Supplemental Digital Content is available in the text.
Keywords: cohort studies, COVID-19, hydroxymethylglutaryl-CoA reductase inhibitors, mortality, propensity score
Abstract
Objective:
Although statins are widely prescribed lipid-lowering drugs, there are concerns about the safety of their use in the context of coronavirus disease 2019 (COVID-19), since statins increase the expression of ACE2 (angiotensin-converting enzyme 2). This study aimed to disclose the association between statins and 60-day COVID-19 mortality.
Approach and Results:
All patients hospitalized with laboratory-confirmed COVID-19 were enrolled in this study from January 19 to April 16, 2020, in Korea. We evaluated the association between the use of statins and COVID-19–related mortality in the overall and the nested 1:2 propensity score–matched study. Furthermore, a comparison of the hazard ratio for death was performed between COVID-19 patients and a retrospective cohort of patients hospitalized with pneumonia between January and June 2019 in Korea. The median age of the 10 448 COVID-19 patients was 45 years. Statins were prescribed in 533 (5.1%) patients. After adjusting for age, sex, and comorbidities, Cox regression showed a significant decrease in hazard ratio associated with the use of statins (hazard ratio, 0.637 [95% CI, 0.425–0.953]; P=0.0283). Moreover, on comparing the hazard ratio between COVID-19 patients and the retrospective cohort of hospitalized pneumonia patients, the use of statins showed similar benefits.
Conclusions:
The use of statins correlates significantly with lower mortality in patients with COVID-19, consistent with the findings in patients with pneumonia.
Highlights.
The use of statins was significantly associated with lower mortality in coronavirus disease 2019 (COVID-19) patients.
The protective effect of statins was similar to pneumonia patients in Korea, in 2019.
Statins can be considered as a part of the supportive regimen during the treatment of COVID-19.
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, Hubei, China, in December 2019 and since then became a pandemic, as declared by the World Health Organization on March 11, 2020.1 As of October 20, >40 118 333 confirmed cases and 1 114 749 deaths have been reported worldwide.2 Although the fatality rate associated with SARS-CoV-2 is lower than that in the context of SARS-CoV or Middle East respiratory syndrome–related coronavirus, the elderly and people experiencing chronic conditions are prone to serious outcomes.1
The most common comorbidities among COVID-19 patients include hypertension, diabetes, and coronary artery disease1; therefore, frequently, such patients are treated with HMG-CoA (hydroxy-methyl-glutaryl-coenzyme A) reductase inhibitors, known as statins. Of note, statin treatment was reported to increase the expression of ACE2 (angiotensin-converting enzyme 2) in the heart of experimental models via epigenetic histone modifications.3 Given that SARS-CoV-2 enters the target cell via ACE2 cell surface receptors,4 the use of statins in the context of COVID-19 initially brought some concerns. In fact, the increased expression of ACE2 was shown to facilitate infection with SARS-CoV5; therefore, statins might increase the risk of COVID-19. In contrast, besides their lipid-lowering activity, statins are well known for pleiotropic effects on inflammation, contributing to their beneficial impact in patients with conditions other than cardiovascular diseases, including autoimmune diseases,6 community-acquired pneumonia, and sepsis.7,8 The immunomodulatory effect of statins depends on the inhibition of the production of isoprenoids and the consequent downregulation of redox-sensitive proinflammatory transcription factors such as NF-κB (nuclear factor-κB).6 Moreover, statins were suggested to potentially inhibit the SARS-CoV-2 main protease—a key coronavirus enzyme—thus exerting antiviral activity.9 Of note, previously, statins were effective in targeting the host response and preventing endothelial barrier damage in patients infected with the Ebola virus during the recent Ebola outbreak in West Africa.10 Therefore, some hospitals included stains in the COVID-19 treatment protocol.11
Korea has registered all SARS-CoV-2–infected patients and managed them either in hospitals or in community treatment centers; therefore, follow-up information is available for all patients.12 Therefore, Korea offers the ideal scenario to comprehensively evaluate the association between the use of statins and the severity of COVID-19. Therefore, in the present study, we investigated the association between the use of statins and COVID-19–related deaths in all COVID-19 patients in Korea. Moreover, the hazard ratio (HR) was compared between COVID-19 patients and a retrospective cohort of patients hospitalized with pneumonia between January and June 2019 in Korea.
