Supplemental Digital Content is available in the text.
Keywords: acute cor pulmonale; cardiac failure; heart-lung interactions; respiratory failure
Objectives:
Changes in right ventricular size and function are frequently observed in patients with severe acute respiratory distress syndrome. The majority of patients who receive venovenous extracorporeal membrane oxygenation undergo chest CT and transthoracic echocardiography. The aims of this study were to compare the use of CT and transthoracic echocardiography to evaluate the right ventricular function and to determine the prevalence of acute cor pulmonale in this patient population.
Design:
Observational, retrospective, single-center, cohort study.
Setting:
Severe respiratory failure and extracorporeal membrane oxygenation center.
Patients:
About 107 patients with severe acute respiratory distress syndrome managed with venovenous extracorporeal membrane oxygenation.
Interventions:
Chest CT to evaluate right ventricular size and transthoracic echocardiography to evaluate right ventricular size and function.
Measurements and Main Results:
All 107 patients had a qualitative assessment of right ventricular size and function on transthoracic echocardiography. Quantitative measurements were available in 54 patients (50%) who underwent transthoracic echocardiography and in 107 of patients (100%) who received CT. Right ventricular dilatation was defined as a right ventricle end-diastolic diameter greater than left ventricular end-diastolic diameter upon visual assessment or an right ventricle end-diastolic diameter/left ventricular end-diastolic diameter and/or right ventricle cavity area/left ventricular cavity area of greater than 0.9. Right ventricle systolic function was visually estimated as being normal or impaired (visual right ventricular systolic impairment). The right ventricle was found to be dilated in 38/107 patients (36%) and in 58/107 patients (54%), using transthoracic echocardiography or CT right ventricle end-diastolic diameter/left ventricular end-diastolic diameter, respectively. When the CT right ventricle cavity/left ventricular cavity area criterion was used, the right ventricle was dilated in 19/107 patients (18%). About 33/107 patients (31%) exhibited visual right ventricular systolic impairment. Transthoracic echocardiography right ventricle end-diastolic diameter/left ventricular end-diastolic diameter showed good agreement with CT right ventricle cavity/left ventricular cavity area (R2 = 0.57; p < 0.01). A CT right ventricle cavity/left ventricular cavity area greater than 0.9 provided the optimal cutoff for acute cor pulmonale on transthoracic echocardiography with an AUC of 0.78. Acute cor pulmonale was defined by the presence of a right ventricle “D-shape” and quantitative right ventricle dilatation on transthoracic echocardiography or a right ventricle cavity/left ventricular cavity area greater than 0.9 on CT. A diagnosis of acute cor pulmonale was made in 9/54 (14% patients) on transthoracic echocardiography and in 19/107 (18%) on CT.
Conclusions:
Changes in right ventricular size and function are common in patients with severe acute respiratory distress syndrome requiring venovenous extracorporeal membrane oxygenation with up to 18% showing imaging evidence of acute cor pulmonale. A CT right ventricular cavity /left ventricular cavity area greater than 0.9 is indicative of impaired right ventricular systolic function.
Patients with severe acute respiratory distress syndrome (ARDS) (1), who are refractory to conventional ventilatory management, may receive venovenous extracorporeal membrane oxygenation (VV-ECMO) to substitute or support inadequate pulmonary gas exchange, while reducing the intensity of mechanical ventilation and, therefore, the likelihood of ventilator-induced lung injury (2). Acute cor pulmonale (ACP) is a morphologic and functional alteration of the right ventricle in response to an acute increase in the right ventricular (RV) afterload due to primary respiratory pathology (3).
ACP can be defined by the presence of RV dilatation and dyskinesia of the intraventricular septum (paradoxical septal motion) (4–6). Most studies assessing ACP in ARDS have used transesophageal echocardiography (4). However, transthoracic echocardiography (TTE) is noninvasive and widely available, and most normal values are validated with TTE (3, 7). It nevertheless comes with challenges of suboptimal acoustic windows in some critically ill patients (8). This has led to the growing interest in the assessment of RV size using CT in patients with acute respiratory failure (particularly in patients with acute pulmonary embolism) (9, 10). ACP is a known complication in patients with severe ARDS who are mechanically ventilated and has been associated with significant mortality and morbidity (11, 12). There is a lack of clinical data on the prevalence of RV morphologic and functional changes in patients with severe ARDS receiving VV-ECMO.
The aims of this study were first to compare RV morphologic and functional changes detected upon CT and TTE and second to describe the prevalence of ACP in patients with severe ARDS receiving VV-ECMO.
