ABSTRACT
Multisystem inflammatory syndrome in children (MIS-C) is a recently identified syndrome that appears to be temporally associated with novel coronavirus 2019 infection. MIS-C presents with fever and evidence of systemic inflammation, which can manifest as cardiovascular, pulmonary, neurologic, and gastrointestinal (GI) system dysfunction. Presenting GI symptoms are seen in the majority, including abdominal pain, diarrhea, and vomiting. Any segment of the GI tract may be affected; however, inflammation in the ileum and colon predominates. Progressive bowel wall thickening can lead to luminal narrowing and obstruction. Most will have resolution of intestinal inflammation with medical therapies; however, in rare instances, surgical resection may be required.
Keywords: coronavirus 2019, histopathology, intestinal inflammation, intestinal resection
What Is Known/What Is New
What Is Known
Novel coronavirus 2019 infection is temporally associated with the development of multisystem inflammatory syndrome in children (MIS-C).
Clinical presentation of multisystem inflammatory syndrome in children commonly involves gastrointestinal signs and symptoms.
What Is New
Radiographic features of bowel inflammation in those with multisystem inflammatory syndrome in children include circumferential bowel wall thickening and luminal narrowing.
Complete bowel obstruction can occur in setting of multisystem inflammatory syndrome in children and may require surgical resection.
The histologic features of intestinal inflammation in multisystem inflammatory syndrome in children appear distinct from chronic inflammatory bowel disease. Features may include venous microthrombi, arteritis, and necrotizing lymphadenitis.
The novel Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), responsible for the Coronavirus Disease 2019 (COVID-19) global pandemic, has caused disproportionately severe disease in adults and elderly populations compared to children. In the largest pediatric series reported to date, 94.1% of 2143 pediatric cases of acute COVID-19 infection were found to have asymptomatic, mild, or moderate clinical disease (1). The reported frequency of gastrointestinal (GI) manifestations at the time of presentation with COVID-19 infection has varied widely (2,3). In contrast, multisystem inflammatory syndrome in children (MIS-C) is a newly described condition observed in children infected with SARS-CoV-2 virus. MIS-C involves early GI symptoms in the vast majority of patients (4). The aim of the present study is to describe the spectrum of intestinal disease associated with MIS-C in this cohort.
METHODS
Approval was obtained from our institutional review board to conduct a retrospective chart review of all cases admitted to our medical center with a diagnosis of MIS-C between April 17 and May 17, 2020. Included patients all met MIS-C diagnostic criteria as updated by the Center for Disease Control and Prevention (CDC) on May 14, 2020 (5). Clinical, radiologic, and histopathologic data were abstracted from the electronic medical record using the Northwell Health pediatric COVID-19 consortium database. Categorical variables were summarized by frequencies, whereas continuous variables were summarized by using mean, median, standard deviation, and range.
RESULTS
Clinical Presentation
Thirty-five patients (22 male, 8.6 median years of age [range 2.2–19]) met the CDC diagnostic criteria for MIS-C during the study period. A majority of children had no significant past medical history. Three patients had underlying asthma and 1 had a congenital atrial septal defect. GI symptoms including abdominal pain, vomiting, and diarrhea occurred in 34 of 35 (97%) patients. Pertinent laboratory values for the patients with GI symptoms at presentation are described in Table 1. Presence of lymphopenia and hypoalbuminemia and absence of thrombocytosis were common, whereas D-dimer and fibrinogen levels were universally elevated regardless of severity of GI symptomatology. Abdominal pain was severe enough to warrant radiographic imaging in 19 of 35 (54.2%) patients (68% male). Admission to the intensive care unit was required in 23 of 35 (65.7%), endotracheal intubation for mechanical ventilation was needed in 3 of 35 (8.6%), and none required extracorporeal membrane oxygenation. Of the patients with moderate to severe GI symptoms, all 19 patients received at least 1 dose of intravenous immunoglobulin (IVIG); corticosteroids and aspirin were added based on clinical severity. One patient with Kawasaki-like disease and persistent fever after IVIG also received a single 5 milligrams per kilogram dose of infliximab. Bowel obstructive symptoms developed in 2 of 19 (10.5%) of these patients; 1 improved with medical therapy, including IVIG and corticosteroids, and the other underwent exploratory laparotomy, ileocolic resection, and diverting ileostomy due to worsening symptoms. There were no deaths and all patients were discharged home. At 2-week posthospital discharge outpatient follow-up, GI symptoms were resolved in all patients.
