Abstract
Sodium-glucose co-transporter 2 Inhibitors (SGLT2i) were initially developed as therapeutic options for patients with type 2 diabetes mellitus (T2DM). Recently, randomized clinical trials have investigated their effects in cardiorenal protection through major adverse cardiovascular event reduction and reductions in diabetic nephropathy. While multiple mechanisms are proposed for this protection, microvascular protection is the primary component of their efficacy. While not primarily emphasized in clinical trials, evidence in other studies suggests that SGLT2i may confer retinoprotective effects via some of the same mechanisms in the aforementioned cardiorenal trials. Diabetic patients are susceptible to vision loss with chronic hyperglycemia promoting inflammation, edema, and retinal pathological changes. Targeting these pathways via SGLT2i may represent opportunities for providers to decrease retinopathy in high-risk T2DM patients, reduce disease progression, and lower drug burden in diabetic retinopathy patients. Further comprehensive clinical trials investigating these associations are needed to establish the potential retinoprotective effects of SGLT2i.
Keywords: diabetes, diabetic, diabetology, microvascular, ophthalmology, retina, retinopathy, sodium-glucose co-transporter 2 inhibitors, translational biology, vision loss
Introduction
Dysregulation of the endocrine system manifests as a disruption of normal physiological function with multifactorial clinical consequences. One prominent example that has garnered interest among public health officials, biomedical researchers, and healthcare providers alike is the pathologic relationship between type 2 diabetes mellitus (T2DM) and retinopathy due to neurovascular damage of the retina secondary to hyperglycemia. Multiple surveys of patients with T2DM report a fear of complications of the eye secondary to T2DM, as well as a decreased quality of life, determined by psychosocial metrics in those with T2DM and already compromised vision [1–3]. The American Diabetes Association cites diabetic retinopathy as the most common causes of new blindness in adults aged 20–74 years [4].
Moreover, with the increase in the number of worldwide patients with T2DM, it is estimated that the total number of individuals with diabetic retinopathy will increase to 191 million by the year 2030 [5]. Modifiable risk factors of diabetic retinopathy include hyperlipidemia, hypertension, obesity, and hyperglycemia [4]. The link between reductions in HbA1c and subsequent reductions in the risk of developing diabetic retinopathy was established by the UK Prospective Diabetes Study (UKPDS) group, with the supporting literature illustrating tight glucose control confers benefits to vision [6–8]. These benefits are summarized in Fig. 1. This evidence supports the clinical practice of controlling diabetic retinopathy via the management of underlying diabetes [9]. The management of T2DM with pharmacotherapy is a clinical decision-making process that involves appropriate selection from multiple agents, a balance of clinical prudence for the severity of T2DM, factoring drug metabolism and tolerance, and considerations of concomitant health disorders that may influence pharmacotherapy implementation [10–12].
Fig. 1.
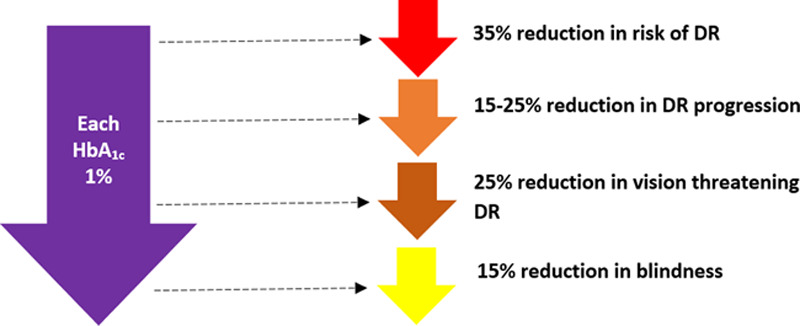
Improvements in glucose control, as measured by decreased HbA1c, confer benefits of interest related to the development of diabetic retinopathy.
Among therapeutic options, sodium glucose co-transporter 2 Inhibitors (SGLT2i), are a class of drugs that have received recent interest in the management of T2DM and reductions in serum glucose. The main SGLT2 inhibitors used in the United States comprise dapagliflozin, empagliflozin, and canagliflozin [13]. Moreover, studies involving SGLT2 inhibitors have revealed that they serve other therapeutic roles including the amelioration of hypertension, weight loss via glycosuria (with an average loss of 3.6–4.5 kg within the first two years of therapy initiation), cardiorenal protective properties, and limited hypoglycemia [14–16]. Noteworthy side effects of these medications include a possibility of mycotic genital infection, polyuria, polyphagia, a decrease in bone mineral density, and euglycemic diabetic ketoacidosis [17–24]. There is evidence that suggests the benefits of glucose reduction may also potentially benefit the current or prospective acquisition of diabetic retinopathy. However, a comprehensive survey of this landscape is scant in the literature. In this review article, we seek to synthesize current developments in SGLT2i therapeutics that draw from clinical trials, case reports, molecular studies, and translational biology to report a multidisciplinary commentary on the role SGLT2i has in the prevention and treatment of diabetic retinopathy.