MATERIALS AND METHODS
We conducted an exploratory retrospective single-center cohort study of patients managed with VV-ECMO by the severe respiratory failure and ECMO service at Guy’s and St Thomas’ Hospital, London (United Kingdom), between January 2011 and March 2015, who had a standardized CT scan as well as a TTE after initiation of ECMO support. The study had institutional approval (institutional research governance reference number: 11145). The need for individual informed consent was waived, as this was a retrospective analysis of data collected prospectively for usual clinical care, with no breach of privacy or anonymity. The study qualified as a service evaluation as defined by the U.K. National Health Service Health Research Authority, and therefore did not require review by the Research Ethics Committee.
Our ECMO center serves a population of ~17 million. Criteria for VV-ECMO are a Murray’s Lung Injury Score greater than or equal to 3 (composite score of lung infiltration quadrants on chest x-ray, lung compliance, Pao2/Fio2, and positive end-expiratory pressure (PEEP) [13]), or a pH less than 7.20 due to hypercapnia refractory to conventional ventilation. Patients with chronic severe pulmonary hypertension, or progressive and nonrecoverable diseases (e.g., advanced cancer, heart diseases, and respiratory diseases) were not deemed suitable for ECMO support. Treatment decisions are made by senior intensivists with specialty training in ECMO. All the patients included in this analysis were commenced on VV-ECMO (Cardiohelp, Getinge, Rastatt, Germany) at the referring hospital (and subsequently transferred to our center). Our mobile ECMO retrieval process has been described previously (14, 15).
In the absence of evidence from clinical trials establishing the best ventilation strategy, all patients were ventilated with the same protocol regardless of the degree of lung recruitability, using pressure-controlled ventilation, with a PEEP of 10 cm H2O, a plateau pressure of 20 cm H2O, with a frequency of 10 breaths × min–1 for the first 24–48 hours, and, subsequently, according to the treating clinician.
Demographic data including comorbidities, respiratory variables, arterial blood gas analysis, and vasopressor support prior to initiation of extracorporeal support were extracted from electronic medical records (CareVue, Koninklijke Philips N.V., Amsterdam, Netherlands).
Transthoracic Echocardiography
TTE was performed with Vingmed system 5, 7, 9 (GE Medical, Milwaukee, WI) and Philips “Epiq 7” (Koninklijke Philips N.V.) cardiac ultrasound machines. All studies were performed by intensivists, cardiologists, or specialized cardiac physiologists trained in echocardiography. For the purposes of this study, we elected to use a basal RV end-diastolic diameter (RVd): left ventricular (LV) end-diastolic diameter (LVd) of greater than 0.9 to define RV dilatation. This threshold has been previously validated as an optimal cutoff for predicting mortality in acute pulmonary embolism (16). The assessment of RV systolic function was visually made by integrating radial contraction with tricuspid annulus long-axis excursion and graded in a binary fashion as being normal or impaired (visual right ventricular systolic impairment [vRVSI]). Where quantitative evaluation was available RV systolic impairment was defined as a reduction in the tricuspid annular plane systolic excursion (TAPSE) (< 17 mm) or peak systolic velocity of tricuspid annulus derived by pulsed-wave Doppler tissue imaging (S’ TDI < 9.5 cm/s) (17). ACP was defined as end-diastolic RV dilatation with paradoxical septal-movement (3).
CT Scan
All patients underwent a standardized CT protocol following ECMO cannulation. This CT protocol is a standard of care in our institution and consists of: a noncontrast brain; a volume-contrast chest, abdomen, and pelvis CT; and a “recruitment CT” performed at low (5 cm H2O) and high (45 cm H2O) airway pressure. This protocol has been previously described (18, 19). All CT scans were reviewed by an independent consultant radiologist.
CT scan measurements at baseline airway pressure were performed using Osirix Version 5.7 64-bit (Pixmeo SARL, Bernex, Switzerland)—a multidimensional image navigation and display software (provided under the GNU open-source licensing agreement) (20). The vascular structures (main pulmonary artery diameter, right and left pulmonary artery diameter, and ascending aorta diameter) were measured in an axial image using abdominal windowing for contrast enhancement (21). From the axial images, multiplanar reformatted reconstructions were made of the four-chamber view. This was achieved by placing the cross hairs over the mitral valve annulus and rotating the axis to the apex of the left ventricle. Once this had been done in the short-axis imaging plane, the cross hairs were rotated to the RV free wall to give simultaneous images of the two-chamber, short-axis, and four-chamber views. From the four-chamber view, the areas of the left ventricular cavity area (LVa) and right ventricular cavity area (RVa) were measured along with the internal dimension from the endocardium at the mid septum to the endocardium of the corresponding RV or LV free wall on the same image (LVd and RVd, respectively). The right-to-left ventricle diameter (RVd/LVd > 0.9 or > 1) or area (RVa/LVa > 0.9) ratio were used as a measure of RV dilatation. Given the lack of data on the best cutoff value for RVd/LVd ratio to predict RV systolic impairment, we explored 0.9 and 1 as potential thresholds against a visual assessment of RV function on TTE. Furthermore, lung volumes were measured in transversal view using lung filter, selecting region of interest, and merging aerated areas at different levels for low and high PEEP, respectively (22).
