Abstract
In some types of meditation, such as mindfulness and Zen, breathing is the focus of attention, whereas during an excessive, short-period of anaerobic exercise, the muscles become the focus of attention. Thus, during both efforts, one’s attention is focused on a certain feature of the body. Both meditation and exercise generally provide mental refreshment to humans. We hypothesized that the same brain regions are activated by both efforts in humans. To examine this hypothesis, we engaged participants in 3 tasks: meditation, exercise, and a control task. After each task, the participants underwent a 2-back test to concentrate their thoughts, while changes in their blood hemoglobin levels were simultaneously monitored using near-infrared spectroscopy (NIRS). Seventeen participants (20–24 years of age; 11 men, 6 women) were enrolled. We applied a fast-Fourier transform (FFT) analysis to the NIRS wave data and calculated the correlation coefficients of the FFT data between (1) meditation and control, (2) exercise and control, and (3) meditation and exercise, at the orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC), brain areas that are generally involved in mental refreshment. A significant difference in the correlation coefficients between the OFC and DLPFC was detected in the meditation and exercise analysis, and signal source analysis confirmed that the NIRS waves spread from the right and left OFC edges (i.e., right and left temples) toward the center. Our results suggest that both meditation and exercise activate the OFC, which is involved in emotional reactions and motivation behavior, resulting in mental refreshment.
Introduction
Humans obtain mental refreshment from meditation and exercise. Mental refreshment is provided by switching one’s attention to a certain feature (e.g., breathing, thought, body part etc.) [1–4]. In fact, mental disorders can be relieved by meditation and exercise [5, 6]. Meditation helps to ameliorate anxiety and depression, resulting in neurobiologic changes in the brain [7]. Attention-deficit/hyperactivity disorder (ADHD), which is associated with cortical thinning, is improved by meditation, which increases cortical thickness [8]. Posttraumatic stress disorder (PTSD) symptoms are also effectively attenuated by meditation [9]. Exercise, too, contributes to the management of mental disorders [10]. Exercise interventions can positively affect PTSD symptoms through modulating internal arousal cues and reducing inflammatory markers [11].
Certain types of meditation, such as mindfulness meditation, actively focus the practitioner’s attention on breathing [12, 13]. Mindfulness is the practice of being aware of one’s body’s sensation, thoughts, and feelings in the present moment. For example, in the neuroscience field, mindfulness meditation practices have come to the attention of many researchers investigating consciousness and affect regulation through mental training and to psychotherapists interested in personal development and interpersonal relationships [14]. On the other hand, an intensive, short-period anaerobic push-up exercise passively focuses the practitioner’s attention on their muscles. Both efforts lead to concentration on one feature of the body. We therefore hypothesized that when attention is focused during meditation or exercise, whether actively or passively, the same brain regions are activated because mental refreshment can be obtained by both efforts. Near-infrared spectroscopy (NIRS) is considered a suitable method for identifying active brain regions [15]. NIRS is a noninvasive neuroimaging method that measures oxy- and deoxy-hemoglobin concentration changes at the brain surface in real-time [16].
To test our hypothesis, we noted the 2 brain regions, the orbitofrontal cortex (OFC) and the dorsolateral prefrontal cortex (DLPFC), and examined whether the effects of meditation and exercise on these regions are similar. The OFC is a region of the prefrontal cortex that contributes to decision-making [17]. The DLPFC is located above the OFC and is most typically associated with executive functions, including selective attention [18]. Chronic and acute stress suppress the function of the prefrontal cortex, including the OFC and DLPFC [19]. Acute and uncontrollable stress evokes an increase in catecholamine release in the prefrontal cortex [20–22], thereby decreasing neuronal firing and cognitive function [19]. When participants look at images that induce mental stress, the activity in the DLPFC is suppressed; such stress is reduced by meditation [23]. Exercise, too, has long been thought to relieve stress [24]. There are some reports showing the effects of meditation on exercise outcomes [25, 26]. Furthermore, some recent studies suggested that the prefrontal cortex is activated by meditation and exercise [27, 28].
Materials and methods
Ethics statement
This study was carried out in accordance with the recommendations of the principles and guidelines of the Declaration of Helsinki. All participants provided their written informed consent to participate in this study. The protocol was approved by Waseda University, Office of Research Ethics (2016–282, 2018–067, 2019–358). The individual in this manuscript has given written informed consent (as outlined in PLOS consent form) to publish these case details.
Participants
Seventeen voluntary participants who declared themselves to be mentally and physically sound on the basis of self-assessment (age 20–24 years; 11 men, 6 women; 2 left-handed and 15 right-handed) from the Tokyo area of Japan were enrolled. To ensure the participants’ sound mental condition, (1) we confirmed that none of the participants self-reported a history of neurologic or psychiatric disorders, or had a disease requiring medical care; and (2) we administered Center for Epidemiologic Studies-Depression Scale (CES-D) tests to exclude persons with an extraordinary pattern of cerebral blood flow [29]. The CES-D is useful for diagnosing depression [30]. This protocol has been widely used in previous studies [31–33]. We set the cut-off point as the scores of CES-D higher than 16 [34]. The mean and SD value of the CES-D for the participants was 9.80 ± 4.17. In the present study, all 17 participants were determined to be in a mentally and physically sound condition.
Tasks
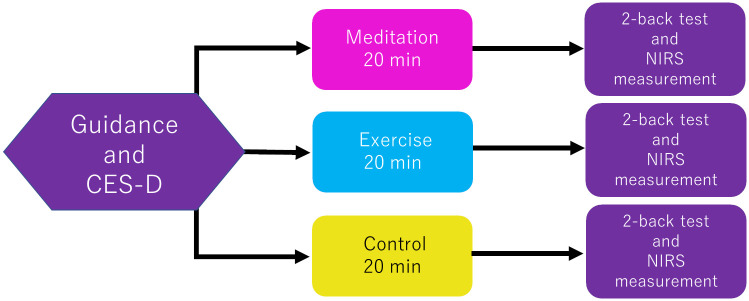
During the tasks, the room temperature was controlled at 22–23°C. The room was kept very quiet and surrounded by the white walls. The following 3 tasks were performed by the participants: meditation, exercise, and a control task, 1 of each was carried out on a different day for a total of 3 days. The order of the tasks was randomly selected for each participant and counter-balanced across participants. In the meditation task, the participants were instructed to sit on a chair, keep their eyes open, and breathe 3 to 4 times a minute for a total of 20 min. They were also asked to pay attention to their respiration. In the exercise task, the participants performed push-ups for no more than 5 min. If the participants felt that it was impossible to continue the push-ups for the entire 5 min, the set was terminated. That is, based on the Rating of Perceived Exertion (RPE) scale [35], we instructed the participants to do push-ups until they felt very tired. After that, they could rest for the remaining time. They repeated this cycle 4 times and the total period was 20 min. The room was large enough to perform the push-ups. Approximately 8 to 23 push-ups were achieved during this period, and thus we considered this an anaerobic exercise. The inter-set interval was 5 min, and 4 sets of pushups were performed. In the control task, participants sat on a chair and watched a movie of scenery with relaxing music for 20 min. After each task, the participants underwent a 2-back test comprising 32 questions while simultaneously undergoing NIRS measurements (Fig 1).
