In a large health system in the United States, investigators examined whether mortality, receipt of mechanical ventilation, and patient acuity changed over time among adult patients with COVID-19–related critical illness admitted to intensive care units.
Visual Abstract. COVID-19–Related Critical Illness.
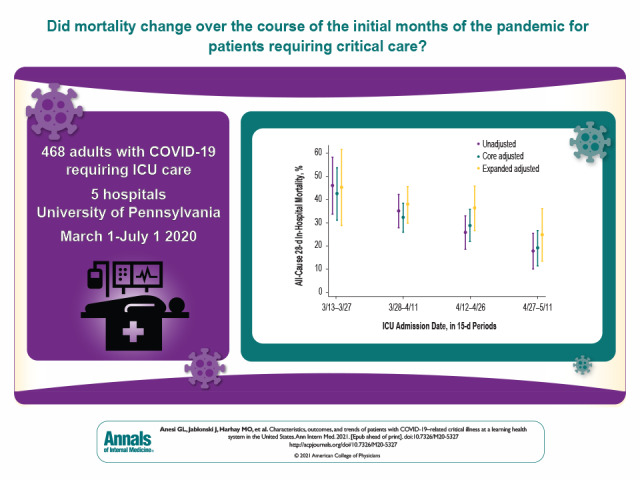
In a large health system in the United States, investigators examined whether mortality, receipt of mechanical ventilation, and patient acuity changed over time among adult patients with COVID-19–related critical illness admitted to intensive care units.
Abstract
Background:
The coronavirus disease 2019 (COVID-19) pandemic continues to surge in the United States and globally.
Objective:
To describe the epidemiology of COVID-19–related critical illness, including trends in outcomes and care delivery.
Design:
Single–health system, multihospital retrospective cohort study.
Setting:
5 hospitals within the University of Pennsylvania Health System.
Patients:
Adults with COVID-19–related critical illness who were admitted to an intensive care unit (ICU) with acute respiratory failure or shock during the initial surge of the pandemic.
Measurements:
The primary exposure for outcomes and care delivery trend analyses was longitudinal time during the pandemic. The primary outcome was all-cause 28-day in-hospital mortality. Secondary outcomes were all-cause death at any time, receipt of mechanical ventilation (MV), and readmissions.
Results:
Among 468 patients with COVID-19–related critical illness, 319 (68.2%) were treated with MV and 121 (25.9%) with vasopressors. Outcomes were notable for an all-cause 28-day in-hospital mortality rate of 29.9%, a median ICU stay of 8 days (interquartile range [IQR], 3 to 17 days), a median hospital stay of 13 days (IQR, 7 to 25 days), and an all-cause 30-day readmission rate (among nonhospice survivors) of 10.8%. Mortality decreased over time, from 43.5% (95% CI, 31.3% to 53.8%) to 19.2% (CI, 11.6% to 26.7%) between the first and last 15-day periods in the core adjusted model, whereas patient acuity and other factors did not change.
Limitation:
Single–health system study; use of, or highly dynamic trends in, other clinical interventions were not evaluated, nor were complications.
Conclusion:
Among patients with COVID-19–related critical illness admitted to ICUs of a learning health system in the United States, mortality seemed to decrease over time despite stable patient characteristics. Further studies are necessary to confirm this result and to investigate causal mechanisms.
Primary Funding Source:
Agency for Healthcare Research and Quality.
A series of reports described the epidemiology and outcomes of patients with coronavirus disease 2019 (COVID-19) requiring hospitalization in the United States and globally (1–7). Reported more recently are the characteristics and outcomes of patients who are critically ill or require admission to an intensive care unit (ICU), as well as trends in critical illness outcomes over time (8–12). In some cases, lack of adequate follow-up has severely clouded the interpretability of early results, especially in light of the prolonged hospital course for patients with severe disease who require mechanical ventilation (MV). Many reports have not placed observed trends in the context of contemporaneous clinical and operational changes in hospitals and health systems.
In this single–health system, multihospital retrospective cohort study, we sought to describe the characteristics and outcomes of patients with COVID-19–related critical illness at a large academic health system in the United States and to evaluate trends in mortality and patient characteristics over time. We hypothesized that mortality would decrease over time for this novel disease, independent of patient-level factors. In addition, we sought to put these findings in the context of a coordinated health system–wide critical care preparedness and response program with rapid-cycle adaptation during all phases of the pandemic to date.
Methods
Study Design
We performed a single–health system, multihospital retrospective cohort study of critically ill patients with COVID-19 admitted to the ICUs of 5 hospitals within the University of Pennsylvania Health System (Penn Medicine). The study protocol was granted exemption by the Institutional Review Board of the University of Pennsylvania (Philadelphia). Analyses were conducted by using Stata (StataCorp), the R language for statistical computing (The R Foundation), and the Python programming language (Python Software Foundation).