Basic science and the diabetic retina
The Centers for Disease Control and Prevention (CDC) monitors eye disease stemming for T2DM, with T2DM implicated as the most frequent cause vision impairment in the United States [25,26]. Currently, it is reported by the CDC that nearly eight million Americans have diabetic retinopathy, and that this number will nearly double to almost 15 million by the year 2050 [27–29]. Diabetic retinopathy can be classified into two separate categories, nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). NPDR is characterized earlier in the disease course, and it is characterized by weakened blood vessels and occlusions due to high glycemic load. PDR is a more advanced form of diabetic retinopathy. The hypoxic state of the retina drives the formation of fragile blood vessels and retinal detachment through changes in cellular genetic response [30]. During both stages of NPDR and PDR, it is possible to develop diabetic macular edema (DME), which is a pathological collection of fluid in the macula due to the breakdown of the blood–retinal barrier [31]. DME is a feared complication due to the deterioration of visual acuity.
The main modality of assessing macular edema is through optical coherence tomography (OCT) a noninvasive imaging modality that uses light to capture retinal cross sections, which are then aggregated to form two-dimension and three-dimensional models of the retina. This tool is useful in the diagnosis and monitoring of treatment response using anatomical features to supplement the clinical picture of a patient.
Current landscape
Current treatments for NPDR and PDR include intravitreal injections of antivascular endothelial growth factor (VEGF) agents, namely bevacizumab, ranibizumab, aflibercept and newly U.S Food and Drug Administration approved brolucizumab. PDR is also typically treated with pan-retinal photocoagulation that destroys regions of hypoxic retina, which decreases the physiological drive to stimulate the production of frail vasculature. If fragile vessels are already present, then they can bleed and accumulate blood in the vitreous. This blood and vitreous can be removed surgically via vitrectomy.
The anti-VEGF era has dramatically improved outcomes in patients with PDR. However, some of the drawbacks of their usage include cost, frequent injection scheduling, risk of vision threatening complications like endophthalmitis, and possible systemic side effects of concern to patients with T2DM that have been reported in cases, such as cerebrovascular accidents, increased proteinuria, exacerbation of hypertension, and possible worsening of glomerular disease [32–38]. In addition, anti-VEGF therapy does not always provide resolution to DME likely due to other pathways of diabetic retinopathy aside from solely VEGF production [39,40]. Currently, there is a paucity in diverse pharmacologic therapeutics that confer protection from the acquisition of diabetic retinopathy in patients with T2DM. Moreover, established therapeutic options do not provide the patient with T2DM much avenues for the amelioration of underlying risk factors commonly seen in these patients, such as chronic kidney disease, cardiovascular disease, and endocrine dysfunction. Therapeutic management of these pathways via SGLT2 inhibition may present an alternative mechanism to prevent retinopathy in high-risk T2DM patients, and in patients with NPDR and DME, an opportunity to reduce disease progression. Finally, total drug burden in those already diagnosed with diabetic retinopathy may be reduced as well. Systemic therapies such as SGLT2i give a potential benefit that extends beyond the retina as outlined by cardiorenal trials that have demonstrated decreased deaths and hospitalizations for major adverse cardiovascular events as well as chronic kidney disease [41–43]. A review of the literature was performed with a focus on the function and pharmacologic application of SGLT2i, with the goal of explaining how the mechanism of action of these drugs conveys empirical clinical effects.
The potential role of sodium-glucose co-transporter 2 therapy
SGLT2i represses renal tubular reabsorption of glucose by inhibiting the SGLT2 protein, a protein expressed on the renal epithelial cells lining the proximal convoluted tubule. This protein serves as a sodium-glucose co-transporter, and it reabsorbs glucose from the glomerular filtrate. This glucose returns to the systemic circulation at a propensity of up to 90% of total renally reabsorbed glucose [44–46]. A reduction in SGLT2 expression promotes increased glucosuria, decreased sodium reabsorption, increased sodium and water excretion, and a reduction in blood pressure. These parameters reduce the burden of the cardiovascular system, with implications in the retina [47]. Notably, systemic hypertension has the propensity to damage microvasculature of the retina. The physiology of SGLT2 receptors in human retinal pericytes is not completely understood. One of the first articles to propose the role of SGLT2 inhibitors in the retinas of cultured bovine retinal pericytes was by Wakisaka et al. [48]. They proposed that a Na+-Ca2+ exchanger that modulates intracellular calcium concentrations partially play a role in the physiology of SGLT2. The retinal pericytes also have an SGLT2 receptor that modulates sodium and glucose entry into the cell. When extracellular glucose concentrations increase, the SGLT2 receptor becomes active, causing an influx of glucose and sodium. An overabundance of sodium in the cell causes a breakdown in the receptors and leads to increased cell swelling, cell contraction, and pericyte loss (Fig. 2) [49]. To relay the significance of this pharmacological phenomenon, a narration of the molecular biology pathogenesis of diabetic retinopathy should be addressed.