TABLE 1.
Pertinent laboratory values
| Lab parameters mean (±SD) | All MIS-C patients presenting with GI symptoms N = 34 | Moderate to severe abdominal pain N = 19 | Mild abdominal pain N = 15 |
| White blood cell count K/μL | 10.26 ± 4.26 | 9.32 ± 3.72 | 11.44 ± 4.72 |
| Absolute lymphocyte count K/μL | 0.96 ± .50 | 0.85 ± .46 | 1.08 ± .54 |
| Absolute neutrophil count K/μL | 8.59 ± 4.03 | 7.84 ± 3.61 | 9.54 ± 4.45 |
| Platelet count K/μL | 170 ± 73 | 165 ± 57 | 175 ± 89 |
| C-reactive protein mg/L | 205.1 ± 119.5 | 219.8 ± 124.9 | 186.5 ± 113.8 |
| Albumin g/dL | 3.4 ± 0.6 | 3.5 ± 0.6 | 3.3 ± 0.6 |
| Ferritin ng/mL | 1009.3 ± 1146.8 | 890.7 ± 1122 | 1153.3 ± 1201.9 |
| D-Dimer ng/mL | 1851.1 ± 1329.4 | 2030.4 ± 1334.8 | 1635.9 ± 1336.1 |
| Fibrinogen mg/dL | 839.5 ± 365.7 | 798.8 ± 256 | 891.9 ± 477.4 |
| Troponin ng/L | 60.9 ± 93.3 | 51.9 ± 88.3 | 72.4 ± 101.2 |
GI = gastrointestinal; MIS-C = multisystem inflammatory syndrome in children; SD = standard deviation.
Radiographic Features
Three patients had unremarkable radiographs. Abdominal ultrasonography was performed in 14 patients, of which 11 (78.6%) were limited to the right lower quadrant (RLQ), primarily to evaluate for appendicitis; a limited assessment of the cecum and terminal ileum was also completed; however, a dedicated bowel ultrasound study was not performed. Mild ileal thickening was noted in 2 patients. Color doppler evaluations were performed in 9 of 14 patients, and all were normal. Of those with imaging studies, computed tomography (CT) scans of the abdomen were performed in 7 of 19 (36.8%); inflammatory bowel changes were noted in 6 of 7 (85.7%), including marked terminal ileitis in 4 of 7 (57.1%). All 4 of these patients also had inflammation in the cecum, with 1 patient having inflammation extending through the ascending colon. One patient without ileocecal involvement had isolated thickening of the splenic flexure and descending colon. Mesenteric fat stranding and RLQ mesenteric lymphadenopathy were each seen in 5 of 7 (71%) patients. Mucosal hyperenhancement was not a prominent feature seen. The patient with obstructive symptoms responsive to medical therapy had severe terminal ileal luminal narrowing with circumferential bowel wall thickening (Fig. 1).
FIGURE 1.