Study Setting
Penn Medicine is a 6-hospital academic health system with centers ranging from rural and suburban community hospitals to urban quaternary referral centers spanning eastern Pennsylvania to southern New Jersey. The system has more than 3000 hospital beds, including more than 380 ICU beds, with variation due to flex and surge spaces. All but 1 health system hospital, which operates a separate electronic health record build, contributed data to the study.
Before the COVID-19 pandemic, Penn Medicine operated a health system–wide Critical Care Alliance comprising leaders from all ICUs across the health system as well as interdisciplinary stakeholders and subject matter experts. The Critical Care Alliance reports to the health system's chief medical officer and has a mandate to effect data-driven quality and value improvement, coordinated across the health system's ICUs. Within this structure, the Critical Care Alliance rapidly formed a COVID-19 Task Force at the onset of the global pandemic, before a single case had presented to the health system, with the mandate to apply the overarching quality goals of the Critical Care Alliance to this novel disease.
Organizational interventions of the Critical Care Alliance COVID-19 Task Force included developing standardized clinical protocols; creating an education program to teach, train, or refresh providers, beginning from an array of critical care competency levels; monitoring quality metrics for emerging points of intervention; and developing novel surge ICUs, including standards for staffing, locations, and equipment. This work, communicated regularly to senior leadership, was accomplished through 6 nimble working groups: guideline development, education, surge planning, palliative care and patient and family experience, telemedicine, and equipment. Over 10 weeks, the task force released 15 clinical practice guidelines, a COVID-19 critical care admission order set for the electronic health record, surge location and staffing templates, equipment surveillance dashboards (including for mechanical ventilators, dialysis machines, and extracorporeal life support machines), and an online curriculum to cross-train non-ICU clinicians being redeployed to surge locations. Supplement Figure 1 details the COVID-19 Task Force interventions and operational timeline.
Study Population
Patients were eligible for enrollment if they were 18 years of age or older and were admitted to an ICU with COVID-19 complicated by either acute respiratory failure or shock. Diagnosis of COVID-19 was ascertained by a positive result on polymerase chain reaction assay performed in our health system or by a COVID-19 flag in the electronic health record, as assigned by hospital infection control to capture positive results of tests performed before hospitalization outside our health system.
Acute respiratory failure was defined clinically as receipt of high-flow nasal cannula (HFNC) with FIo 2 of 50% or greater; noninvasive ventilation (NIV), including helmet continuous positive airway pressure (CPAP); or MV at any time. Shock was defined clinically as the receipt of continuous-infusion vasopressors at any dose, at any time. These clinical definitions, beyond ICU admission alone, were deployed to standardize populations facing different ICU admission thresholds both among and within hospitals over time, because such thresholds may have changed during the course of the pandemic. We enrolled patients meeting this definition who were admitted to an ICU during the initial surge of the pandemic, from 1 March to 11 May 2020; the last day of follow-up was 1 July 2020.
Patients who were transferred between ICUs of different hospitals were considered to have a single hospitalization, and ICU presentation data were collected at the first ICU admission. Because only a few patients (n = 39) were transferred from outside the health system, these patients were not analyzed separately.
Data Collection and Definitions
Clinical and demographic variables, therapies, and outcomes were extracted from the Epic electronic health record (Epic Systems Corporation). Acute physiology vital signs and laboratory values were measured during the 24 hours before and 24 hours after the initial ICU admission, with the most extreme value or the value temporally closest to ICU admission used, as clinically appropriate. Chronic comorbidity burden was measured by using the International Classification of Diseases, 10th Revision, diagnosis coding–based Charlson Comorbidity Index (13).
ICU Therapies, Hospital Outcomes, and Survivorship
We report ICU therapy utilization rates for HFNC; NIV, including helmet CPAP; MV; vasopressors; acute renal replacement therapy; and extracorporeal life support. Among patients requiring renal replacement therapy, chart review by a nephrologist (D.N.) distinguished acute renal replacement therapy (that is, for acute or acute-on-chronic kidney injury) from end-stage kidney disease with preexisting long-term dialysis dependence. Chart review also determined recovery from acute renal replacement therapy, defined as receipt of acute renal replacement therapy during the hospitalization with no renal replacement therapy requirement at the time of hospital discharge.
The primary outcome was all-cause 28-day in-hospital mortality, with all patients either dying in the hospital or surviving to hospital discharge by day 28, or having at least 28 days of in-hospital follow-up (considered survivors). This was selected as the primary outcome to allow all patients to have either an outcome or, if not, equal and adequate in-hospital follow-up. In addition, we report all-cause death at any time (secondary outcome defined as also including out-of-hospital deaths at any time, in-hospital deaths after hospital day 28, and hospice discharges), hospital length of stay, ICU length of stay, MV duration, recovery from acute renal replacement therapy, and tracheostomy, using the complete follow-up time in the data set.
