/29.5.587. [DOI] [PubMed] [Google Scholar]
- European Commission. (2018). The 2018 ageing report: Economic and budgetary projections for the EU member states (2016–2070). Institutional Paper No. 79. Luxembourg: Publications Office of the European Union.
- Franceschi C, Bonafè M. Centenarians as a model for healthy aging. Biochemical Society Transactions. 2003;31(2):457–461. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- Gilleard, C., & Higgs, P. (2016). Gerontology versus geriatrics: Different ways of understanding ageing and old age. In The Palgrave handbook of the philosophy of aging (pp. 31–48). Palgrave Macmillan, London.
- Gjødsbøl IM, Koch L, Svendsen MN. Resisting decay: Disposing (of) and (re)valuing people with late stage dementia in the nursing home. Social Science and Medicine. 2017;184:116–123. doi: 10.1016/j.socscimed.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Green S, Andersen H. Systems science and the art of interdisciplinary integration. Systems Research and Behavioral Science. 2019;36(5):727–743. [Google Scholar]
- Green S, Vogt H. Personalizing medicine: Disease prevention in silico and in socio Humana. Mente Journal of Philosophical Studies. 2016;9(30):105–145. [Google Scholar]
- Greene JA. Prescribing by Numbers: Drugs and the Definition of Disease. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- Hacking, I. (1995). The looping effects of human kinds. In D. Sperber, D. Premack, & A. J. Premack (Eds.), Symposia of the Fyssen Foundation. Causal cognition: A multidisciplinary debate (p. 351–394). Clarendon Press/Oxford University Press.
- Hájek A. The reference class problem is your problem too. Synthese. 2007;156(3):563–585. [Google Scholar]
- Hilgartner S. The social sonstruction of risk objects: Or, how to pry open networks of risk. In: Short JF, Clarke L, editors. Organizations, Uncertainties, and Risk. Boulder: Westview Press; 1992. [Google Scholar]
- Hogle LF. Accounting for accountable care: Value-based population health management. Social Studies of Science. 2019;49(4):556–582. doi: 10.1177/0306312719840429. [DOI] [PubMed] [Google Scholar]
- Hood, L., & Siegel, A. F. (2011). U.S. Patent No. 8,036,834. Washington, DC: U.S. Patent and Trademark Office.
- Jecker N. Ending midlife bias: New values for old age. Oxford: Oxford University Press; 2020. [Google Scholar]
- Jecker NS. The time of one's life: Views of aging and age group justice. History and Philosophy of the Life Sciences. 2021;43:24. doi: 10.1007/s40656-021-00377-8. [DOI] [PubMed] [Google Scholar]
- Jørgensen T, Jacobsen RK, Toft U, Aadahl M, Glümer C, Pisinger C. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ. 2014;348:g3617. doi: 10.1136/bmj.g3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. Disciplining Old Age: The Formation of Gerontological Knowledge. Charlottesville: University of Virginia Press; 1996. [Google Scholar]
- Katz S, Marshall B. New sex for old: Lifestyle, consumerism, and the ethics of aging well. Journal of Aging Studies. 2003;17(1):3–16. [Google Scholar]
- Kaufman SR. Ordinary Medicine: Extraordinary Treatments, Longer Lives, and Where to Draw the Line. Durham: Duke University Press; 2015. [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Rando TA. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjøller M, Davidsen M, Juel K. Ældrebefolkningens Sundhedstilstand i Danmark—Analyser baseret på Sundheds—og sygelighedsundersøgelsen 2005 og udvalgte registre København: Sundhedsstyrelsen. Systems Research and Behavioral Science. 2010;36(5):727–743. [Google Scholar]
- Lamb S, editor. Successful Aging As a Contemporary Obsession: Global Perspectives. New Brunswick: Rutgers University Press; 2017. [Google Scholar]
- Lassen, A. J. (2014). Active ageing and the unmaking of old age: The knowledge productions, everyday practices, and policies of the good late life. PhD dissertation, September 19, Faculty of Humanities, University of Copenhagen.
- Lassen, A. J., & Jespersen, A. P. (2017). Getting old and keeping going: The motivation technologies of active aging in Denmark. In Successful aging? Global perspectives on a contemporary obsession (pp. 141–153). Rutgers University Press.
