Abstract
Objective:
The epidemiology of chronic critical illness is not well characterized. We sought to determine the incidence, outcomes and associated costs of chronic critical illness in the United States.
Design:
Population-based cohort study using data from the United States Healthcare Costs and Utilization Project from 2004 to 2009.
Setting:
Acute care hospitals in Massachusetts, North Carolina, Nebraska, New York and Washington.
Patients:
Adult and pediatric patients meeting a consensus-derived definition for chronic critical illness, which included one of 6 eligible clinical conditions (prolonged acute mechanical ventilation, tracheotomy, stroke, traumatic brain injury, sepsis or severe wounds) plus at least 8 days in an intensive care unit.
Interventions:
None.
Measurements and Main Results:
Out of 3,235,741 admissions to an intensive care unit during the study period, 246,151 (7.6%) met the consensus definition for chronic critical illness. The most common eligibility conditions were prolonged acute mechanical ventilation (72.0% of eligible admissions) and sepsis (63.7% of eligible admissions). Among patients meeting chronic critical illness criteria through sepsis, the infections were community-acquired in 48.5% and hospital acquired in 51.5%. In-hospital mortality was 30.9% with little change over the study period. The overall population-based incidence was 34.4 per 100,000. The incidence varied substantially with age, peaking at 82.1 per 100,000 individuals aged 75-79 but then declining coincident with a rise in mortality before day 8 in otherwise eligible patients. Extrapolating to the entire United States, for 2009 we estimated a total of 380,001 cases; 107,880 in-hospital deaths and $26 billion in hospital-related costs.
Conclusions:
Using a consensus-based definition, the incidence, hospital mortality and costs of chronic critical illness are substantial. Chronic critical illness is particularly common in the elderly, although in very old patients the incidence declines, in part due to an increase in early mortality among potentially eligible patients.
Keywords: intensive care units, mechanical ventilation, long-term care, sepsis
INTRODUCTION
Many critically ill patients survive their initial acute illness but go on to experience persistent organ failures necessitating prolonged intensive care, a syndrome known as chronic critical illness (CCI) (1). CCI is characterized by high hospitalization costs, frequent post-acute care use and poor long-term survival (2). The clinical and financial burden of CCI is expected to increase in the coming years, due to both an aging population and advances in the early management of critical illness leading to more long-term survivors (3, 4). As a consequence, it is increasingly important to develop to strategies to prevent CCI, as well as improve outcomes and reduce costs for patients who develop CCI.
Despite the importance of CCI, robust information on the incidence, outcomes and associated costs of this syndrome are lacking. Prior epidemiological evaluations used data from small numbers of hospitals and limited geographic areas, and are over 10 years old (5-7). Prior studies also used widely varying definitions of CCI, reflecting a lack of consensus about the defining characteristics of this complex syndrome (7). To address this knowledge gap, we conducted a retrospective cohort study of the epidemiology of CCI using recent data from a broad sample of United States (US) hospitals. We used a newly developed, multi-stakeholder definition that reflects current consensus about both the underlying conditions and the duration of the episode (8). Our goals were to quantify the incidence, outcomes and costs of CCI in the United States; examine the clinical characteristics of patients with CCI; and determine whether the epidemiology of CCI is changing over time.
MATERIALS AND METHODS
Study design and data.
We performed a retrospective cohort study using 2004 – 2009 hospital discharge data from five US states: Massachusetts, North Carolina, Nebraska, New York and Washington. These states were chosen because of the size and quality of their state discharge databases, which include sufficiently granular information to identify ICU admission and quantify ICU length of stay. The data were obtained from the Agency for Healthcare Research and Quality’s Healthcare Costs and Utilization Project, a national clearinghouse of hospital discharge records. Together these states contain 14.3% of the US population and represent a wide range of urban and rural areas. The datasets contain patient-level data on all hospitalizations within each state, including demographic information, admission and discharge locations, and International Classification of Diseases, version 9.0—clinical modification (ICD-9-CM) diagnosis and procedure codes. We supplemented the hospital discharge data with data on hospital characteristics from the Centers for Medicare and Medicaid Services (CMS) Healthcare Cost Reporting Information System (HCRIS) and population data from the US Census (Geolytics, Inc., Somerville, New Jersey).