Fig. 2.
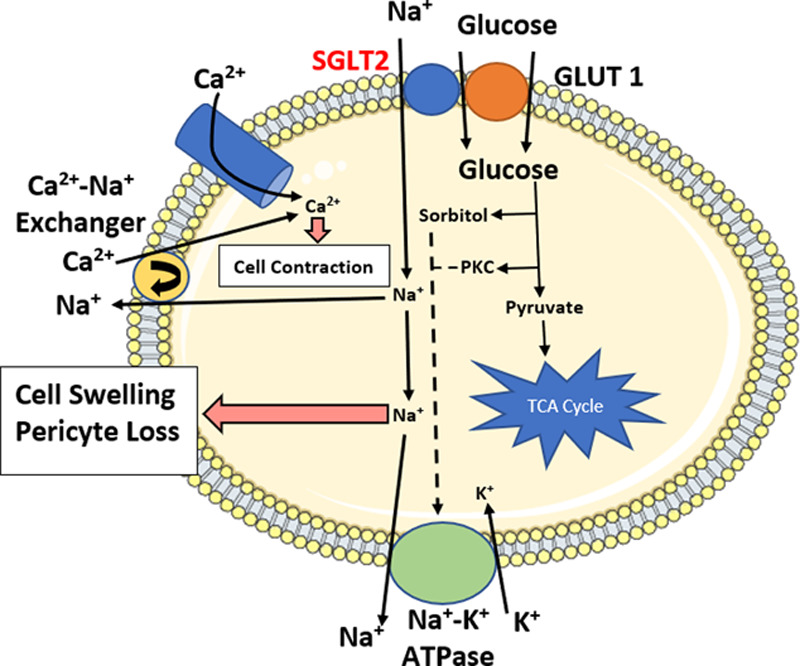
A review of the intracellular dynamics in the retinal cell of the hyperglycemic patient with implications for SGLT2 inhibition. Basic lipid bilayer template adapted from Servier Medical Art by Servier, which is licensed under a Creative Commons Attribution 3.0 Unported License. Physiological dynamics and artwork created by authors with adaptations from Wakisaka et al. [50,51]. SGLT2, sodium-glucose co-transporter 2.
Diabetic retinopathy: from molecules to pathology
Long-standing hyperglycemia is the fundamental etiology in the pathogenesis of diabetic retinopathy. There are a multitude of mechanisms reported delineating the loss of vision seen in patients with T2DM. The main theories that explain compromised sight include perturbed retinal blood flow autoregulatory mechanisms, increased sorbitol in retinal cells with resultant osmotic effects, advanced glycosylation end products formation, and growth-factor production [52–55]. Uncontrolled hyperglycemia can lead to inflammation of hypoxic origin leading to tissue dysfunction [56,57]. Advanced glycation-end products (AGEs) form from long-standing hyperglycemia. Insulin resistance, as seen in patients with T2DM, mitigates the trafficking of peripheral glucose and exacerbates this problem by increasing the exposure time to hyperglycemia. Eventually, AGEs promote a cellular phenotype that encourages flow of glucose and proteins into the basement membrane. This leakage then hyalinizes and thickens the tissue–vasculature barrier, specifically in endoneurial microvessels [58]. By Fick’s law, the increased cross cellular thickness as a result of this hyalinization will decrease oxygen flux to the tissue, especially those related to ocular function, and serve as an interface that mitigates the utilization of oxygen for metabolic purposes. Hyperglycemia is also associated with qualitative red blood cell defects, as is shown by scanning electron studies that reveal glycosylated hemoglobin in patients with T2DM, resulting in impaired oxygen transfer. Multiple studies have proven the value of assessing hematologic features in assessing the severity of diabetic retinopathy [59–61].
