Remdesivir is being studied for the treatment of COVID-19. This first update of a living rapid review used to inform American College of Physicians Practice Points includes information from a newly published large multinational randomized controlled trial (RCT) as well as the final results of an RCT previously published as a preliminary report. This update presents the results of new meta-analyses on the effect of remdesivir on mortality and nonmortality outcomes and determines aggregate certainty of evidence on the effectiveness of 5 versus 10 days of remdesivir therapy on the basis of previously published data.
Abstract
Background:
Remdesivir is being studied and used for treatment of coronavirus disease 2019 (COVID-19).
Purpose:
To update a previous review of remdesivir for adults with COVID-19, including new meta-analyses of patients with COVID-19 of any severity compared with control.
Data Sources:
Several sources from 1 January 2020 through 7 December 2020.
Study Selection:
English-language, randomized controlled trials (RCTs) of remdesivir for COVID-19. New evidence is incorporated by using living review methods.
Data Extraction:
1 reviewer abstracted data; a second reviewer verified the data. The Cochrane Risk of Bias Tool and GRADE (Grading of Recommendations Assessment, Development and Evaluation) method were used.
Data Synthesis:
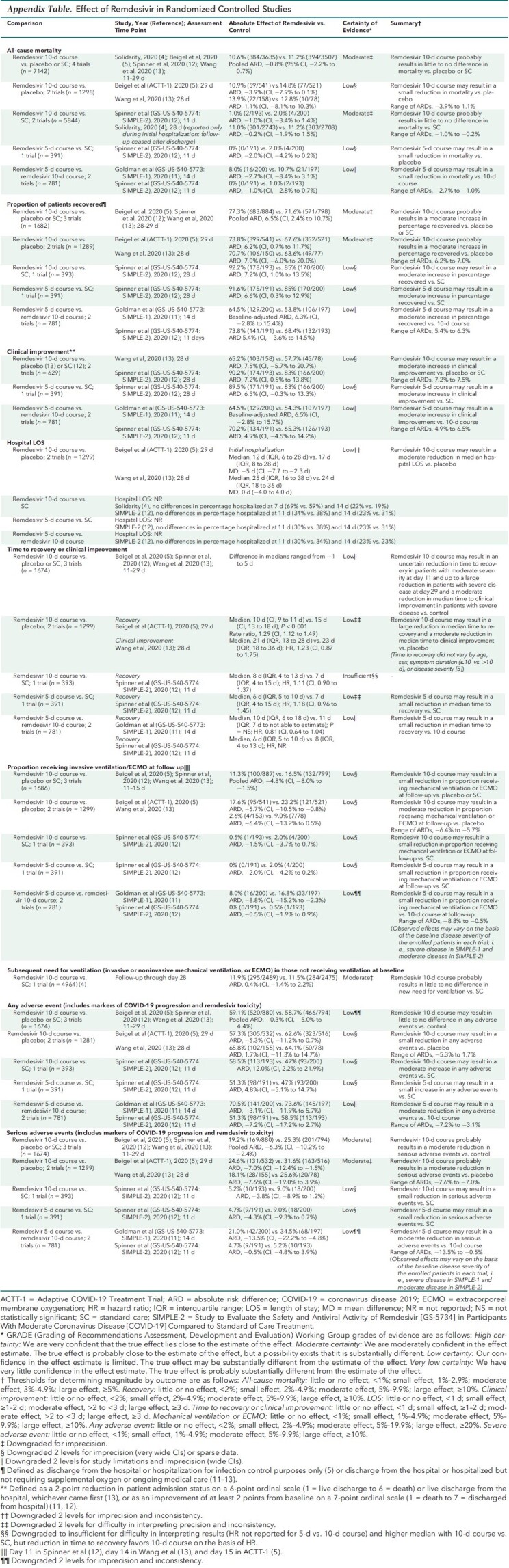
The update includes 5 RCTs, incorporating data from a new large RCT and the final results of a previous RCT. Compared with control, a 10-day course of remdesivir probably results in little to no reduction in mortality (risk ratio [RR], 0.93 [95% CI, 0.82 to 1.06]; 4 RCTs) but may result in a small reduction in the proportion of patients receiving mechanical ventilation (RR, 0.71 [CI, 0.56 to 0.90]; 3 RCTs). Remdesivir probably results in a moderate increase in the percentage of patients who recovered and a moderate decrease in serious adverse events and may result in a large reduction in time to recovery. Effect on hospital length of stay or percentage remaining hospitalized is mixed. Compared with a 10-day course for those not requiring ventilation at baseline, a 5-day course may reduce mortality, the need for ventilation, and serious adverse events while increasing the percentage of patients who recovered or clinically improved.
Limitation:
Summarizing findings was challenging because of varying disease severity definitions and outcomes.
