Abstract
INTRODUCTION:
Despite heterogeneity, an increased prevalence of psychological comorbidity and an altered pronociceptive gut microenvironment have repeatedly emerged as causative pathophysiology in patients with irritable bowel syndrome (IBS). Our aim was to study these phenomena by comparing gut-related symptoms, psychological scores, and biopsy samples generated from a detailed diarrhea-predominant IBS patient (IBS-D) cohort before their entry into a previously reported clinical trial.
METHODS:
Data were generated from 42 patients with IBS-D who completed a daily 2-week bowel symptom diary, the Hospital Anxiety and Depression score, and the Patient Health Questionnaire-12 Somatic Symptom score and underwent unprepared flexible sigmoidoscopy. Sigmoid mucosal biopsies were separately evaluated using immunohistochemistry and culture supernatants to determine cellularity, mediator levels, and ability to stimulate colonic afferent activity.
RESULTS:
Pain severity scores significantly correlated with the daily duration of pain (r = 0.67, P < 0.00001), urgency (r = 0.57, P < 0.0005), and bloating (r = 0.39, P < 0.05), but not with psychological symptom scores for anxiety, depression, or somatization. Furthermore, pain severity scores from individual patients with IBS-D were significantly correlated (r = 0.40, P < 0.008) with stimulation of colonic afferent activation mediated by their biopsy supernatant, but not with biopsy cell counts nor measured mediator levels.
DISCUSSION:
Peripheral pronociceptive changes in the bowel seem more important than psychological factors in determining pain severity within a tightly phenotyped cohort of patients with IBS-D. No individual mediator was identified as the cause of this pronociceptive change, suggesting that nerve targeting therapeutic approaches may be more successful than mediator-driven approaches for the treatment of pain in IBS-D.
INTRODUCTION
Chronic abdominal pain and loose stools is a debilitating condition and one of the most common causes of presentation to a gastroenterologist. After excluding inflammatory or infectious disease, most cases are diagnosed as irritable bowel syndrome with diarrhea (IBS-D) (1). However, the cause of the characteristic pain remains obscure. Pain has been attributed to visceral hypersensitivity to otherwise non-noxious stimuli, found in between 50% and 90% of patients (2). This may arise from a range of abnormalities including enhanced nociception, augmented central pain processing (3), and impaired adaptation to pain (4), which may explain the heterogeneity of pathophysiology within patients with irritable bowel syndrome (IBS).
Although central factors are undoubtedly important, recognition that IBS could arise after acute infectious gastroenteritis, or postinfectious IBS (PI-IBS), and the chronic changes in mucosal cellularity and mediator content associated with PI-IBS (5,6) has focused attention on local mucosal abnormalities. Subsequent studies across different subgroups of patients with IBS have broadly documented altered mast cell and endocrine cell numbers and mediators (7). However, such findings are not universal and the link between mucosal changes, visceral hypersensitivity, and pain symptomology in unselected patients with IBS is variable (8–10). These disparities may be due to studying unselected, and hence heterogeneous patients with IBS, rather than specific subtypes.
The commonest subtype of PI-IBS is diarrhea predominant (11), a group in whom several studies have shown alterations in tight junctions (12) and increased permeability (13). These features are linked to visceral hypersensivity by promoting exposure to luminal content and local immune cell activation (14). Evidence for these changes have come from biopsy studies that have documented mast cell hyperplasia and elevated levels of mediators such as histamine, serotonin, and tryptase, which contribute to activation of enteric nerves by biopsy supernatants (8,15,16). In some studies, these changes have been shown to correlate with pain scores or pain threshold assessed by rectal barostat, thereby providing a link between local changes in the gut mucosa with pain symptomology (17).
We have previously reported the results of a large proof of concept clinical trial in patients with IBS-D (18). Nested within the prescreening period of the trial was a mechanistic study that is reported here. We first examined the correlation between symptoms of pain with related sensory abnormalities of urgency, bloating, and psychological factors known to influence pain processing (19). We then focused on the histology of the sigmoid colonic mucosa and mediator release from incubated biopsies to identify mediators and cell types contributing to IBS pain. Finally, we tested the effect of sigmoid biopsy supernatant on colonic afferent activity and correlated this with pain symptomology. We found considerable heterogeneity in our patient group, with evidence that locally generated mediators are associated with the severity of abdominal pain, with a stronger effect than central psychological factors in this subtype of IBS.