Autoregulation and hemodynamics
As mentioned previously in our discussion of the basic science outlining diabetic retinopathy, the loss of autoregulation also results in the disruption of locally produced factors that maintain and regulate homeostatic blood flow. It is widely believed that multiple indicators implicate the loss of neurovascular function on the subclinical level which play as an integral role in this loss of autoregulation. This is supported by studies examining neuroretinal layer thickness, electroretinography studies for nonvisible neurovascular changes in the retina, and other methods such as the evaluation of protein expression on neurovasculature [62]. From a histopathological perspective, this may be appreciated by the presence of glial cell hyperplasia, indicating chronic neurological damage, as well as neuronal apoptosis [63]. Retinal glial cells are highly dependent upon oxidative phosphorylation and, therefore, require many mitochondria to meet the metabolic demand. Hyperglycemia-induced mitochondrial damage therefore renders the retinal glial cells susceptible to damage in T2DM, and thereby represents the bulk of neuronal deficit seen in diabetic retinopathy [64]. More evidence for the role of neural involvement can be appreciated by studies that show the neural architecture of the retina is highly embedded within microvasculature and losses in contrast vision precede vision loss in diabetic retinopathy [65,66]. The mechanism of contrast delineation is highly neurologic in nature.
As a result of this loss of autoregulation, the retinal blood flow is increased with an accompanying rise in shear stress on retinal microvasculature, reactive oxygen species (ROS) synthesis from endothelial cells due to damage from mass effect, inflammatory modulation, and hyperglycemia in the retinal mitochondria [67]. Inflammation stemming from the influx of ROS activates cytokine release, which plays a role in promoting vascular pliability, namely through inducing a vascular environment that is conducive to the extravasation of lymphocytes. This same vascular pliability is implicated in the edema that results in vision compromise [68]. The accumulation of ROS has been shown to influence the epigenetic regulation of mitochondrial protein expression, with pathological changes noted in the mitochondria that resemble those seen in tissue undergoing prolonged anaerobic metabolism and chronic inflammation [69]. For example, mitofusin-2 plays a role in configuring the architecture of the mitochondria by establishing connections within cellular endoplasmic reticula membranes and the mitochondria itself, playing a vital role in keeping the integrity of insulin signaling pathways and thus maintaining the balance of anabolic and catabolic pathways in the mitochondria [70]. This theme of shifting in the metabolic economy of the mitochondria seen in retinopathy secondary to hyperglycemia culminates in hypoxia and ATP depletion from dysfunctional mitochondria. Early in diabetic retinopathy, this can be appreciated as retinal capillary microangiopathy in the form of microaneurysms and ‘cotton wool spots’, which are believed to be histopathologic indicators of hypoxia from obstruction of the microvasculature and lack of oxygen-rich perfusion [71]. Later, this hypoxia serves as a stimulus for the production of growth factors, with VEGF being one of the more prominent growth factors. One of the primary stimuli for VEGF production is hypoxia-inducible growth factor 1 (HIF-1), which is supported by the evidence that increased levels of HIF-1 DNA binding both in space and time in the ischemic retina is associated with a rise in VEGF transcription [72,73].
VEGF serves as a signaling molecule that binds to tyrosine kinase receptors. It promotes neovascularization through changes in gene expression as a mechanism to create new blood vessels in an attempt to provide new avenues to supply oxygen flux to counteract hypoxia. However, these vessels are fragile, thin, and not capable of adequate retinal tissue perfusion. These vessels lack proper structural integrity and exhibit the propensity to leak fluid. This fluid accumulates and results in macular edema, as a direct consequence of neovascularization [74,75]. Potential pathologic sequelae of neovascularization include tractional retinal detachment, rubeosis, and intraocular pressure elevation potentially resulting in glaucoma and acute vision loss [76].
In a manner similar to increased retinal blood flow, an influx of volume flow from the resultant fluid accumulation and macular edema in a restricted compartment such as the retina promotes further shear stress. This shear stress induces vascular adaptations via subsequent prostacyclin and nitric oxide synthesis as a result of pressure-induced gene expression changes of prostacyclin synthase and endothelial nitric oxide synthase, respectively [77]. These factors serve as vasodilators, and this retinal vessel vasodilation results in further blood flow influx and shear stress, forming a positive feed-back loop of damage [52].