Methods
Study Design
We report a population-based cohort study supported by the Korea Disease Control and Prevention Agency, the National Health Insurance Service, and the Korean Society of Hypertension. The study was approved by the Institutional Review Board of Seoul National University Hospital (No. 2003-102-1109). Informed consent was waived by the institutional review board. All authors reviewed the manuscript for the accuracy and completeness of the data.
Data Collection
Data related to all of the 10 448 patients, with laboratory-confirmed infection with SARS-CoV-2 since the first case on January 19 up to April 16, 2020, in Korea, were retrieved from the compiled information available at the National Health Insurance Service. A case designated confirmed for COVID-19 was defined as one whose nasal and pharyngeal swab specimens tested positive in the context of high-throughput sequencing or real-time reverse transcriptase polymerase chain reaction assays.13,14 Diagnoses, prescription records, and information related to medical utilization were extracted from the national health information database—a public database compiled by the National Health Insurance Service.15 Furthermore, for the National Health Insurance Service claim data analysis, operational definitions using the ICD-10-CM (International Classification of Diseases, Tenth Revision, Clinical Modification) codes were applied (Table I in the Data Supplement).
The association between the use of statins and the severity of pneumonia in hospitalized Korean patients between January and June 2019 was investigated using the information retrieved from the national health information database.15 Of note, the national health information database holds information related to health care utilization, health screening, sociodemographic variables, and mortality of the entire Korean population; overall, the information available refers to data gathered during the process of claiming health care services. It includes information related to the records of inpatient and outpatient usage (diagnosis, length of stay, treatment costs, and services received) and prescription records (drug code, days prescribed, and daily dosage). Importantly, in our analysis, all potential identifiers in the national health information database data were removed, and the information was linked with new unidentifiable codes representing individual patients.
Study Outcomes and Definitions
In the present study, the association between the use of statins and COVID-19–related mortality within 60 days was investigated. All commercially available statins (atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin) in Korea were included in the analysis. Dichotomous variables were created to identify the use of statins defined as a filled prescription with >60% of the proportion of days covered during 3 months before the diagnosis of COVID-19.16
Statistical Analysis
The association of the clinical characteristics with mortality within 60 days was tested using bivariate statistics. Continuous variables were analyzed using t test, and categorical variables were analyzed with the χ2 test. The time to occurrence of the COVID-19–related death in patients prescribed with statins was analyzed using Kaplan-Meier survival curves displaying the failure functions. Statistical significance was assessed using the log-rank test. Moreover, a Cox proportional hazard model was used for the estimation of unadjusted HRs and 95% CIs for statins. The additional factors included in the model as covariates were age group, sex, comorbidities including hypertension, type 2 diabetes, coronary heart diseases, heart failure, stroke, chronic obstructive pulmonary disorder (COPD), cancer, and chronic kidney diseases. To control confounding biases, we performed propensity score matching (PSM) using sex, age at diagnosis, and history of comorbidities (hypertension, diabetes, coronary artery disease, and COPD) in both study populations. Each statin user was matched with 2 nonstatin users; the details are provided in Tables II and VII in the Data Supplement. We diagnosed the proportional hazard assumption using the goodness-of-fit test and the Schoenfeld residual plots. Furthermore, we similarly analyzed with pneumonia 2019 patients. We standardized the difference of the coefficient of prior statin use for risk of death between COVID-19 patients and pneumonia patients in 2019. If the absolute value of Z score is >1.96, we regarded that the comparative analyses show the significant difference. P<0.05 was considered statistically significant. All analyses were performed using SAS, version 7.15 (SAS Institute, Inc, Cary, NC), and R, version 4.0.0 (R Development Core Team, Vienna, Austria).