METHODS
Patient details
Patients with IBS-D were recruited into a multicentered, parallel group, randomized placebo-controlled trial as previously reported (18) (ClinicalTrial.gov ISRCTN76612274). Nested within the main trial was a mechanistic study of 42 patients who were recruited in the Nottingham center and consented for sigmoid biopsy before randomization. In addition to stool consistency and frequency, other bowel-related symptoms comprising pain severity and daily duration, urgency, and bloating were recorded daily over the 14-day screening period. Pain severity was recorded on a 0–10 scale (0 = no pain and 10 = the most severe pain ever experienced) along with daily pain duration in hours per day. Urgency and bloating were also reported on a similar 0–10 scale, stool frequency as bowel movements/day and stool consistency recorded daily using the Bristol Stool Form scale. Symptoms were recorded each evening documenting the preceding 24 hours.
All randomization patients completed the Hospital Anxiety and Depression Scale (20) along with the Patient Health Questionnaire-12 Somatic Symptom score, a measure of nongastroenterological somatic symptoms (21).
Sigmoid biopsy and assessments
After symptom screening to confirm eligibility for the trial, mucosal biopsies were obtained at 30 cm from the anus during an unprepared, unsedated flexible sigmoidoscopy in the left lateral position. Two biopsies were taken and processed for immunohistochemistry and 2 were cultured to obtain supernatants. Biopsy processing is detailed in the Supplemental Methods (see Supplementary Digital Content 2, http://links.lww.com/CTG/A510).
Two biopsies were weighed and placed into 2 mL of Hanks balanced salt solution in a polystyrene organ culture dish and incubated for two 30 minute periods at 37 °C, in 5% CO2. Fresh Hanks was used for the second, 30-minute incubation period. The supernatant collected in the first 30 minutes was used to assess released mediators, whereas the supernatant collected in the second 30-minute period was used for testing in colonic afferent preparations. Supernatants were aliquoted and stored at −80 oC. Samples were transported on dry ice and aliquots were thawed on the day of use in electrophysiological or laboratory studies.
Biopsy supernatant levels of histamine, tryptase, chymase, and carboxypeptidase 3 (CPA3) were measured using sandwich ELISA assays provided by the Immunopharmacology Research Group, the University of Southampton, as described previously (22,23). Histamine was measured using a commercially available enzyme immunoassay kit (Neogen, Lexington, KY) as directed by the manufacturer.
Ex-vivo recordings of colonic afferent fiber activity
Few fiber afferent activities were recorded from teased lumbar splanchnic nerve bundles in a flat sheet colorectal preparation (male 12-weeks old, wild-type C57BL/6 mice, or NaV1.9 −/− mice, as previously described (24)) using suction electrodes. Receptive fields were identified and characterized based on the criteria developed by Brierley et al. (25–27). Experiments were only performed on the receptive fields of vascular (or serosal) afferents. Once characterized mechanosensitivity was determined by probing with 0.6 g and 1.0 g von Frey hairs (vFh). Thereafter, a brass ring was placed around the receptive field and the indwelling buffer replaced with biopsy supernatant (100 μL) for 12 minutes, and mechanosensitivity retested after removal of the ring and supernatant. The individual single unit discharge of the receptive field tested was discriminated using template matching software within Spike 2 software (Cambridge Electronic Design, Cambridge, UK) performed over the period of vFh probing. Mechanosensitivity was determined for each weight of vFh before and after supernatant application, and the difference was calculated and expressed in action potentials (spikes) per second. Chemosensitivity to biopsy supernatant was expressed as the increase in afferent discharge over the 12-minute application period (see Supplemental Methods, Supplementary Digital Content 2, http://links.lww.com/CTG/A510).
Power and statistical analysis
All analysis was performed using Graphpad Prism Version 7 and above (GraphPad Software, San Diego, CA). Unless otherwise stated, data are expressed as mean ± SD. Normality of data was tested by using the D'Agostino and Pearson omnibus normality tests, and comparisons were made between parameters for individual patients using Pearson or Spearman correlation coefficients or between group data using a Student t-test or Mann-Whitney U test for parametric and nonparametric data, respectively. Adjustments were made to significant data sets for greater than 3 multiple comparisons using a Bonferroni correction and false discoveries highlighted. Significance was set at P < 0.05 or smaller.
