Supplemental Digital Content is available in the text.
Keywords: critical care, end-of-life care, intracerebral hemorrhage, ischemic stroke, subarachnoid hemorrhage
Abstract
Objectives:
The determinants of decisions to limit life support (withholding or withdrawal) in ventilated stroke patients have been evaluated mainly for patients with intracranial hemorrhages. We aimed to evaluate the frequency of life support limitations in ventilated ischemic and hemorrhagic stroke patients compared with a nonbrain-injured population and to determine factors associated with such decisions.
Design:
Multicenter prospective French observational study.
Setting:
Fourteen ICUs of the French OutcomeRea network.
PATIENTS:
From 2005 to 2016, we included stroke patients and nonbrain-injured patients requiring invasive ventilation within 24 hours of ICU admission.
INTERVENTION:
None.
MEASUREMENTS AND MAIN RESULTS:
We identified 373 stroke patients (ischemic, n = 167 [45%]; hemorrhagic, n = 206 [55%]) and 5,683 nonbrain-injured patients. Decisions to limit life support were taken in 41% of ischemic stroke cases (vs nonbrain-injured patients, subdistribution hazard ratio, 3.59 [95% CI, 2.78–4.65]) and in 33% of hemorrhagic stroke cases (vs nonbrain-injured patients, subdistribution hazard ratio, 3.9 [95% CI, 2.97–5.11]). Time from ICU admission to the first limitation was longer in ischemic than in hemorrhagic stroke (5 [3–9] vs 2 d [1–6] d; p < 0.01). Limitation of life support preceded ICU death in 70% of ischemic strokes and 45% of hemorrhagic strokes (p < 0.01). Life support limitations in ischemic stroke were increased by a vertebrobasilar location (vs anterior circulation, subdistribution hazard ratio, 1.61 [95% CI, 1.01–2.59]) and a prestroke modified Rankin score greater than 2 (2.38 [1.27–4.55]). In hemorrhagic stroke, an age greater than 70 years (2.29 [1.43–3.69]) and a Glasgow Coma Scale score less than 8 (2.15 [1.08–4.3]) were associated with an increased risk of limitation, whereas a higher nonneurologic admission Sequential Organ Failure Assessment score was associated with a reduced risk (per point, 0.89 [0.82–0.97]).
Conclusions:
In ventilated stroke patients, decisions to limit life support are more than three times more frequent than in nonbrain-injured patients, with different timing and associated risk factors between ischemic and hemorrhagic strokes.
The prognosis of mechanically ventilated stroke patients is poor, with 1-year mortality rates ranging from 60% to 92% (1–5). In this subset of extremely severe cases with high fatality rates, a high incidence of limitation (withholding or withdrawal) of life support has been reported, ranging from 30% to 40% (6–8) compared with 9–14% in large multicenter observational studies in the general ICU population (9–15). Compared with nonbrain-injured critically ill patients, the decision to limit life support in brain-injured patients may have more serious consequences, as the continuation of organ support could result in months or years of life in a state of disability that may be against the patient’s wishes (16). Assessing long-term vital and functional outcomes in these patients is difficult, and current prognostic models are based on datasets including a significant proportion of patients with treatment restriction. In turn, these life support limitations affect the outcomes of the populations in which the models were developed (17).
The high incidence of life support limitation in mechanically ventilated stroke patients and the potential confounding impact on prognostication models suggest that determinants of limitation of life support should be thoroughly investigated. In this observational multicenter study, we sought to describe the incidence, timing, and factors associated with life support limitation in critically ill patients, with either ischemic or hemorrhagic stroke, requiring invasive mechanical ventilation (IMV).
MATERIALS AND METHODS
Patient Data Source
This observational cohort study was conducted using data from the French prospective multicenter (n = 28 ICUs) OutcomeRea database. Patients admitted between 2005 and 2016 were considered for this study. We chose 2005 as the beginning of the study period as important end-of-life legislation was acted in France that year (18). Per this law, withholding or withdrawal of treatments is authorized when they appear “useless, disproportionate or having no other effect than solely the artificial preservation of life.” However, the decision to withdraw or withhold a treatment from a patient unable to express their will has to consider the wishes they might have expressed through advance directives and/or the wishes of a trusted person or, last, of the family. Furthermore, before making any decision, physicians have to respect a collegial medical procedure. Finally, euthanasia in France remains illegal. The OUTCOMEREA database, described in previous publications (19), has been approved by the French Advisory Committee for Data Processing in Health Research and the French Informatics and Liberty Commission (registration number 8999262). The database protocol was submitted to the Institutional Review Board of the Clermont-Ferrand University Hospital (Clermont-Ferrand, France), who waived the need for informed consent (Institutional Review Board number 5891). The datasets used during the current study are available from the corresponding author on reasonable request.
Study Populations and Definitions
The stroke population included all adult patients with acute stroke and requiring IMV within 24 hours of ICU admission. ICU stays were considered as related to acute stroke in cases of: 1) direct ICU admission following stroke onset or 2) ICU admission during the initial acute care hospital stay following stroke onset. We excluded patients without hospitalization reports. From the same ICUs where the stroke population was selected, we defined a nonbrain-injured population comprised of nonstroke adult patients requiring IMV within 24 hours of ICU admission and without admission diagnoses associated with brain injury: cardiac arrest, status epilepticus, meningitis/encephalitis, and traumatic brain injury. We also chose to exclude subdural hematomas from our analysis, as it was retrospectively difficult to ascertain their nontraumatic nature.
Intracranial hemorrhages (ICHs) and subarachnoid hemorrhages (SAHs) were merged as “hemorrhagic strokes” (20). Limitations of life support were categorized as either withholding or withdrawing. Withholding of life support was defined as a decision not to start or increase a life-sustaining intervention. Life-sustaining interventions comprised organ support (mechanical ventilation invasive or not, vasopressors and dialysis) and acute phase stroke therapies if there was a theoretical indication for it. Withdrawal of life support was defined as a decision to actively stop a life-sustaining intervention presently underway (21). If more than one limitation decision occurred for a single patient, the most active limitation (withdrawing > withholding) defined the limitation category. End-of-life outcomes were categorized as follows: 1) death without limitation if death occurred in absence of any decision to limit life support, 2) death following limitation if death occurred after any limitation of life support, and 3) brain death, in cases of documented cessation of cerebral function (21). The severity of illness was graded at ICU admission with the use of the Simplified Acute Physiology Score II (22) and the Sequential Organ Failure Assessment (SOFA) score (23). The nonneurologic SOFA was defined as the SOFA score without the neurologic component. Coma was defined as a Glasgow Coma Scale (GCS) score less than 8 (24). We used the Charlson comorbidities index to assess the burden of comorbid conditions (25).