Among survivors, we report hospital discharge disposition, weight change, prevalence of persistent lymphopenia, and all-cause 30-day rehospitalizations (among at-risk survivors, defined as patients with 30 days of postdischarge follow-up and excluding those who transitioned to hospice or who remained in the hospital at the end of follow-up).
Therapy, Acuity, and Mortality Trends Over Time
We evaluated whether all-cause 28-day in-hospital mortality changed over time by using logistic regression. We used an unadjusted model; a core multivariable model adjusted for age, Charlson Comorbidity Index, Sequential (Sepsis-Related) Organ Failure Assessment (SOFA) score (14), and hospital or hospital-specific measures of COVID-19 patient occupancy of pre–COVID-19 ICU and ward beds; and then an expanded multivariable model further adjusted for patient-level factors—including demographic characteristics, comorbid conditions, and measures of acute physiology, such as vital signs and laboratory values measured surrounding ICU admission—selected based on existing literature and hypothesized associations with mortality (1–7). These included body mass index, Glasgow Coma Score, oxygen saturation, respiratory rate, platelet count, and Pao 2–FIo 2 ratio. These analyses were repeated only for patients who received MV and for the secondary outcome of all-cause death at any time. Time of ICU admission was categorized into 4 15-day periods across the study period (of 63, 165, 140, and 96 patients, respectively, with 4 early and temporally separate ICU admissions excluded). We separately evaluated whether mortality changed over intervals of cumulative COVID-19 patient volume (in equal increments of 78 consecutive ICU admissions across the study period) as a related but different proxy of health system COVID-19 experience. Finally, using linear and logistic regression, we evaluated whether ICU admission date was associated with rates of MV use (unadjusted and adjusted for Pao 2–FIo 2 ratio) and with the aforementioned patient-level factors.
Role of the Funding Source
The funding sources had no role in the study design, conduct, or analysis, or in manuscript submission.
Results
Patients and Patient Characteristics
Among 1470 patients with COVID-19 admitted to the 5 study hospitals from 1 March to 11 May 2020, 480 (32.7%) were admitted to an ICU. Of these patients, 468 (97.5%) met inclusion criteria for critical illness due to acute respiratory failure or shock (Appendix Figure). Characteristics of patients with COVID-19–related critical illness are shown in Table 1, Supplement Table 1, and Supplement Figure 2. These patients had a median age of 65 years (interquartile range [IQR], 54 to 74 years), were more likely to be male (57.7%), were more likely to be Black (52.8%), and had a high comorbidity burden (71.8% with ≥2 points on the Charlson Comorbidity Index).
Appendix Figure 1. CONSORT (Consolidated Standards of Reporting Trials) diagram.
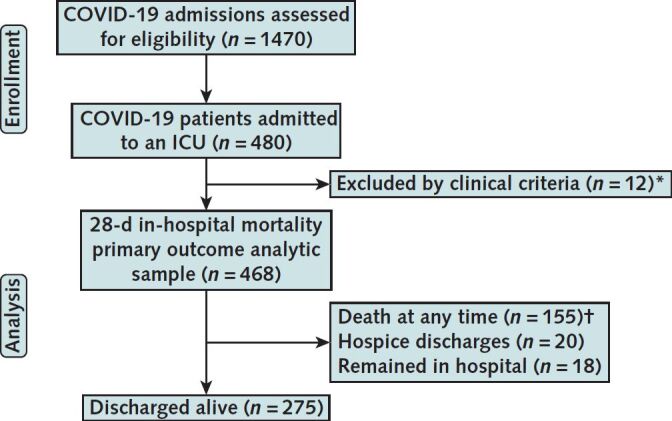
ICU = intensive care unit.
* 12 patients admitted to the ICU but who did not meet clinical criteria for acute respiratory failure or shock, defined as receiving ≥1 of the following interventions at any time during hospitalization: high-flow nasal cannula with FIo 2 ≥50%; noninvasive ventilation, including helmet noninvasive ventilation; mechanical ventilation; or vasopressors.
† 140 patients died in hospital within 28 days; 15 patients died in hospital after 28 days.
Table 1. Characteristics of Patients With COVID-19–Related Critical Illness (n = 468)*.