Conclusion:
In hospitalized adults with COVID-19, remdesivir probably results in little to no mortality difference but probably improves the percentage recovered and reduces serious harms and may result in a small reduction in the proportion receiving ventilation. For patients not receiving ventilation, a 5-day course may provide greater benefits and fewer harms with lower drug costs than a 10-day course.
Primary Funding Source:
U.S. Department of Veterans Affairs.
This is the first update of our living rapid review on the effects of remdesivir for adults with coronavirus disease 2019 (COVID-19). Remdesivir, a nucleotide analogue prodrug that inhibits viral RNA, is being studied for treating patients with COVID-19 (1, 2). Our original rapid review assessing the benefits and harms of remdesivir for adults hospitalized with COVID-19 included 4 randomized controlled trials (RCTs) (3).
Our update includes information on remdesivir from a newly published, large, multinational RCT (4), as well as the final results of an RCT previously published as a preliminary report (5). In this update, we assess the new evidence, revise previous analyses and certainty of evidence (COE), conduct new meta-analyses on the effect of remdesivir on mortality (overall and by subgroups) and nonmortality outcomes, and determine aggregate COE on the effectiveness of 5 versus 10 days of remdesivir therapy on the basis of previously published data.
Methods
We used methods similar to those described previously (3). Our literature search was updated to include publications from 1 September through 7 December 2020 and used the original search strategies and inclusion criteria. Tools to assess risk of bias (6) and estimate COE (7) were unchanged (Supplement Tables 1 to 9). The definitions of critical and important outcomes and our a priori–established thresholds for estimating effect magnitude for these outcomes were also unchanged.
Data Synthesis and Analysis
We pooled outcomes reported in at least 3 trials to calculate relative and absolute measures of effect with corresponding 95% CIs. We used a fixed-effects model because of the small number of trials. Data were analyzed in R (The R Foundation) (8). The magnitude of statistical heterogeneity was assessed with the I 2 statistic (I 2 > 75% may indicate substantial heterogeneity) (9).
Our original review mainly compared the effectiveness of remdesivir versus placebo. However, our new primary analyses, developed in consultation with the American College of Physicians Scientific Medical Policy Committee, focused on comparing a 10-day course of remdesivir with a combined control of placebo and standard care for all outcomes in patients with COVID-19 of any severity. We also provide results for the different controls (placebo and standard care) separately and the COE for outcomes we could not pool. We conducted sensitivity analyses by baseline COVID-19 severity and by combining remdesivir 5- and 10-day courses.
We include new analyses and data on all-cause mortality, recovery or improvement, need for ventilation, hospital length of stay, and percentage of patients hospitalized between days 7 and 14. We also provide new mortality analyses by subgroups defined by baseline respiratory support requirements: no oxygen, supplemental oxygen but not ventilation, and ventilation. These categories approximate National Institutes of Health and World Health Organization (WHO) definitions of moderate, severe, and critical COVID-19, respectively (Supplement Table 10.
We reanalyzed aggregate COE on the effectiveness of a 5- versus 10-day course of remdesivir by combining RCTs that included patients with moderate and severe COVID-19 on the basis of previously published data. These reanalyses were done in consultation with the American College of Physicians because they were deemed clinically reasonable and useful.
Role of the Funding Source
This work is based on a living rapid review done for the U.S. Department of Veterans Affairs Evidence Synthesis Program (10). Funding for that review was provided by the Veterans Health Administration Office of Research and Development, Health Services Research and Development Service. The funding source assigned the topic but was not involved in data collection, analysis, manuscript preparation, or submission.
Results
The updated literature search identified 476 citations (Appendix Figure). We identified 2 new eligible publications: final results from previously published preliminary findings of a placebo-controlled RCT (ACTT-1 [Adaptive COVID-19 Treatment Trial]) (5) and interim published results of an open-label RCT of remdesivir versus standard care (Solidarity) (4). Therefore, a total of 5 RCTs were eligible for inclusion in this update (4, 5, 11–13).
Appendix Figure. Evidence search and selection.