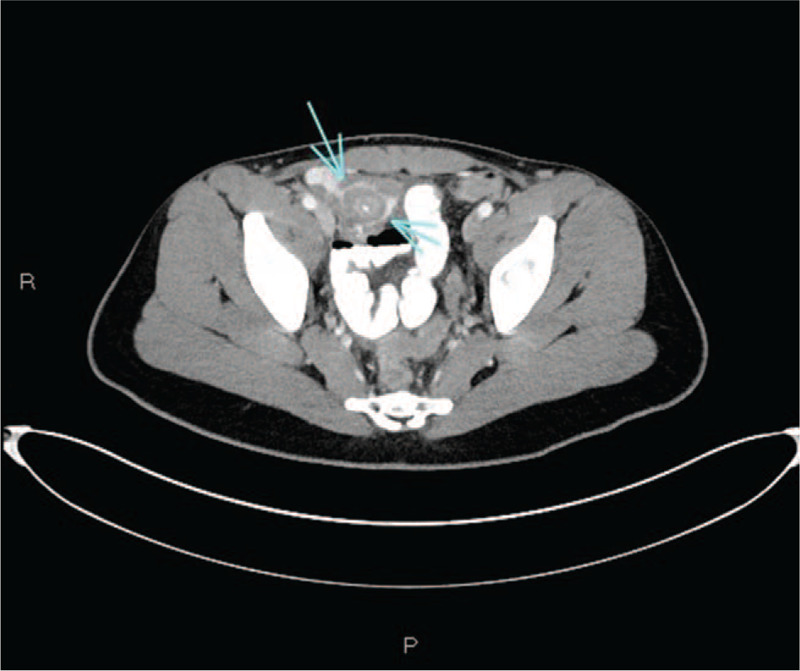
Axial image from a computed tomography scan of the abdomen and pelvis with IV and oral contrast. The terminal ileum demonstrates marked circumferential thickening and luminal narrowing (arrows).
Histopathologic Features
One patient underwent ileocolic resection. On gross examination (Fig. 2A), there was a 6 cm mass in the ileocolic pedicle and a 3 cm span of granular, thickened terminal ileal mucosa. Microscopic examination with hematoxylin and eosin (H&E) stain yielded a spectrum of findings. There was marked transmural lymphocytic inflammation (Fig. 2B) and focal acute enteritis. Venous microthrombi (Fig. 2C,D) involved the mucosa, submucosa, and subserosa of the terminal ileum extensively; these often appeared to originate in the subendothelial space. The mesentery was markedly inflamed; arteritis with subendothelial and transmural lymphocytes, and endothelial fibrin deposition (Fig. 2E,F), was often present adjacent to unremarkable arteries (Fig. 2G). The ileocolic mass consisted of coalescent lymph nodes with necrotizing lymphadenitis (Fig. 2H,I), sinus histiocytosis, and erythrophagocytosis. No viral cytopathic effects were present on H&E stain and Epstein-Barr encoding region in situ hybridization was negative. Electron microscopy on small intestinal mucosa did not yield any definite viral particles. No other features of inflammatory bowel disease (IBD) were present, and the appendix and cecum were histologically unremarkable.
FIGURE 2.
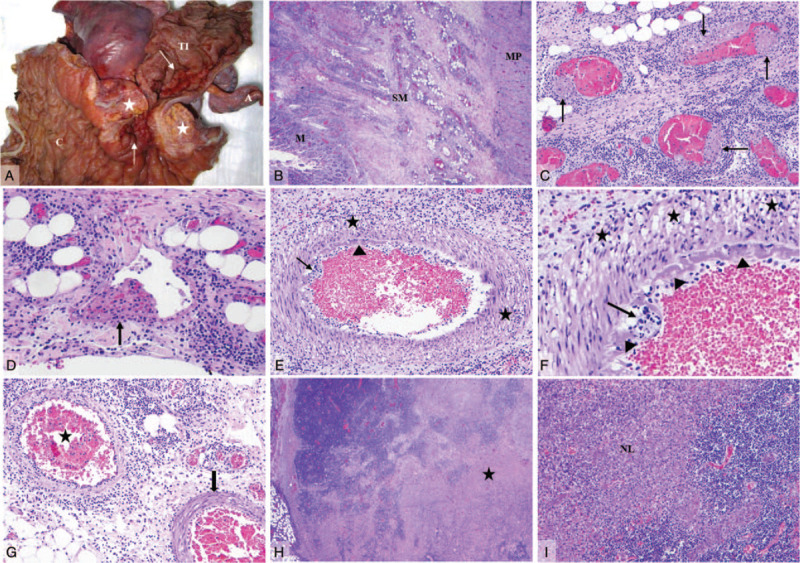
Gross and histology composite. (A) Ileocolic specimen. A 6 cm mass in the ileocolic pedicle (asterisk) and granular, thickened terminal ileum mucosa (arrow). (B) Transmural chronic inflammation. (C, D) Extensive venous microthrombi (arrows) present in mucosa, submucosa (pictured) and subserosa of terminal ileum. (E, F) Markedly inflamed mesentery with arterial transmural (asterisk) and subendothelial (arrows) lymphocytes, and endothelial fibrin deposition (arrowheads). (G) Inflamed arteries (asterisk) adjacent to unremarkable arteries (arrow). (H, I) Coalescent lymph nodes (ileocolic pedicle mass) with necrotizing lymphadenitis (asterisk). A = appendix; C = colon; M = mucosa; MP = muscularis propria; NL = necrotizing lymphadenitis; SM = submucosa; TI = terminal ileum.