Statistical Analysis
The mean and sd were used for all normally distributed data and the median and interquartile range for nonparametrically distributed data. A chi-square test was used to evaluate the frequencies among categorical variables and the Mann-Whitney U test to compare independent measures of nonparametrically distributed variables. We used the Spearman rank correlation to explore the association between the TTE and CT findings. Furthermore, linear regression and the Bland-Altman analysis for assessing agreement between two methods of clinical measurements, a parametric approach based on exploration of variance, were employed to assess agreement in identifying ACP. The related results were expressed as bias and limits of agreement (23). Receiver-operating-characteristic (ROC) curves were constructed using logistic regression models for the CT RVa/LVa and the CT RVd/LVd ratio, respectively, to determine the cutoff value that yielded the largest discriminating power in terms of combined sensitivity and specificity for RV systolic impairment as well as ACP on TTE. A p value of less than 0.05 was considered to be statistically significant. Statistical analyses were performed using STATA v 16 (StataCorp LLC, College Station, TX).
RESULTS
A total of 183 consecutive patients with severe ARDS who were established on ECMO during the study period were screened for eligibility. Seventy-four patients were excluded as they had either lacking or incomplete CT imaging (i.e., only on low pressures) or did not undergo TTE. Consequently, 107 patients with both TTE and CT were included in the study. The demographic data, comorbidities, and clinical variables prior to initiation of VV-ECMO are presented in Table 1.
TABLE 1.
Baseline Demographic and Physiologic Data of Patients Subsequently Commenced on Venovenous Extracorporeal Membrane Oxygenation Support
| Baseline at Referral | Mean (sd) or n (%) |
|---|---|
| n | 107 |
| Age | 45.3 (13.7) |
| Sex (female) | 42 (39%) |
| Body mass index (kg/m2) | 29.4 (8.8) |
| Plateau pressure (cm H2O) | 33.6 (5.2) |
| Positive end-expiratory pressure (cm H2O) | 13.4 (3.7) |
| Tidal volume (mL) | 423 (115) |
| Compliance (mL/cm H2O) | 22.5 (9.7) |
| Fio2 | 0.94 (0.11) |
| Pao2 (mm Hg) | 65 (20) |
| Pao2/Fio2 ratio (mm Hg) | 70 (29) |
| Paco2 (mm Hg) | 73 (28) |
| Noradrenaline (µg/kg/min) | 0.41 (0.34) |
| pH | 7.17 (0.14) |
| Base excess (mEq/L) | –2.3 (8.5) |
| Lactate (mmol/L) | 3.2 (3.5) |
| Baseline at admission | |
| Lactate (mmol/L) | 4.4 (4.6) |
| C-reactive protein (mg/dL) | 229 (131) |
| Troponin (ng/L) (n = 47) | 333.7 (630.5) |
| Comorbidities | |
| Asthma/chronic obstructive pulmonary disease (n = 74) | 21 (29%) |
| Current smoker (n = 66) | 40 (61%) |
| Hypertension (n = 74) | 17 (23%) |
| Diabetes (n = 74) | 19 (26%) |
| Obesity (n = 74) | 39 (53%) |
| Ischemic heart disease (n = 74) | 3 (4%) |
| Etiology of acute respiratory failure (n = 106) | |
| Pulmonary ARDS | 93 (88%) |
| Pneumonia | 80 (75%) |
| Aspiration of gastric content | 5 (5%) |
| Other | 7 (7%) |
| Extrapulmonary ARDS | 13 (12%) |
| Nonpulmonary sepsis | 6 (6%) |
| Major trauma | 2 (2%) |
| Pancreatitis | 1 (1%) |
| Other | 5 (5%) |
ARDS = acute respiratory distress syndrome.
Variables are given as mean with sd or absolute numbers with percentage.