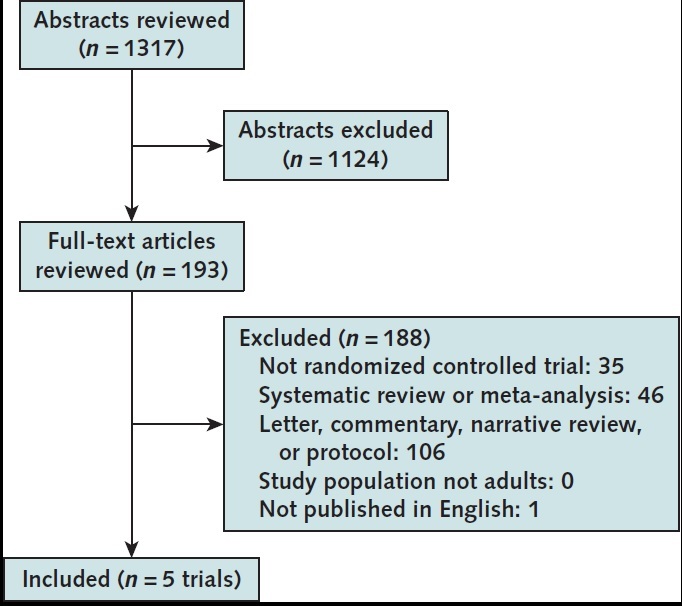
Overview of All Randomized Trials (5 trials)
All studies evaluated remdesivir administered intravenously, with 200 mg on day 1 and 100 mg on subsequent days. Studies excluded patients who were pregnant or had severe kidney or liver dysfunction. Four RCTs included patients with severe COVID-19 (4, 5, 11, 13); 1 included only patients with moderate COVID-19 (12). Four RCTs compared remdesivir with control (placebo or standard care) (4, 5, 12, 13), and 1 RCT compared a 5- versus a 10-day remdesivir course (11). The primary outcome in 4 RCTs was time to clinical improvement or symptom recovery; in 1 RCT (Solidarity) (4), it was in-hospital all-cause mortality through 28 days. Details about study characteristics, outcomes, and harms are reported in Supplement Table 3-Table 4-Table 5-Table 6-Table 7-Table 8, and information on risk of bias is presented in Supplement Table 9.
New Findings From ACTT-1 and Solidarity
ACTT-1 (Final Report)
Final results from ACTT-1 (n = 1062), a double-blind, placebo-controlled study (5), were generally similar to those of the preliminary report and provide additional mortality information through 29 days. Review of interim results had prompted trial investigators to end the placebo group early. Compared with the placebo group, patients who were randomly assigned to receive up to a 10-day course of remdesivir had a shorter time to recovery. Remdesivir increased the percentage of patients who recovered and resulted in a numerically lower mortality rate at 29 days. It reduced the percentage of patients receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) on day 15. Remdesivir reduced serious adverse events and resulted in a moderate, nonsignificant reduction in any adverse event compared with placebo. The effectiveness of remdesivir in shortening time to recovery did not vary by prespecified subgroups of age (categories), sex, symptom duration (≤10 vs. >10 days), disease severity (mild or moderate vs. severe), or concomitant corticosteroid use (23% of all participants). However, in the subset of patients with severe disease who were receiving invasive mechanical ventilation or ECMO (critical COVID-19, n = 285 [27% of enrollees]), time to recovery was not improved with remdesivir.
Solidarity
The WHO's Solidarity trial, the largest published RCT of remdesivir (n = 5472), was a simple, open-label, adaptive study comparing a 10-day course of remdesivir with a “standard-of-care” control in adults hospitalized with COVID-19 (4). The primary outcome was in-hospital mortality through 28 days. Among all participants, 65% were older than 50 years and 63% were men; 24% of participants were receiving no oxygen, 67% were receiving supplemental oxygen, and 9% were receiving ventilation (invasive or noninvasive mechanical ventilation or ECMO) at baseline. Compared with standard care, remdesivir did not reduce in-hospital 28-day mortality, either overall or in any subgroup. The effects of remdesivir on mortality did not vary by age, sex, comorbid conditions, current smoking status, geographic location, presence of bilateral pulmonary infiltrates, concomitant corticosteroid use (48% of participants), duration of hospitalization before randomization (0, 1, or ≥2 days), or respiratory support at baseline, although there was a suggestion of increased mortality with remdesivir in patients receiving ventilation at baseline. Among participants not receiving ventilation at baseline, remdesivir did not reduce the subsequent need for ventilation. Remdesivir did not decrease the percentage of persons hospitalized at day 7 or 14.
Summary Findings
See Figures 1 and 2, the Table, and the Appendix Table).
Figure 1. Mortality for remdesivir 10-d course vs.
![Figure 1. Mortality for remdesivir 10-d course vs. control (placebo or standard care). The black diamonds reflect pooled results from randomized controlled trials (listed above) that enrolled patients in the corresponding respiratory support subgroups. ACTT-1 = Adaptive COVID-19 Treatment Trial; ECMO = extracorporeal membrane oxygenation; RR = risk ratio; SIMPLE-2 = Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment. Top. Overall. Bottom. Results by initial respiratory status.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3b14/7901604/ff89c9933f6a/aim-olf-M208148-M208148ff1.jpg)
control (placebo or standard care). The black diamonds reflect pooled results from randomized controlled trials (listed above) that enrolled patients in the corresponding respiratory support subgroups. ACTT-1 = Adaptive COVID-19 Treatment Trial; ECMO = extracorporeal membrane oxygenation; RR = risk ratio; SIMPLE-2 = Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment. Top. Overall. Bottom. Results by initial respiratory status.