Fig 1. Experimental protocol.
First, the CES-D was administered to the participants, and then 3 tasks: meditation, exercise, and a control task, were performed by the participants in a randomly selected order. After each task, a 2-back test was administered while the NIRS measurement was simultaneously performed.
In the 2-back test, the numbers (1–9) were displayed one after another randomly on the monitor in front of the participant. The participant answered the number displayed 2 steps earlier in the sequence on the monitor using the keyboard. When the participant answered, the next number was displayed. The 2-back test was performed to let the participants concentrate on something in order to stabilize their eyes and their body movements and then to stabilize their thoughts. Because the order in which each participant underwent the meditation, exercise, and control experiments was randomly selected, any potential learning effect on the 2-back test was canceled out across the cohort in the present study.
NIRS measurement
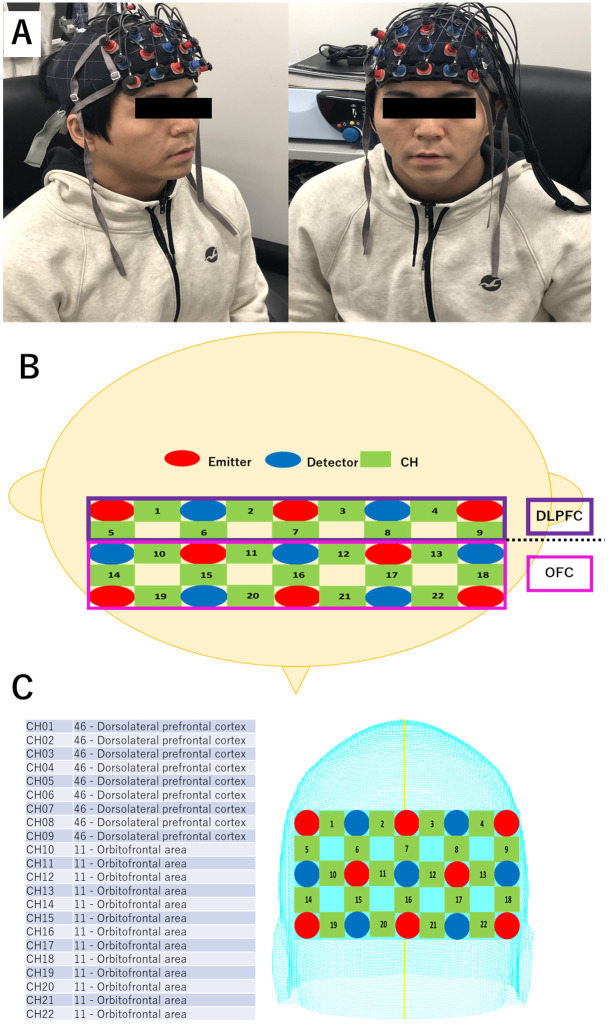
NIRS (ETG-4000/OT-R40, Hitachi, Tokyo, Japan) was used to detect blood flow over the DLPFC and the OFC. NIRS channels (CH) 1–9 were assigned to the DLPFC, and CH 10–22 were assigned to the OFC (Fig 2A and 2B).
Fig 2. NIRS measurement.
A. A typical picture of a participant equipped with NIRS optodes. B. The NIRS comprised 8 elements for the NIR light emitter (red) and 7 elements for the detector (blue). The numbers indicate the channels (green) recorded. The NIRS channels were placed above the dorsolateral prefrontal cortex (DLPFC) and the orbitofrontal cortex (OFC). C. The channels between the optodes measured on the skull were assigned to the brain regions using the virtual registration method.
The NIRS data is the measured relative concentration of oxyhemoglobin. The sampling rate of the NIRS was 10 Hz. All NIRS data were processed using high band-pass (0.001 Hz) and low band-pass (1 Hz) filters to remove artifacts, such as heartbeat and body movements. The NIRS optodes were placed in the proper position for each participant according to the international 10–20 system [36]. This system shows the location of scalp electrodes (i.e., NIRS optodes), particularly in electroencephalography (EEG) researches. This method ensures that human subject’s studies can be compiled, reproduced, analyzed and compared well each other. The numbers, ‘10’ and ‘20’, refer to the fact that the actual distances between adjacent optodes are either 10% or 20% of the total front-back or right-left distance of the skull. The points (i.e., channels) between the optodes measured on the skull were assigned to the brain regions using the virtual registration method [37, 38] (Fig 2C). We note that the OFC consists of the Brodmann areas 10 and 11 [39]. The responses in OFC can be measured with NIRS, because the OFC, at least the part of this region, is located within 3 cm under the skull [40–42]. The NIRS data were analyzed with MATLAB (2018b, MathWorks, Natick, MA, USA). Fast-Fourier transform (FFT) was applied to the NIRS data to analyze the frequency distribution of the NIRS data. To minimize the edge effects, we used the rectangular window for FFT, which was prepared as a default in MATLAB. Correlation coefficients of the FFT-analyzed NIRS signal frequencies were calculated among each pair of tasks, meditation-exercise, meditation-control, and exercise-control, for each channel of the DLPFC and OFC. After finding some channels on the left and right temples in which there were significantly larger correlation coefficients for the meditation and exercise pair than the other pairs, we performed signal source analysis as described below.