Right Ventricular Morphology and Function on TTE
The mean time to TTE after ECMO cannulation was 1.3 (±2.3) days (all CT scans were performed on the day of ECMO cannulation as per protocol). RV abnormalities were found in 50 of 107 patients (47%) using TTE. The findings were of RV dilatation and/or impairment of RV systolic function (i.e., vRVSI, RVd/LVd of > 0.9, TAPSE < 17 mm, and/or S’ TDI < 9.5 cm/s). In 54 patients who had quantitative measurements of RV size, nine (17%) had an RVd/LVd > 0.9. RV systolic function was visually impaired (vRVSI) in 33 of the 107 patients (31%). There was a significant difference in TAPSE between the patients who had and who had no RV impairment based on binary visual assessment (vRVSI) 1.6 cm (1.2–1.95 cm) versus 2 cm (1.75–2.4 cm) (median [95% CI]—p = 0.03). Patients with vRVSI had significantly lower S’ TDI: 0.09 (0.07–0.13) versus 0.14 (0.13–0.19) (median [95% CI]—p = 0.011). Of the 49 patients with quantitative data, seven (14%) satisfied the criteria of ACP (Table 2).
TABLE 2.
Prevalence of Right Ventricular Abnormalities on Transthoracic Echocardiography and CT
| Echocardiogram (TTE) | n (%) |
|---|---|
| TTE, any sign of RV dilatationa (n = 107) | 38 (36) |
| TTE, any sign of RV impairment (n = 107) | 50 (47) |
| TTE tricuspid annular plane systolic excursion < 17 mm (n = 35) | 13 (37) |
| TTE RVd/LVd > 0.9 (n = 54) | 9 (17) |
| TTE D-shapesystolic (n = 73) | 14 (19) |
| TTE tissue Doppler velocity tricuspid valve annulus < 9.5 (n = 30) | 10 (33) |
| TTE acute cor pulmonale (n = 49) | 7 (14) |
| TTE visual right ventricular systolic impairment (n = 107) | 33 (31) |
| CT | n (%) |
| CT RVd/LVd > 0.9 (n = 107) | 58 (54) |
| CT RVd/LVd > 1.0 (n = 107) | 36 (34) |
| CT RVa/LVa > 0.9 (n = 107) | 19 (18) |
| CT RVa/LVa > 1.0 (n = 107) | 9 (8) |
LVa = left ventricular area, LVd = left-ventricle end-diastolic diameter, RV = right ventricle, RVa = right ventricular area, RVd = right-ventricle end-diastolic diameter, TTE = transthoracic echocardiography.
aRV dilatation defined as an RVd > LVd upon visual assessment, or an RVd/LVd of > 0.9.
Right Ventricular Morphology on CT
Quantification of the RV and LV was possible in all 107 patients on CT (Table 2). Using a cutoff value of RVd/LVd > 0.9, RV dilatation was present in 58 patients (54%). This proportion decreased to 36 patients (34%), when a higher threshold of greater than 1 was used. When thresholds for cavity area measurements were used, RV dilatation was less frequent compared with using thresholds for diameter: 19% and 9% if using an RVa/LVa > 0.9 or RVa/LVa > 1, respectively. The additional measurements made from the CT scans are shown in the Supplemental Digital Content (http://links.lww.com/CCX/A507).
The Relationship Between Echocardiographic and CT Markers of RV Dysfunction
Linear regression curves for RVd/LVd on TTE, and RVa/LVa and RVd/LVd ratios on CT, respectively, were constructed (Figs. 1A and 2A). Bland-Altman analysis comparing RVd/LVd ratio on CT and TTE showed a bias of –0.13 and limits of agreement –0.35 to 0.22 (n = 52) (Fig. 1B). The ratio between the right and the left ventricle areas (RVa/LVa) measured on CT scan correlated with TTE-measured RVd/LVd ratio (R2 = 0.57; p < 0.01) and Bland-Altman analysis of these showed a bias of 0.0079 (limits of agreement 0.416 to 0.431) (n = 52) (Fig. 2B). A univariate logistic model (n = 107) showed an RVa/LVa ratio of 0.8260 as the best cutoff for the prediction of vRVSI on TTE, with an area under the ROC curve of 0.64. The odds ratio (OR) for predicting vRVSI from the CT RVa/LVa was 3.11 (95% CI, 0.62–5.60; p = 0.014). The given cutoff provided a sensitivity of 48.5% and a specificity of 75.7% (positive predictive value [PPV] 47.1%, negative predictive value [NPV] 76.7%). Furthermore, univariate logistic regression (n = 49) assessing the diagnostic value of the RVa/LVa ratio measured by CT to predict the diagnosis of ACP on TTE showed an RVa/LVa ratio of 0.8807 (subsequently rounded to 0.9) as the best cutoff with an area under the ROC curve of 0.78. The OR for predicting ACP on TTE from the CT RVa/LVa was 8.03 (95% CI, 2.19–13.87; p = 0.007). The given cutoff provided a sensitivity of 57.1% and a specificity of 90% (PPV 50.0%, NPV 92.7%). Subsequently, an RVa/LVa ratio of 0.9 was employed as a marker of ACP on CT in this population. Of the 107 patients, 19 (18%) exceeded this threshold and were attributed as having ACP on CT. Eleven of these 19 patients (58%) showed signs of RV impairment on TTE.