RESULTS
Patient details
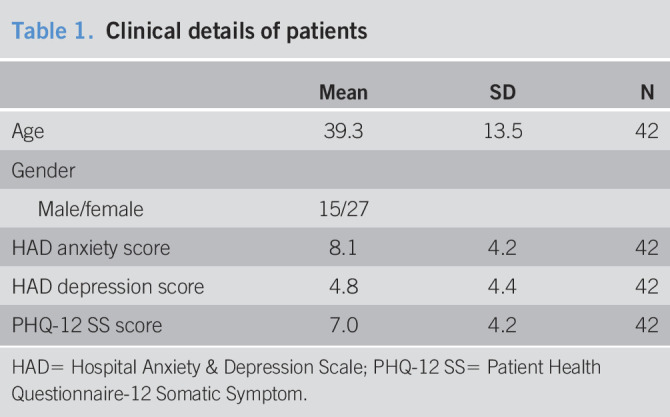
Symptom scores, psychological tests, and sigmoid biopsies were obtained from 42 patients with IBS-D. Clinical details are shown in Table 1.
Table 1.
Clinical details of patients
HAD= Hospital Anxiety & Depression Scale; PHQ-12 SS= Patient Health Questionnaire-12 Somatic Symptom.
Symptom scores
Bowel-related symptoms and their correlation with pain severity.
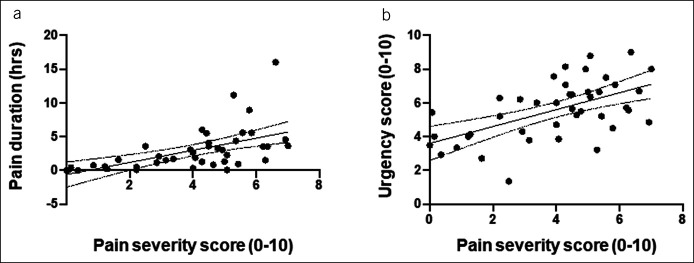
As required for trial entry, patients recorded frequent loose stools (Table 1). Pain severity and bloating scores lay within the mild-to-moderate ranges with slightly higher urgency scores. The mean daily duration of pain experienced was 2.9 ± 3.2 hours, with the wide SD highlighting the heterogeneous nature of pain experienced by patients, ranging from brief periods of pain to more prolonged periods of pain. Pain severity was strongly correlated with daily pain duration and urgency and to a lesser degree with bloating and stool frequency, but not with stool consistency (Table 1 and Figure 1).
Figure 1.
Scatter plots of data for individual patients illustrating the correlation between pain severity scores with (a) daily pain duration and (b) symptom scores for urgency.
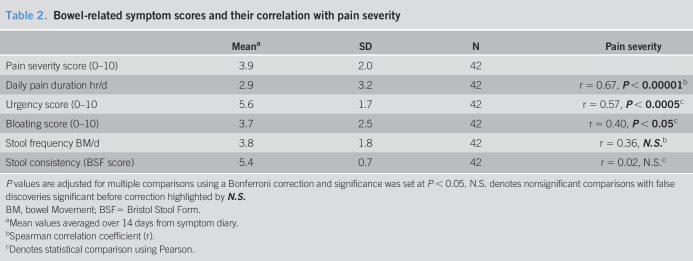
As expected, patient symptom scores for urgency also correlated significantly with stool consistency (Figure 2), but not with stool frequency or bloating. There was no significant correlation between symptom scores for bloating, stool frequency, or stool consistency (Table 2).
Figure 2.