Diabetic retinopathy and metabolism
The role of sorbitol in the formation of diabetic retinopathy is a topic of current debate. One primary belief is centered around glucose, which is intracellularly converted to sorbitol through aldose reductase, an enzyme expressed in the retinal epithelium. Sorbitol may then undergo conversion to soluble fructose via sorbitol dehydrogenase (SDH) using NAD+ as a cofactor. Certain cells, such as those found in the retina, lack adequate SDH expression, placing them at risk from osmotic damage and subsequent cell necrosis secondary to sorbitol accumulation in hyperglycemic patients [78–81]. This enzymatic economy of the retina and the manifestations it has on the pathology of diabetic retinopathy can be further appreciated when this model is applied as noted in observational population studies. One study, which investigated 3000 Han Chinese individuals with SDH polymorphisms, noted that certain polymorphisms were associated with statistically significant differential risks of diabetic retinopathy [22,82]. Another study, which investigated retinal glucose metabolism from the perspective of aldose reductase polymorphisms in an Indian population of 926 subjects, reported that certain alleles are associated with diabetic retinopathy when compared to wild genotypes of aldose reductase of T2DM controls [83]. Furthermore, polymorphisms of alleles affecting the promotor region of the aldose reductase gene have been investigated with reported associations with diabetic retinopathy in Japanese patients with T2DM, giving further evidence that the polyol pathway can be validated beyond biochemistry and has clinical context [84].
The effects of osmotic damage secondary to sorbitol accumulation are summarized in Fig. 3.
Fig. 3.
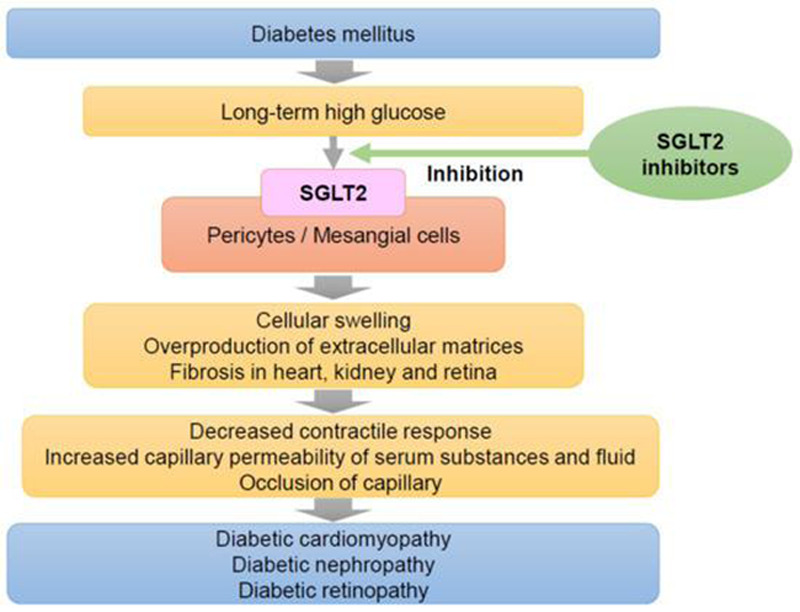
Effects of prolonged hyperglycemia on systemic physiology with an emphasis on ocular manifestations. This is an open access article distributed under the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited [48,50].
AGE products that bind to proteins and lipids in the basement membrane of retinal cells can disrupt cellular architecture, affecting adequate trafficking of cellular products and the ability to maintain homeostasis. In addition, the conversion of sorbitol through aldose reductase is a process that consumes NADPH. This depletion of NADPH is proposed to exacerbate oxidative injury in retinal cells, and the increased level of the intracellular fluid from osmotic damage may alter normal metabolic cellular pathways, promoting cellular swelling, injury, and cell death [85–87]. The sequence of pathologic hallmarks seen in diabetic retinopathy is theorized to occur throughout the stages of disease progression. However, more credence is being given to the notion that each of the topics discussed (metabolism, neovascularization, loss of autoregulation, etc.) exhibits a more robust impact in different stages of the disease, potentially giving an avenue for disease staging and targeting [88].
Translational biology and preliminary evidence – bridging sodium-glucose co-transporter 2 and the retina
Animal models of retinal physiology in the context of metabolic dysregulation represented some of the earliest reported findings regarding the ocular expression of SGLT2. Of interest was the discovery of SGLT2 expression in the retinal pericytes of bovine origin, which supported the notion that SGLT2 plays a role in retinal physiology [49,50]. Early studies also show a statistically significant increase in SGLT2 mRNA levels in the retina of diabetic mice based on quantitative reverse transcription PCR (qT-PCR) [89]. Unfortunately, formal histological studies assessing the expression of SGLT2 directly in human retinas have not been widely reported in the literature.