Acute Physiology at ICU Admission
Table 2 and Supplement Table 2 report acute physiology parameters of patients with COVID-19–related critical illness at the time of ICU admission. Median Pao 2–FIo 2 ratio was 103 (IQR, 65 to 191), and 57.8% of patients had a Pao 2–FIo 2 ratio of 300 or less (including 73.7% of those who received MV), which would meet the Berlin definition of acute respiratory distress syndrome, in the presence other qualifying criteria (15). Median SOFA score was 5.0 (IQR, 2.5 to 7.0), which carries an unadjusted mortality rate of approximately 15% (16) and was driven substantially by the respiratory subscore, with a median of 2 (IQR, 0 to 4).
Table 2. Acute Physiology of Patients With COVID-19–Related Critical Illness at ICU Admission (n = 468)*.
Altered mental status, on the basis of a Glasgow Coma Score less than 15, was present in 139 patients (33.5%), and 265 patients (56.6%) were febrile. At the time of ICU admission, patients with COVID-19–related critical illness had tachycardia (median maximum heart rate, 112 beats/min [IQR, 97 to 128 beats/min]), hypotension (median minimum systolic blood pressure, 90 mm Hg [IQR, 80 to 103 mm Hg]), tachypnea (median maximum respiratory rate, 35 breaths/min [IQR, 29 to 41 breaths per minute]), and hypoxemia (median minimum oxygen saturation, 86% [IQR, 78% to 91%]). Leukocytosis was present in 148 patients (31.6%), whereas 19 (4.1%) had leukopenia, and patients generally were lymphopenic (median absolute lymphocyte count, 600 cells/L [IQR, 400 to 990 cells/L]) (17). Electrolyte levels and liver study results were within normal limits. Levels of D-dimer (median, 8.93 nmol/L [IQR, 4.82 to 25.08 nmol/L]) and fibrinogen (median, 5.5 g/L [IQR, 4.2 to 7.1 g/L]) were elevated. There was evidence of systemic inflammation on the basis of elevated levels of procalcitonin (≥1.42 ng/mL in 25% of patients), C-reactive protein (median, 97.1 mg/L [IQR, 48.1 to 130.1 mg/L]), ferritin (median, 761 µg/L [IQR, 361 to 1828 µg/L]), lactate dehydrogenase (median, 7.0 µkat/L [IQR, 5.2 to 9.7 µkat/L]), and interleukin-6 (median, 16 pg/mL [IQR, 8 to 77 pg/mL]).
ICU Therapies
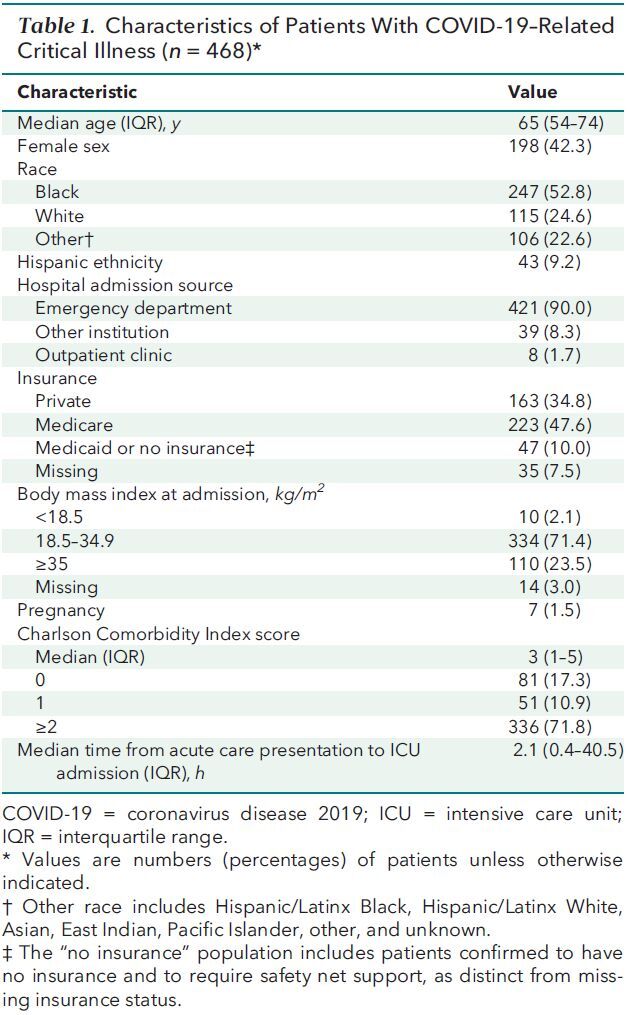
Table 3 lists ICU therapies received by patients with COVID-19–related critical illness. Among the 468 patients with critical illness, 241 (51.5%) received treatment with HFNC at least once during their hospitalization, 107 (22.9%) received NIV (including helmet CPAP), 35 (7.5%) received helmet CPAP, 319 (68.2%) received MV, and 121 (25.9%) received vasopressors. Among 450 patients without preexisting end-stage kidney disease, 44 (9.8%) required acute renal replacement therapy. Extracorporeal life support was used for 15 patients (3.2%). The proportion of patients treated with MV decreased over time, from 85.7% (95% CI, 77.1% to 94.4%) to 54.2% (CI, 44.2% to 64.1%) between the first and last 15-day periods (Supplement Table 3 and Supplement Figure 3. Compared with the first 15-day period, the odds ratio (OR) for receiving MV decreased stepwise across the second (OR, 0.49 [CI, 0.22 to 1.07]; P = 0.074), third (OR, 0.29 [CI, 0.13 to 0.64]; P = 0.002), and fourth (OR, 0.20 [CI, 0.09 to 0.44]; P < 0.001) 15-day periods. This trend persisted but was attenuated with adjustment for Pao 2–FIo 2 ratio (Supplement Table 3 and Supplement Figure 3).