Figure 2. Nonmortality outcomes for remdesivir 10-d course vs.
![Figure 2. Nonmortality outcomes for remdesivir 10-d course vs. control (placebo or standard care). ACTT-1 = Adaptive COVID-19 Treatment Trial; ECMO = extracorporeal membrane oxygenation; RR = risk ratio; SIMPLE-2 = Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment. Top. Proportion of patients recovered. Middle. Need for invasive ventilation/ECMO. Bottom. Patients with ≥1 serious adverse event. * For the pooled trials, defined as proportion receiving invasive ventilation/ECMO (new vs. continued from baseline) at follow-up (ACTT-1 on day 15, Wang et al on day 14, and SIMPLE-2 on day 11). † Unpooled Solidarity trial, defined as subsequent need for ventilation in those not receiving ventilation at baseline (through day 28).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3b14/7901604/d7c9f0398944/aim-olf-M208148-M208148ff2.jpg)
control (placebo or standard care). ACTT-1 = Adaptive COVID-19 Treatment Trial; ECMO = extracorporeal membrane oxygenation; RR = risk ratio; SIMPLE-2 = Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment. Top. Proportion of patients recovered. Middle. Need for invasive ventilation/ECMO. Bottom. Patients with ≥1 serious adverse event.
* For the pooled trials, defined as proportion receiving invasive ventilation/ECMO (new vs. continued from baseline) at follow-up (ACTT-1 on day 15, Wang et al on day 14, and SIMPLE-2 on day 11).
† Unpooled Solidarity trial, defined as subsequent need for ventilation in those not receiving ventilation at baseline (through day 28).
Table. Summary of Conclusions and Updated Findings for Randomized Trials of Remdesivir.
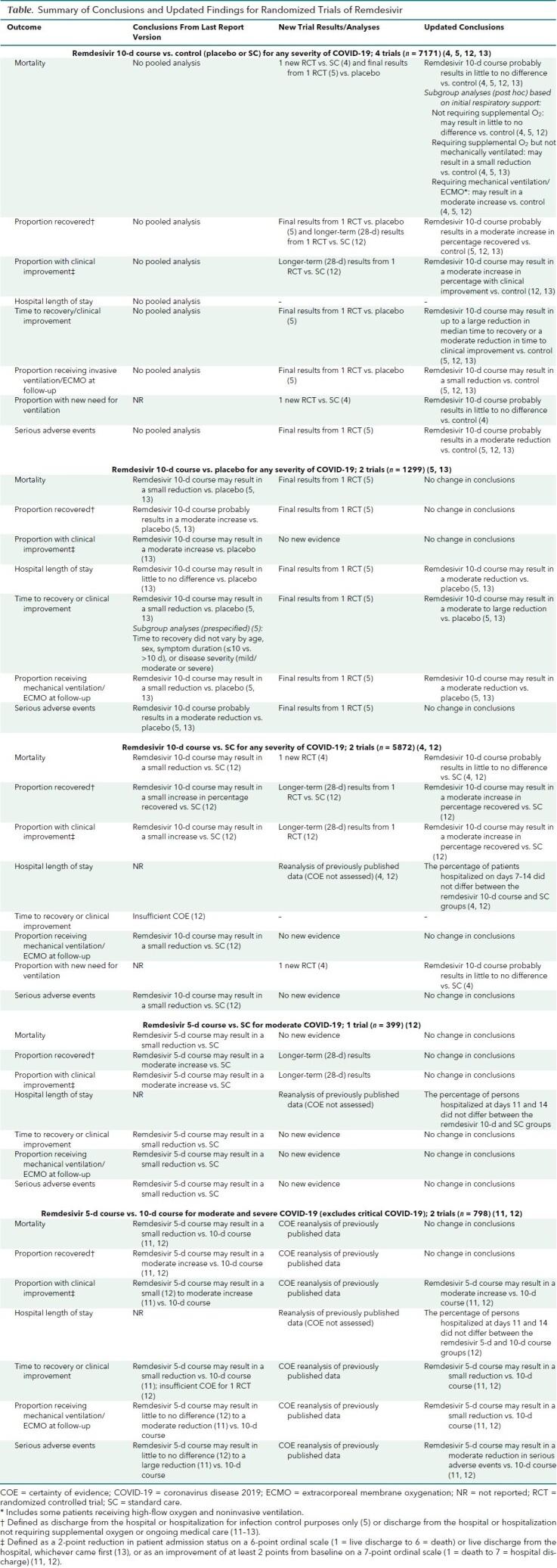
Appendix Table. Effect of Remdesivir in Randomized Controlled Studies.