Figure 1.
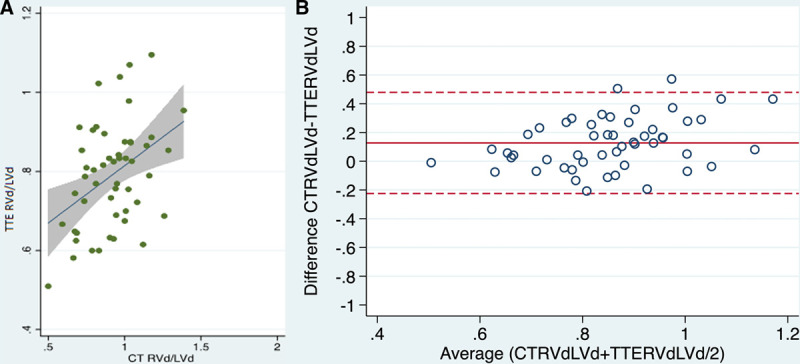
A, Linear regression and (B) Bland-Altman analysis comparing right-ventricle end-diastolic diameter (RVd)/left-ventricle end-diastolic diameter (LVd) ratio on CT and transthoracic echocardiography (TTE) (n = 52).
Figure 2.
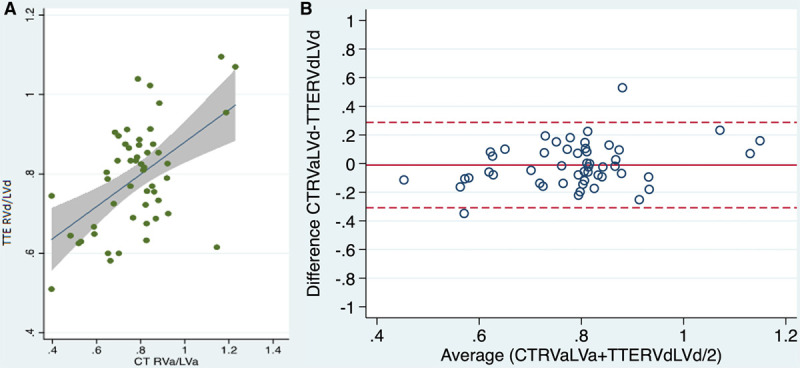
A, Linear regression and (B) Bland-Altman analysis comparing right ventricular area (RVa)/left ventricular area (LVa) ratio on CT and right-ventricle end-diastolic diameter (RVd)/left-ventricle end-diastolic diameter (LVd) ratio on transthoracic echocardiography (TTE) (n = 52).
DISCUSSION
The main findings of this study are as follows. First, RV morphologic and functional changes occur in up to 50% of patients with ARDS receiving VV-ECMO. Second, ACP occurs in up to 18% of patients with severe ARDS who receive VV-ECMO irrespective of the technique used. Third, quantitative TTE parameters are rarely acquired and cardiac chamber evaluation on CT may provide a useful alternative method for RV evaluation.
Prevalence of ACP (TTE and CT)
In our study, the prevalence of ACP in patients with severe ARDS receiving VV-ECMO was around 14% when defined by the RVd/LVd greater than 0.9 combined with the presence of D-shape on TTE, and it was around 18% when defined by a ventricular area ratio greater than 0.9 on 3D CT scan reconstruction. These two approaches showed a good agreement in this patient cohort and both seem valid to define the presence of ACP in patients with severe ARDS on VV-ECMO.
The prevalence of ACP in our population was 14–18% depending on the imaging modality used. Differences in the prevalence of ACP depend on the population studied as much as the definition criteria employed. For example, the prevalence of 14–18% in our cohort is slightly lower than the 22% reported by Mekontso Dessap et al (24) in a population of mechanically ventilated patients with moderate-severe ARDS. The other source of difference is that Mekontso Dessap et al (24) used transesophageal echocardiography to report ACP, which was defined as septal dyskinesia with an RVa/LVa > 0.6. The French Pulmonary Artery Catheter Study Group found a right ventricle failure prevalence of 9.6% in 145 patients with early ARDS when right ventricle failure was defined by the concomitant presence of mean pulmonary artery pressure greater than 25 mm Hg, central venous pressure higher than pulmonary artery occlusion pressure, and a stroke volume index less than 30 mL/m2 (25).