Patients.
We defined CCI using a recently advanced consensus definition developed by the Research Triangle Institute (RTI) under contract from CMS (8). This definition evolved out of the need to standardize the payment methods and sites of care for these patients, whom often receive care in both acute care hospitals and long-term acute care hospitals (LTACHs), which are hospitals that specialize in the care of complex patients recovering from severe acute illness (9). To develop the definition, RTI combined quantitative patient-level clinical data from a project evaluating the appropriateness of post-acute care among Medicare beneficiaries and qualitative data from site visits to acute care hospitals to characterize the subset of critically ill patients requiring prolonged acute care. Based on this characterization RTI developed a preliminary CCI definition, which was then reviewed with clinical experts and revised based on their feedback. A complete description of this process can be found in the cited RTI report beginning on page 21 and available at www.cms.gov (8). This definition was not intended as a strictly clinical definition of CCI, but as a broadly encompassing definition meant to simultaneously serve both clinicians and policy makers.
The final definition consisted of one of 5 eligible clinical conditions plus at least 8 days in an ICU during an acute care hospitalization (8). The 5 eligibility conditions were: prolonged acute mechanical ventilation (i.e. mechanical ventilation for at least 96 hours in a single episode); tracheotomy; sepsis and other severe infections; severe wounds; and multiple organ failure, ischemic stroke, intercerebral hemorrhage or traumatic brain injury. For our analysis, we recategorized these eligible conditions into 6 simple groups: prolonged acute mechanical ventilation, tracheotomy, sepsis, severe wounds, stroke (including both ischemic stroke and intercerebral hemorrhage), and traumatic brain injury. Multiple organ failure did not remain as one of these six groups because it was the least well defined and was largely subsumed by the other groups. We then defined CCI as an ICU length of stay of at least 8 days combined with at least one eligible diagnosis during the hospitalization.
To identify patients with these conditions in our administrative data, we mapped each condition to set of ICD-9-CM diagnosis and procedure codes. In most cases we used codes that were used in the RTI report and were validated against clinical data (10-15), with two exceptions. First, we favored the use of ICD-9-CM codes over diagnosis related group codes, since diagnosis related groups are less commonly subject to clinical validation. Second, for severe wounds, for which validated ICD-9-CM procedure codes do not presently exist, we selected codes through a literature review and the consensus of the authors. A complete listing of the codes used for each condition is provided in the supplementary digital content.
Of note, this definition contrasts with previously used definitions for chronic critical illness, which either required a longer period of mechanical ventilation, up to 21 days (16), or required a tracheostomy (17). However, these definitions are older and do not necessarily reflect modern critical care, in which long durations of mechanical ventilation less common and many patients with chronic critical illness have persistent organ dysfunctions other that respiratory failure (1).
Variables.
For each eligible admission we defined demographic, clinical, resource utilization, and outcome variables using the hospital discharge record. Demographic data included age, gender and primary payer; and were obtained directly from the discharge record. Clinical data included comorbidities (based on ICD-9-CM diagnosis codes (15), and, for patients with sepsis, whether or not their infection was community-acquired or hospital-acquired (based on previously validated present-on-admission codes) (18). Resource utilization variables included ICU and hospital length of stay (obtained directly from the discharge record), and hospital costs (obtained by multiplying total charges in the discharge record with hospital-specific cost-to-charge ratios from HCRIS (19). Outcome variables included in-hospital mortality and discharge location, categorized as home, transfer to another hospital, transfer to a skilled nursing or inpatient rehabilitation facility, transfer to an LTACH, transfer to hospice, or dead. Information about post-discharge mortality and costs were not available in our data and were not included in the analysis.
Analysis.