Remdesivir 10-Day Course Compared With Control (Placebo or Standard Care [4 trials])
Of the 4 RCTs comparing remdesivir with control (placebo or standard care), 2 used a placebo (5, 13) and 2 used standard care as the control (4, 12) (Table and Appendix Table). One RCT included only patients with moderate disease (12), but the other 3 RCTs included patients with more severe COVID-19, including critical disease (4, 5, 13). We used 28-day (instead of 11-day) data from SIMPLE-2 (Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment) (12) to allow pooling with studies that had longer follow-up.
All-Cause Mortality
Our updated analyses, including new results from Solidarity, show that remdesivir, compared with control, probably results in little to no difference in mortality (risk ratio [RR], 0.93 [95% CI, 0.82 to 1.06]; absolute risk difference [ARD], −0.8% [CI, −2.2% to 0.7%]; 4 RCTs) (moderate COE) (Figure 1, top). Mortality results varied little when we did sensitivity analyses that included results of a 5-day course of remdesivir.
Based on pooled post hoc subgroup analyses, remdesivir's effect on mortality may vary by baseline respiratory support requirements (Figure 1, bottom). Compared with control, remdesivir may result in little to no decrease in mortality in patients not requiring supplemental oxygen (RR, 0.78 [CI, 0.41 to 1.50]; ARD, −0.5% [CI, −0.2% to 0.8%]; 3 RCTs), a small decrease in patients receiving supplemental oxygen but not needing ventilation (RR, 0.81 [CI, 0.68 to 0.96]; ARD, −2.3% [CI, −4.2% to −0.4%]; 3 RCTs), and a moderate increase in patients receiving ventilation (RR, 1.19 [CI, 0.98 to 1.46]; ARD, 4.9% [CI, −0.6% to 10.3%]; 3 RCTs). Remdesivir's effect on mortality may not vary by other patient, disease, or treatment factors, including age, sex, race/ethnicity, current smoking status, comorbid conditions, geographic location, presence of bilateral pulmonary infiltrates, concomitant corticosteroid use, duration of hospitalization before randomization (0, 1, or ≥2 days) (4), or symptom duration (≤10 vs. >10 days) (13).
Proportion Recovered
New analyses show that remdesivir probably results in a moderate increase in the percentage of patients who recovered (ARD, 6.5% [CI, 2.4% to 10.7%]; 3 RCTs) (moderate COE) (Figure 2, top). Recovery was defined as discharge from the hospital or hospitalization for infection control purposes only (5), or discharge from the hospital or hospitalized but not requiring supplemental oxygen or ongoing medical care (11–13).
Proportion Clinically Improved
New analyses show that remdesivir versus control may result in a moderate increase in the proportion clinically improved (range of ARDs, 7.2% to 7.5%; 2 RCTs) (low COE). Clinical improvement was defined as a 2-point reduction in patients' admission status on a 6-point ordinal scale (1 = live discharge to 6 = death) or live discharge from the hospital, whichever came first (13), or as an improvement of at least 2 points from baseline on a 7-point ordinal scale (1 = death to 7 = discharged from hospital) (12).
Hospital Length of Stay and Percentage Hospitalized
New analyses show that remdesivir versus placebo may moderately reduce median hospital length of stay (mean difference, −5 days [CI, −7.7 to −2.3 days] and 0 days [CI, −4.0 to 4.0 days]; 2 RCTs) (low COE). However, remdesivir probably does not decrease the percentage of patients hospitalized between days 7 and 14 compared with standard care (4, 12).
Time to Recovery or Clinical Improvement
New analyses show that compared with control, remdesivir may result in a large reduction in time to recovery for patients with severe disease (5) and an uncertain reduction for patients with moderate disease (12). Remdesivir may result in a moderate reduction in median time to clinical improvement versus control (low COE) (13). The effect of remdesivir on time to recovery may not vary by age category, sex, symptom duration (≤10 days vs. >10 days or median duration, ≤9 days vs. >9 days) (5, 13), disease severity (mild or moderate vs. severe [including critical]), or concomitant corticosteroid use (5). However, in patients with severe disease who are receiving invasive mechanical ventilation or ECMO at baseline (that is, those with critical COVID-19), remdesivir may not reduce time to recovery (5).
Proportion Receiving Invasive Ventilation or ECMO at Follow-up or With Subsequent New Need for Ventilation
New analyses show that remdesivir may result in a small reduction in the percentage of patients receiving invasive mechanical ventilation or ECMO (new need or continued use from baseline) at specific follow-up time points between days 11 and 15 (11.3% [100 of 887] vs. 16.5% [132 of 799]; ARD, −4.8% [CI, −8.0% to −1.5%]; 3 RCTs). However, on the basis of Solidarity data, remdesivir probably results in little to no difference in subsequent new need for ventilation (invasive or noninvasive mechanical ventilation or ECMO) (11.9% [295 of 2489] vs. 11.5% [284 of 2475]; ARD, 0.4% [CI, −1.4% to 2.2%]; 1 RCT) (low COE) (Figure 2, middle).