Role of the Funding Source
This study was supported by a research grant from the Korea Disease Control and Prevention Agency (No. 4838-330-320-01) and by the Seoul National University Hospital (No. 04-2020-0030). The Korea Disease Control and Prevention Agency supported the organization of the National Committee for Clinical Management of Emerging Infectious Diseases, which conducted this study.
Results
The present study includes data related to a total of 10 448 COVID-19 patients who were hospitalized in Korea from January 19, 2020, through April 16, 2020. As of April 24, 2020, 228 of these patients (2.18%) died. Among them, 533 statin and 1066 nonstatin users were examined in the nested case-control study.
The demographic and clinical characteristics of the patients in the cohort and the nested 1:2 matched study are summarized in Table 1. Of the patients, 60% were women. The median age of all patients was 45 years; of note, 4.8% were aged ≤19 years, 20.3% were aged 60 to 79 years, and 5.1% were aged ≥80 years. Furthermore, 38.1% had at least one preexisting comorbidity; 20.6% had hypertension, 17.9% had diabetes, 14.2% had COPD, and 4.5% had cancer. Patients prescribed with statins were >20 years older with a higher number of comorbidities than that of the nonusers and accounted for 5.1% of the overall cases. On the other hand, most of variables in the nested matched study are balanced, compared with the overall cohort (Table II in the Data Supplement).
Table 1.
Clinical Characteristics of COVID-19 Patients
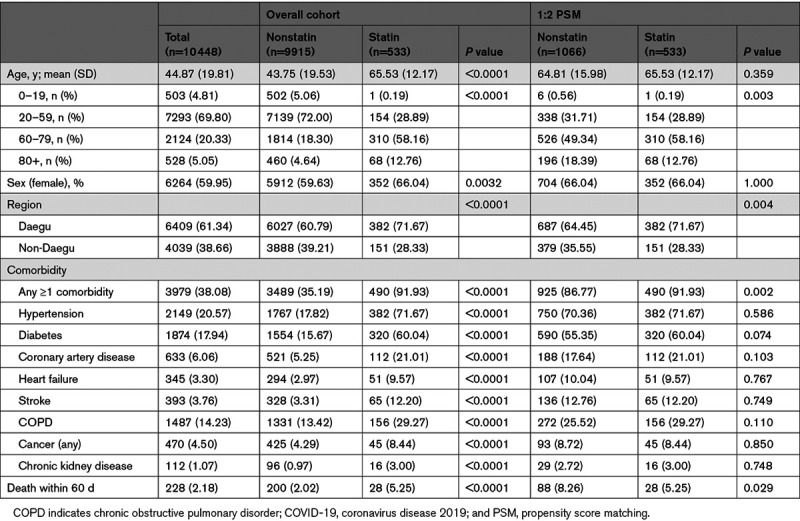
The crude HRs of elderly (>65 years) and hypertension were 36.5 ([95% CI, 24.6–54.2] P<0.0001) and 14.1 ([95% CI, 10.3–19.2] P<0.0001) in the overall cohort, respectively (Table 2). Even in the context of multivariate regression analysis, old age (>65 years) was by far the most important predictor of COVID-19–related mortality in patients. After adjusting for age, sex, and the history of comorbidities (hypertension, diabetes, cancer, COPD, stroke, coronary artery disease, heart failure, and chronic renal disease) before the diagnosis of COVID-19, Cox regression showed a significant decrease in HR associated with the use of statins (HR, 0.637 [95% CI, 0.425–0.953]; P=0.0283; Figure 1A). Next, the association of statins was stratified by age, sex, and comorbidities. A decreased risk of death with statin use was consistent across all subgroups (Figure 2). After PSM, Cox regression showed a more significant decrease in HR by 45% associated with the use of statins (HR, 0.553 [95% CI, 0.360–0.852]; P=0.0071; Table III in the Data Supplement).
Table 2.