Signal source analysis
We observed CH14 and CH18 at the right and left OFC edges (right and left temples, respectively). Using continuous wavelet transformation, we obtained the maximum correlation coefficients at the time delay of τ between each CH and CH 14/CH18. The time delay was obtained by continuous wavelet transformation. If the delay time was less than 0.5 s, and it increased associated with the distance from CH14 and CH18, we decided that the signals spread from CH14 and CH18 to the other regions. We mapped the time for each channel at which the peak values of the correlation coefficients in the meditation-exercise pair emerged within 0.0 to 0.5 s after the peak emergence at CH14 or CH18. We then calculated the cross-correlation values between CH14 or CH18 and another channel every 0.1 s, which was the NIRS resolution time. When the maximum value of this cross-correlation was obtained in a channel during the period of 0.1 to 1 s, the delay time was plotted. If the delay time increased as the distance increased from CH14 or CH18, then that channel (CH14 or CH18) was judged to be the signal source.
Statistical analysis
The relation between the correlation coefficients of DLPFC and those of OFC is expressed in a box plot. This relation was analyzed by a Mann-Whitney U-test after an F test for equal variance. The relation between the correlation coefficients in each of the channels was analyzed as follows. The mean value of the correlation coefficients was subtracted from the correlation coefficients obtained for the meditation, exercise, and control tasks, and the value was referred to as a ‘correlation value’. In each channel, one-way analysis of variance (ANOVA) followed by a post-hoc Tukey test was performed to assess any differences in these correlation values. The Mann-Whitney U-test and ANOVA were performed with JSTAT for Windows (ver. 22.0J). A p of less than 0.05 was considered to indicate a significant difference. The 95% confidence interval (CI) was calculated using the Clopper-Pearson exact confidence interval method. The difference between groups was considered significant when the 95% CI of one group did not overlap with that of another group [43].
Results
NIRS signals and FFT-analyzed frequency
We first found that the performance scores of the participants in the 2-back test were not significantly different following the meditation, exercise, and control tasks (Fig 3).
Fig 3. Scores (correct answer rate) in the 2-back tests.
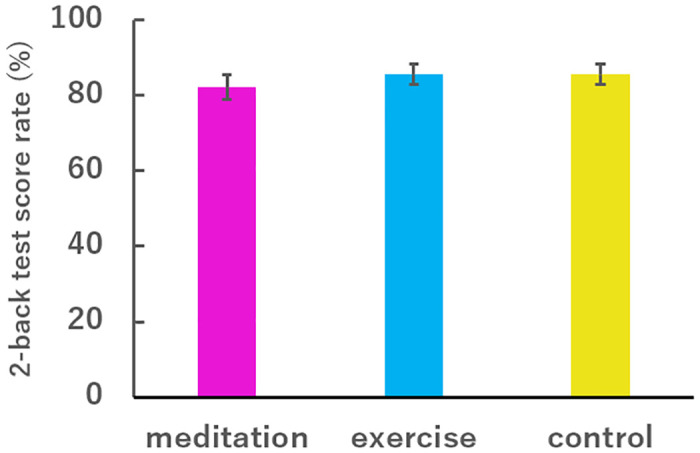
All participants performed the 2-back tests after meditation (red), exercise (blue), and control (yellow) tasks. The scores are shown as mean ± SEM. There is no significant difference.
The oxyhemoglobin concentration changes measured with NIRS are thought to correspond to neuronal activity [44, 45]. Thus, relative concentrations of oxyhemoglobin were measured by NIRS during the 2-back tests after the meditation, exercise, and control tasks (Fig 4A).
Fig 4. Representative NIRS signals and their FFT data.
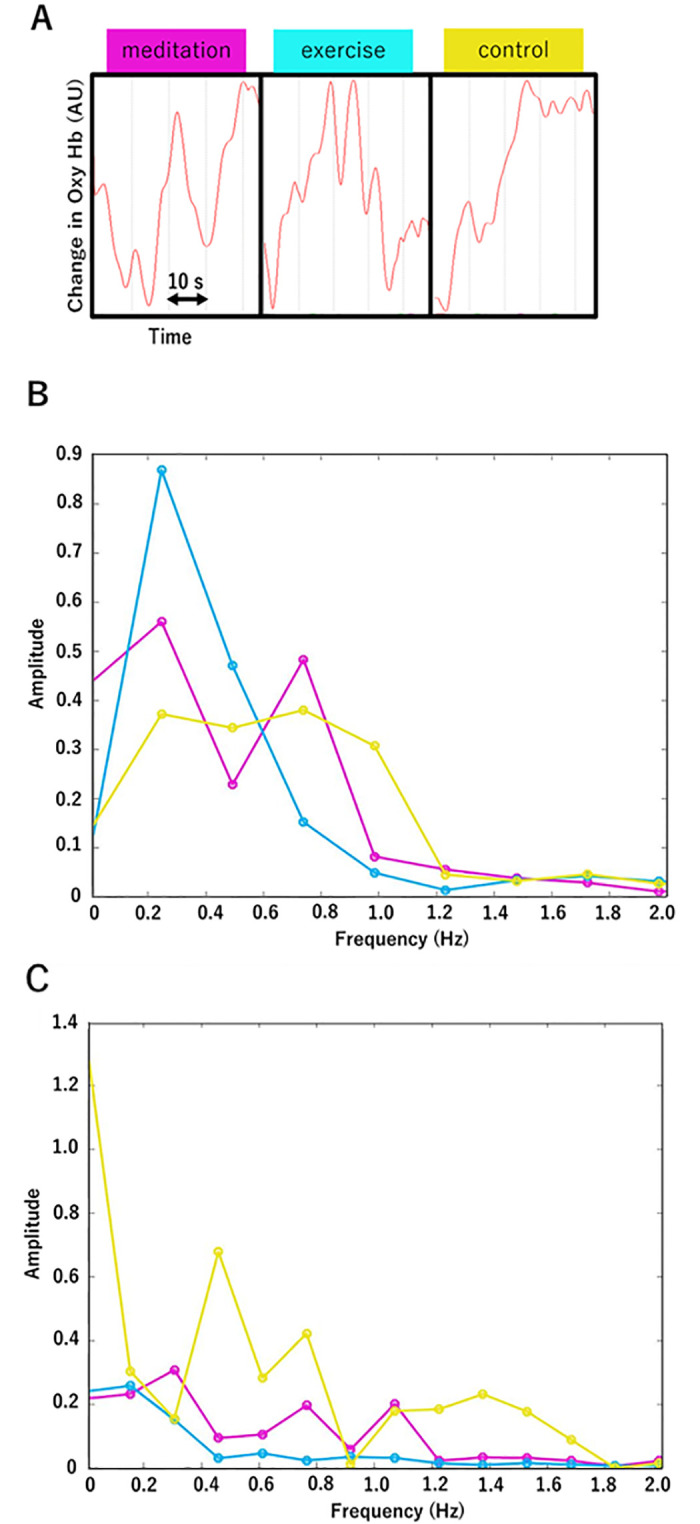
A. At CH14 in a participant, the oxyhemoglobin NIRS signals (red lines) were obtained during the 2-back tests after meditation, exercise, and a control task. These figures show only typical signal changes as examples. B. FFT values calculated from the data of a left-handed participant. C. FFT values calculated from the data of a right-handed participant. Red line indicates the meditation data; blue line indicates those of exercise; and yellow line indicates those of control.