Adverse Events
New analyses show that remdesivir probably reduces serious adverse events by a moderate amount (ARD, −6.3% [CI, −10.2% to −2.4%]; 3 RCTs) (moderate COE) (Figure 2, bottom) and may result in little to no difference in any adverse event (ARD, −0.3% [CI, −5.0% to 4.4%]; 3 RCTs) (low COE). Of note, serious adverse events reported in trials included a combination of clinical findings resulting from COVID-19 progression (such as respiratory failure and need for endotracheal intubation) and direct remdesivir toxicity.
Remdesivir 10-Day Course Compared With Placebo
The inclusion of final results from ACTT-1 led to updated conclusions that a 10-day course of remdesivir compared with placebo may result in a moderate reduction in hospital length of stay, a large reduction in time to recovery, and a moderate reduction in the proportion of patients receiving invasive mechanical ventilation or ECMO at follow-up (5). Our other conclusions remain unchanged (Table and Appendix Table).
Remdesivir 10-Day Course Compared With Standard Care
The inclusion of Solidarity results led to updated conclusions that compared with standard care, remdesivir probably results in little to no difference in mortality (4, 12). Remdesivir probably does not decrease the subsequent need for ventilation in patients not receiving it at baseline (4) or the percentage of persons hospitalized between days 7 and 14 (4, 12). Using 28-day data from SIMPLE-2 (12), we now conclude that remdesivir may result in a moderate increase in the proportions recovered and clinically improved. Our other conclusions remain unchanged (Table and Appendix Table).
Duration of Remdesivir Therapy: 5 Versus 10 Days (2 trials)
For the 2 previously published RCTs comparing 5 versus 10 days of remdesivir therapy in patients with moderate and severe COVID-19 (11, 12), we determined aggregate COEs.
Our reanalysis of previous publications shows that for hospitalized patients not requiring invasive mechanical ventilation or ECMO, a 5-day course of remdesivir, compared with a 10-day course, may result in a moderate increase in the proportion clinically improved, a small reduction in time to recovery, a small reduction in the percentage of patients receiving mechanical ventilation or ECMO, and a moderate reduction in serious adverse events, but may not decrease the percentage of persons hospitalized between days 11 and 14 (Table).
Results from a previously reported post hoc analysis found that among patients whose condition worsened clinically so that they needed invasive mechanical ventilation or ECMO despite a 5-day course of remdesivir, continuing remdesivir through 10 days resulted in a lower mortality rate (ARD, 23.0% [CI, 1.0% to 45.0%]) (3, 11). However, we downgraded the COE from our previous assessment of low certainty to insufficient because of the post hoc nature of analysis, a small sample size (n = 66), and relatively few events.
Discussion
Our updated living review and meta-analyses are derived from 5 RCTs of remdesivir enrolling 7767 patients worldwide. Information from Solidarity, the largest RCT, contributes 78% of all deaths to our mortality analyses. The substantive new evidence changes the nature and strength of our earlier conclusions.
For our main analysis, we selected the effect of a 10-day course of remdesivir (intervention) on mortality compared with a placebo or standard care control (comparator). All-cause mortality is an important unbiased measure of treatment effectiveness and allowed us to pool findings from both the placebo and standard-of-care studies. A 10-day course of remdesivir was chosen as the primary intervention because it was used in 4 of the 5 RCTs (4, 5, 12, 13).
The results of the 2 largest trials (ACTT-1 and Solidarity), both of which enrolled patients with all severities of COVID-19 (including severe and critical), vary slightly in their point estimate on the effect of remdesivir on mortality. However, neither trial showed a statistically significant lower mortality rate with remdesivir, and the numerically lower mortality rate seen with remdesivir in ACTT-1 is fairly consistent with results from the larger Solidarity trial (which was powered for mortality). Furthermore, differences in geographic location, prevalence of concomitant steroid use, or control (placebo vs. standard care) seem unlikely to account for the slightly varied results. Therefore, for adults hospitalized with COVID-19, a 10-day course of remdesivir compared with placebo or standard care probably results in little to no mortality difference.
New information allowed us to assess the effect of remdesivir on mortality in clinically meaningful subgroups. A 10-day course of remdesivir versus control may result in little to no mortality reduction in patients not receiving supplemental oxygen, a small mortality reduction in those receiving supplemental oxygen and not needing ventilation, and a moderate increase in mortality in patients receiving ventilation. However, effects of remdesivir on mortality may not vary by other patient or disease characteristics, including symptom duration (≤10 vs. >10 days). Furthermore, the effect of remdesivir on mortality and time to recovery did not vary by concomitant corticosteroid use in sensitivity analyses, an important finding given that treatment with dexamethasone reduces mortality in patients with severe or critical COVID-19 (14). Our subgroup results are based on post hoc analyses, which limits the credibility of their findings. However, the results are consistent across studies and are useful for clinical decision making. Furthermore, because the U.S. Food and Drug Administration has approved remdesivir (15), it is unlikely that substantial new information comparing remdesivir with control will be published (16–18).