Results of Univariable and Multivariable Regression Analysis for the COVID-19 Mortality Within 60 d (Overall Cohort)
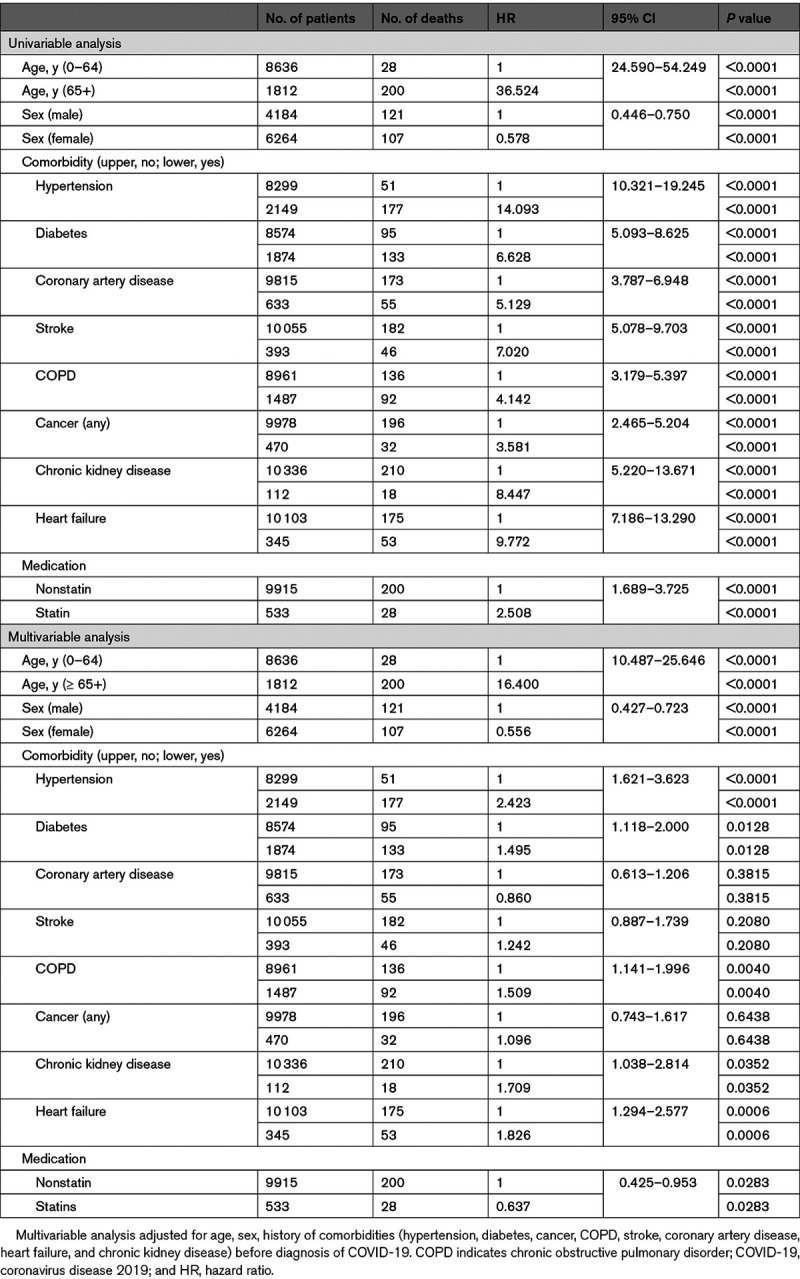
Figure 1.