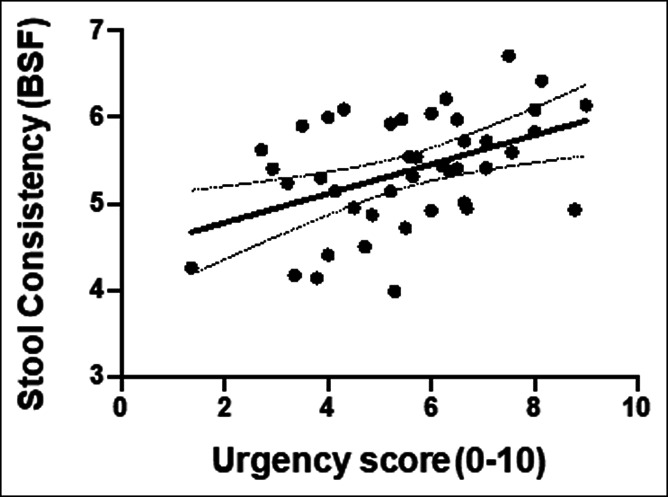
Scatter plot of data for individual patients illustrating the correlation between patient symptom scores for urgency and stool consistency.
Table 2.
Bowel-related symptom scores and their correlation with pain severity
P values are adjusted for multiple comparisons using a Bonferroni correction and significance was set at P < 0.05. N.S. denotes nonsignificant comparisons with false discoveries significant before correction highlighted by N.S.
BM, bowel Movement; BSF= Bristol Stool Form.
Mean values averaged over 14 days from symptom diary.
Spearman correlation coefficient (r).
Denotes statistical comparison using Pearson.
Psychological scores and their correlation with pain severity and bowel-related symptoms.
Anxiety, depression, and Patient Health Questionnaire-12 Somatic Symptom scores showed significant psychological distress with 24/42 (57%), 10/42 (24%), and 13/42 (31%) of patients having values above the normal cutoffs (7, 7, and 6, respectively) (20,21). However, no significant correlation was found between these and pain severity (Table 3) or other bowel-related symptoms (see Table S1, Supplementary Digital content 1, http://links.lww.com/CTG/A509); although as expected, these psychological measures were highly correlated with each other (see Figure S1, Supplementary Digital content 3, http://links.lww.com/CTG/A508 and Table S2, Supplementary Digital content 1, http://links.lww.com/CTG/A509).
Table 3.
Correlation of patient symptom scores for urgency, bloating, stool frequency, or stool consistency
Significant results shown in bold text.
Denotes statistical comparison using Pearson.
Spearman correlation coefficient (r). P values are adjusted for multiple comparisons using a Bonferroni correction, and significance was set at P < 0.05. N.S. denotes nonsignificant comparisons.
Effect of age and sex on bowel-related and psychological symptom scores.
No difference was observed in the magnitude of bowel-related symptoms or psychological scores between male and female patients. Furthermore, no correlation was observed between any symptom score and patient age (see Table S3, Supplementary Digital content 1, http://links.lww.com/CTG/A509).
Sigmoid biopsies
Correlation of pain severity scores with biopsy mediator levels and histology.
No significant correlation was observed between pain severity scores and biopsy supernatant levels of histamine, tryptase, chymase, and CPA3 nor with biopsy mast cell or CD68 positive cell counts (see Table S4, Supplementary Digital content 1, http://links.lww.com/CTG/A509). Furthermore, no significant correlation was found between biopsy mediator levels or histology and other bowel-related symptoms (see Table S5, Supplementary Digital content 1, http://links.lww.com/CTG/A509) or psychological scores (see Table S6, Supplementary Digital content 1, http://links.lww.com/CTG/A509). It is perhaps worth noting that there was a correlation between CPA3 and urgency and stool consistency (Table S5, Supplementary Digital content 1, http://links.lww.com/CTG/A509), but after correction for multiple comparisons, this failed to reach conventional significance.
Correlation of biopsy-evoked colonic afferent activity with pain severity scores.