The NIRS wave data exhibited large individual differences. We therefore analyzed the NIRS data by FFT and compared the spectra of the FFT data to overcome individual differences. Further, we compared the results of the 2 left-handed participants with those of the right-handed participants (n = 15) (Figs 4B, 4C and 5).
Fig 5. Correlation coefficients of the FFT-analyzed NIRS frequency at the DLPFC and the OFC for the meditation-control pair (red), the exercise-control pair (green), and the meditation-exercise pair (blue).
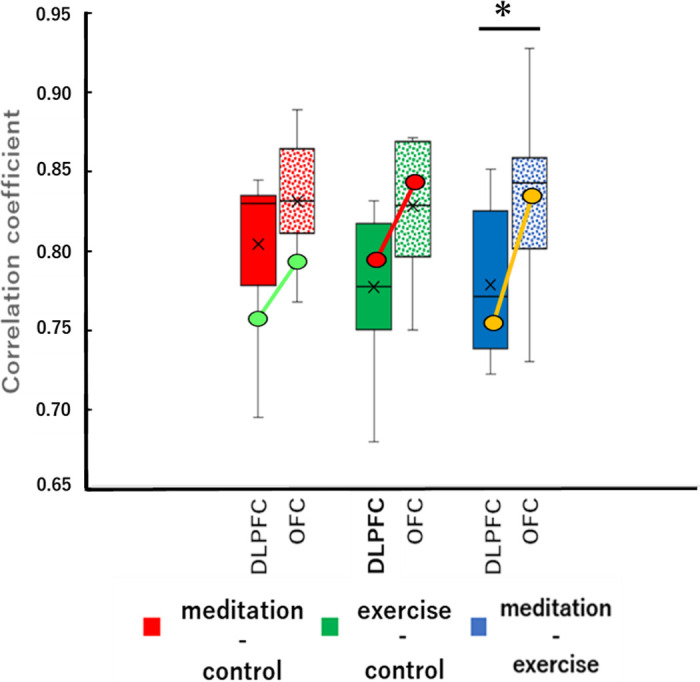
A Mann-Whitney U-test was performed after an F test for equal variance. *p < 0.05. The diagonal lines show the changes in mean values obtained from the 2 left-handed participants.
As these figures indicate, the results showed almost the same trend. Thus, we combined the results obtained from the left-handed participants and right-handed participants.
Correlation coefficients of FFT-analyzed NIRS frequency in the DLPFC and OFC
Although the raw NIRS data appeared too varied to compare between tasks, the FFT data could easily be compared. Thus, the correlation coefficients of the FFT data were calculated and collected for the DLPFC and OFC (Fig 5). The results demonstrated that the correlation coefficients in the meditation-exercise pair were significantly larger in the OFC than in the DLPFC (p < 0.05).
As the next step, the correlation coefficients of the FFT-analyzed NIRS frequencies at each channel were compared among the 3 pairs of tasks: meditation-control, exercise-control, and meditation-exercise. For this purpose, we calculated the ‘correlation values’ (see Materials and methods). At CH14 and CH18, which were the right and left OFC edges (i.e., right and left temples), the correlation coefficients were significantly larger for the meditation-exercise pair than for the meditation-control and exercise-control pairs [CH14: F(2,48) = 3.407, p < 0.05; CH18: F(2,48) = 3.207, p < 0.05] (Fig 6).
Fig 6. Coefficient correlations of FFT-analyzed NIRS frequencies at each channel among the meditation-control pair (red), the exercise-control pair (green), and the meditation-exercise pair (blue) analyses.
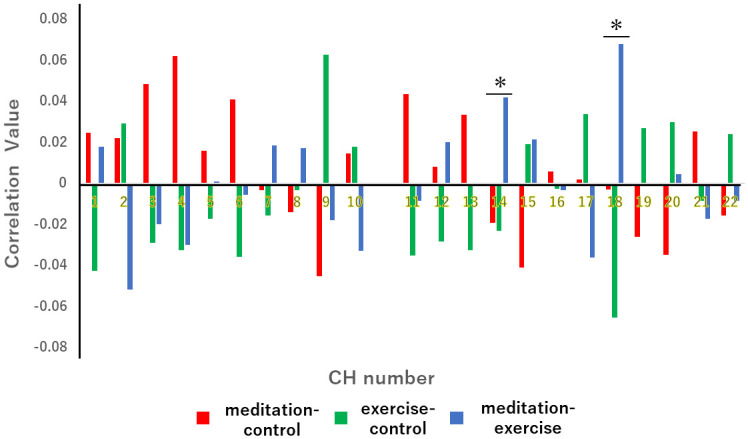
The CH14 and CH18 showed significant differences only in the meditation-exercise pair (blue). One-way analysis of variance (ANOVA) followed by a post-hoc Tukey test was performed. For CH14, F(2,48) = 3.407, *p < 0.05; for CH18, F(2,48) = 3.207, *p < 0.05.
Thus, the edges of the OFC seem to play an important role in the brain activity that occurs during both meditation and exercise.
Signal source analysis
We mapped the time at each of the channels where the peak values of the correlation coefficients in the meditation-exercise pair emerged within 0.0 to 0.5 s after the peak activity emerged at CH14 or CH18 (Fig 7A).
Fig 7. Signal source analysis for CH14 and CH18.