We conducted three primary analyses. First, we examined the characteristics of patients meeting the consensus definition by summarizing demographic variables, clinical variables, resource utilization variables and outcomes using standard summary statistics. To determine whether the admissions with CCI differed across hospital types we compared these variables across small, medium and large hospitals defined using total beds categorized using natural cut-points (<100 beds, 100 – 250 beds, and >250 beds, respectively). To determine whether outcomes changed over time we compared discharge destinations across study years. We used one way ANOVA or chi-square tests with the Bonferroni correction for multiple comparisons to assess statistical significance. However, given our large sample size we suspected that many differences with little clinical relevance would achieve statistical significance. Therefore, although we report p-values for these comparisons, we preferentially judged differences by their clinical rather than statistical significance.
Second, to understand the population burden of CCI we generated overall and age-specific incidence rates in the 5 state sample, obtaining year-specific population denominators from the US Census. Since the CCI definition includes a minimum length of stay in the ICU, and many patients with clinical conditions that might lead to CCI could die prior to 8 days, we also calculated the age-specific incidence of patients with an eligible condition but who died prior 8 days in an ICU. The goal of this analysis was to understand the degree to which the incidence of CCI was potentially related to differential early mortality among age groups.
Third, to understand the national burden of CCI we extrapolated national estimates of the incidence, mortality and total costs based on the age and gender distribution of the population from the US Census using direct standardization.
For analyses that included community-acquired versus hospital-acquired infection in patients with sepsis, we limited this analysis only to states and years with present-on-admission codes (Massachusetts, Nebraska, New York and Washington; 2007-2009); all other analyses were performed in the entire sample. All costs were adjusted for inflation to 2009 dollars using the consumer price index from the US Bureau of Labor Statistics (www.bls.gov/cpi). Data management and analysis were conducted in SAS 9.2 (SAS Institute, Cary, North Carolina). This research involved the use of previously collected de-identified data sets and was considered exempt from human subjects review by the University of Pittsburgh Institutional Review Board.
RESULTS
Out of 3,235,741 ICU admissions during the study period, a total of 246,151 (7.6%) met the consensus definition for CCI. Characteristics of these patients are shown in Supplementary Digital Content Table 1. The most common eligibility conditions were prolonged acute mechanical ventilation (72.0% of cases) and sepsis (63.7% of cases), with no other eligibility condition occurring in more than 10% of cases. A majority of patients had at least 3 co-morbid conditions (55.5% of cases). Medicare was the most common payer (58.0% of cases), followed by private insurance (23.0% of cases). Despite statistical differences, patients with CCI generally looked similar across different hospital types, although patients in larger hospitals tended to be younger and were more likely to have Medicaid as the primary payer.
For patients with sepsis as an eligible condition in states with present-on-admission codes (n=63,262), 51.5% of infections were hospital-acquired while 48.5% of infections were community-acquired (Figure 1). Hospital-acquired infections were substantially more common in small hospitals (64.9% of sepsis cases) compared to large hospitals (49.3% of sepsis cases).
Figure 1.
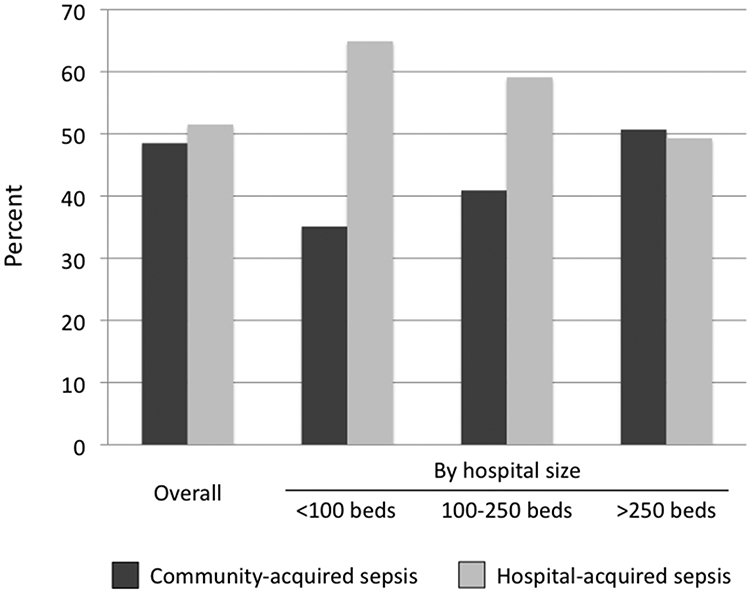
Distribution of community-acquired versus hospital-acquired sepsis, both overall and by hospital size. Data limited to states and years with present-on-admission codes (n=63,262). P<0.05 for the comparison across hospital types.