Table 3. ICU Therapies and Hospital Outcomes of Patients With COVID-19–Related Critical Illness (n = 468).
Hospital Outcomes and Mortality Over Time
Table 3 shows hospital outcomes for patients with COVID-19–related critical illness. These patients had an observed all-cause 28-day in-hospital mortality rate of 29.9% (37.0% among those who received MV and 14.8% among those who did not), with 92 patients (19.7%) remaining hospitalized after 28 days. All-cause death at any time, including 15 in-hospital deaths after 28 days and 20 hospice discharges considered to be deaths, occurred in 33.1% of patients (18 [3.9%] remained hospitalized at the time follow-up ended or had been transferred to another hospital and were lost to follow-up). Patients had a median ICU stay of 8 days (IQR, 3 to 17 days) and median hospital stay of 13 days (IQR, 7 to 25 days). Among patients who received MV, median duration of MV was 12 days (IQR, 6 to 20); 74 patients (15.8%) underwent tracheostomy (23.2% of patients who required MV).
All-cause 28-day in-hospital mortality decreased over time in the core adjusted model, from 43.5% (CI, 31.3% to 53.8%) to 19.2% (CI, 11.6% to 26.7%) (Figure 1 and Supplement Table 4). Compared with the first 15-day period, the adjusted OR for mortality decreased stepwise across the second (OR, 0.57 [CI, 0.28 to 1.15]; P = 0.119), third (OR, 0.47 [CI, 0.23 to 0.97], P = 0.042), and fourth (OR, 0.24 [CI, 0.10 to 0.57]; P = 0.001) 15-day periods. Results were similar in the unadjusted and expanded adjusted models (Figure 1 and Supplement Table 4) and persisted when restricted to patients who received MV (Supplement Figure 4 and Supplement Table 5) and when adjusted for COVID-19 patient occupancy of pre–COVID-19 ICU and ward beds (Supplement Figures 5 and 6 and Supplement Table 6). Results were similar—but overall higher, as expected—for all-cause death at any time (Supplement Table 7 and Supplement Figure 7). Mortality also decreased over intervals of cumulative COVID-19 patient volume (Supplement Figure 8) and trended toward a decrease among the 19.4% patients (n = 91) aged 50 years or younger, who would be expected to be minimally affected by trends related to assisted living facilities (Supplement Figure 9 and Supplement Table 8).
Figure 1. All-cause 28-day in-hospital mortality over time.
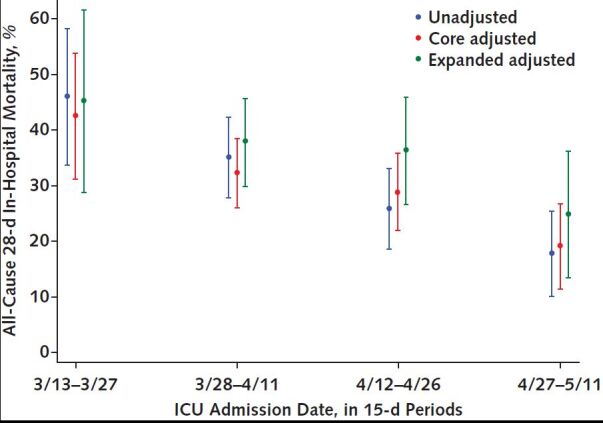
All-cause 28-day in-hospital mortality decreased over ICU admission dates in 15-day periods in the unadjusted (observed), core adjusted, and expanded adjusted models. The core adjusted model includes age, Charlson Comorbidity Index score, SOFA score, and hospital; the expanded adjusted model also includes body mass index, Glasgow Coma Score, oxygen saturation, respiratory rate, platelet count, and Pao2–FIo2 ratio. ICU = intensive care unit; SOFA = Sequential Organ Failure Assessment.