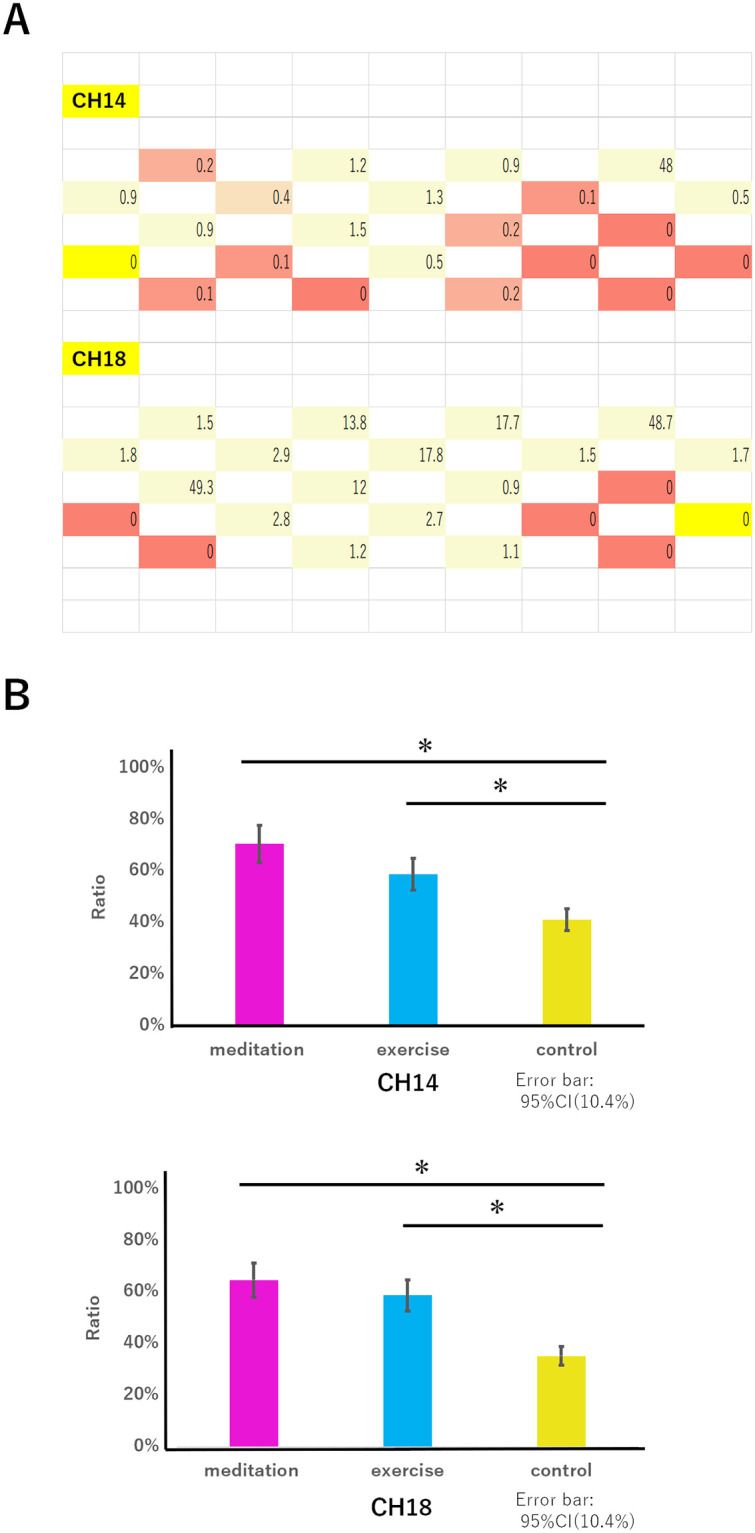
A. Representative data of spread of signals originating in CH14 and CH18 are shown. Numbers indicate the time at the maximum peak value of the correlation coefficients after the peak emergence of CH14 or CH18. B. Ratios of CH14 or CH18 recognized as the signal source are shown for the 17 participants. Error bars indicate 95% CI (e.g., 10.4% for both CH14 and CH18), and if the error bars did not overlap, we determined that there was a significant difference between the tasks. * indicates a significant difference.
The results obtained from the typical data (i.e., 1 participant) showed that the oxyhemoglobin concentration changes (i.e., a wave of neuronal activity) spread from the right and left OFC edges (i.e., right and left temples) to the midline of the brain. As far as we measured, this spread was observed once, not repeatedly, during each measurement. We then calculated the cross-correlation values between CH14 or CH18 and another channel every 0.1 s. When the maximum value of this cross-correlation was obtained in a channel, the delay time was plotted. A delay of more than 1 s was not observed in the NIRS imaging and wave data. Among the 17 participants, nearly 70% of those performing the meditation task and nearly 60% of those performing the exercise task showed this trend, whereas less than 40% of those performing the control task showed this trend (Fig 7B). The 95% CI was 10.4% for CH14 and 10.4% for CH18.
Discussion
Our findings demonstrated that both meditation and exercise activate the right and left OFC edges (i.e., right and left temples), and the signal waves spread from these 2 specific sites toward the midline in the OFC. Our results seem reliable because (1) the OFC is involved in emotional reaction and motivation behavior [46]; and (2) the insular cortices, which are involved in both meditation and exercise [47–49], locate under the temples [50]. In particular, the change in insular cortices has been vigorously examined in the studies of mindfulness meditation [51]. Therefore, the temples are reasonable starting points of neuronal activity during both meditation and exercise. The correlation coefficients of the FFT-analyzed NIRS data differed significantly between the DLPFC and OFC in the meditation and exercise pair. This result showed that the NIRS channels most responsive to both meditation and exercise were localized more in the OFC than the DLPFC, indicating that the insular cortex (i.e., OFC) is an important region involved in both meditation and exercise.
An important difference between NIRS and other brain imaging systems, e.g., functional magnetic resonance imaging (fMRI), is that participants can maintain a sitting or standing position for NIRS, whereas they must assume a supine position for fMRI. Assuming supine position is thought to induce large changes in the autonomic nervous system [52]. With regard to the NIRS sampling time, neural activity triggers regional vasodilation in the brain. Although the mechanisms underlying activity-dependent vascular responses are complex and controversial, the extensive innervation of brain blood vessels suggests direct neuronal control of regional vasodilation [53]. This vasodilation mainly depends on the production of nitric oxide [54]. Previous studies demonstrated that the nitric oxide-induced vasodilation is observed with a time scale ranging from a few hundred milliseconds to a few seconds after stimulation [55]. Because the NIRS sampling time was 10 Hz, we can conclude that NIRS assesses brain function through the intact skull by detecting changes in blood hemoglobin concentrations associated with neural activity via vasodilation.