Patient outcomes over time are shown in Table 1. In-hospital mortality was 33.1% in 2004 and declined slightly over time, to 28.7% in 2009. LTACH transfer increased from 1.9% to 4.9%. Other discharge destinations remained relatively constant.
Table 1.
Hospital discharge disposition by year
| Year | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|---|
| Outcomes | ||||||
| Home | 7880 (21.2) | 7800 (20.2) | 8245 (20.6) | 8603 (20.8) | 9315 (21.1) | 9713 (21.8) |
| Other hospital | 1402 (3.8) | 1505 (3.9) | 1455 (3.6) | 1501 (3.6) | 1526 (3.5) | 1579 (3.5) |
| SNF | 14446 (38.9) | 15480 (40.2) | 16157 (40.5) | 16293 (39.5) | 16989 (38.5) | 17099 (38.3) |
| Dead | 12284 (33.1) | 12487 (32.4) | 12401 (31.1) | 12555 (30.4) | 13420 (30.4) | 12818 (28.7) |
| LTACH | 722 (1.9) | 743 (1.9) | 976 (2.4) | 1530 (3.7) | 1948 (4.4) | 2207 (4.9) |
| Hospice | 369 (1.0) | 540 (1.4) | 704 (1.8) | 800 (1.9) | 979 (2.2) | 1174 (2.6) |
Values are frequency (percent)
P<0.05 for all pair wise comparisons across years.
SNF = Skilled Nursing Facility; LTACH= long-term acute care hospital
The overall population incidence rate of CCI in the five-state sample was 34.4 per 100,000. The incidence steadily rose with age in persons younger than 75, peaking at 82.1 per 100,000 in persons aged 75-79, then declined in persons aged 80 and older (Figure 2). In contrast, the incidence of patients who have an eligible clinical condition for CCI but who died prior to day 8 increased steadily with age across all age groups, suggesting that the declining incidence of CCI among older persons is due in part to early mortality among otherwise eligible patients.
Figure 2.
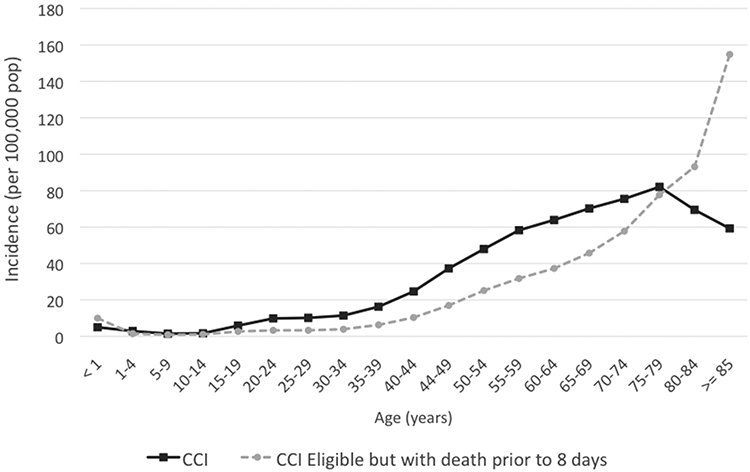
Age-specific population-based incidence of chronic critical illness (dark line) and chronic critical illness eligible conditions but with death prior to 8 days (dashed line). Data are for the five state sample, all years. CCI = chronic critical illness
Extrapolating to national estimates, there were 302,173 CCI cases in 2004, increasing to 380,001 in 2009 (Table 2). The number of in-hospital decedents increased from 100,552 in 2004 to 107,880 in 2009. Inflation-adjusted in-hospital costs for these patients increased out of proportion to both these figures, rising from $15.6 billion in 2004 to $26.0 billion in 2009.