Data Collection
Data were prospectively collected at admission and daily throughout the ICU stay, through an anonymized electronic case report form using Vigirea, Rhea, and e-Rhea software (OutcomeRea, Aulnay-sous-Bois, France). Long-term survival after hospital discharge was collected by each local investigator. We retrospectively collected the following data in medical charts: date of stroke, location, and acute phase therapy (i.e., thrombolysis or endovascular thrombectomy for ischemic strokes and neurosurgery or embolization for hemorrhagic strokes).
Statistical Analysis
Quantitative variables are presented as medians, first and third quartiles and compared between groups with the Wilcoxon test. Qualitative variables are presented as frequencies and corresponding percentages and compared with the chi-square test or Fisher exact test, as appropriate.
To compare the risk of life support limitation between stroke and nonbrain-injured populations, we used an adjusted Fine and Gray subdistribution competing risk model (26) to estimate the subdistribution hazard of stroke as a class variable (ischemic stroke/hemorrhagic strokes/no stroke) and considering ICU death without limitation as the competing event. For each stroke subgroup, factors associated with the occurrence of a limitation of life support were evaluated using a Fine and Gray model (26), with the same competing event. All models were adjusted on clinically relevant factors or factors associated (p < 0.2) with the outcome of interest in univariate analysis. In the presence of collinear variables, the most clinically relevant one was retained. Variables were selected using a backward selection procedure with a threshold of p value of less than 0.1. The log-linearity of quantitative variables included in the models was tested. When this was not the case, variables were binarized using the median as the cutoff. To account for variability in practice of life support limitation across ICUs (12, 27), models were stratified on center (centers with < 10% of the cohort were combined into one stratum). Two-by-two clinically relevant interactions were tested in each model. Missing data were all completely at random with less than 10% missing values per variable and were handled by simple imputation with the median/most frequent method (28). For each stroke subgroup, we conducted a sensitivity analysis by forcing in the models the period of study inclusion, arbitrarily divided into 4-year time intervals.
All statistical analyses were carried out with SAS 9.4 (SAS Institute, Cary, NC). A p value of 0.05 and lower was considered statistically significant.
RESULTS
Among 17,520 ICU admissions over the study period, we identified 373 acute stroke patients from 14 ICUs where IMV was initiated within 24 hours of admission. In the same 14 ICUs, we identified 5,683 nonbrain-injured patients (Supplemental Fig. 1, Supplemental Digital Content 1 http://links.lww.com/CCX/A505). Stroke patients were predominantly male (59%), age 68.7 years (58.2–76.5 yr) old, with strokes classified as ischemic (n = 167, 45%) and hemorrhagic (n = 206, 55%). The reasons for intubation and mechanical ventilation were coma (n = 271, 73%), respiratory failure (n = 46, 12%), seizures (n = 27, 7%), cardiac arrest (n = 17, 5%), and elective procedure (n = 12, 3%). Patients’ characteristics according to stroke type or absence of brain injury are presented in Table 1. Ischemic stroke patients were admitted to university hospitals in 92 of 167 cases (55%), hospitals with a stroke unit in 160 of 167 cases (96%), and hospitals with a neurosurgery unit and interventional radiology in 80 of 167 cases (48%). Hemorrhagic stroke patients were admitted to university hospitals in 131 of 206 cases (64%), hospitals with a stroke unit in 182 of 206 cases (88%), and hospitals with a neurosurgery unit and interventional radiology in 107 of 206 cases (48%).
TABLE 1.
Population Characteristics According to Stroke Subtype or Absence of Brain Injury
| Variables | Nonbrain-Injured Patients, n = 5,683 | Ischemic Stroke Patients, n = 167 | Hemorrhagic Stroke Patientsa, n = 206 | p b |
|---|---|---|---|---|
| Demographics/history | ||||
| Age, yr, median (quartile 1–quartile 3) | 62.4 (49.4–74) | 69.6 (61.2–77.2) | 67 (56.6–76.4) | 0.09 |
| Male sex, n (%) | 3,506 (61.7) | 112 (67.1) | 109 (52.9) | < 0.01 |
| Charlson comorbidity index ≥ 1, n (%) | 3,810 (67) | 103 (61.7) | 97 (47.1) | < 0.01 |
| ICU characteristics | ||||
| University affiliated ICU, n (%) | 3,846 (67.7) | 92 (55.1) | 131 (63.6) | 0.10 |
| Glasgow Coma Scale score at admission, median (quartile 1–quartile 3) | 12 (5–15) | 6 (3–10) | 3 (3–6) | < 0.01 |
| Simplified Acute Physiology Score II, median (quartile 1–quartile 3) | 50 (37–64) | 56 (45–67) | 61 (52–77) | < 0.01 |
| ICU length of stay, d, median (quartile 1–quartile 3) | 6 (3–13) | 7 (4–13) | 3 (2–8) | < 0.01 |
| Life support limitations | ||||
| Any life support limitationc, n (%) | 695 (12.2) | 69 (41.3) | 68 (33) | 0.10 |
| Limitation categoriesc, n (%) | < 0.01 | |||
| Withholding | 504 (8.9) | 31 (18.6) | 16 (7.8) | . |
| Withdrawal | 314 (5.5) | 38 (22.8) | 52 (25.2) | . |
| Time from ICU to first limitation, d, median (quartile 1–quartile 3) | 6 (2–15) | 5 (3–9) | 2 (1–6) | < 0.01 |
| Outcomes, n (%) | ||||
| ICU mortality | 1,322 (23.3) | 92 (55.1) | 145 (70.4) | < 0.01 |
| End-of-life outcome | < 0.01 | |||
| Brain death | 0 | 21 (22.8) | 68 (46.9) | . |
| Death without limitation of life support | 755 (57.1) | 7 (7.6) | 12 (8.3) | . |
| Death following a limitation of life support | 567 (42.9) | 64 (69.6) | 65 (44.8) | . |
aIntracranial hemorrhage and subarachnoid hemorrhage.
bComparison of acute ischemic stroke patients and hemorrhagic stroke patients.
cIf more than one limitation of life support occurred, the most active limitation (withdrawing > withholding) defined the limitation category.
During their ICU stay, 137 of 373 stroke patients (37%) and 695 of 5,683 nonbrain-injured patients (12%) underwent a limitation of life support. The frequency of such limitation was 41% (69/167 patients) for ischemic strokes and 33% (68/206 patients) for hemorrhagic strokes (p = 0.1). In a Fine and Gray subdistribution multivariable competing risk model adjusted on age, comorbidities, and severity at ICU admission, we found that having an ICU admission diagnosis of ischemic stroke was associated with a 3.6-fold increased (95% CI (CI) [2.78—4.65]) risk of undergoing a limitation of life support, as compared to the nonbrain-injured population. Similarly, having an ICU admission diagnosis of hemorrhagic stroke was associated with a 3.9-fold increased (95% CI [2.97—5.11]) risk of qualifying for limitation of life support, as compared to the nonbrain-injured population (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A505).