Although there are few data in ECMO patients, in a small single-center cohort study (n = 21), Lazzeri et al (26) showed a prevalence of ACP around 9% in patients with refractory ARDS just before ECMO treatment. Having found a lower prevalence of ACP when compared with conventionally managed ARDS patients, Lazzeri et al (26) hypothesized that there was not enough time for ACP to develop because of the early initiation of VV-ECMO. In our cohort, CT and TTE were performed after initiation of VV-ECMO when RV afterload is likely to be reduced by means of a synergy of reduction in ventilator pressure, and improvement of Po2, Pco2, and pH (2). We show that the prevalence of RV dysfunction using various parameters (vRVSI, or TAPSE, or RV TDI) is approximately double the prevalence of ACP. It is likely that either this reflects the persistence of RV systolic dysfunction while the signs of increased afterload had already reversed or the early reduction in afterload attenuated the development of ACP. A study on acute pulmonary embolism in 848 hemodynamically stable patients showed similar findings with 63% of patients having RV enlargement on CT and only 23% showing RV overload on TTE (27). In addition, similar to our study, CT imaging was obtained first, whereas TTE followed slightly delayed, hence, after initiation of therapy. It is possible that in addition to an actual difference in imaging modality, this discrepancy represents a resolution of right ventricle failure in a subpopulation of these patients.
Tricuspid Annular Plane Systolic Excursion
TAPSE has been extensively studied in critically ill patients (8). Fichet et al (28) compared TAPSE with S’ TDI in patients with ARDS; they found TAPSE (with a cutoff of 12 mm) may be more suitable. TAPSE was found to be an independent predictor of intraoperative cardiopulmonary resuscitation and death in patients undergoing emergent pulmonary embolectomy (29). A correlation between TAPSE and length of stay in ICU and postdischarge mortality was observed (30). We used a TAPSE of less than 17 mm to define RV dysfunction (17) and we found a reduction in TAPSE in 37% of patients. In a study focused on patients with ARDS before ECMO initiation, Lazzeri et al (31) found a higher proportion of patients with TAPSE less than 16 mm (47.6%).
Visual Right Ventricular Systolic Impairment
The assessment of RV systolic function can be made visually by grading it in a binary fashion as being either normal or impaired. Although vRVSI has been shown to be associated with outcome in the International Cooperative Pulmonary Embolism Registry (32), its value and accuracy have been challenged previously (33). However, a recent study in which 52 intensivists trained in echocardiography reviewed 80 TTE studies, found a fair agreement between visual assessment of RV function and TAPSE, S’ TDI, and RV fractional area change, respectively (34). Our study is in keeping with this as we found a significant difference of TAPSE and S’ TDI, respectively, between the patients who had and who had no RV impairment based on binary visual assessment (vRVSI). Finally, Park et al (35) found vRVSI to be in agreement with CT-derived RV measurements in patients with pulmonary embolism.
CT and TTE
CT is widely used in patients with suspected or confirmed ARDS to diagnose its etiology, assess extent and distribution of consolidations, pleural effusions or pneumothoraces, assess lung recruitability, and guide respiratory treatment (36). It would be desirable if information acquired on this scan could also be used to provide additional information on RV function that may be valuable in decision-making or to validate echocardiographic findings.
The use of CT imaging for an evaluation of RV size requires a standardized approach and the use of multiplanar formatted images to obtain the true dimensions of the LV and RV. We found a good correlation between CT RV/LV area ratio and the TTE RV/LV diameter ratio. Prior studies show good agreement between echocardiography and CT with a similar approach in patients with acute pulmonary embolism (10). The principle theoretical limitations with CT relate to the image acquisition and also the lack of any cardiac-cycle–specific data. The dimensions are measured in systole or diastole owing to the lack of cardiac gating, and hence, ratios of internal diameters and areas are preferred. These in themselves can be influenced by loading conditions and, hence, only serve as a potential surrogate to TTE. However, data from clinical practice show only minimal diagnostic yield of electrocardiogram-gating for evaluation of RV measurements (37).