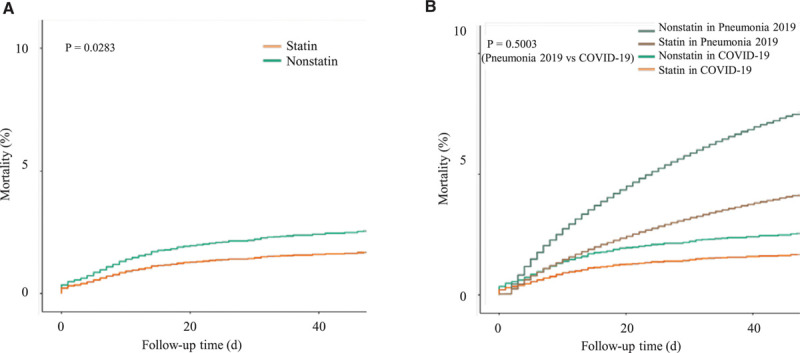
Survival within 60 d in severe acute respiratory syndrome coronavirus 2–infected patients and retrospective cohort of hospitalized pneumonia patients between January and June 2019 (overall cohort). A, Comparison of statin-treated vs statin-nontreated coronavirus disease 2019 (COVID-19) patients (adjusted). B, Comparison of statin-treated vs statin-nontreated COVID-19 and hospitalized pneumonia patients between January and June 2019 (retrospective cohort; adjusted).
Figure 2.
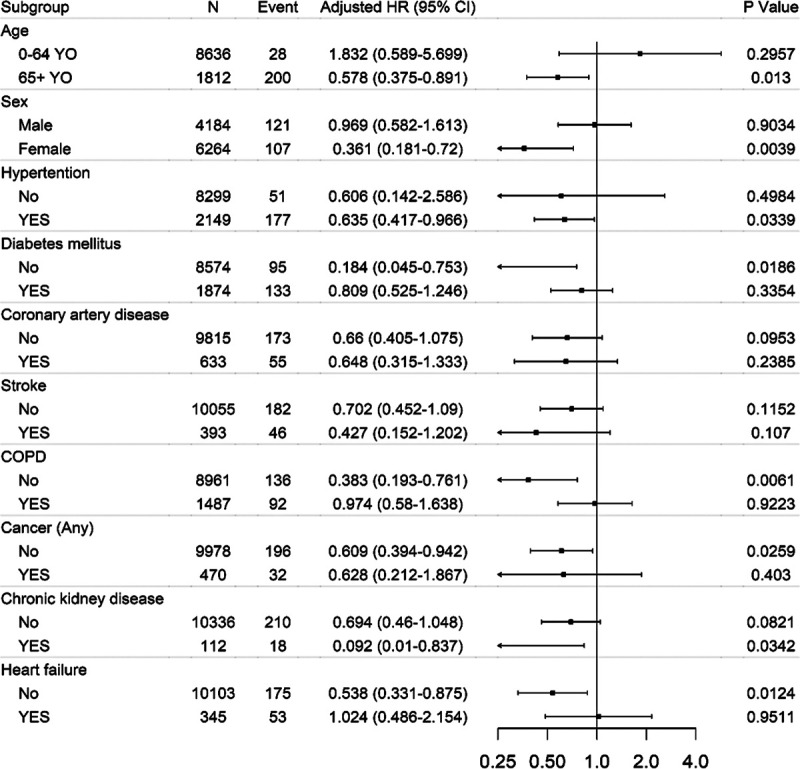
Hazard ratio (HR) for 60-d survival in the context of prior statin treatment: stratified subgroup analysis (overall cohort). COPD indicates chronic obstructive pulmonary disorder.
Then, the HR of COVID-19 patients and a retrospective cohort of patients hospitalized with pneumonia between January and June 2019 was compared. Importantly, in 2019, there was no viral pneumonia epidemic in Korea, for example, due to severe acute respiratory syndrome or Middle East respiratory syndrome infection. Therefore, the 2019 pneumonia cohort was composed of bacterial and viral pneumonia patients combined. This retrospective cohort included 179 265 patients with a mean age of 39 years, of whom 49.4% were women. The most common comorbidity in the cohort was hypertension (33.3%), followed by COPD (25.1%), diabetes (23.4%), and any cancer (6.9%; Table IV in the Data Supplement). The mortality rate at 60 days was 7.4% and sharply increased among elderly patients (≥60 years); of note, it was even higher than that in the context of COVID-19 (Figure 1B). Moreover, after considering secondary factors, the results of the present study indicated a significantly lower risk of 60-day mortality in the context of the use of statins (HR, 0.519 [95% CI, 0.495–0.544]; P<0.0001; Tables V and VI in the Data Supplement both in the overall cohort and the 1:2 PSM). Of note, the HR values were comparable between COVID-19 patients and the retrospective cohort of patients hospitalized with pneumonia (2019) in Korea, in the context of the use of statins (P=0.3244 in the overall cohort, P=0.7162 in the 2:1 PSM; Table VIII in the Data Supplement).