Among patients who underwent life support limitation, withdrawal was the predominant limitation category in the stroke population (ischemic strokes, 38/69 [55%]; hemorrhagic strokes, 52/68 [76%]), whereas withholding was the most frequent category in the nonbrain-injured population (381/695 [55%]). The daily ICU incidence rate of life support limitation according to stroke subtype or absence of brain injury is presented in Figure 1 and shows different time patterns between stroke and nonbrain-injured patients. Time from ICU admission to the first limitation of life support was the shortest in hemorrhagic stroke, both compared with ischemic strokes (2 [1–6] vs 5 d [3–9 d]; p < 0.01) and nonbrain-injured patients (2 [1–6] vs 6 d [2–15 d]; p < 0.01). There was no difference in time from ICU admission to the first limitation between ischemic stroke nonbrain-injured patients (5 [3–9] vs 6 d [2–15 d]; p = 0.67).
Figure 1.
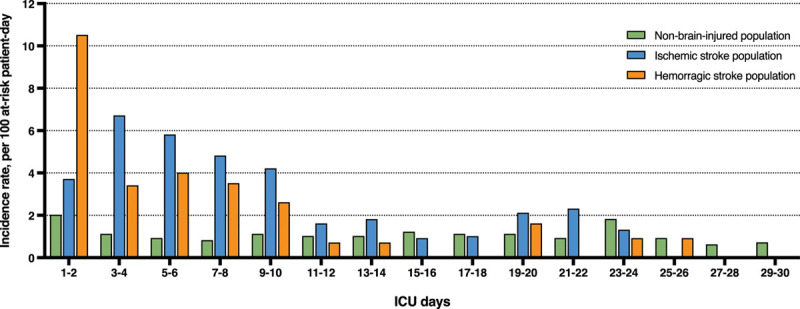
Daily ICU occurence rate of life support limitations according to stroke subtype or absence of brain injury.
ICU mortality was 92 of 167 (55%), 145 of 206 (70%), and 1,322 of 5,683 (23%) for ischemic stroke, hemorrhagic stroke, and nonbrain-injured populations, respectively (Table 1). In the nonbrain-injured population, death following life support limitation occurred in 567 of 1,322 cases (43%) (Table 1). In the stroke population, death following a limitation of life support occurred in 129 of 237 (54%) patients, including 64 of 92 ischemic stroke patients (70%) and 65 of 145 hemorrhagic stroke patients (45%)(p < 0.01). Brain death occurred in 21 of 92 ischemic stroke patients (23%) and 68 of 145 hemorrhagic stroke (47%) (p < 0.01). End-of-life outcomes according to the time from ICU admission and by stroke subtype are presented in Figure 2. From the fifth day of ICU stay and beyond, the rate of death following life support limitation exceeded 80% in ischemic stroke patients and 70% in hemorrhagic stroke patients.
Figure 2.
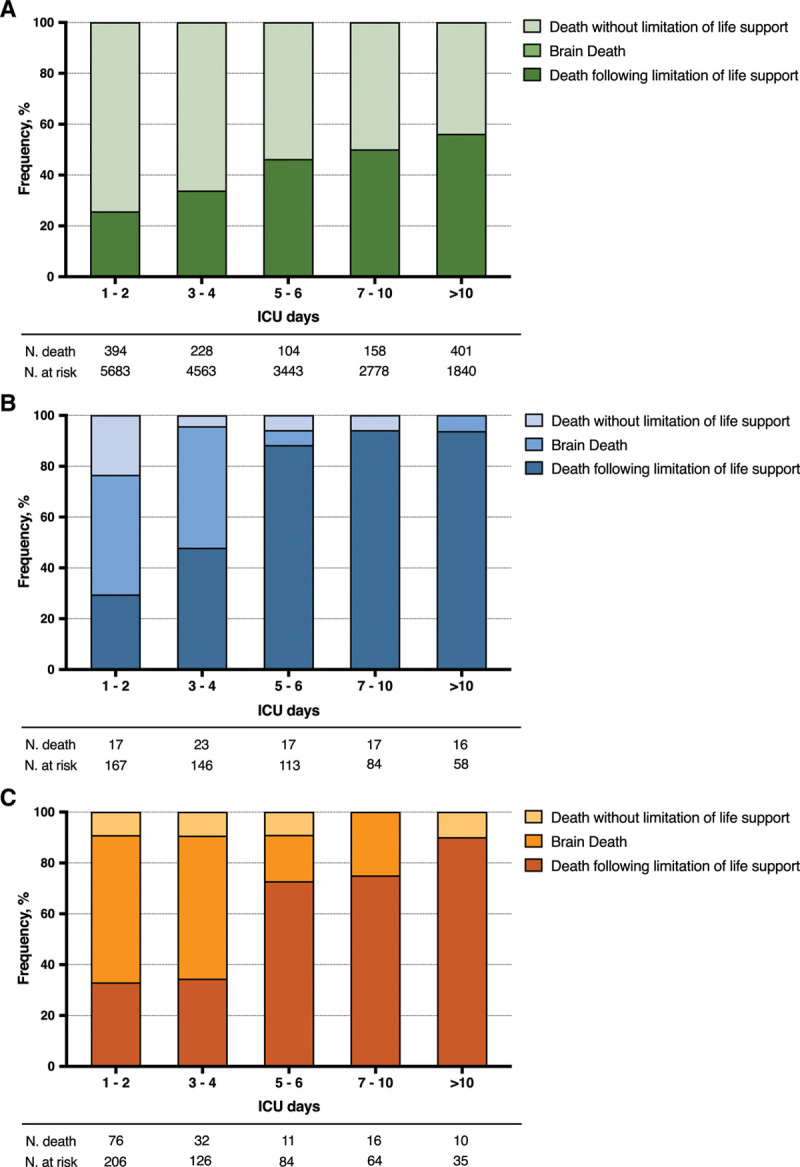
End-of-life outcome according to length of ICU stay (d): comparison between nonbrain-injured patients and stroke subtypes. A, Nonbrain-injured patients. B, Ischemic stroke patients. C, Hemorrhagic stroke patients.