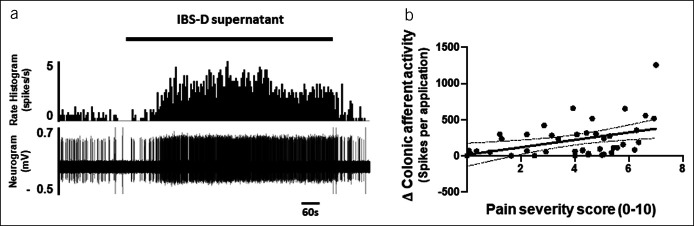
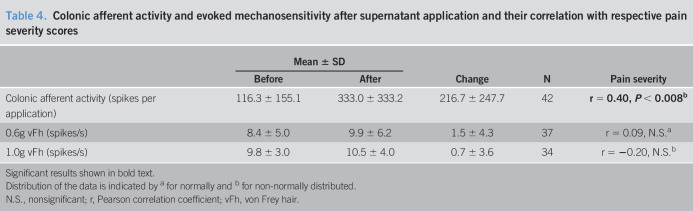
By contrast, application of biopsy supernatant to the receptive field of colonic afferents produced colonic afferent responses that correlated significantly (P < 0.008) with the severity of pain experienced by the patient from which the biopsy was obtained (Figure 3, Table 4). No correlation was found between the change in colonic afferent mechanosensitivity (0.6 g and 1.0 g vFh) after supernatant application and pain severity scores (Table 4).
Figure 3.
Effect of supernatant on afferent nerve activity a) raw trace illustrating the stimulatory effect of IBS-D biopsy supernatant on colonic afferent fiber activity and b) scatter plot of data for individual patients illustrating the correlation between biopsy mediated colonic afferent firing and pain severity scores.
Table 4.
Colonic afferent activity and evoked mechanosensitivity after supernatant application and their correlation with respective pain severity scores
Significant results shown in bold text.
Distribution of the data is indicated by a for normally and b for non-normally distributed.
N.S., nonsignificant; r, Pearson correlation coefficient; vFh, von Frey hair.
Correlation of colonic afferent activity with biopsy mediator levels.
No significant correlation was observed between biopsy supernatants mediator levels or biopsy histology findings and respective magnitudes of colonic afferent activation or change in colonic afferent mechanosensitivity (see Table S7, Supplementary Digital content 1, http://links.lww.com/CTG/A509). This suggests that multiple mediators may be responsible for the pronociceptive potential of biopsy supernatants, and these mediators may vary from patient to patient.
Effect of age and sex on biopsy responses.
Furthermore, no significant difference was found in the colonic afferent response to the application of biopsy supernatant or subsequent change in mechanosensitivity based on the sex of the patient from which the biopsy was taken (see Table S8, Supplementary Digital content 1, http://links.lww.com/CTG/A509). In addition, no effect of patient sex was found on biopsy mediator levels or histology, and no correlation was found between the patients' age and the effect of biopsy supernatant on colonic afferent activity and mechanosensitivity or age and biopsy mediator release or histology (see Table S8, Supplementary Digital content 1, http://links.lww.com/CTG/A509).
Effect of NaV1.9 deletion on biopsy-mediated colonic afferent responses.
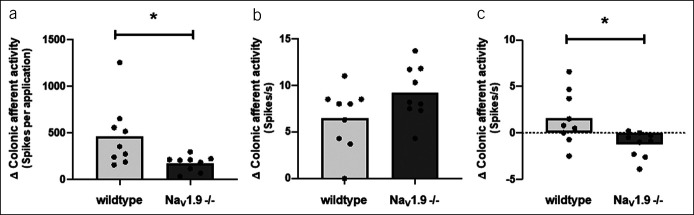
Given the lack of correlation of nerve response with individual mediators in the supernatant, an alternative strategy to the treatment of abdominal pain in IBS-D may be to target ion channels responsible for the activation of colonic afferents by multiple mediators. NaV1.9 is one such channel that is responsible for the sensitization of colonic afferents in response to inflammatory and algogenic mediators (24,28) consistent with the observation that gain of function human NaV1.9 mutants display episodic abdominal pain and diarrhea (28). To highlight the therapeutic potential of NaV1.9, we also evaluated the effect of biopsy supernatants from patients with the highest pain scores (severity score of 5 or greater) on colonic afferent activity in tissue from NaV1.9 −/− mice. We demonstrated a reduced afferent response by comparison to the responses observed when supernatants were tested in wild type tissue (Figure 4a). Furthermore, although colonic afferent mechanosensitivity to von Frey probing was comparable in tissue from C7B6 mice or NaV1.9 −/− mice (Figure 4b), the change in mechanosensitivity after supernatant application was also significantly reduced in tissue from NaV1.9 −/− mice (Figure 4c).