Patient-Level Factors Over Time
In univariate analyses, ICU admission SOFA score (P = 0.141), age (P = 0.41), body mass index (P = 0.091), platelet count (P = 0.087), Glasgow Coma Score (P = 0.67), respiratory rate (P = 0.42), oxygen saturation (P = 0.46), and Pao 2–FIo 2 ratio (P = 0.99) were not associated with ICU admission date (Figure 2).
Figure 2. Patient-level factors over time.
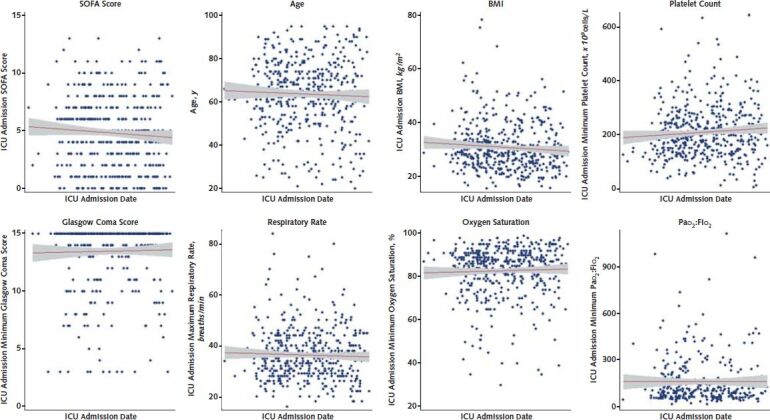
In univariate linear regression models, ICU admission SOFA score (P = 0.141), age (P = 0.41), BMI (P = 0.091), platelet count (P = 0.087), Glasgow Coma Score (P = 0.67), respiratory rate (P = 0.42), oxygen saturation (P = 0.46), and Pao2–FIo2 ratio (P = 0.99) were not associated with ICU admission date. BMI = body mass index; ICU = intensive care unit; SOFA = Sequential Organ Failure Assessment.
Survivorship
As shown in Table 3, among patients who survived to hospital discharge, the most common discharge disposition was home with services (32.2%), followed by home without services (23.4%), skilled nursing facility (18.3%), acute rehabilitation (12.2%), long-term acute care hospital (6.1%), and hospice (6.8%). In total, 55.6% of discharged patients went home. At hospital discharge, COVID ICU survivors had a median weight loss of 2.3 kg (greater with longer hospital stay) and 10.2% had severe, persistent lymphopenia (that is, absolute lymphocyte count ≤600 cells/L) (17). Among 250 at-risk survivors (that is, those with 30 days of postdischarge follow-up and excluding patients discharged to hospice or those who remained hospitalized at the end of follow-up), 27 patients were rehospitalized within 30 days, for an all-cause 30-day hospital readmission rate of 10.8%.
Discussion
Our study of more than 450 patients with COVID-19–related critical illness across 5 hospitals within an academic health system during the initial surge of the pandemic adds support to many findings published by other centers in the United States and globally (1–10, 12), and offers some important additions to our rapidly evolving understanding of the epidemiology of this novel disease. These additions, as well as those in reports from other centers that continue to be released, are particularly important as the United States and other nations combat ongoing and new surges related to opening economies, reduced mitigation strategies, and seasonal changes, among other factors (18).
Our study adds additional strength to the previously reported findings of male and Black race predominance, older age, high comorbidity burden (including cardiac, pulmonary, vascular, and metabolic conditions), obesity, hypoxemia, and systemic inflammation in patients presenting with or developing severe COVID-19 (1–7, 19–22). We examined all-cause 28-day in-hospital mortality over 2 measures of health system COVID-19 experience: intervals of time and intervals of cumulative COVID-19 patient volume. Other studies have reported a learning effect (5, 10, 12, 23). Our results support the notion that outcomes improved during the pandemic independent of patient-level factors. Although we could not evaluate a causal relationship between organizational modifications and changing outcomes in this study, we sought to put these mortality trend findings in temporal context with our preparedness and response strategy (Supplement Figure 1) to the extent that it may assist others in this ongoing pandemic and to help identify foci of future observational or prospective studies. One potential mechanism for improved mortality over time in the face of unchanging acuity was better adherence to evidence-based standard-of-care therapies for critical illness—such as HFNC to avert intubation, as well as prone positioning—which initially proved challenging in the setting of isolation precautions, including personal protective equipment requirements for bedside clinicians. Likewise, our rates of MV use decreased over time (Supplement Table 3, Supplement Figure 1, and Supplement Figure 3) as, along with other centers (10), we moved away from an early intubation strategy toward more liberal use of noninvasive oxygenation via HFNC, because of greater understanding about aerosol generation and risk to health care workers (24). In addition, we transitioned mid-surge from dry ventilator circuits (that is, use of heat and moisture exchangers) to heated airway humidification in response to identified safety events related to endotracheal tube obstruction and high airway resistance (Supplement Figure 1) (25). These results and experiences may be particularly important for regions with ongoing or recurrent surges, where health care staff must continue to deliver high-quality care under newly adverse and unusual circumstances. We should mention that even with a robust preparedness program in place before our first COVID-19 cases, we observed a clear learning curve (Figure 1, Supplement Figures 3 to 4 and 6 to 9, and Supplement Tables 3 to 8) and had to make frequent real-time iterative updates to our approach, including to our clinical treatment guidelines (Supplement Figure 1), which continue to this day (25).