Univariate analysis of factors associated with any life support limitation is presented in Table 2 (for univariate analysis by stroke subset see Supplemental Tables 2 and 3, Supplemental Digital Content 1, http://links.lww.com/CCX/A505). In the subset of ischemic stroke patients, variables significantly associated with a decision to limit life support in multivariate analysis were stroke location (vertebrobasilar vs anterior circulation location, subdistribution hazard ratio [sHR], 1.61 [1.01–2.59]) and a modified Rankin score greater than 2 before stroke onset (sHR, 2.38 [1.27–4.55]) (Fig. 3). In the subset of hemorrhagic stroke patients, variables independently associated with a decision to limit life support in multivariate analysis were age greater than 70 years (sHR, 2.29 [1.43–3.69]), a GCS score less than 8 at ICU admission (sHR, 2.15 [1.08–4.3]), and the nonneurologic SOFA score at ICU admission (sHR, 0.89 [0.82–0.97]) (Fig. 3). The period of inclusion in the study, when forced into each model, was not significantly associated with a decision to limit life support (Supplemental Tables 4 and 5, Supplemental Digital Content 1, http://links.lww.com/CCX/A505). Among the 137 patients with a limitation of life support, univariate analysis of factors associated with a choice of withholding rather than withdrawal of life support is presented in the Supplemental Table 6 (Supplemental Digital Content 1, http://links.lww.com/CCX/A505).
TABLE 2.
Stroke Population Characteristics, With or Without Limitation of Life Support (n = 373)
| Variables | Limitation of Life Support | p | |
|---|---|---|---|
| No Limitation, n = 236 | Any Limitation, n = 137 | ||
| Demographics/history | |||
| Age, yr, median (quartile 1–quartile 3) | 65.5 (56.3–74.1) | 72.8 (62.6–79.6) | < 0.01 |
| Male sex, n (%) | 134 (56.8) | 87 (63.5) | 0.20 |
| Charlson comorbidity index ≥ 1, n (%) | 120 (50.8) | 80 (58.4) | 0.16 |
| Hospital characteristics, n (%) | |||
| University hospital | 137 (58.1) | 86 (62.8) | 0.37 |
| Stroke unit on-site | 220 (93.2) | 122 (89.1) | 0.16 |
| Neurosurgery unit on-site | 119 (50.4) | 68 (49.6) | 0.88 |
| ICU type | 0.64 | ||
| Medical | 129 (54.7) | 79 (57.7) | |
| Mixed | 104 (44.1) | 55 (40.1) | |
| Surgical | 3 (1.3) | 3 (2.2) | |
| ICU authorized for organ donation | 161 (68.2) | 84 (61.3) | 0.18 |
| Ischemic stroke characteristics (n = 167) | |||
| Location, n (%) | 0.11 | ||
| Anterior circulation | 67/98 (68.4) | 38/69 (55.1) | |
| Vertebrobasilar circulation | 31/98 (31.6) | 31/69 (44.9) | |
| Acute phase therapya, n (%) | 26/98 (26.5) | 8/69 (11.6) | 0.02 |
| Time from stroke to ICU admission, d, median (quartile 1–quartile 3) | 2 (1–7) | 1 (1–2) | 0.02 |
| Hemorrhagic stroke b characteristics (n = 206) | |||
| Location, n (%) | 0.62 | ||
| Deep | 32/138 (23.2) | 14/68 (20.9) | |
| Lobar | 85/138 (61.6) | 40/68 (58.8) | |
| Infratentorial | 21/138 (15.2) | 14/68 (20.6) | |
| Acute phase therapyc, n (%) | 27/138 (19.6) | 7/68 (10.3) | 0.09 |
| Time from stroke to ICU admission, d, median (quartile 1–quartile 3) | 1 (1–2) | 1 (1–1) | 0.09 |
| ICU characteristics | |||
| Glasgow Coma Scale score at admission, median (quartile 1–quartile 3) | 5 (3–9) | 3 (3–6) | < 0.01 |
| Simplified Acute Physiology Score II, median (quartile 1–quartile 3) | 56 (45–68.5) | 65 (53–77) | < 0.01 |
| Duration of mechanical ventilation, d, median (quartile 1–quartile 3) | 3 (2–8) | 5 (2–8) | 0.02 |
| Vasopressor support, n (%) | 127 (53.8) | 52 (38) | < 0.01 |
| ICU length of stay, d, median (quartile 1–quartile 3) | 4 (2–11) | 6 (3–9) | 0.24 |
| Outcomes, n (%) | |||
| ICU mortality | 108 (45.8) | 129 (94.2) | < 0.01 |
| Hospital mortality | 126 (53.4) | 134 (97.8) | < 0.01 |
| 1 yr mortalityd | 138/215 (64.2) | 136/136 (100) | < 0.01 |
aThrombolysis or endovascular thrombectomy.
bIntracranial hemorrhage and subarachnoid hemorrhage.
cNeurosurgery or embolization.
dTwenty-two of 373 stroke patients (6%) were lost to follow-up and censored at 47 d (23–153 d).
Figure 3.
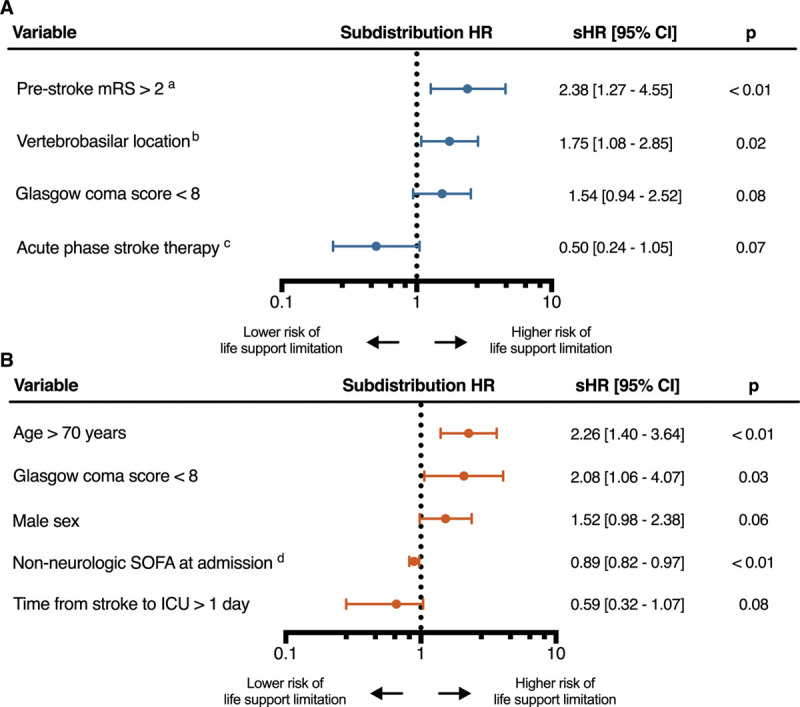
Fine and Gray subdistribution hazard analysis for the occurrence of life support limitations, and death without such limitation as the competing event. A, Ischemic stroke patients. B, Hemorrhagic stroke patients. amodified Rankin Score, bversus anterior circulation location, cthrombolysis or endovascular thrombectomy, dper SOFA point. HR = hazard ratio, sHR = subdistribution hazard ratio, SOFA = Sequential Organ Failure Assessment.