The effects of remdesivir on nonfatal outcomes generally showed a benefit. Compared with control, remdesivir may result in a small reduction in the percentage of patients receiving invasive mechanical ventilation or ECMO at specific follow-up time points (11 to 15 days). However, Solidarity results suggest that it probably results in little to no difference in new need for ventilation. These varied results across trials are probably the result of differences in study population and outcome ascertainment. Although 3 RCTs followed all participants, including those receiving mechanical ventilation at baseline, to determine the proportion receiving mechanical ventilation at specific time points, Solidarity reported only new need for ventilation in patients not receiving ventilation at baseline. Remdesivir compared with control probably results in moderate to large reductions in median time to clinical improvement or recovery and a moderate increase in percentage recovered or clinically improved. The effect of remdesivir on hospitalization status (length of stay or percentage hospitalized between days 7 and 14) is mixed, perhaps in part because of continued hospitalization while remdesivir is being administered. Remdesivir probably reduces serious adverse events, some of which include measures of COVID-19 progression, by a moderate amount.
Updated results strengthen our previous findings that among patients not requiring mechanical ventilation or ECMO, a 5-day course of remdesivir versus a 10-day course may have net benefit. Furthermore, a 5-day course would lower drug and resource costs associated with remdesivir infusion.
The U.S. Food and Drug Administration recently approved remdesivir for patients with COVID-19 who are older than 12 years, weigh more than 40 kg, and are hospitalized or in a facility that can provide care similar to that of an acute care hospital (15). Recommendations on the use of remdesivir for COVID-19 vary. Whereas the WHO conditionally recommends against the use of remdesivir (19), the National Institutes of Health and Infectious Diseases Society of America continue to recommend remdesivir for selected patients on the basis of disease severity (20, 21). The Scientific Medical Policy Committee used our updated systematic review to develop a new Practice Points document to guide clinicians on remdesivir use (22).
The Institute for Clinical and Economic Review recently updated its assessment on pricing models for remdesivir to include findings from Solidarity (23). It concluded that remdesivir does not meet a key cost-effectiveness threshold of $50 000 per quality-adjusted life-year for any severity of COVID-19 as currently priced ($3120 for a 5-day [6-vial] course). If remdesivir were priced at $2470 for the 9-vial patient treatment course similar to the average course in ACTT-1, it would meet the cost-effectiveness benchmark for treating patients similar to those enrolled in ACTT-1 (5) (that is, patients receiving nasal oxygen, mechanical ventilation, or ECMO [severe or critical COVID-19]). However, remdesivir is unlikely to be priced at $70 per 5-day treatment course, the price needed to meet cost-effectiveness for patients similar to those enrolled in SIMPLE-2 (12) (that is, patients not needing respiratory support [mild or moderate COVID-19]). Gilead has an ongoing placebo-controlled RCT evaluating the efficacy and safety of intravenous remdesivir (3-day course) for outpatients with mild or moderate, early-stage COVID-19 (≤7 days of symptoms) who are at risk for progression to severe disease (24). Given the logistic challenges of administering intravenous therapies to outpatients with COVID-19, Gilead is also investigating an inhaled solution of remdesivir for such patients (25, 26).
The evidence base for effectiveness is derived from 5 RCTs; Solidarity was published with interim results, and 3 studies were open-label. Important differences exist across RCTs, including various countries of enrollment; differences in control (placebo vs. standard care); heterogeneity in definitions of COVID-19 severity at baseline that did not fully align with those from the National Institutes of Health, WHO, or U.S. Food and Drug Administration; and diverse outcomes. Given these differences, decisions on pooling results across trials, including for subgroups, were made after discussions between review authors and the Scientific Medical Policy Committee. Studies were pooled when they were deemed to be clinically similar, with the goal of providing useful analyses. We also provide information separately for individual trials, placebo, and standard care control groups, and 5- and 10-day treatment durations.
The evidence base for remdesivir harms is derived from 3 RCTs. Because adverse events reported in RCTs included both adverse effects of remdesivir and indicators of COVID-19 disease progression, our conclusion that remdesivir probably reduces serious adverse events may not accurately differentiate the effect of remdesivir on these physiologically disparate events. Furthermore, the largest study, Solidarity, has recorded serious adverse reactions (limited to suspected drug-related events considered rare, life-threatening, and unexpected), but the published interim results do not report these results. Future availability of this information may affect our conclusions. Of note, pregnant women, children younger than 12 years, and persons with severe kidney or liver dysfunction were excluded from studies, and these results cannot be extrapolated to those populations.