Centers should anticipate a growing population of survivors of COVID-19–related critical illness as the pandemic continues (26). In our study, 68.8% of survivors received post–acute care services (75.6% if hospice discharges are included), and among at-risk survivors (that is, those not discharged to hospice), all-cause 30-day rehospitalization was relatively uncommon, at 10.8%. In contrast to COVID-19 survivorship, among sepsis ICU survivors, for example, 44% received post–acute care services or placement and 20% were rehospitalized (27–29). Potential explanations for the low COVID-19 readmission rate include increased use of acute rehabilitation, given the relationship between acute rehabilitation and improved outcomes among sepsis survivors (30); increased use of home health services, given data suggesting that early and intense home health care is associated with fewer rehospitalizations (31); unprecedented support at home in the setting of the pandemic; and a new phenotype of sepsis survivors who may have fewer subsequent infections (29). However, given the rate of persistent lymphopenia observed and known increased risk for 1-year mortality after sepsis (32, 33), studies designed to examine the long-term health of COVID-19 survivors are needed. Of note, COVID-19 survivorship and disposition outcomes may be biased because of challenges with post–acute care placement due to infection control concerns.
The results of this study should be interpreted in the context of important limitations. First, this was a single–health system study, and the results should be put in context with the totality of epidemiologic data that have emerged and are emerging from other centers in the United States and globally. Second, our secondary outcomes of all-cause death at any time and all-cause 30-day readmissions may miss some events occurring outside and not reported to our health system during follow-up. Of importance, we also did not have access to information on prehospital dwelling type (such as nursing homes or skilled nursing facilities), an important epidemiologic factor, or on initial ICU admissions for patients who transferred directly from other institutions. Third, we required ICU admission for cohort entry and therefore did not study the subgroup of patients who required relatively high oxygen support (such as HFNC) but remained on a ward. Finally, although we report mortality trends over time and temporally related health system organizational interventions, these cannot offer causal arguments or explain differences among hospitals, and are instead hypothesis generating. Likewise, we did not evaluate the use of clinical interventions—such as antiviral therapies, corticosteroids, convalescent plasma, anticoagulants, lung-protective ventilation, or ventilator circuits—or highly dynamic trends in these interventions, nor did we assess related complications, such as thromboembolic events.
In conclusion, among patients with COVID-19–related critical illness admitted to the ICU at an academic health system in the United States, mortality seemed to decrease over time despite stable patient characteristics. Further studies are necessary to confirm this result and to investigate causal mechanisms.
An abstract of this work has been accepted to the Society of Critical Care Medicine Critical Care Congress 2021.
Supplementary Material
Footnotes
This article was published at Annals.org on 19 January 2021.
* Dr. Anesi and Ms. Jablonski are co–first authors.
† Drs. Anderson and Gutsche are co–senior authors.