Table 2.
Estimated annual number of cases, mortality and hospital costs of chronic critical illness in the United States, by year.
| Year | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|---|
| Cases (N) | 302173 | 317451 | 324790 | 339930 | 365817 | 380001 |
| Mortality (N, %) | 100552 (33.3) |
103357 (32.6) |
102018 (31.4) |
104067 (30.6) |
111348 (30.4) |
107880 (28.3) |
| Costs (billions)* | $15.6 | $17.5 | $19.4 | $21.8 | $24.7 | $26.0 |
Costs are inflation adjusted to 2009 dollars using the consumer price index
DISCUSSION
In a population-based sample of 5 US states, we found that the incidence of CCI based on a consensus definition is substantial and increasing over time, with over 100,000 in-hospital deaths and associated in-hospital costs exceeding $25 billion per year. These findings underscore the importance of CCI to the field of critical care, not only in the clinical arena but also for health care policy and planning.
The large clinical and economic burden of CCI we observe is only a fraction of the true burden, since we could not observe post-discharge mortality and could not account for post-discharge costs. Prior studies estimate that the one-year mortality approaches 50% (20), a figure that is consistent with our in-hospital mortality findings. Indeed, the rising use of LTACs in our population, and the finding that LTAC utilization can shift the mortality burden of critically ill patients to other hospitals (21), strongly suggests that even the short-term mortality of this population is higher than we estimate. Prior studies also suggest that in-hospital costs are approximately 75% of one-year costs (2), suggesting that the true costs of CCI are closer to $35 billion, or 1.4% of all health care spending in the US (22). Spending on CCI is likely to rise further as the population ages, since, similar to other critical illness syndromes like sepsis and the acute respiratory distress syndrome (23, 24), the incidence of CCI increases dramatically with age. These results underscore the necessity of efforts to prevent CCI and reduce the associated costs of care, lest CCI account for an ever greater proportion of limited health care resources in the coming years.
Although the incidence of CCI generally rises with age, it declines after age 80 concomitant with an increase in the number of patients who have a clinical condition making them eligible for CCI but die before day 8 in an ICU. This finding could be due to age-related differences in severity of illness. However, given that the vast majority of ICU deaths are preceded by a decision to withdraw or withhold life support (25), it may also be due to age-related differences in patient preferences for intensive care near the end-of-life. If so, this pattern suggests that CCI is “preference sensitive condition”, necessitating a decision (either implicit or explicit) to undergo prolonged life support on the part of patients or their surrogate decision makers. An important next step is to determine what portion of CCI is a reflection of patient preferences for aggressive treatment versus what portion reflects excessive treatment that may be inconsistent with patients’ preferences (26). Such efforts may lead to interventions that better align care with patients’ preferences, not only improving the quality of death and dying for some intensive care patients, but also curbing the growth of CCI.
By far the most common eligibility conditions were acute respiratory failure requiring mechanical ventilation and sepsis. These conditions are characterized by an excessive pro-inflammatory state that can lead to prolonged organ failures, neuromuscular weakness and neurocognitive dysfunction, all of which are the hallmarks of CCI (27). This finding suggests that the early treatment of sepsis and acute respiratory failure is an important target for CCI prevention. By preventing organ failures early in the course of critical illness, novel treatments like early mobility (28), early resuscitation (29), decreased tidal volumes (30), conservative fluid management after shock resolution (31), and avoidance of excessive sedation (32) may be powerful levers for stopping CCI before it occurs, minimizing the population burden of this syndrome.
Among sepsis cases, we found that nearly half were related to infections acquired in the hospital, and that this proportion was greater in smaller hospitals. This finding does not necessarily imply that hospital-acquired infections directly cause CCI—these patients all had relatively long lengths of stay, putting them at increased risk of infections after onset of CCI. Yet, at the very least, our study suggests that hospital-acquired infections play an important role in this condition. Hospital-acquired infections are known to be large contributors to mortality among hospitalized patients and now also appear to contribute to the burden of CCI (33). Thus there may be an opportunity to reduce the incidence of CCI by preventing common hospital-acquired infections such as catheter-associated blood stream infections and pneumonia. Recent policy initiatives such as non-payment for hospital-acquired infections are one such strategy. The potential impact of these policies on the incidence of CCI warrants further study.