Figure 4.
Effect of biopsy supernatants (from patients with IBS-D with pain scores >5) in tissue from NaV1.9 −/− compared with wild type mice. Bar charts illustrating (a) the reduced colonic afferent response to supernatant, (b) the comparable magnitude of evoked mechanosensitivity in wild type and NaV1.9 −/− tissue before supernatant application, and (c) the lower change mechanosensitivity after supernatant application in tissue from NaV1.9 −/− compared with wild type mice.
DISCUSSION
The aim of this study was to gain insight into putative mechanisms of visceral pain in IBS-D. Although previous studies have shown separately that central psychological factors such as mood and somatization (2) and peripheral mediators (8,9) can contribute to visceral pain, we have assessed these factors within a single study. An additional strength of our study is that it has been conducted in a single subtype of IBS, using bowel symptom scores recorded in a daily diary rather than retrospective symptom scores across a mixture of IBS subtypes. We have been able to show that although variable, reported pain severity was highly correlated with the overall daily duration of pain. Urgency, a key feature of IBS-D, was also found to be strongly correlated with pain severity, which is perhaps unsurprising, given that urgency is also believed to be driven by colorectal hypersensitivity and points toward a common pathology.
However, we found no correlation between pain severity scores and either anxiety, depression, nor somatic sensitivity as assessed by the PHQ-12SS. Our sample was representative of all patients with IBS because the mean scores and the proportion scoring above the upper limit of normal for anxiety and depression were very similar to the published data from a much larger IBS-D patient cohort (21). As a consequence, our findings indicate that such psychological factors are not the major determinant of pain severity in our IBS-D patient cohort. This does not however exclude a contribution from psychological factors to pain in some patients with more marked psychological disturbances who may not be selected for clinical trials.
Our data instead point to a consistent contribution of peripheral factors to pain severity in patients with IBS-D. We examined the effect of biopsy supernatants on colonic afferent activity in a population of lumbar splanchnic afferents classified as vascular afferents and previously shown to display a nociceptor phenotype (26). Recordings were performed from the lumbar splanchnic nerve because this pathway has previously been shown to be responsible for the transmission of pain from the sigmoid colon (29), our site of biopsy collection. Consistent with our hypothesis, we found a strong correlation between biopsy-mediated colonic afferent activation and patient pain severity. Although we found no correlation between individual biopsy mediator levels and pain scores, we speculate that this reflects the range of possible mediators (e.g., histamine, serotonin, PGE2, and tryptase) (8,9,15) that may differ from patient to patient. An alternative explanation for the lack of correlation would be that other unmeasured mediators are important, such as bile acid derivatives, short-chain fatty acids, lipopolysaccharide, or other microbial metabolites. One possible therapeutic approach would be to generally suppress neural activation in the periphery while avoiding the side effects associated with actions on higher centers (30). To illustrate the utility of this approach, we also examined the effect of biopsy supernatants from patients with high pain severity scores on colonic afferent activity in tissue from NaV1.9 −/− mice, a channel highly expressed in colonic afferents (31), and possibly implicated in IBS-D visceral nociception because episodic abdominal pain and diarrhea has been reported in the gain of function human mutants (28). Consistent with our previous findings that colonic afferent response to algogenic mediators and supernatants generated from inflammatory bowel disease (IBD) patient tissue are attenuated in tissue from NaV1.9 −/− mice (24,28), we also observed a significant reduction in the response to IBS-D biopsy supernatants in NaV1.9 mouse tissue.
A further observation from this study was the significant correlation between urgency scores and stool consistency, suggesting that looser stools may contain mediators such as bile acids (32) and fecal proteases (33,34) that could promote urgency (35) by stimulating colorectal afferents (36,37). This concept is supported by previous reports that urgency correlates with fecal tryptase (38). Future studies are now warranted to explore these possibilities and the effect of biopsy supernatants on afferent fiber subtypes responsible for the perception of urgency such as pelvic afferents fibers within the colorectum.