In conclusion, in hospitalized adults with COVID-19, remdesivir probably results in little to no difference in mortality and no more than a small reduction in the need for ventilation, but it probably improves the percentage recovered and reduces serious adverse events. For patients not receiving ventilation, a 5-day course may provide greater benefits and fewer harms, with lower drug costs, than a 10-day course.
Supplementary Material
Footnotes
This article was published at Annals.org on 9 February 2021.
References
- 1. Mulangu S , Dodd LE , Davey RT Jr , et al; PALM Writing Group. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-303. [PMID: ] doi: 10.1056/NEJMoa1910993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sheahan TP , Sims AC , Leist SR , et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. [PMID: ] doi: 10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilt TJ , Kaka AS , MacDonald R , et al. Remdesivir for adults with COVID-19: a living systematic review for an American College of Physicians Practice Points. Ann Intern Med. 2020. doi: 10.7326/M20-5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan H , Peto R , Henao-Restrepo AM , et al; WHO Solidarity Trial Consortium. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity trial results. N Engl J Med. 2020. [PMID: ] doi: 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beigel JH , Tomashek KM , Dodd LE , et al; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. [PMID: ] doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration 2011. Accessed at http://handbook.cochrane.org on 24 July 2020.
- 7. Schunemann H, Brozek J, Guyatt G, et al, eds. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Accessed at https://gdt.gradepro.org/app/handbook/handbook.html on 2 June 2020.
- 8. Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1-48.
- 9. Higgins JP , Thompson SG , Deeks JJ , et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilt TJ, Kaka AS, MacDonald R, et al. Rapid Response: COVID-19: Remdesivir for Hospitalized Adults. Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs; 2020. VA ESP Project 09-009.
- 11. Goldman JD , Lye DCB , Hui DS , et al; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827-37. [PMID: ] doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spinner CD , Gottlieb RL , Criner GJ , et al; GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048-57. [PMID: ] doi: 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y , Zhang D , Du G , et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-78. [PMID: ] doi: 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horby P , Lim WS , Emberson JR , et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020. [PMID: ] doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Food and Drug Administration. FDA approves first treatment for COVID-19. Accessed at www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 on 3 November 2020.
- 16. Harrington DP , Baden LR , Hogan JW . A large, simple trial leading to complex questions [Editorial]. N Engl J Med. 2020. [PMID: ] doi: 10.1056/NEJMe2034294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu J . Covid-19: what now for remdesivir. BMJ. 2020;371:m4457. [PMID: ] doi: 10.1136/bmj.m4457 [DOI] [PubMed] [Google Scholar]
- 18. McCreary EK , Angus DC . Efficacy of remdesivir in COVID-19 [Editorial]. JAMA. 2020;324:1041-2. [PMID: ] doi: 10.1001/jama.2020.16337 [DOI] [PubMed] [Google Scholar]
- 19. Siemieniuk R , Rochwerg B , Agoritsas T , et al. A living WHO guideline on drugs for Covid-19. BMJ. 2020;370:m3379. [PMID: ] doi: 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 20. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Accessed at www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-8 on 21 December 2020. [DOI] [PMC free article] [PubMed]
- 21. National Institutes of Health. Therapeutic management of patients with COVID-19. Accessed at www.covid19treatmentguidelines.nih.gov/therapeutic-management on 21 December 2020.
- 22. Abraham GM, Etxeandia-Ikobaltzeta I, Jokela JA, et al. Should remdesivir be used for the treatment of patients with COVID-19? Rapid, living practice points from the American College of Physicians (version 2). Ann Intern Med. 9 February 2020. [Epub ahead of print]. doi:10.7326/M20-8101 [DOI] [PMC free article] [PubMed]
- 23. Institute for Clinical and Economic Review. ICER provides second update to pricing models for remdesivir as a treatment for COVID-19. Accessed at https://icer.org/pressreleases/icer-provides-second-update-to-pricing-models-for-remdesivir-as-a-treatment-for-covid-19 on 8 December 2020.
- 24.Study to Evaluate the Efficacy and Safety of Remdesivir (GS-5734™) Treatment of Coronavirus Disease 2019 (COVID-19) in an Outpatient Setting [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04501952 on 21 December 2020.
- 25.Study in Participants With Early Stage Coronavirus Disease 2019 (COVID-19) to Evaluate the Safety, Efficacy, and Pharmacokinetics of Remdesivir Administered by Inhalation [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04539262 on 21 December 2020.
- 26. Gilead Sciences. Gilead's response to COVID-19. Accessed at www.gilead.com/purpose/advancing-global-health/covid-19 on 21 December 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