Limitations
This study has the intrinsic limitations of a retrospective observation analysis. Furthermore, all our patients belonged to the severe class of ARDS and were selected by the fact of receiving VV-ECMO. Although this selection is fully justified by our specific interest to describe ACP in the VV-ECMO population, this may limit the comparability of our findings with studies describing RV function in the general ARDS population. TTE can be used to confidently assess the RV; however, windows may be difficult to obtain and suboptimal in some critically ill patients (8), and only in half of our studies, endocardial definition was sufficient to allow objective measurement of RV and LV diameters. Thus, we did also not attempt to obtain area measurements on TTE. An option would have been the use of transesophageal echocardiography in this subpopulation, although extrapolation of TTE derived normal values is subject to uncertainty (7). Coagulopathy and thrombocytopenia are considered relative contraindications to transesophageal echocardiography (38), but small cohorts show a reasonable safety profile in patients anticoagulated for ECMO (39). Furthermore, we compared the ability of two different techniques to identify ACP at two different time points with a mean time to TTE after CT of 1.3 (±2.3) days. Ventilatory settings remained unchanged during this time and there was no significant difference in vasopressor requirement. Despite allowing speculations on the dynamic change of RV function over time, the effect of such temporal uncoupling on the direct comparability between CT and TTE assessment is unknown. This study was not designed to evaluate the prognostic implications of ACP and its influencing factors; however, this is subject to ongoing work by our group.
CONCLUSIONS
Changes in RV size and function are common in patients with severe ARDS requiring VV-ECMO with up to 18% showing imaging evidence of ACP. A CT RVa/LVa > 0.9 is indicative of impaired RV systolic function.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 2.Fan E, Gattinoni L, Combes A, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory failure: A clinical review from an international group of experts. Intensive Care Med. 2016; 42:712–724 [DOI] [PubMed] [Google Scholar]
- 3.Jardin F, Dubourg O, Bourdarias JP. Echocardiographic pattern of acute cor pulmonale. Chest. 1997; 111:209–217 [DOI] [PubMed] [Google Scholar]
- 4.Repessé X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: Rationale for protecting the right ventricle. Chest. 2015; 147:259–265 [DOI] [PubMed] [Google Scholar]
- 5.Vieillard-Baron A, Prin S, Chergui K, et al. Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med. 2002; 166:1310–1319 [DOI] [PubMed] [Google Scholar]
- 6.Price LC, Wort SJ, Finney SJ, et al. Pulmonary vascular and right ventricular dysfunction in adult critical care: Current and emerging options for management: A systematic literature review. Crit Care. 2010; 14:R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts SM, Klick J, Fischl A, et al. A comparison of transesophageal to transthoracic echocardiographic measures of right ventricular function. J Cardiothorac Vasc Anesth. 2020; 34:1252–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SJ, Nalos M, Smith L, et al. The use of echocardiographic indices in defining and assessing right ventricular systolic function in critical care research. Intensive Care Med. 2018; 44:868–883 [DOI] [PubMed] [Google Scholar]
- 9.Kang DK, Thilo C, Schoepf UJ, et al. CT signs of right ventricular dysfunction: Prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011; 4:841–849 [DOI] [PubMed] [Google Scholar]
- 10.Mansencal N, Joseph T, Vieillard-Baron A, et al. Diagnosis of right ventricular dysfunction in acute pulmonary embolism using helical computed tomography. Am J Cardiol. 2005; 95:1260–1263 [DOI] [PubMed] [Google Scholar]
- 11.Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013; 39:1725–1733 [DOI] [PubMed] [Google Scholar]
- 12.Vieillard-Baron A, Charron C, Caille V, et al. Prone positioning unloads the right ventricle in severe ARDS. Chest. 2007; 132:1440–1446 [DOI] [PubMed] [Google Scholar]
- 13.Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988; 138:720–723 [DOI] [PubMed] [Google Scholar]
- 14.Burns J, Cooper E, Salt G, et al. Retrospective observational review of percutaneous cannulation for extracorporeal membrane oxygenation. ASAIO J. 2016; 62:325–328 [DOI] [PubMed] [Google Scholar]
- 15.Sherren PB, Shepherd SJ, Glover GW, et al. Capabilities of a mobile extracorporeal membrane oxygenation service for severe respiratory failure delivered by intensive care specialists. Anaesthesia. 2015; 70:707–714 [DOI] [PubMed] [Google Scholar]