DISCUSSION
The recent surge of young patients developing MIS-C follows the peak of acute COVID-19 infection in the community by approximately 1 month (5). The SARS-CoV-2 virus has been implicated in the pathogenesis of MIS-C, although the exact pathophysiology remains uncertain. The GI tract of children with MIS-C appears especially prone to inflammatory damage reflected by >95% of children in our cohort presenting with GI symptoms and >50% of those with CT imaging having terminal ileitis with bowel wall thickening. This presentation, particularly if it predates other organ system involvement, may raise suspicion for early IBD in the differential diagnosis. Most patients had diffuse abdominal pain, with a subset having RLQ pain. We found CRP levels to be typically higher than levels seen in children at presentation with more classical IBD (6), whereas platelet counts were often normal or low. Laboratory values were similar in patients with mild GI symptoms compared to those with moderate to severe symptoms at presentation. MIS-C may be further distinguished from early IBD by abnormalities in other inflammatory biomarkers, such as D-dimer, fibrinogen, and troponin (4). Several patients had radiographic features similar to those seen in IBD, such as bowel wall thickening and mesenteric fat stranding. Features of more advanced IBD, including fibrostenotic or penetrating disease, were notably absent reflecting the acute inflammatory process of MIS-C. Although there was transmural chronic inflammation in the resected specimen, there was only focal active inflammation and no granulomatous inflammation, or evidence of chronic mucosal injury as would be seen with IBD. There was no evidence of an acute appendicitis, and the striking venous microthrombi and accompanying necrotizing lymphadenitis are not features of IBD.
Conventional features of acute ischemia such as loss of epithelium, hyalinization of the lamina propria, or mucosal necrosis were not seen. The presence of microthrombi is as striking as the vasculitis, and these vascular findings may represent a response to overwhelming inflammation. Whether in turn this induces true ischemic bowel injury is uncertain. It is possible with the lapse of a few more days, changes may have evolved to more recognizable morphologic evidence of bowel ischemia.
Within the GI tract, the absorptive enterocytes of the small intestine and colon have the greatest density of angiotensin converting enzyme 2 receptors, the receptor used by the SARS-CoV-2 virus to gain cell entry (7). It is unknown whether the inflammatory disease in these areas of bowel is due to direct viral induced cellular damage or is the end organ damage of a systemic inflammatory process – the intestinal resection evaluated in this case series was notable for absence of viral cytopathic effect or detectable viral particles. Follow-up studies will be important to understand any lasting implications and risk of future bowel disease in patients recovered from MIS-C.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020; 145: [DOI] [PubMed] [Google Scholar]
- 2.Xiong XL, Wong K, Chi SQ, et al. Comparative study of the clinical characteristics and epidemiological trend of 244 COVID-19 infected children with or without GI symptoms. Gut 2020; May 19. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Chen A, Agarwal A, Ravindran N, et al. Are gastrointestinal symptoms specific for coronavirus 2019 infection? A prospective case-control study from the United States. Gastroenterology 2020; 159:1161–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capone CA, Subramony A, Sweberg T, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of children associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr 2020; 224:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC Health Alert Network. The Centers for Disease Control and Prevention. Published May 14, 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp Accessed May 30, 2020. [Google Scholar]
- 6.Tsampalieros A, Griffiths AM, Barrowman N, et al. Use of C-reactive protein in children with newly diagnosed inflammatory bowel disease. J Pediatr 2011; 159:340–342. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Li HB, Lyu JR, et al. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis 2020; 96:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