We found that only one fifth of patients with CCI were discharged home at the end of their hospitalization. Among survivors, the majority of others were transferred to a skilled nursing facility, with a small but increasing group transferred to an LTACH. Determining the optimal post-acute care setting for patients with CCI is of great importance to Medicare and other health care payers. Early data suggest that LTACHs provide similar quality of care compared to skilled nursing facilities or acute care ICUs for most conditions (34). Yet LTACs may also reduce downstream costs by preventing re-hospitalizations and downstream admissions to skilled nursing facilities (34). Our finding that the vast majority CCI patients do not go to LTACs highlights the uncertain role that these facilities play in the care of CCI. More research is needed both to determine which CCI patients are best served by an LTACH and the optimal way to care for CCI patients in the LTACH setting (35).
Our study has several important limitations. Primarily, we used only one of several possible definitions for CCI, and our definition included some diseases that vary widely in their severity, such as severe wounds. Critical illness syndromes are inherently difficult to define in way that meets the needs of all stakeholders, and we acknowledge that different definitions may produce different estimates of incidence and costs. Nonetheless, our definition was developed by a rigorous consensus process, reflects state-of-the art thinking about the pathophysiology of CCI, and carries the preliminary endorsement of CMS, making it of strong clinical and policy relevance.
Second, we identified CCI in administrative data, which are subject to coding errors. However with few exceptions our coding has been validated in external clinical datasets. Third, we examined only a limited geographic area, although our states were diverse in nature and represented a large portion of the nation as a whole. Fourth, we could not account for the possibility that single patients were admitted multiple times to study hospitals over the year, potentially inflating our incidence figures, albeit to a minor degree. Finally, we were unable to assess clinical outcomes or health care spending after hospital discharge, although if anything this would cause us to underestimate the clinical and financial burden of CCI.
CONCLUSIONS
Our study provides the first robust, national data on the incidence and outcomes of patients with CCI. As such, our results provide new guidance to clinicians and researchers seeking strategies to prevent and treat CCI, as well as policy makers seeking novel ways to organize and finance care for this large and growing segment of patients with critical illness.
Supplementary Material
Acknowledgments
Financial support: National Institutes of Health (R01HL096651).
REFERENCES
- 1.Nelson JE, Cox CE, Hope AA, et al. : Chronic critical illness. American Journal of Respiratory and critical care medicine 2010; 182:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unroe M, Kahn JM, Carson SS, et al. : One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann. Intern. Med 2010; 153:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angus DC, Shorr AF, White A, et al. : Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit. Care Med 2006; 34:1016–1024 [DOI] [PubMed] [Google Scholar]
- 4.Erickson SE, Martin GS, Davis JL, et al. : Recent trends in acute lung injury mortality: 1996-2005. Crit. Care Med 2009; 37:1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson SS, Bach PB: The epidemiology and costs of chronic critical illness. Crit Care Clin 2002; 18:461–476 [DOI] [PubMed] [Google Scholar]
- 6.Carson SS, Cox CE, Holmes GM, et al. : The changing epidemiology of mechanical ventilation: a population-based study. J Intensive Care Med 2006; 21:173–182 [DOI] [PubMed] [Google Scholar]
- 7.Carson SS: Definitions and epidemiology of the chronically critically ill. Respir Care 2012; 57:848–56– discussion 856–8 [DOI] [PubMed] [Google Scholar]
- 8.Kandilov AM, Ingber MJ, Morley M, et al. : Chronically Critically Ill Population Payment Recommendations. Research Triangle Park, NC: RTI International; 2014. [Google Scholar]
- 9.Kahn JM, Benson NM, Appleby D, et al. : Long-term acute care hospital utilization after critical illness. JAMA 2010; 303:2253–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan H, Parsons GA, Ghali WA: Validity of Procedure Codes in International Classification of Diseases, 9th revision, Clinical Modification Administrative Data. Medical care 2004; 42:801. [DOI] [PubMed] [Google Scholar]