- 16.Frémont B, Pacouret G, Jacobi D, et al. Prognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: Results from a monocenter registry of 1,416 patients. Chest. 2008; 133:358–362 [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16:233–270 [DOI] [PubMed] [Google Scholar]
- 18.Camporota L, Caricola EV, Bartolomeo N, et al. Lung recruitability in severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation. Crit Care Med. 2019; 47:1177–1183 [DOI] [PubMed] [Google Scholar]
- 19.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006; 354:1775–1786 [DOI] [PubMed] [Google Scholar]
- 20.Rosset A, Spadola L, Ratib O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004; 17:205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumsden AB, Karmonik C, Smolock CJ, et al. Advanced aortic imaging: Future directions. Methodist Debakey Cardiovasc J. 2011; 7:28–31 [DOI] [PubMed] [Google Scholar]
- 22.Meinel FG, Nance JW, Jr, Schoepf UJ, et al. Predictive value of computed tomography in acute pulmonary embolism: Systematic review and meta-analysis. Am J Med. 2015; 128:747–759.e2 [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310 [PubMed] [Google Scholar]
- 24.Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: Prevalence, predictors, and clinical impact. Intensive Care Med. 2016; 42:862–870 [DOI] [PubMed] [Google Scholar]
- 25.Osman D, Monnet X, Castelain V, et al. ; French Pulmonary Artery Catheter Study Group. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009; 35:69–76 [DOI] [PubMed] [Google Scholar]
- 26.Lazzeri C, Cianchi G, Bonizzoli M, et al. Pulmonary vascular dysfunction in refractory acute respiratory distress syndrome before veno-venous extracorporeal membrane oxygenation. Acta Anaesthesiol Scand. 2016; 60:485–491 [DOI] [PubMed] [Google Scholar]
- 27.Barrios D, Morillo R, Lobo JL, et al. ; PROTECT Investigators. Assessment of right ventricular function in acute pulmonary embolism. Am Heart J. 2017; 185:123–129 [DOI] [PubMed] [Google Scholar]
- 28.Fichet J, Moreau L, Genée O, et al. Feasibility of right ventricular longitudinal systolic function evaluation with transthoracic echocardiographic indices derived from tricuspid annular motion: A preliminary study in acute respiratory distress syndrome. Echocardiography. 2012; 29:513–521 [DOI] [PubMed] [Google Scholar]
- 29.Schmid E, Hilberath JN, Blumenstock G, et al. Tricuspid annular plane systolic excursion (TAPSE) predicts poor outcome in patients undergoing acute pulmonary embolectomy. Heart Lung Vessel. 2015; 7:151–158 [PMC free article] [PubMed] [Google Scholar]
- 30.Gajanana D, Seetha Rammohan H, Alli O, et al. Tricuspid annular plane systolic excursion and its association with mortality in critically ill patients. Echocardiography. 2015; 32:1222–1227 [DOI] [PubMed] [Google Scholar]
- 31.Lazzeri C, Cianchi G, Bonizzoli M, et al. Right ventricle dilation as a prognostic factor in refractory acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation. Minerva Anestesiol. 2016; 82:1043–1049 [PubMed] [Google Scholar]
- 32.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999; 353:1386–1389 [DOI] [PubMed] [Google Scholar]
- 33.Ling LF, Obuchowski NA, Rodriguez L, et al. Accuracy and interobserver concordance of echocardiographic assessment of right ventricular size and systolic function: A quality control exercise. J Am Soc Echocardiogr. 2012; 25:709–713 [DOI] [PubMed] [Google Scholar]
- 34.Orde S, Slama M, Yastrebov K, et al. ; College of Intensive Care Medicine of Australia and New Zealand [CICM] Ultrasound Special Interest Group [USIG]. Subjective right ventricle assessment by echo qualified intensive care specialists: Assessing agreement with objective measures. Crit Care. 2019; 23:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JR, Chang SA, Jang SY, et al. Evaluation of right ventricular dysfunction and prediction of clinical outcomes in acute pulmonary embolism by chest computed tomography: Comparisons with echocardiography. Int J Cardiovasc Imaging. 2012; 28:979–987 [DOI] [PubMed] [Google Scholar]
- 36.Pesenti A, Musch G, Lichtenstein D, et al. Imaging in acute respiratory distress syndrome. Intensive Care Med. 2016; 42:686–698 [DOI] [PubMed] [Google Scholar]
- 37.Lu MT, Cai T, Ersoy H, et al. Comparison of ECG-gated versus non-gated CT ventricular measurements in thirty patients with acute pulmonary embolism. Int J Cardiovasc Imaging. 2009; 25:101–107 [DOI] [PubMed] [Google Scholar]
- 38.Hilberath JN, Oakes DA, Shernan SK, et al. Safety of transesophageal echocardiography. J Am Soc Echocardiogr. 2010; 23:1115–1127; quiz 1220–1221 [DOI] [PubMed] [Google Scholar]
- 39.Nowak-Machen M, Schmid E, Schlensak C, et al. Safety of transesophageal echocardiography during extracorporeal life support. Perfusion. 2016; 31:634–639 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