DISCUSSION
In this reanalysis of a prospective database, including 6,056 critically ill patients requiring mechanical ventilation within 24 hours of admission, we showed that 37% of stroke patients (n = 373) underwent life support limitation, representing more than a three-fold increase in the risk of receiving a decision to limit life support compared with nonbrain-injured patients (n = 5683). Although there was no difference in the global risk of limitation of life support between ischemic and hemorrhagic strokes, limitations occurred earlier in hemorrhagic stroke patients. Factors associated with life support limitation differed between stroke types, including mainly stroke location and prestroke modified Rankin score for ischemic strokes and mainly age and organ failure for hemorrhagic strokes.
The 37% rate of life support limitation observed in our cohort is consistent with rates reported in previous studies conducted in ICH patients, ranging from 34% to 43% (7, 8). Of note, our study provides unique data regarding the limitation rate in the specific population of ischemic stroke patients requiring IMV. Furthermore, we present accurate estimates, as they integrate the competitive risk of dying without receiving a decision of limitation. These models are particularly relevant in populations with very high case fatality rates where death precludes the occurrence of the outcome of interest (26, 29). Our results confirm that stroke patients under IMV are a population submitted to a high incidence of end-of-life decisions and thus deserve a more thorough evaluation (16, 30). A prospective multicenter study investigating 1-year outcomes, ethical issues, and care pathways of acute stroke patients requiring IMV in the ICU is ongoing (NCT 03335995) (31).
We found that 54% of stroke patient ICU deaths and 43% of those of nonbrain-injured patients were preceded by a decision to limit life support. These rates are consistent with those reported in the general ICU population, ranging from 47% to 53% (10, 15, 32). When evaluating end-of-life outcomes by stroke subtype, it is interesting to note that ischemic stroke patients had a higher proportion of death following a decision to limit life support than hemorrhagic stroke patients, probably because hemorrhagic stroke patients had a higher proportion of brain death.
The highest incidence of life support limitation during ICU stay occurred during the first 4 days. For hemorrhagic strokes in particular, the incidence of limitation was highest within the 48 hours following ICU admission, with a more than two-fold incidence than any other period of the ICU stay. This result could notably be explained by a higher rate of direct ICU admission from home or the emergency department in hemorrhagic stroke patients, where physicians might initiate IMV without knowing neither the patient’s medical history nor the extent of brain injury. Early decisions of life support limitation have been associated with a higher risk of short-term mortality independently of patient factors, suggesting that some of these decisions may be undue (7, 8, 33). Inappropriate prognostic pessimism and premature limitations of life support define the mechanism by which self-fulfilling prophecies occur (34). Unfortunately, the design of our study and available data did not allow us to neither quantify the effect of self-fulfilling prophecies nor explore further this issue. Currently, life support limitation within 48 hours of ICU admission is not recommended in ICH patients (35), and time-limited ICU trials should be proposed in severe stroke patients. Bias that may result in underuse of life support in severe stroke patients include erroneous prognostic estimates (36–38), misunderstanding patient’s values and expectation (39) and undervaluing the future patient’s health state (disability paradox) (30, 40). The influence of cognitive bias in the decision-making process must also be acknowledged and may be as important as patient factors (41).
The most commonly described risk factors for receiving a decision to limit life support in the general ICU population are age, the presence of chronic diseases, and clinical severity at ICU admission (11–13, 42, 43). In critically ill brain-injured patients, age and a low GCS are the most frequently reported (6, 44). It is interesting to note that in our study, risk factors appear to differ between ischemic and hemorrhagic stroke patients. For the latter, the usual patient-related risk factors were found (i.e., age and neurologic severity), with the notable addition of nonneurologic organ failure that appeared to play a protective role. We hypothesize that intensivists would be more inclined to continue aggressive care in these patients because nonneurologic organ failure may be more reversible and without obvious impact on functional outcome, as compared to neurologic failure. For ischemic stroke patients, however, neither age nor comorbidities were associated with life support limitation. We hypothesize that age and comorbidities are variables strongly associated with a decision of life support limitation even before referring the patients to an ICU (i.e., left censoring) and that this phenomenon may have mitigated the effect of these variables in our dataset (45, 46).
The strengths of our study include a multicenter population from a high-quality prospective database. The relatively small number of patients included, considering the study period and the 14 ICUs, is due to the fact that several ICUs did not contribute throughout the 12 years, and some used only a fraction of their beds to feed the database. Our study also has limitations. First, the OUTCOMEREA database was not built specifically for stroke studies, and all data regarding stroke are retrospective, collected from hospitalization records. As a result, specific severity scores are lacking: National Institutes of Health Stroke Scale (47) for ischemic strokes, ICH score for ICHs (48), and World Federation of Neurosurgical Societies or Hunt and Hess scores (49, 50) for SAH. For the same reason, data regarding the modality of treatments withheld or withdrawn, the reason for undertaking a life support limitation, and the presence or absence of advanced directives were not available. Second, end-of-life decision-making is a complex process, and we did not explore all the determinants that lead to a limitation of life support, which may include patient- or surrogate-centered determinants and physicians’ determinants (personal beliefs, religion, medical specialty, etc.) (16, 30). Third, our study population excluded stroke patients that were critically ill but were not referred to the ICU because of care-limiting decisions made by the neurologist or the emergency physician in charge. Fourth, when analyzing hemorrhagic strokes, we decided to merge ICH and SAH patients for analytical purposes. Although merging these two clinical entities has previously been done in the literature (20), a separate analysis of ICH and SAH could have brought additional information. Fifth, our results and conclusion may apply only for the setting and culture we recruited the patients from, as this is an exclusively French cohort including only medical and mixed ICUs. As only 50% of the cohort were treated with on-site neurosurgery and interventional radiology, we may have selected a population with a high proportion of patients not eligible for acute phase stroke therapy. However, as all multivariate models were stratified on centers of inclusion, we believe that this effect was accounted for. Sixth, due to the recent rise of mechanical thrombectomy following the publication of important randomized controlled trials (51–53), the current proportion of stroke patients intubated for an elective procedure is likely to be higher than reported in our study, thus limiting the generalizability of our results. Seventh, data on functional outcomes in survivors could not be reported despite being a more relevant endpoint than mortality for stroke studies.