- Lassen AJ, Moreira T. Unmaking old age: Political and cognitive formats of active ageing. Journal of Aging Studies. 2014;30:33–46. doi: 10.1016/j.jaging.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Law J. Aircraft stories: Decentering the Object in Technoscience. Durham: Duke University Press; 2002. [Google Scholar]
- Lazer D, Kennedy R, King G, Vespignani A. The parable of Google Flu: traps in big data analysis. Science. 2014;343(6176):1203–1205. doi: 10.1126/science.1248506. [DOI] [PubMed] [Google Scholar]
- Lehallier B, Gate D, Schaum N, Nanasi T, Lee SE, Yousef H, Sathyan S. Undulating changes in human plasma proteome profiles across the lifespan. Nature Medicine. 2019;25(12):1843–1850. doi: 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin N. Multivariate statistics and the enactment of metabolic complexity. Social Studies of Science. 2014;44(4):555–578. doi: 10.1177/0306312714524845. [DOI] [PubMed] [Google Scholar]
- Longino H. The Fate of Knowledge. Princeton: Princeton University Press; 2002. [Google Scholar]
- Mackenzie J. The Future of Medicine. London: Frowde, Hodder & Stoughton; 1919. [Google Scholar]
- MacLeod M. What makes interdisciplinarity difficult? Some consequences of domain specificity in interdisciplinary practice. Synthese. 2018;195(2):697–720. [Google Scholar]
- Magis AT, Earls JC, Glusman G, Omenn GS, Lovejoy JC, Price ND, Hood L. Reply to Precision medicine in the clouds. Nature biotechnology. 2018;36(8):680–682. doi: 10.1038/nbt.4211. [DOI] [PubMed] [Google Scholar]
- Mau S. The Metric Society. On the Quantification of the Social. Cambridge: Polity Press; 2019. [Google Scholar]
- Mol A. The Body Multiple: Ontology in Medical Practice. Durham: Duke University Press; 2002. [Google Scholar]
- Monsonis-Centelles S, Hoefsloot HC, Engelsen SB, Smilde AK, Lind MV. Repeatability and reproducibility of lipoprotein particle profile measurements in plasma samples by ultracentrifugation. Clinical Chemistry and Laboratory Medicine (CCLM) 2019;58(1):103–115. doi: 10.1515/cclm-2019-0729. [DOI] [PubMed] [Google Scholar]
- Moreira T. Science, Technology and the Again Society. London: Routledge; 2017. [Google Scholar]
- Moskalev A, editor. Biomarkers of Human Aging. Cham: Springer International Publishing; 2019. [Google Scholar]
- Mulherin SA, Miller WC. Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Annals of Internal Medicine. 2002;137(7):598–602. doi: 10.7326/0003-4819-137-7-200210010-00011. [DOI] [PubMed] [Google Scholar]
- Nathan, M. (this issue). Does anybody really know what time it is? From biological age to biological time. History and Philosophy of the Life Sciences. [DOI] [PMC free article] [PubMed]
- Nersessian NJ. Systems biology modeling practices: Reflections of a philosopher-ethnographer. In: Green S, editor. Philosophy of systems biology Perspectives from scientists and philosophers. Cham: Springer; 2017. pp. 215–226. [Google Scholar]
- OECD. (1998). Maintaining prosperity in an ageing society. Policy Brief. OECD Observer.
- Oxlund B, Whyte SR. Measuring and managing bodies in the later life course. Journal of Population Ageing. 2014;7(3):217–230. [Google Scholar]
- Plutynski A. Ethical issues in cancer screening and prevention. Journal of Medicine and Philosophy. 2012;37(3):310–323. doi: 10.1093/jmp/jhs017. [DOI] [PubMed] [Google Scholar]
- Plutynski A. Safe or sorry? Cancer screening and inductive risk. In: Elliot KC, Richards T, editors. Exploring inductive risk: Case studies of values in science. Oxford: Oxford University Press; 2017. pp. 149–169. [Google Scholar]
- Putin E, Mamoshina P, Aliper A, Korzinkin M, Moskalev A, Kolosov A, Zhavoronkov A. Deep biomarkers of human aging: application of deep neural networks to biomarker development. Aging. 2016;8(5):1021. doi: 10.18632/aging.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach H. The Theory of Probability. Berkeley: University of California Press; 1949. [Google Scholar]
- Reiser SJ. Medicine and the Reign of Technology. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- Ruscica M, Macchi C, Pavanello C, Corsini A, Sahebkar A, Sirtori CR. Appropriateness of statin prescription in the elderly. European Journal of Internal Medicine. 2018;50:33–40. doi: 10.1016/j.ejim.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Russo F, Vineis P. Opportunities and challenges of molecular epidemiology. In: Boniolo G, Nathan M, editors. Philosophy of Molecular Medicine Foundational Issues in Research and Practice. New York: Routledge; 2016. pp. 260–290. [Google Scholar]
- Scrinis G. Nutritionism: the science and politics of dietary advice. New York: Columbia University Press; 2015. [Google Scholar]
- Sholl, J. (this issue). Can aging research generate a theory of health? History and Philosophy of the Life Sciences. [DOI] [PubMed]
- Sholl J, Rattan SI. Biomarkers of health and healthy ageing from the outside-in. In: Moskalev A, editor. Biomarkers of Human Aging. Springer: Cham; 2019. pp. 37–46. [Google Scholar]
- Sprott RL. Biomarkers of aging and disease: introduction and definitions. Experimental Gerontology. 2010;45(1):2–4. doi: 10.1016/j.exger.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Veytsman B, Cui T, Baranova A. Practical detection of biological age: Why it is not a trivial task. In: Moskalev A, editor. Biomarkers of Human Aging. Cham: Springer; 2019. pp. 7–21. [Google Scholar]
- Vogt H, Hofmann B, Getz L. Personalized medicine: evidence of normativity in its quantitative definition of health. Theoretical Medicine and Bioethics. 2016;37(5):401–416. doi: 10.1007/s11017-016-9379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt H, Hofmann B, Getz L. The new holism: P4 systems medicine and the medicalization of health and life itself. Medicine, Health Care and Philosophy. 2016;19(2):307–323. doi: 10.1007/s11019-016-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt H, Green S, Ekstrøm CT, Brodersen J. How precision medicine and screening with big data could increase overdiagnosis. BMJ. 2019;366:l5270. doi: 10.1136/bmj.l5270. [DOI] [PubMed] [Google Scholar]
- Wareham, C. S. (this issue). Between hoping to die and longing to live longer. History and Philosophy of the Life Sciences. [DOI] [PubMed]
- Wareham CS. What is the ethics of ageing? Journal of Medical Ethics. 2018;44(2):128–132. doi: 10.1136/medethics-2017-104374. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Active ageing: A policy framework. Geneva: World Health Organization; 2002. [PubMed] [Google Scholar]
- World Health Organization . World report on ageing and health. Geneva: World Health Organization; 2015. [Google Scholar]