Mental refreshment or happiness in humans involves 5-hydroxytryptamine (5-HT) and oxytocin, among other neurotransmitters [56–58]. Both 5-HT and oxytocin are found in the insular cortex [59, 60]. Some studies report that 5-HT increases in participants who experience exercise-induced euphoria [61, 62]. In addition, intranasal administration of oxytocin boosts participants’ experience of specific positive emotions during meditation [63], and saliva oxytocin levels increase during meditation [64]. Although these articles did not refer to the insular cortex, the findings provide sufficient evidence that the same neurotransmitters or hormones play an important role in the same brain regions activated by meditation and exercise.
Previous NIRS studies examined brain regions that were activated when participants paid attention to their breathing movements in the lower abdomen during Zen meditation [65]. The oxyhemoglobin level increased symmetrically in the anterior prefrontal cortex when the participant’s attention was focused, and the participants reported a reduction in feelings of a negative mood compared with before the meditation session. On the other hand, alpha band activity is increased and theta band activity is decreased during and after the focused attention in EEG. These EEG changes correlated with a significant increase in blood 5-HT levels. Therefore, activation of the anterior prefrontal cortex and 5-HT system is considered to play an important role in the improvement of a negative mood. Measurements of 5-HT in blood should be examined in a future study. Another EEG study showed asymmetric changes in anterior brain activation during mindfulness meditation [66]. The meditators showed a significant increase in activation only in the left-side anterior temporal region. Thus, a left-right asymmetry of activated brain regions is likely a property of mindfulness. In the present study, we monitored function only in the forehead area of brain, because this area is uncovered by hair and easily measured with NIRS. Other apparatuses can examine various regions even in hair-covered areas.
Previous studies using EEG measurements revealed a peak at a specific EEG frequency in the prefrontal cortex and the left temporo-parietal area in participants with extensive experience in meditation [67]. Further, the EEG frequency varied with the type of meditation [68]. In the present study, all the participants were new learners of meditation, resulting in the demonstration that both meditation and exercise activated the same brain region. In the future, we will perform experiments similar to the EEG studies, and deepen our understanding of the activation of the OFC and DLPFC.
We finally discuss the limitation of our present study. Many researches using NIRS has not used the analysis method of power spectral density or FFT. Thus, FFT is brand-new for the analysis of NIRS data. As the first step in NIRS data analysis, we selected the FFT method rather than the power spectral density. Our NIRS (Hitachi ETG-4000) can set only 0.001, 0.01, and 0.1 frequency (Hz) as the cut-off value. We thought that the high pass filter of 0.001 Hz was the best, because the others may cutoff the essential frequency range of data. Hitachi ETG-4000 automatically obtains the 5-s moving average data to remove artifacts. Furthermore, as described earlier, we used the rectangular window for FFT. The divergence of the data is also known after FFT due to Gibbs phenomenon, which is caused by an inappropriate application of the window function to discontinuous graphs [69]. However, it is unlikely to be caused by Gibbs phenomenon, because we also take a 5-s moving average and the measured data are smooth. We believe that there are no problems associated with this issue in the post-FFT data.
In conclusion, the present study showed that the edges of the OFC are activated by both meditation and exercise. These regions are temporal areas reflecting insular cortex activity. Activation of the insular cortex by both meditation and exercise has been reported previously. The findings of our NIRS study implicate the involvement of this cortical region in the mental refreshment experienced by human beings when attention is focused on a specific body feature, such as breathing or muscle activity.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This work was supported by a Grant-in-Aid (19H00633) for Scientific Research (A) from the Japan Society for the Promotion of Science to F.K. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stone AA, Neale JM. New measure of daily coping: Development and preliminary results. J Pers Soc Psychol. 1984; 46: 892–906. 10.1037/0022-3514.46.4.892 [DOI] [Google Scholar]
- 2.Morrow J, Nolen-Hoeksema S. Effects of responses to depression on the remediation of depressive affect. J Pers Soc Psychol. 1990; 58: 519–527. 10.1037//0022-3514.58.3.519 [DOI] [PubMed] [Google Scholar]
- 3.Lyubomirsky S, Nolen-Hoeksema S. Effects of self-focused rumination on negative thinking and interpersonal problem solving. J Pers Soc Psychol. 1995; 69: 176–190. 10.1037//0022-3514.69.1.176 [DOI] [PubMed] [Google Scholar]
- 4.Blagden JC, Craske MG. Effects of active and passive rumination and distraction: A pilot replication with anxious mood. J Anxiety Disord. 1996; 10: 243–252. 10.1016/0887-6185(96)00009-6 [DOI] [Google Scholar]
- 5.Alderman BL, Olson RL, Brush CJ, Shors TJ. MAP training: combining meditation and aerobic exercise reduces depression and rumination while enhancing synchronized brain activity. Transl Psychiatry 2016; 6: e726 10.1038/tp.2015.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards MK, Loprinzi PD. Comparative effects of meditation and exercise on physical and psychosocial health outcomes: a review of randomized controlled trials. Postgrad Med J. 2018; 130: 222–228. 10.1080/00325481.2018.1409049 [DOI] [PubMed] [Google Scholar]