In conclusion, using a tightly defined patient group, we were able to show a strong correlation between pain severity and the stimulation of colonic afferent activity by biopsy supernatants, suggesting that in this patient group, the peripheral influences are more significant that central ones. However, we found no evidence for the dominant role of any one of the mediators examined suggesting that either there is another, as yet unmeasured, mediator or that targeting multiple mediator pathways may be a better strategy than targeting a single specific pathway. Future studies should include larger panels of potential mediators that may be present in the stool of patients with IBS.
CONFLICTS OF INTEREST
Guarantor of the article: Robin Spiller, FRCP.
Specific author contributions: Vincent Cibert-Goton, PhD, and Ching Lam, PhD, MRCP, are joint first author. David C. Bulmer, PhD, and Robin Spiller, FRCP, are joint senior author. All authors contributed important intellectual content during manuscript drafting and revision and approved the final draft. Research idea and study design C.L., R.S., D.C.B., and V.C.-G. Data collection and generation of transgenic mice C.L., V.C.-G., M.Y., Y.F., and J.N.W. Data analysis C.L., R.S., D.C.B., and V.C.-G.
Financial support: The clinical trial was funded by the Efficacy and Evaluation Mechanism (EME) program grant number 09-20-16 and supported by the NIHR Nottingham Biomedical Research Center. The afferent recording work was supported by an unrestricted educational grant from Neusentis (D.C.B.).
Potential competing interest: R.S. has received research grants from Sanofi-Aventis and Zespri International and speaker fees from Alfawasserman. D.C.B. receives research funding from Astra Zeneca, Heptares and Life Arc.
Disclaimer: This is a summary of independent research funded by the National Institute for Health Research Biomedical Research Unit. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Study Highlights.
WHAT IS KNOWN
✓ Peripheral and psychological factors contribute to symptomology in irritable bowel syndrome (IBS).
✓ Peripheral factors contribute to pain in IBS by stimulating sensory nerves.
✓ Psychological factors contribute to pain in IBS by promoting hypervigilance and increasing stress responses.
WHAT IS NEW HERE
✓ Pain severity scores correlated with daily duration of pain, and urgency, but not with anxiety, depression, or somatization in patients with IBS-D.
✓ Pain severity scores correlated with biopsy-mediated colonic afferent firing.
✓ Individual biopsy mediator levels did not predict pain severity.
TRANSLATIONAL IMPACT
✓ Nerve targeting therapies may be more effective than specific mediator antagonists.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the Sister Andrea Bennet and research nurses of the Nottingham Biomedical Research Unit for their invaluable support for these studies and Dr. Andrew Walls and the Immunopharmacology Group, University of Southampton, who performed the mediator assays.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A510, http://links.lww.com/CTG/A509, http://links.lww.com/CTG/A508
Contributor Information
Vincent Cibert-Goton, Email: v.cibert-goton@imperial.ac.uk.
Ching Lam, Email: chinglam@nhs.net.
John N. Wood, Email: j.wood@ucl.ac.uk.
David C. Bulmer, Email: dcb53@cam.ac.uk.
REFERENCES
- 1.Guagnozzi D, Lucendo AJ, Angueira-Lapena T, et al. Prevalence and incidence of microscopic colitis in patients with diarrhoea of unknown aetiology in a region in central Spain. Dig Liver Dis 2012;44:384–8. [DOI] [PubMed] [Google Scholar]
- 2.Azpiroz F, Bouin M, Camilleri M, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil 2007;19:62–88. [DOI] [PubMed] [Google Scholar]
- 3.Farzaei MH, Bahramsoltani R, Abdollahi M, et al. The role of visceral hypersensitivity in irritable bowel syndrome: Pharmacological targets and novel treatments. J Neurogastroenterol Motil 2016;22:558–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albusoda A, Ruffle JK, Friis KA, et al. Systematic review with meta-analysis: Conditioned pain modulation in patients with the irritable bowel syndrome. Aliment Pharmacol Ther 2018; 48:797–806. [DOI] [PubMed] [Google Scholar]
- 5.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999; 44:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000; 47:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol.Motil. 2018;30 (doi: 10.1111/nmo.13192). [DOI] [PubMed] [Google Scholar]
- 8.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007;132:26–37. [DOI] [PubMed] [Google Scholar]
- 9.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 2009; 137:1425–34. [DOI] [PubMed] [Google Scholar]