CONCLUSIONS
In this secondary data use of a prospective multicenter cohort study of critically ill patients requiring IMV, we showed that life support limitation was more than three times more frequent in stroke patients than in nonbrain-injured patients. There were significant differences in timing and risk factors for limitation of life support between ischemic and hemorrhagic strokes. In ventilated stroke patients, early decisions to limit life support are frequent, and a high proportion of deaths follow such decisions. These findings warrant further investigations to clarify the impact of life support limitation on prognostication models.
ACKNOWLEDGMENTS
Members of the OUTCOMEREA Study Group: Scientific Committee: Jean-François Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm –Paris Diderot university IAME, F75018, Paris); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Maïté Garrouste-Orgeas (Paliative care, Institut franco Britanique, Paris, France); Jean-Ralph Zahar (Infection Control Unit, Avicenne Hospital, Bobigny, France); Bruno Mourvillier (Medical ICU, CHU Reims,France); Michael Darmon (Medical ICU, APHP Saint Louis hospital Paris, France). Biostatistical and information system expertise: Jean-Francois Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm –Paris Diderot University IAME, F75018, Paris); Corinne Alberti (Medical Computer Sciences and Biostatistics Department, Robert Debré Hospital, Paris, France); Stephane Ruckly (OUTCOMEREA Organization and Inserm UMR 1137 IAME, F75018, Paris); Sébastien Bailly (Grenoble University Hospital Inserm UMR 1137 IAME, F75018, Paris) and Aurélien Vannieuwenhuyze (Tourcoing, France). Investigators of the OUTCOMEREA database: Christophe Adrie (ICU, CH Melun, and Physiology, Cochin Hospital, Paris, France); Carole Agasse (Medical ICU, University Hospital Nantes, France); Bernard Allaouchiche (ICU, Hospices civils de Lyon, Lyon sud, Lyon, France); Olivier Andremont (ICU, Bichat Hospital, Paris, France); Pascal Andreu (CHU Dijon, Dijon, France); Laurent Argaud (Medical ICU, Hospices Civils de Lyon, Lyon, France); Claire Ara-Somohano (Medical ICU, University Hospital, Grenoble, France); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Francois Barbier (Medical-Surgical ICU, Orleans, France); Jean-Pierre Bedos (ICU, Versailles Hospital, Le Chesnay, France); Thomas Baudry (Medial ICU, Edouard Herriot Hospital, Lyon France); Jérome Bedel (ICU, Versailles Hospital, Le Chesnay, France); Julien Bohé (ICU, Hôpital Pierre Benite, Lyon France); Lila Bouadma (ICU, Bichat Hospital, Paris, France); Jeremy Bourenne (Réanimation des Urgences, Timone-2; APHM, Marseille, France); Noel Brule (Medical ICU, University Hospital Nantes, France); Frank Chemouni (Polyvalent ICU, Grand Hôpital de l’Est Francilien, Jossigny, France); Julien Carvelli (Réanimation des Urgences, Timone-2; APHM, Marseille, France); Elisabeth Coupez (ICU, G Montpied Hospital, Clermont-Ferrand, France); Martin Cour (Medial ICU, Edouard Heriot Hospital, Lyon France); Michael Darmon (ICU, APHP St louis, Paris France); Claire Dupuis (ICU, G Montpied Hospital, Clermont-Ferrand, France); Etienne de Montmollin (ICU, Bichat Hospital, Paris, France); Loa Dopeux (ICU, G Montpied Hospital, Clermont-Ferrand, France); Anne-Sylvie Dumenil (Antoine Béclère Hospital, Clamart, France); Jean-Marc Forel (AP HM, Medical ICU, Hôpital Nord Marseille); Marc Gainnier (Réanimation des Urgences, Timone-2; APHM, Marseille, France); Charlotte Garret (Medical ICU, University Hospital Nantes, France); Dany Goldgran-Tonedano (CH le Raincy-Montfermeil; France); Steven Grangé (ICU, CHU Rouen, France); Antoine Gros (ICU, Versailles Hospital, Versailles, France); Hédia Hammed (CH le Raincy-Montfermeil); Akim Haouache (Surgical ICU, H Mondor Hospital, Creteil, France); Tarik Hissem (ICU, Eaubonne, France); Vivien Hong Tuan Ha (ICU, CH Meaux, France); Sébastien Jochmans (ICU, CH Melun); Jean-Baptiste Joffredo (ICU, G Montpied Hospital, Clermont-Ferrand, France); Hatem Kallel (ICU, Cayenne General Hospital, Cayenne, France); Guillaume Lacave (ICU, Versailles Hospital, Le Chesnay, France); Virgine Laurent (ICU, Versailles Hospital, Le Chesnay, France); Alexandre Lautrette (ICU, G Montpied Hospital, Clermont-Ferrand, France); Clément Le Bihan (ICU, Bichat Hospital, Paris, France); Virgine Lemiale (Medical ICU, Saint Louis Hospital, Paris, France); David Luis (Médecine Intensive et Réanimation, CH Simone Veil, Beauvais, France); Guillaume Marcotte (Surgical ICU, Hospices Civils de Lyon, Lyon, France); Jordane Lebut (ICU, Bichat Hospital, Paris, France); Bruno Mourvillier (ICU, CHU Reims, Reims, France); Mathild Neuville (ICU, Foch Hospital, Paris, France); Laurent Nicolet (Medical ICU, University Hospital Nantes, France); Johanna Oziel (Medico-Surgical ICU, Hôpital Avicenne APHP, Bobigny, France); Laurent Papazian (Hopital Nord, Marseille, France); Juliette Patrier (ICU, Bichat Hospital, Paris, France); Benjamin Planquette (Pulmonology ICU, George Pompidou Hospital Hospital, Paris, France); Aguila Radjou (ICU, Bichat Hospital, Paris, France); Marie Simon (Medial ICU, Edouard Heriot Hospital, Lyon France); Romain Sonneville (ICU, Bichat Hospital, Paris, France); Jean Reignier (Medical ICU, University Hospital Nantes, France); Bertrand Souweine (ICU, G Montpied Hospital, Clermont-Ferrand, France); Carole Schwebel (ICU, A Michallon Hospital, Grenoble, France); Shidasp Siami (ICU, Etampes Hospital, France); Nicolas Terzi (ICU, A Michallon Hospital, Grenoble, France); Gilles Troché (ICU, André Mignot Hospital, Le Chesnay, France); Fabrice Thiollieres (ICU, Hospices civils de Lyon, Lyon sud, Lyon, France); Guillaume Thierry (ICU, St Etienne, France); Guillaume Van Der Meersch (Medical-Surgical ICU, University Hospital Avicenne); Marion Venot (Medical ICU, Saint Louis Hospital, Paris, France); Florent Wallet (ICU, Hospices Civils de Lyon, Lyon sud, Lyon, France); Sondes Yaacoubi (CH le Raincy-Montfermeil); Olivier Zambon (Medical ICU, University Hospital Nantes, France); Jonathan Zarka (Reanimation Polyvalente, Centre Hospitalier de Marne la Vallée, France). Study Monitors: Mireille Adda, Vanessa Vindrieux, Marion Provent, Sylvie de la Salle, Pauline Enguerrand, Vincent Gobert, Stéphane Guessens, Helene Merle, Nadira Kaddour, Boris Berthe, Samir Bekkhouche, Kaouttar Mellouk, Mélaine Lebrazic, Carole Ouisse, Diane Maugars, Christelle Aparicio, Igor Theodose, Manal Nouacer, Veronique Deiler, Fariza Lamara, Myriam Moussa, Atika Mouaci, Nassima Viguier.