- 7.Simkin DR, Black NB. Meditation and mindfulness in clinical practice. Child Adolesc Psychiatr Clin N Am. 2014; 23: 487–534. 10.1016/j.chc.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Grant JA, Duerden EG, Courtemanche J, Cherkasova M, Duncan GH, Rainville P. Cortical thickness, mental absorption and meditative practice: possible implications for disorders of attention. Biol Psychol. 2013; 92: 275–281. 10.1016/j.biopsycho.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 9.Gallegos AM, Crean HF, Pigeon WR, Heffner KL. Meditation and yoga for posttraumatic stress disorder: a meta-analytic review of randomized controlled trials. Clin Psychol Rev. 2017; 58: 115–124. 10.1016/j.cpr.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashdown-Franks G, Sabiston CM, Stubbs B. The evidence for physical activity in the management of major mental illnesses: a concise overview to inform busy clinicians’ practice and guide policy. Curr Opin Psychiatry 2019; 32: 375–380. 10.1097/YCO.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 11.Hegberg NJ, Hayes JP, Hayes SM. Exercise intervention in PTSD: A narrative review and rationale for implementation. Front Psychiatry 2019; 10: 133 10.3389/fpsyt.2019.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doll A, Hölzel BK, Mulej Bratec S, Boucard CC, Xie X, Wohlschläger AM, et al. Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage 2016; 134: 305–313. 10.1016/j.neuroimage.2016.03.041 [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa H, Mieda T, Oshio A, Koshikawa F. The relationship between decentering and adaptiveness of response styles in reducing depression. Mindfulness 2018; 9: 556–563. 10.1007/s12671-017-0797-8 [DOI] [Google Scholar]
- 14.Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA 2008; 300: 1350–1352. 10.1001/jama.300.11.1350 [DOI] [PubMed] [Google Scholar]
- 15.Saito N, Shima R, Yamada Y, Nagaoka M, Ito E, Yoshioka T. A proposed molecular mechanism for physical analgesia in chronic pain. Neural Plast. 2018; 2018: 1260285. 10.1155/2018/1260285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CH, Sugiyama T, Kataoka A, Kudo A, Fujino F, Chen YW, et al. Analysis for distinctive activation patterns of pain and itchy in the human brain cortex measured using near infrared spectroscopy (NIRS). PLoS One 2013; 8: e75360 10.1371/journal.pone.0075360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007; 30: 31–56. 10.1146/annurev.neuro.30.051606.094334 [DOI] [PubMed] [Google Scholar]
- 18.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trend Cogn Sci. 2003; 7: 415–423. 10.1016/s1364-6613(03)00197-9 [DOI] [PubMed] [Google Scholar]
- 19.Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015; 18: 1376–1385. 10.1038/nn.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990; 85: 367–403. 10.1016/s0079-6123(08)62691-6 [DOI] [PubMed] [Google Scholar]
- 21.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996; 16: 4787–4798. 10.1523/JNEUROSCI.16-15-04787.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996; 93: 1325–1329. 10.1073/pnas.93.3.1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascoe MC, Thompson DR, Ski CF. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology 2017; 86: 152–168. 10.1016/j.psyneuen.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 24.Eliot RS, Forker AD, Robertson RJ. Aerobic exercise as a therapeutic modality in the relief of stress. Adv Cardiol. 1976; 18: 231–242. 10.1159/000399527 [DOI] [PubMed] [Google Scholar]
- 25.Kerr CE, Sacchet MD, Lazar SW, Moore CI, Jones SR. Mindfulness starts with the body: somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Front Hum Neurosci. 2013; 7: 12 10.3389/fnhum.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bühlmayer L, Birrer D, Röthlin P, Faude O, Donath L. Effects of mindfulness practice on performance-relevant parameters and performance outcomes in sports: A meta-analytical review. Sports Med. 2017; 47: 2309–2321. 10.1007/s40279-017-0752-9 [DOI] [PubMed] [Google Scholar]
- 27.Kwak S, Kim SY, Bae D, Hwang WJ, Cho KIK, Lim KO, et al. Enhanced attentional network by short-term intensive meditation. Front Psychol. 2020; 10: 3073 10.3389/fpsyg.2019.03073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roux-Mallouf TLE, Vallejo A, Pelen F, Halimaoui I, Doutreleau S, Verges S. Synergetic effect of NO precursor supplementation and exercise training. Med Sci Sports Exerc. 2020; 52: 2437–2447. 10.1249/MSS.0000000000002387 [DOI] [PubMed] [Google Scholar]
- 29.Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for depression in the general population with the center for epidemiologic studies depression (CES-D): a systematic review with meta-analysis. PLoS One 2016; 11: e0155431 10.1371/journal.pone.0155431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takizawa R, Fukuda M, Kawasaki S, Kasai K, Mimura M, Pu S, et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage 2014; 85: 498–507. 10.1016/j.neuroimage.2013.05.126 [DOI] [PubMed] [Google Scholar]
- 31.Kahlaoui K, Di Sante G, Barbeau J, Maheux M, Lesage F, Ska B, et al. Contribution of NIRS to the study of prefrontal cortex for verbal fluency in aging. Brain Lang. 2012; 121: 164–173. 10.1016/j.bandl.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 32.Byun K, Hyodo K, Suwabe K, Ochi G, Sakairi Y, Kato M, et al. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: an fNIRS study. Neuroimage 2014; 98: 336–345. 10.1016/j.neuroimage.2014.04.067 [DOI] [PubMed] [Google Scholar]
- 33.Shin J, Müller KR, Hwang HJ. Eyes-closed hybrid brain-computer interface employing frontal brain activation. PLoS One 2018; 13: e0196359 10.1371/journal.pone.0196359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977; 1: 385–401. [Google Scholar]
- 35.Williams N. The Borg Rating of Perceived Exertion (RPE) scale. Occup Med. 2017; 67: 404–405. 10.1093/occmed/kqx063 [DOI] [Google Scholar]
- 36.Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage 2004; 21: 99–111. 10.1016/j.neuroimage.2003.08.026 [DOI] [PubMed] [Google Scholar]
- 37.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000; 12: 191–200. 10.1155/2000/421719 [DOI] [PubMed] [Google Scholar]
- 38.Tsuzuki D, Jurcak V, Singh AK, Okamoto M, Watanabe E, Dan I. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage 2007; 34: 1506–1518. 10.1016/j.neuroimage.2006.10.043 [DOI] [PubMed] [Google Scholar]
- 39.Kringelbach ML. The orbitofrontal cortex: linking reward to hedonic experience. Nature Rev Neurosci. 2005; 6: 691–702. 10.1038/nrn1747 [DOI] [PubMed] [Google Scholar]