Discussion
In the present study, we showed that statins are not associated with COVID-19–related fatal outcomes. In fact, the use of statins correlated with significantly lower mortality in COVID-19 patients (by 36%).
Higher event rates in elderly patients with comorbidities are a common finding reported previously.17 Of note, in this study, patients with comorbidities were much older than those without comorbidities. For example, the mean age of hypertensive patients was >20 years older than that of nonhypertensive patients. In fact, after adjusting for secondary factors such as age and sex, our results revealed that the association between comorbidities and mortality was much reduced. However, still, patients with hypertension, diabetes, chronic kidney disease, or heart failure showed higher mortality compared with those without comorbidities.
The most important finding of our study was that prior use of statins was significantly associated with lower mortality in COVID-19 patients. There are a few recently published studies investigating the effect of the use of statins on COVID-19,18,19 showing results consistent with those in our study. Moreover, this finding was consistent with previous meta-analyses in pneumonia patients16,20 and also with pneumonia patients in Korea, in 2019, evaluated in this study. Lastly, this protective effect was supported by the potential inhibitory effect in the context of the SARS-CoV-2 main protease—a key coronavirus enzyme.9 In spite of the controversy regarding the role ACE2, the detailed effect of statins has not been comprehensively evaluated.21,22 As a result, the prescription of statins was reportedly reduced during the COVID-19 pandemic in the United States.23 The concern is that the use of statins in COVID-19 patients may be further reduced due to possible drug interaction with antiviral/antibacterial agents and lower cholesterol levels in the acute stress condition.24,25 However, our findings suggested that statins need not be discontinued during the COVID-19 treatment.
The current study has several limitations. First, data related to drug exposure were measured based on the claims data. Hence, detailed information about drug exposure, such as adherence to the medication or discontinuation during COVID-19 hospitalization, remains unknown. Second, the follow-up duration of the study was limited to a short period of time. However, most outcomes (86%) occurred within 2 weeks of the COVID-19 treatment. Third, although we evaluated a total of 10 448 COVID-19 patients who were hospitalized in Korea from January 19, 2020, through April 15, 2020, the interpretation of our findings is influenced by the limited sample size. To overcome this limitation, we compared the results with those obtained with a retrospective cohort of >170 000 pneumonia patients hospitalized from January to June 2019. The results indicated similar trends in COVID-19 and pneumonia cases. Lastly, although we performed PSM and multivariate analyses, there might be still a healthy user bias, for example, due to the healthy adherer effect or selective prescribing,26 potentially influencing the association between the use of statins and lower mortality in COVID-19 patients. The healthy adherer effect might affect the observational study result, which reported that patients who showed complete compliance to antihypertensive medication regardless of either calcium channel blockers or angiotensin II receptor blockers had a lower risk of COVID-19.27 Therefore, we cannot exclude the possibility that the healthy adherer effect confounded the report’s finding.
Conclusions
Statins were associated with significantly lower mortality of COVID-19, consistent with usual pneumonia patients. Overall, the findings of this article suggest that statins might be considered as a part of the supportive regimen during the treatment of COVID-19. The prospective randomized studies might be warranted to confirm the benefit of statins in COVID-19.