- 10.Braak B, klooker tk, Wouters MM, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: Is there any relationship? Am J Gastroenterol 2012;107:715–26. [DOI] [PubMed] [Google Scholar]
- 11.Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: A systematic review and meta-analysis. Gastroenterology 2017; 152:1042–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez C, Vicario M, Ramos L, et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol 2012;107:736–46. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006;101:1288–94. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009;146:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004;126:693–702. [DOI] [PubMed] [Google Scholar]
- 16.Valdez-Morales EE, Overington J, Guerrero-Alba R, et al. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: A role for PAR2. Am J Gastroenterol 2013;108:1634–43. [DOI] [PubMed] [Google Scholar]
- 17.Buhner S, Braak B, Li Q, et al. Neuronal activation by mucosal biopsy supernatants from irritable bowel syndrome patients is linked to visceral sensitivity. Exp Physiol 2014;99:1299–311. [DOI] [PubMed] [Google Scholar]
- 18.Lam C, Tan W, Leighton M, et al. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut 2016;65:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma A, Van OL, Paine P, et al. Anxiety increases acid-induced esophageal hyperalgesia. Psychosom Med 2010;72:802–9. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 21.Spiller RC, Humes DJ, Campbell E, et al. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther 2010;32:811–20. [DOI] [PubMed] [Google Scholar]
- 22.Walls AF, Bennett AR, Godfrey RC, et al. Mast cell tryptase and histamine concentrations in bronchoalveolar lavage fluid from patients with interstitial lung disease. Clin Sci (Lond) 1991;81:183–8. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Buckley MG, Lau LC, et al. Mast cell carboxypeptidase as a new clinical marker for anaphylaxis. J Allergy Clin Immunol 2006;117:S85. [Google Scholar]
- 24.Hockley JR, Boundouki G, Cibert-Goton V, et al. Multiple roles for NaV1.9 in the activation of visceral afferents by noxious inflammatory, mechanical, and human disease-derived stimuli. Pain 2014;155:1962–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brierley SM, Jones RC, III, Gebhart GF, et al. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 2004; 127:166–78. [DOI] [PubMed] [Google Scholar]
- 26.Brierley SM, Page AJ, Hughes PA, et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 2008;134:2059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song X, Chen BN, Zagorodnyuk VP, et al. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 2009;137:274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockley JR, Winchester WJ, Bulmer DC. The voltage-gated sodium channel NaV 1.9 in visceral pain. Neurogastroenterol Motil 2016;28:316–26. [DOI] [PubMed] [Google Scholar]
- 29.Ray BS, Neill CL. Abdominal visceral sensation in man. Ann.Surg 1947;126:709–23. [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Ilham SJ, Feng B. Pharmacological approach for managing pain in irritable bowel syndrome: A review article. Anesth Pain Med 2017;7:e42747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hockley JRF, Taylor TS, Callejo G, et al. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 2019;68:633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijayvargiya P, Camilleri M, Burton D, et al. Bile and fat excretion are biomarkers of clinically significant diarrhoea and constipation in irritable bowel syndrome. Aliment Pharmacol Ther 2019;49:744–58. [DOI] [PubMed] [Google Scholar]
- 33.Gecse K, Roka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: A colonic lumenal factor impairing colonic permeability and sensitivity. Gut 2008;57:591–9. [DOI] [PubMed] [Google Scholar]
- 34.Annahazi A, Gecse K, Dabek M, et al. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain 2009;144:209–17. [DOI] [PubMed] [Google Scholar]
- 35.Edwards CA, Brown S, Baxter AJ, et al. Effect of bile acid on anorectal function in man. Gut 1989;30:383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro J, Harrington AM, Lieu T, et al. Activation of pruritogenic TGR5, MrgprA3, and MrgprC11 on colon-innervating afferents induces visceral hypersensitivity. JCI Insight 2019;4 (doi: 10.1172/jci.insight.131712). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jimenez-Vargas NN, Pattison LA, Zhao P, et al. Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc Natl Acad Sci U.S.A 2018;115:E7438–E7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tooth D, Garsed K, Singh G, et al. Characterisation of faecal protease activity in irritable bowel syndrome with diarrhoea: Origin and effect of gut transit. Gut 2014;63:753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.