Because of their role in promoting glucose entry, excessive glucose entry as a result of increased SGLT2 expression represents both another example in the pathogenesis of diabetic retinopathy as well as a potential therapeutic target. The same experiment by Leley et al. [89] that assessed SGLT2 mRNA levels in the retina of diabetic mice also investigated the application of an SLGT2 inhibitor, dapagliflozin, on human retinal endothelial cells (HRECs). They used 0.1, 1, and 10 nM formulations with a prospective glucose uptake assay in hopes of determining the resultant glycemic influx on mRNA expression of SGLT2 [89]. The application of HRECs with 10 nM dapagliflozin was marked by a statistically significant decrease in SGLT2 mRNA expression (P < 0.05), with dapagliflozin treatment demonstrating a reduction in glucose uptake within HRECs at all concentrations of formulations. There is also an impact by inflammatory mediators on the retina. A study by Taher et al. [90] reviewed the effect of dapagliflozin in the reduction of diabetic retinopathy and specifically the impact on mediators such as IL-6 and H2O2 on mice models controls (n = 10), with diabetes mellitus (n = 10), and diabetes mellitus with dapagliflozin treatment (n = 10). Results showed a statistically significant decrease in the serum IL-6 (P < 0.05), a marker of inflammation, in diabetes mellitus treated with dapagliflozin in comparison to only diabetes mellitus mice. Retinal H2O2 was also reviewed and was shown to exhibit a statistically significant increase (P < 0.05) in mice with untreated T2DM and T2DM treated with dapagliflozin in comparison to the normal control group. However, there was a statistically significant (P < 0.05) decrease in retinal H2O2 in the mice with T2DM treated with dapagliflozin in comparison to mice with untreated T2DM. Additionally, the histological comparison showed that dapagliflozin decreased effusion of the retinal capillaries and decreased structural integrity loss to the arterioles, which often leads to diabetic retinopathy. Thus, this study demonstrated that dapagliflozin treated diabetes mellitus led to a smaller increase oxidative stress (via H2O2 as a marker) and less inflammation (via IL-6 levels as a marker) in comparison to the diabetes mellitus untreated group [90,91].
A case report by Yoshizumi et al. [92] demonstrated that a woman with T2DM and DME was successfully treated with SGLT2 inhibitor, ipragliflozin (25 mg/day) after being refractory to a dipeptidyl peptidase-4 inhibitor, sitagliptin (50 mg daily), and metformin (250 mg daily), and corticosteroids. They reported an improvement in her visual acuity within 4 weeks as well as amelioration of DME was noted in 16 weeks with use of the ipragliflozin (25 mg/day) [92]. This study lends support that SGLT2 inhibitors improved DME in an avenue that extends beyond chronic glycemic control.
This postulated that the secondary effect of SGLT2 inhibition on VEGF production is supported by previous experiments in which canagliflozin was shown to mitigate aortic endothelial cell proliferation via changes in gene expression on male C57BL/6 mice in a dose-dependent manner [93]. A similar phenomenon with canagliflozin was noted in human aortic endothelial cells. Furthermore, it was elucidated that canagliflozin arrested endothelial cell proliferation specifically in the G0/G1 phase of the cell cycle, supported by statistically significant distributions of cells treated with SGLT2 versus control and phosphorylation of cell cycle progression proteins [93,94]. This association points to credence of the proposed mechanism mentioned earlier that phlorizin decreased the contraction and dysfunction of pericytes and mesangial cells in the retina which are under high stress from extracellular glucose concentrations. VEGF modulation has also been implicated as a potential pathway of mitigation of renal fibrosis when SGLT2 was administered with the intent of renal capillaries [95]. The overall effect of SGLT2i on the full spectrum of VEGF pathways in multiple physiological settings within the human body is something that still needs to be investigated further. This represents a potential limitation in the integration of SGLT2i requiring further clinical studies that address the implications of retinal capillary physiology directly.
An experiment conducted by Maekawa et al. [96] used the spontaneously diabetic torii (SDT), which is a fatty rat T2D model, to assess the effect of Phlorizin on diabetic retinopathy. After the use of 100–150 mg/kg/daily, decreased intraepidermal nerve fiber density and decreased histopathological markers of mitochondrial damage and inflammation were noted, supporting the notion that SGLT2 therapy played a role in improving the physiological outcomes related to the loss of neural autoregulation and inflammasome function in the hyperglycemic rat [96]. A separate study also using ipragliflozin on SDT rats reported that SGLT2 therapy prevented oscillation peaks in rats assessed with electroretinogram, implying a reduction in the deterioration of motor nerve conduction velocity and thus loss of neural regulatory mechanisms in the retina [97]. The latter shows that SGLT2 applications may have physiological consequences related to retinal physiology that can be measured empirically with metrics that may be quantified, offering an avenue to formally study the effects of SGLT2 inhibitors on the neural component in the pathophysiology of diabetic retinopathy.