Acknowledgments
This study was supported by the Korean Society of Hypertension and the National Committee for Clinical Management of Emerging Infectious Diseases. M.-d. Oh and H.-Y. Lee conceived and designed the study, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. H.-Y. Lee, J. Ahn, D. Wook Kim, and C. Kyung Kang drafted the paper. S.-H. Won, J. Ahn, D. Wook Kim, J. Park, and J.-H. Park did the analysis, and C. Hee Kang, K.-H. Chung, J.-S. Joh, J. Bang, W. Bum Pyun, and M.-d. Oh contributed to data collection and interpretation. All authors critically revised the manuscript for relevant intellectual content and gave final approval for the version to be published. All authors agree to be accountable for all aspects of the work and will answer any questions related to the accuracy or integrity of the work. We declare no competing interests. We acknowledge all health care workers involved in the diagnosis and treatment of patients in Korea. We thank all the hospital staff members for their effort in collecting the information used in this study: Hongsang Oh, Junghan Kim (Armed Forces Caital Hospital), Yeonjae Kim, Ji Hwan Bang, Bumsik Jin (National Medical Center), Sangjun Park, Wangjun Lee, Kangwon Choe, Kiduk Lee, Yumin Kang (Myongji Hospital), Jinwon Jung, Jeeyoung Park (Chung-Ang University Hospital), Eu Suk Kim, Hong Bin Kim, Kyoung-Ho Song (Bundang Seoul National University Hospital), Jeeyoung Seo (Samsung Medical Center), Nam Joong Kim, Chang Kyung Kang, Myoung-don Oh, Wan Beom Park, Pyeong Gyun Choe (Seoul National University Hospital), Jaephil Choi, Donghyun Oh (Seoul Medical Center), Jinyong Kim (Incheon Medical Center), Jaehoon Lee (Wonkwang University Hospital), Sookin Jung, Kyungwha Park, Sungen Kim, Taehoon Oh (Chonnam National University Hospital), Hyunah Jang (Kyungpook National University Hospital), Younghee Jung (Hallym University Medical Center), Hyejine Jung (Daegu Medical Center), Dongmin Kim, Nara Yoon, Joonwon Suh (Chosun University Hospital), Boram Koh (Gyeonggi Provincial Medical Center Ansung Hospital), Woonjung Nam (Daenam Hospital), Joonsup Yeom (Korean Society of Infectious Diseases), Donggun Lee, and Yeonsook Kim (Korean Society for Antimicrobial Therapy). We thank all of the organizing committee members of the Korean Society of Hypertension for the critical review and the endorsement of this study: Sungha Park, Kwang-il Kim, Jinho Shin, Ki Chul Sung, Hyeon Chang Kim, Sang-Hyun Ihm, Eun Joo Cho, Juhan Kim, Dae-Hee Kim, Il-Suk Sohn, Wook Jin Chung, Sung Kee Ryu, Myeong-Chan Cho, and Wook Bum Pyun (President).
Sources of Funding
This research was supported by research grant from the Korea Centers for Disease Control and Prevention (No. 4838-330-320-01) and Seoul National University Hospital (No. 04-2020-0030).
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- COPD
- chronic obstructive pulmonary disorder
- COVID-19
- coronavirus disease 2019
- HMG-CoA
- hydroxy-methyl-glutaryl-coenzyme A
- HR
- hazard ratio
- PSM
- propensity score matching
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
These authors contributed equally to this article.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.315551.
For Sources of Funding and Disclosures, see page e181.
Contributor Information
Hae-Young Lee, Email: hylee612@snu.ac.kr.
Juhee Ahn, Email: ahnjuhee23@gmail.com.
Juhong Park, Email: parkjh@nhis.or.kr.
Chang Kyung Kang, Email: zeptemiger@hanmail.net.
Sung-Ho Won, Email: sunghow@gmail.com.
Dong Wook Kim, Email: kimdw2269@gmail.com.
Jong-Heon Park, Email: parkjh@nhis.or.kr.
Ki-Hyun Chung, Email: ckhyun56@nmc.or.kr.
Joon-Sung Joh, Email: ssabana777@gmail.com.
Ji Hwan Bang, Email: roundbirch@gmail.com.
Cheong Hee Kang, Email: chkangmd@nhis.or.kr.