- 11.Zilberberg MD, Luippold RS, Sulsky S, et al. : Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit. Care Med 2008; 36:724–730 [DOI] [PubMed] [Google Scholar]
- 12.Andaluz N, Zuccarello M: Recent trends in the treatment of spontaneous intracerebral hemorrhage: analysis of a nationwide inpatient database. J. Neurosurg 2009; 110:403–410 [DOI] [PubMed] [Google Scholar]
- 13.Selassie AW, Zaloshnja E, Langlois JA, et al. : Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil 2008; 23:123–131 [DOI] [PubMed] [Google Scholar]
- 14.Iwashyna TJ, Odden A, Rohde J, et al. : Identifying Patients With Severe Sepsis Using Administrative Claims: Patient-Level Validation of the Angus Implementation of the International Consensus Conference Definition of Severe Sepsis. Medical care 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care 2005; 43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 16.MacIntyre NR, Epstein SK, Carson S, et al. : Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. 2005. p. 3937–3954. [DOI] [PubMed] [Google Scholar]
- 17.Cox CE, Carson SS, Holmes GM, et al. : Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993-2002. Crit. Care Med 2004; 32:2219–2226 [DOI] [PubMed] [Google Scholar]
- 18.Seymour CW, Rea TD, Kahn JM, et al. : Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. American Journal of Respiratory and critical care medicine 2012; 186:1264–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley GF: Administrative and claims records as sources of health care cost data. Medical care 2009; 47:S51–5 [DOI] [PubMed] [Google Scholar]
- 20.Carson SS, Kahn JM, Hough CL, et al. : A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit. Care Med 2012; 40:1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall WB, Willis LE, Medvedev S, et al. : The implications of long-term acute care hospital transfer practices for measures of in-hospital mortality and length of stay. American Journal of Respiratory and critical care medicine 2012; 185:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Organisation for Economic Cooperation and Development: Health at a glance: OECD indicators. Paris: OECD Publishing; 2011. [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, et al. : Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med 2001; 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 24.Rubenfeld GD, Caldwell E, Peabody E, et al. : Incidence and outcomes of acute lung injury. N. Engl. J. Med 2005; 353:1685–1693 [DOI] [PubMed] [Google Scholar]
- 25.Prendergast TJ, Luce JM: Increasing incidence of withholding and withdrawal of life support from the critically ill. American Journal of Respiratory and critical care medicine 1997; 155:15–20 [DOI] [PubMed] [Google Scholar]
- 26.White DB, Braddock CH, Bereknyei S, et al. : Toward shared decision making at the end of life in intensive care units: opportunities for improvement. Arch. Intern. Med 2007; 167:461–467 [DOI] [PubMed] [Google Scholar]
- 27.Villar J, Blanco J, Zhang H, et al. : Ventilator-induced lung injury and sepsis: two sides of the same coin? Minerva Anestesiol 2011; 77:647–653 [PubMed] [Google Scholar]
- 28.Schweickert WD, Pohlman MC, Pohlman AS, et al. : Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ProCESS Investigators, Yealy DM, Kellum JA, et al. : A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med 2014; 370:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 31.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. : Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med 2006; 354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 32.Girard TD, Kress JP, Fuchs BD, et al. : Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134 [DOI] [PubMed] [Google Scholar]
- 33.Burgmann H, Hiesmayr JM, Savey A, et al. : Impact of nosocomial infections on clinical outcome and resource consumption in critically ill patients. Intensive Care Med 2010; 36:1597–1601 [DOI] [PubMed] [Google Scholar]
- 34.Kahn JM, Werner RM, David G, et al. : Effectiveness of Long-term Acute Care Hospitalization in Elderly Patients With Chronic Critical Illness. Medical care 2013; 51:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carson SS: Know your long-term care hospital. Chest 2007; 131:2–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