- 40.Kida T, Shinohara K. Gentle touch activates the anterior prefrontal cortex: an NIRS study. Neurosci Res. 2013; 76: 76–82. 10.1016/j.neures.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 41.Feng K, Shen CY, Ma XY, Chen GF, Zhang ML, Xu B, et al. Effects of music therapy on major depressive disorder: A study of prefrontal hemodynamic functions using fNIRS. Psychiatry Res. 2019; 275: 86–93. 10.1016/j.psychres.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 42.Maharjan A, Peng M, Cakmak YO. Non-invasive high frequency median nerve stimulation effectively suppresses olfactory intensity perception in healthy males. Front Hum Neurosci. 2019; 12: 533 10.3389/fnhum.2018.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cumming G, Fidler F, Vaux DL. Error bars in experimental biology. J Cell Biol. 2007; 177: 7–11. 10.1083/jcb.200611141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoshi Y, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol. 2001; 90: 1657–1662. 10.1152/jappl.2001.90.5.1657 [DOI] [PubMed] [Google Scholar]
- 45.Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 2002; 17: 719–731. 10.1006/nimg.2002.1227 [DOI] [PubMed] [Google Scholar]
- 46.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004; 55: 11–29. 10.1016/S0278-2626(03)00277-X [DOI] [PubMed] [Google Scholar]
- 47.Williamson JW, Nobrega AC, McColl R, Mathews D, Winchester P, Friberg L, et al. Activation of the insular cortex during dynamic exercise in humans. J Physiol. 1997; 503: 277–283. 10.1111/j.1469-7793.1997.277bh.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williamson JW, McColl R, Mathews D, Ginsburg M, Mitchell JH. Activation of the insular cortex is affected by the intensity of exercise. J Appl Physiol. 1999; 87: 1213–1219. 10.1152/jappl.1999.87.3.1213 [DOI] [PubMed] [Google Scholar]
- 49.Manna A, Raffone A, Perrucci MG, Nardo D, Ferretti A, Tartaro A, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res Bull. 2010; 82: 46–56. 10.1016/j.brainresbull.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 50.Motomura K, Terasawa Y, Natsume A, Iijima K, Chalise L, Sugiura J, et al. Anterior insular cortex stimulation and its effects on emotion recognition. Brain Struct Funct. 2019; 224: 2167–2181. 10.1007/s00429-019-01895-9 [DOI] [PubMed] [Google Scholar]
- 51.Tang Y-Y, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015; 16: 213–225. 10.1038/nrn3916 [DOI] [PubMed] [Google Scholar]
- 52.McLaughlin LJ, Goldman H, Kleinman KM, Korbol B. The influence of body position on autonomic nervous system function. Pavlov J Biol Sci. 1978; 13: 29–41. 10.1007/BF03005155 [DOI] [PubMed] [Google Scholar]
- 53.Krimer LS, Muly EC 3rd, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nature Neurosci. 1998; 1: 286–289. 10.1038/1099 [DOI] [PubMed] [Google Scholar]
- 54.Szabó C. Physiological and pathophysiological roles of nitric oxide in the central nervous system. Brain Res Bull. 1996; 41: 131–141. 10.1016/0361-9230(96)00159-1 [DOI] [PubMed] [Google Scholar]
- 55.Kitaura H, Uozumi N, Tohmi M, Yamazaki M, Sakimura K, Kudoh M, et al. Roles of nitric oxide as a vasodilator in neurovascular coupling of mouse somatosensory cortex. Neurosci Res. 2007; 59: 160–171. 10.1016/j.neures.2007.06.1469 [DOI] [PubMed] [Google Scholar]
- 56.Esch T, Stefano GB. The neurobiology of love. Neuroendocrinol Lett. 2005; 26: 175–192. 10.1016/j.febslet.2007.03.094 [DOI] [PubMed] [Google Scholar]
- 57.Ito E, Shima R, Yoshioka T. A novel role of oxytocin: oxytocin-induced well-being in humans. Biophys Physicobiol. 2019; 16: 132–139. 10.2142/biophysico.16.0_132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sirgy MJ. Positive balance: a hierarchical perspective of positive mental health. Qual Life Res. 2019; 28: 1921–1930. 10.1007/s11136-019-02145-5 [DOI] [PubMed] [Google Scholar]
- 59.Sun H, Xu S, Yi L, Chen Y, Wu P, Cao Z, et al. Role of 5-HT1A receptor in insular cortex mediating stress—induced visceral sensory dysfunction. Neurogastroenterol Motil. 2016; 28: 1104–1113. 10.1111/nmo.12815 [DOI] [PubMed] [Google Scholar]
- 60.Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci. 2018; 21: 404–414. 10.1038/s41593-018-0071-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basso JC, Suzuki WA. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Brain Plast. 2017; 2: 127–152. 10.3233/BPL-160040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamedinia M, Sharifi M, Hosseini-Kakhak A. The effect of eight weeks of aerobic, anaerobic and resistance training on some factor of endocannabinoid system, serotonin, beta-endorphin and BDNF in young men. Biosci Biotech Res Asia 2017; 14: 1201–1210. 10.13005/bbra/2562 [DOI] [Google Scholar]
- 63.Van Cappellen P, Way BM, Isgett SF, Fredrickson BL. Effects of oxytocin administration on spirituality and emotional responses to meditation. Soc Cogn Affect Neurosci. 2016; 11: 1579–1587. 10.1093/scan/nsw078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Machida S, Sunagawa M, Takahashi T. Oxytocin release during the meditation of altruism and appreciation (Arigato-Zen). Int J Neurology Res. 2018; 4: 364–370. [Google Scholar]
- 65.Yu X, Fumoto M, Nakatani Y, Sekiyama T, Kikuchi H, Seki Y, et al. Activation of the anterior prefrontal cortex and serotonergic system is associated with improvements in mood and EEG changes induced by Zen meditation practice in novices. Int J Psychophysiol. 2011; 80: 103–111. 10.1016/j.ijpsycho.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 66.Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003; 65: 564–570. 10.1097/01.psy.0000077505.67574.e3 [DOI] [PubMed] [Google Scholar]
- 67.Dentico D, Bachhuber D, Riedner BA, Ferrarelli F, Tononi G, Davidson RJ, et al. Acute effects of meditation training on the waking and sleeping brain: Is it all about homeostasis? Eur J Neurosci. 2018; 48: 2310–2321. 10.1111/ejn.14131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braboszcz C, Cahn BR, Levy J, Fernandez M, Delorme A. Increased gamma brainwave amplitude compared to control in three different meditation traditions. PLoS One 2017; 12: e0170647 10.1371/journal.pone.0170647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan C. Gibbs phenomenon removal and digital filtering directly through the fast Fourier transform. IEEE Trans Process. 2001; 49: 444–448. 10.1109/78.902128 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.