Supplementary Material
Footnotes
This work was performed at Université de Paris, UMR 1137, IAME, Team DESCID, Paris, France.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Dr. Thiéry has received honoraria from Gilead-Kite. The remaining authors have disclosed that they do not have any conflicts of interest.
Contributor Information
Collaborators: Jean-François Timsit, Elie Azoulay, Jean-Ralph Zahar, Bruno Mourvillier, Michael Darmon, Jean-Francois Timsit, Corinne Albert, Stephane Ruckly, Sébastien Bailly, Aurélien Vannieuwenhuyze, Christophe Adrie, Carole Agasse, Bernard Allaouchiche, Olivier Andremont, Pascal Andreu, Laurent Argaud, Claire Ara-Somohano, Elie Azoulay, Francois Barbier, Jean-Pierre Bedos, Thomas Baudry, Jérome Bedel, Julien Bohé, Lila Bouadma, Jeremy Bourenne, Noel Brule, Frank Chemouni, Julien Carvelli, Elisabeth Coupez, Martin Cour, Michael Darmon, Claire Dupuis, Etienne de Montmollin, Loa Dopeux, Anne-Sylvie Dumenil, Jean-Marc Forel, Marc Gainnier, Charlotte Garret, Dany Goldgran-Tonedano, Steven Grangé, Antoine Gros, Hédia Hammed, Akim Haouache, Tarik Hissem, Vivien Hong Tuan Ha, Sébastien Jochman, Jean-Baptiste Joffredo, Hatem Kallel, Guillaume Lacave, Virgine Laurent, Alexandre Lautrette, Clément Le Bihan, Virgine Lemiale, David Luis, Guillaume Marcotte, Jordane Lebut, Bruno Mourvillier, Mathild Neuville, Laurent Nicolet, Johanna Oziel, Laurent Papazian, Juliette Patrier, Benjamin Planquette, Aguila Radjou, Marie Simon, Romain Sonneville, Jean Reignier, Bertrand Souweine, Carole Schwebel, Shidasp Siami, Nicolas Terzi, Gilles Troché, Fabrice Thiollieres, Guillaume Thierry, Guillaume Van Der Meersch, Marion Venot, Florent Wallet, Sondes Yaacoubi, Olivier Zambon, Jonathan Zarka, Mireille Adda, Vanessa Vindrieux, Marion Provent, Sylvie de la Salle, Pauline Enguerrand, Vincent Gobert, Stéphane Guessens, Helene Merle, Nadira Kaddour, Boris Berthe, Samir Bekkhouche, Kaouttar Mellouk, Mélaine Lebrazic, Carole Ouisse, Diane Maugars, Christelle Aparicio, Igor Theodose, Manal Nouacer, Veronique Deiler, Fariza Lamara, Myriam Moussa, Atika Mouaci, and Nassima Viguier
REFERENCES
- 1.Santoli F, De Jonghe B, Hayon J, et al. Mechanical ventilation in patients with acute ischemic stroke: Survival and outcome at one year. Intensive Care Med. 2001; 27:1141–1146 [DOI] [PubMed] [Google Scholar]
- 2.Schielke E, Busch MA, Hildenhagen T, et al. Functional, cognitive and emotional long-term outcome of patients with ischemic stroke requiring mechanical ventilation. J Neurol. 2005; 252:648–654 [DOI] [PubMed] [Google Scholar]
- 3.Milhaud D, Popp J, Thouvenot E, et al. Mechanical ventilation in ischemic stroke. J Stroke Cerebrovasc Dis. 2004; 13:183–188 [DOI] [PubMed] [Google Scholar]
- 4.de Montmollin E, Terzi N, Dupuis C, et al. ; OUTCOMEREA Study Group. One-year survival in acute stroke patients requiring mechanical ventilation: A multicenter cohort study. Ann Intensive Care. 2020; 10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonneville R, Gimenez L, Labreuche J, et al. What is the prognosis of acute stroke patients requiring ICU admission? Intensive Care Med. 2017; 43:271–272 [DOI] [PubMed] [Google Scholar]
- 6.Diringer MN, Edwards DF, Aiyagari V, et al. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001; 29:1792–1797 [DOI] [PubMed] [Google Scholar]
- 7.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007; 68:1651–1657 [DOI] [PubMed] [Google Scholar]
- 8.Creutzfeldt CJ, Becker KJ, Weinstein JR, et al. Do-not-attempt-resuscitation orders and prognostic models for intraparenchymal hemorrhage. Crit Care Med. 2011; 39:158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azoulay E, Metnitz B, Sprung CL, et al. ; SAPS 3 investigators. End-of-life practices in 282 intensive care units: Data from the SAPS 3 database. Intensive Care Med. 2009; 35:623–630 [DOI] [PubMed] [Google Scholar]
- 10.Ferrand E, Robert R, Ingrand P, et al. ; French LATAREA Group. Withholding and withdrawal of life support in intensive-care units in France: A prospective survey. French LATAREA group. Lancet. 2001; 357:9–14 [DOI] [PubMed] [Google Scholar]
- 11.Wunsch H, Harrison DA, Harvey S, et al. End-of-life decisions: A cohort study of the withdrawal of all active treatment in intensive care units in the United Kingdom. Intensive Care Med. 2005; 31:823–831 [DOI] [PubMed] [Google Scholar]
- 12.Quill CM, Ratcliffe SJ, Harhay MO, et al. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014; 146:573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo SM, De Simoni FHB, Jakob SM, et al. ; ICON investigators. Decision-making on withholding or withdrawing life support in the ICU: A worldwide perspective. Chest. 2017; 152:321–329 [DOI] [PubMed] [Google Scholar]
- 14.Lautrette A, Garrouste-Orgeas M, Bertrand PM, et al. ; Outcomerea Study Group. Respective impact of no escalation of treatment, withholding and withdrawal of life-sustaining treatment on ICU patients’ prognosis: A multicenter study of the outcomerea research group. Intensive Care Med. 2015; 41:1763–1772 [DOI] [PubMed] [Google Scholar]
- 15.Lesieur O, Leloup M, Gonzalez F, et al. ; EPILAT study group. Withholding or withdrawal of treatment under French rules: A study performed in 43 intensive care units. Ann Intensive Care. 2015; 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geurts M, Macleod MR, van Thiel GJ, et al. End-of-life decisions in patients with severe acute brain injury. Lancet Neurol. 2014; 13:515–524 [DOI] [PubMed] [Google Scholar]