References
- 1. Bhatraju PK , Ghassemieh BJ , Nichols M , et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382:2012-22. [PMID: ] doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Docherty AB , Harrison EM , Green CA , et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [PMID: ] doi: 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grasselli G , Zangrillo A , Zanella A , et al; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574-1581. [PMID: ] doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewnard JA , Liu VX , Jackson ML , et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ. 2020;369:m1923. [PMID: ] doi: 10.1136/bmj.m1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petrilli CM , Jones SA , Yang J , et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [PMID: ] doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richardson S , Hirsch JS , Narasimhan M , et al; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052-9. [PMID: ] doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D , Hu B , Hu C , et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-9. [PMID: ] doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grasselli G , Greco M , Zanella A , et al; COVID-19 Lombardy ICU Network. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345-55. [PMID: ] doi: 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta S , Hayek SS , Wang W , et al; STOP-COVID Investigators. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020. [PMID: ] doi: 10.1001/jamainternmed.2020.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Auld SC , Caridi-Scheible M , Robichaux C , et al; Emory COVID-19 Quality and Clinical Research Collaborative. Declines in mortality over time for critically ill adults with coronavirus disease 2019 [Letter]. Crit Care Med. 2020;48:e1382-4. [PMID: ] doi: 10.1097/CCM.0000000000004687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kristinsson B , Kristinsdottir LB , Blondal AT , et al. Nationwide incidence and outcomes of patients with coronavirus disease 2019 requiring intensive care in Iceland. Crit Care Med. 2020;48:e1102-5. [PMID: ] doi: 10.1097/CCM.0000000000004582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asch DA , Sheils NE , Islam MN , et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2020. [PMID: ] doi: 10.1001/jamainternmed.2020.8193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quan H , Li B , Couris CM , et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676-82. [PMID: ] doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 14. Seymour CW , Liu VX , Iwashyna TJ , et al. Assessment of clinical criteria for sepsis: for the Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762-74. [PMID: ] doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranieri VM , Rubenfeld GD , Thompson BT , et al; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526-33. [PMID: ] doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 16. Sakr Y , Lobo SM , Moreno RP , et al; SOAP Investigators. Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care. 2012;16:R222. [PMID: ] doi: 10.1186/cc11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drewry AM , Samra N , Skrupky LP , et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42:383-91. [PMID: ] doi: 10.1097/SHK.0000000000000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johns Hopkins Coronavirus Resource Center. Daily confirmed new cases (3-day moving average). Outbreak evolution for the 50 states, D.C., and Puerto Rico. Accessed at https://coronavirus.jhu.edu/data/new-cases-50-states on 24 June 2020.
- 19. Cunningham JW , Vaduganathan M , Claggett BL , et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020. [PMID: ] doi: 10.1001/jamainternmed.2020.5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katz MH . Regardless of age, obesity and hypertension increase risks with COVID-19. JAMA Intern Med. 2020. [PMID: ] doi: 10.1001/jamainternmed.2020.5415 [DOI] [PubMed] [Google Scholar]
- 21. Tartof SY , Qian L , Hong V , et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773-81. doi: 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson MR , Geleris J , Anderson DR , et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection : a retrospective cohort study. Ann Intern Med. 2020;173:782-90. doi: 10.7326/M20-3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Armstrong RA , Kane AD , Cook TM . Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75:1340-9. [PMID: ] doi: 10.1111/anae.15201 [DOI] [PubMed] [Google Scholar]
- 24. Li J , Fink JB , Ehrmann S . High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion [Letter]. Eur Respir J. 2020;55. [PMID: ] doi: 10.1183/13993003.00892-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ginestra JC, Atkins JH, Mikkelsen ME, et al. The I-READI quality and safety framework: a health system's response to airway complications in mechanically ventilated patients with Covid-19. NEJM Catalyst Innovations in Care Delivery. 2021;2. doi:10.1056/CAT.20.0305
- 26. Chopra V , Flanders SA , O'Malley M , et al. Sixty-day outcomes among patients hospitalized with COVID-19 [Letter]. Ann Intern Med. 2020. doi: 10.7326/M20-5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones TK , Fuchs BD , Small DS , et al. Post-acute care use and hospital readmission after sepsis. Ann Am Thorac Soc. 2015;12:904-13. [PMID: ] doi: 10.1513/AnnalsATS.201411-504OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JT , Mikkelsen ME , Qi M , et al. Trends in post-acute care use after admissions for sepsis [Letter]. Ann Am Thorac Soc. 2020;17:118-21. [PMID: ] doi: 10.1513/AnnalsATS.201905-368RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maley JH , Mikkelsen ME . Short-term gains with long-term consequences: the evolving story of sepsis survivorship. Clin Chest Med. 2016;37:367-80. [PMID: ] doi: 10.1016/j.ccm.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 30. Chao PW , Shih CJ , Lee YJ , et al. Association of postdischarge rehabilitation with mortality in intensive care unit survivors of sepsis. Am J Respir Crit Care Med. 2014;190:1003-11. [PMID: ] doi: 10.1164/rccm.201406-1170OC [DOI] [PubMed] [Google Scholar]
- 31. Deb P , Murtaugh CM , Bowles KH , et al. Does early follow-up improve the outcomes of sepsis survivors discharged to home health care. Med Care. 2019;57:633-40. [PMID: ] doi: 10.1097/MLR.0000000000001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Courtright KR , Jordan L , Murtaugh CM , et al. Risk factors for long-term mortality and patterns of end-of-life care among Medicare sepsis survivors discharged to home health care. JAMA Netw Open. 2020;3:e200038. [PMID: ] doi: 10.1001/jamanetworkopen.2020.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prescott HC , Angus DC . Enhancing recovery from sepsis: a review. JAMA. 2018;319:62-75. [PMID: ] doi: 10.1001/jama.2017.17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.