Clinical evidence
While empirical and experimental evidence showing SGLT2 inhibition and improvements in retinopathy in smaller cohorts was presented earlier, newer evidence supporting differential SGLT2 functionality that has implications in the prevalence of diabetic retinopathy in humans on a genetic level was noted as recently as 2019 by Klen et al. [98]. It was shown that individuals with polymorphisms in the SLC5A2 gene, the gene that codes for the SGLT2 protein, exhibited not only impaired glycemic control but even statistically significant differential microvascular changes such as an increased risk for retinopathy when controlled for duration of T2DM [odds ratio (OR) = 7.62, 95% confidence interval (CI) = 1.68–35.28, P = 0.009] [98,99]. The same authors report that these alleles of SLC5A2 are associated with increased fasting blood glucose levels. It can be implied that long-term outcomes of this physiological status may manifest similarly to prolonged hyperglycemia. The significance of the study by Klen et al. [98] shows that functionality in SGLT2 expression on the genetic level has implications in diabetic retinopathy. This mechanism raises interest into the notion that inhibition via SGLT2 may confer the same beneficial clinical outcomes in diabetic retinopathy as was seen in multiple recent cardiorenal trials using SGLT2i [14,17,99,100].
Exploratory analyses by Dziuba et al. [101] used the Archimedes model to predict the 20-year cardiovascular and microvascular events in patients with T2DM. They found that patients taking dapagliflozin could expect to have a relative decrease of 9.8% of incidence of diabetic retinopathy compared to standard of care, and dapagliflozin had a number needed to treat of only 15 patients to avoid an outcome similar to the standard of care of patients with uncontrolled T2DM [101]. Moreover, a network meta-analysis by Tang et al. [102] found that SGLT2i were not associated with a greater risk of diabetic retinopathy when compared to a placebo (OR = 0.79), and they had the smallest likelihood of complications from diabetic retinopathy. The authors support the idea that there are many mechanisms that may benefit patients with diabetic retinopathy, and endorse the notion that further research to elucidate the parameters of beneficial outcomes in patients with T2DM and diabetic retinopathy should be conducted [102].
As mentioned previously, current therapeutic options for those with diabetic retinopathy and DME include anti-VEGF agents. The Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis study is an example of a study illustrating the benefits of such agents. Ranibizumab, a mAb with antiangiogenic effects through VEGF receptor inhibition, was initiated in this trial [103]. The rationale of this therapeutic option to target diabetic retinopathy was centered on repression of angiogenesis, resulting in a decrease in retinal blood flow, and an ensuing reprieve of the retina from damage secondary to the mass effect of volume flow [104]. A reduction in central retinal thickness (CRT) by 61.3 μm measured via OCTwas observed at a 12-month assessment time point following therapy. Given the established role of SGLT2 inhibition has on microvascular changes and even possible VEGF pharmacology, the potential of these drugs in improving vision in patients with DME and T2DM is promising, and small-scale studies using OCT to assess ocular changes and CRT in a similar manner have recently been conducted [105].
SGLT2 administration on five patients by Mieno et al. [105] with concurrent visual acuity testing and measurement of CRT from baseline at 3, 6, and 12 months was one of the first studies to use this method of associating SGLT2i therapy with anatomical changes pertinent to diabetic retinopathy and DME. The results of this small-scale study was compared retroactively, with CRT significantly improving after 12 months of treatment and beneficial outcomes seen as early as 3 months after treatment (Figs. 4 and 5) [105].
Fig. 4.
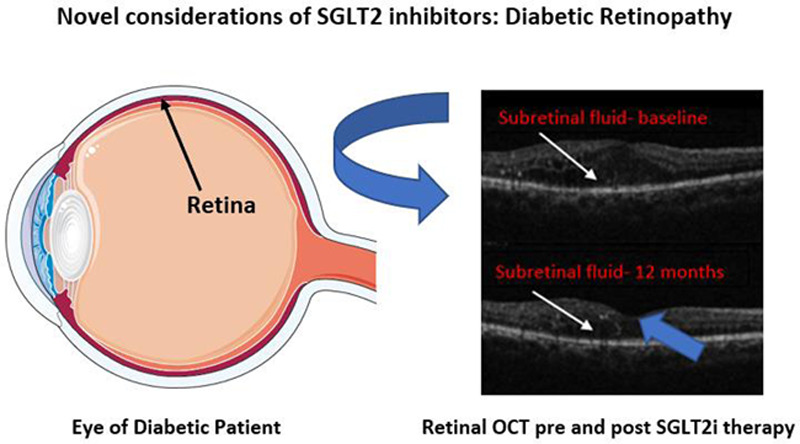
SGLT2 inhibitors exhibit potential in reducing macular edema. This phenomenon can be appreciated when noting improvements in macular edema seen on optical coherence tomography (see Mieno et al. [105] human scans). A reduction in macular edema and visual acuity in patients with T2DM was found to be statistically significant (P < 0.01). Image of the retina created using Servier Medical Art images (http://smart.servier.com) under Creative Commons License. OCT images were adapted from Mieno et al. [105] under Creative Commons License 5.5. Subretinal fluid representing macular edema is noted by white arrows, while the fovea is oriented using the blue arrow for orientation. OCT, optical coherence tomography; SGLT2, sodium-glucose co-transporter 2.