- 17.Wartenberg KE, Hwang DY, Haeusler KG, et al. Gap analysis regarding prognostication in neurocritical care: A joint statement from the German Neurocritical Care Society and the Neurocritical Care Society. Neurocrit Care. 2019; 31:231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann A, Audibert G, Claudot F, et al. Ethics review: End of life legislation–the French model. Crit Care. 2009; 13:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truche AS, Darmon M, Bailly S, et al. ; OUTCOMEREA Study Group. Continuous renal replacement therapy versus intermittent hemodialysis in intensive care patients: Impact on mortality and renal recovery. Intensive Care Med. 2016; 42:1408–1417 [DOI] [PubMed] [Google Scholar]
- 20.The GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018; 379:2429–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprung CL, Ricou B, Hartog CS, et al. Changes in end-of-life practices in European intensive care units from 1999 to 2016. JAMA. 2019; 322:1692–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993; 270:2957–2963 [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 24.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974; 2:81–84 [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 26.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009; 170:244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mark NM, Rayner SG, Lee NJ, et al. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: A systematic review. Intensive Care Med. 2015; 41:1572–1585 [DOI] [PubMed] [Google Scholar]
- 28.Vesin A, Azoulay E, Ruckly S, et al. Reporting and handling missing values in clinical studies in intensive care units. Intensive Care Med. 2013; 39:1396–1404 [DOI] [PubMed] [Google Scholar]
- 29.de Montmollin E, Ruckly S, Schwebel C, et al. ; OUTCOMEREA Study Group. Pneumonia in acute ischemic stroke patients requiring invasive ventilation: Impact on short and long-term outcomes. J Infect. 2019; 79:220–227 [DOI] [PubMed] [Google Scholar]
- 30.Holloway RG, Benesch CG, Burgin WS, et al. Prognosis and decision making in severe stroke. JAMA. 2005; 294:725–733 [DOI] [PubMed] [Google Scholar]
- 31.Sonneville R, Mazighi M, Bresson D, et al. ; SPICE investigators. Outcomes of acute stroke patients requiring mechanical ventilation: Study protocol for the SPICE multicenter prospective observational study. Neurocrit Care. 2020; 32:624–629 [DOI] [PubMed] [Google Scholar]
- 32.Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998; 158:1163–1167 [DOI] [PubMed] [Google Scholar]
- 33.Mohammed MA, Mant J, Bentham L, et al. Process of care and mortality of stroke patients with and without a do not resuscitate order in the West Midlands, UK. Int J Qual Health Care. 2006; 18:102–106 [DOI] [PubMed] [Google Scholar]
- 34.Rabinstein AA, Diringer MN. Withholding care in intracerebral hemorrhage: Realistic compassion or self-fulfilling prophecy? Neurology. 2007; 68:1647–1648 [DOI] [PubMed] [Google Scholar]
- 35.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; 46:2032–2060 [DOI] [PubMed] [Google Scholar]
- 36.Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001; 56:766–772 [DOI] [PubMed] [Google Scholar]
- 37.Frick S, Uehlinger DE, Zuercher Zenklusen RM. Medical futility: Predicting outcome of intensive care unit patients by nurses and doctors–a prospective comparative study. Crit Care Med. 2003; 31:456–461 [DOI] [PubMed] [Google Scholar]
- 38.Geurts M, de Kort FAS, de Kort PLM, et al. Predictive accuracy of physicians’ estimates of outcome after severe stroke. PLoS One. 2017; 12:e0184894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried TR, Bradley EH, Towle VR. Valuing the outcomes of treatment: Do patients and their caregivers agree? Arch Intern Med. 2003; 163:2073–2078 [DOI] [PubMed] [Google Scholar]
- 40.Albrecht GL, Devlieger PJ. The disability paradox: High quality of life against all odds. Soc Sci Med. 1999; 48:977–988 [DOI] [PubMed] [Google Scholar]
- 41.Rohaut B, Claassen J. Decision making in perceived devastating brain injury: A call to explore the impact of cognitive biases. Br J Anaesth. 2018; 120:5–9 [DOI] [PubMed] [Google Scholar]
- 42.Sprung CL, Cohen SL, Sjokvist P, et al. ; Ethicus Study Group. End-of-life practices in European intensive care units: The Ethicus study. JAMA. 2003; 290:790–797 [DOI] [PubMed] [Google Scholar]
- 43.Roger C, Morel J, Molinari N, et al. ; AzuRea Group. Practices of end-of-life decisions in 66 southern French ICUs 4 years after an official legal framework: A 1-day audit. Anaesth Crit Care Pain Med. 2015; 34:73–77 [DOI] [PubMed] [Google Scholar]
- 44.Kowalski RG, Chang TR, Carhuapoma JR, et al. Withdrawal of technological life support following subarachnoid hemorrhage. Neurocrit Care. 2013; 19:269–275 [DOI] [PubMed] [Google Scholar]
- 45.Qureshi AI, Adil MM, Suri MF. Rate of utilization and determinants of withdrawal of care in acute ischemic stroke treated with thrombolytics in USA. Med Care. 2013; 51:1094–1100 [DOI] [PubMed] [Google Scholar]
- 46.Parry-Jones AR, Paley L, Bray BD, et al. ; SSNAP Collaborative Group. Care-limiting decisions in acute stroke and association with survival: Analyses of UK national quality register data. Int J Stroke. 2016; 11:321–331 [DOI] [PubMed] [Google Scholar]
- 47.Luengo-Fernandez R, Paul NL, Gray AM, et al. ; Oxford Vascular Study. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford vascular study. Stroke. 2013; 44:2854–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemphill JC, 3rd, Bonovich DC, Besmertis L, et al. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001; 32:891–897 [DOI] [PubMed] [Google Scholar]
- 49.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968; 28:14–20 [DOI] [PubMed] [Google Scholar]
- 50.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: Report of a committee of the world federation of neurosurgical societies. J Neurol Neurosurg Psychiatry. 1988; 51:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731 [DOI] [PubMed] [Google Scholar]
- 52.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