Wook Bum Pyun, Email: pwb423@ewha.ac.kr.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: Summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease (COVID-2019) situation reports. Accessed March 27, 2020. https://www.Who.Int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 3.Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Liang M, Li Y, Guo J, Fei D, Wang L, He L, Sheng C, Cai Y, Li X, et al. Mental health care for medical staff in china during the COVID-19 outbreak. Lancet Psychiatry. 2020;7:e15–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regla-Nava JA, Jimenez-Guardeño JM, Nieto-Torres JL, Gallagher TM, Enjuanes L, DeDiego ML. The replication of a mouse adapted SARS-CoV in a mouse cell line stably expressing the murine SARS-CoV receptor mACE2 efficiently induces the expression of proinflammatory cytokines. J Virol Methods. 2013;193:639–646. doi: 10.1016/j.jviromet.2013.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154:69–75. doi: 10.1111/imm.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–435. doi: 10.1016/j.antiviral.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 8.Pertzov B, Eliakim-Raz N, Atamna H, Trestioreanu AZ, Yahav D, Leibovici L. Hydroxymethylglutaryl-CoA reductase inhibitors (statins) for the treatment of sepsis in adults - A systematic review and meta-analysis. Clin Microbiol Infect. 2019;25:280–289. doi: 10.1016/j.cmi.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Reiner Ž, Hatamipour M, Banach M, Pirro M, Al-Rasadi K, Jamialahmadi T, Radenkovic D, Montecucco F, Sahebkar A. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16:490–496. doi: 10.5114/aoms.2020.94655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe COVID-19 infection. mBio. 2020;11:e00398-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massachusetts General Hospital. Massachusetts general hospital (mgh) COVID-19 treatment guidance. 2020. https://www.Massgeneral.Org/news/coronavirus/treatment-guidance. Accessed May 26, 2020
- 12.Park PG, Kim CH, Heo Y, Kim TS, Park CW, Kim CH. Out-of-hospital cohort treatment of coronavirus disease 2019 patients with mild symptoms in Korea: an experience from a single community treatment center. J Korean Med Sci. 2020;35:e140 doi: 10.3346/jkms.2020.35.e140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park WB, Kwon NJ, Choi SJ, Kang CK, Choe PG, Kim JY, Yun J, Lee GW, Seong MW, Kim NJ, et al. Virus isolation from the first patient with SARS-CoV-2 in Korea. J Korean Med Sci. 2020;35:e84 doi: 10.3346/jkms.2020.35.e84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brunink S, Schneider J, Schmidt ML, et al. Detection of 2019 novel coronavirus (2019-ncov) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the National health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu A, Good C, Downs JR, Fine MJ, Pugh MJ, Anzueto A, Mortensen EM. The association of cardioprotective medications with pneumonia-related outcomes. PLoS One. 2014;9:e85797 doi: 10.1371/journal.pone.0085797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, Chung CW, Trelles-Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24:429 doi: 10.1186/s13054-020-03154-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry C, Zaizafoun M, Stock E, Ghamande S, Arroliga AC, White HD. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018;31:419–423. doi: 10.1080/08998280.2018.1499293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in COVID-19. N Engl J Med. 2020;382:2582. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020;395:1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O’Donnell CJ, Warraich HJ. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA. 2020;323:2524–2526. doi: 10.1001/jama.2020.9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlow A, Landolf KM, Barlow B, Yeung SYA, Heavner JJ, Claassen CW, Heavner MS. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40:416–437. doi: 10.1002/phar.2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chien YF, Chen CY, Hsu CL, Chen KY, Yu CJ. Decreased serum level of lipoprotein cholesterol is a poor prognostic factor for patients with severe community-acquired pneumonia that required intensive care unit admission. J Crit Care. 2015;30:506–510. doi: 10.1016/j.jcrc.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 26.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26:546–550. doi: 10.1007/s11606-010-1609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Kim DW, Kim KI, Kim HB, Kim JH, Lee YG, Byeon KH, Cheong HK; Korean Society of Hypertension. Compliance of antihypertensive medication and risk of coronavirus disease 2019: a cohort study using big data from the korean national health insurance service. J Korean Med Sci. 2020;35:e232 doi: 10.3346/jkms.2020.35.e232 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