Fig. 5.
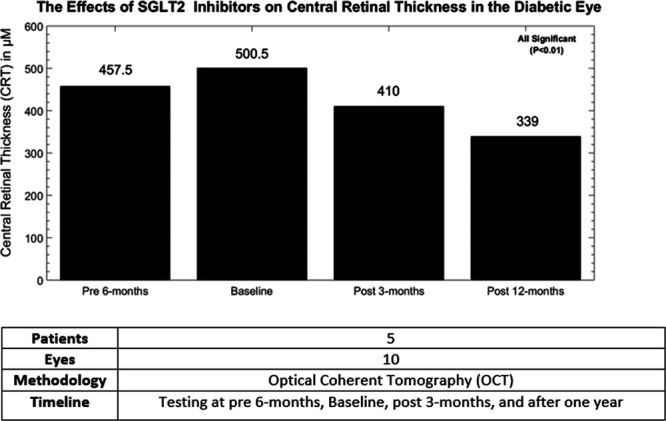
The following chart is a representation of a five-patient cohort with T2DM and diabetic retinopathy who underwent treatment with SGLT2 inhibitors. A statistically significant reduction from baseline in CRT was observed within a year. While this phenomenon should be investigated with a larger sample size, current data show a meaningful potential avenue in treating compromised vision in the patient with T2DM. Image adapted from Silva-Cardoso et al. [99]. CRT, central retinal thickness; SGLT2, sodium-glucose co-transporter 2; T2DM, type 2 diabetes mellitus.
Nevertheless, it should be underlined that this experiment is a retrospective analysis that recruited a small number of patients and is lacking the significant volume of participants that would increase the reliability of these findings. For clinical context, an increase in CRT is classically seen in pathologies that involve a compromised blood–retinal barrier, such as macular edema and diabetic retinopathy, with worsening CRT implying worse clinical outcomes [106–108]. Impeding the progression of CRT by abating the magnitude of retinal glycemic load through the reduction of reabsorbed glucose from the kidney is a plausible avenue in which SGLT2i are believed to attenuate CRT. Considerations in retinal capillary contractility through decreased calcium flux as shown in Fig. 2 should not be ignored. We should note that this type of experimental model has not been consistently reproduced on a larger scale in the literature. Therefore, larger studies involving a similar format as conducted by Mieno et al. [105] would give more insight in the association between SGLT2i and anatomical markers of diabetic retinopathy such as CRT, which would in turn give more clinical validation for these drug therapies.
Studies showing the association between SGLT2i therapy and diabetic retinopathy have shown mostly mixed results. However, diabetic retinopathy and DME has not been a primary outcome or elucidated composite defined in most of these trials, highlighting the need for large-scale randomized clinical trials dedicated to SGLT2 therapy and diabetic retinopathy given the array of data on the genetic, molecular, experimental, and even limited clinical levels showing promise. Empagliflozin when compared with placebo with considerations of a composite of risk in diabetic retinopathy outcome (HR 0.78; 95% CI, 0.54, 1.12; P = 0.1732) in the EMPA-REG OUTCOME studies showed no statistically significant difference [109]. Retinopathy as a composite was not reported in the CANVAS-Renal (canagliflozin) study or the Declare-TIMI58 study (dapagliflozin), which are the two studies that showed marked cardiorenal benefits [110]. The latter is noteworthy considering the promise canagliflozin and dapagliflozin had an modulating endothelial cell expression in molecular biology experiments [89,93].
A prospective, placebo-controlled, double-blinded randomized crossover phase IIIb trial conducted by Ott et al. [111,112] involving dapagliflozin administered among 59 patients has shown that SGLT2 inhibition has beneficial outcomes in retinal arteriole remodeling and retinal capillary flow after 6 weeks (308 versus 318 arbitrary units, P = 0.028) assessed with Doppler flowmetry, with arbitrary units defined using previous trials in a similar experiment by Ott et al.
The differential efficacy of SGLT2i relative to other drugs is also another question of interest when trying to assess the clinical value of such pharmacotherapies. It was shown that in a review of the medical records of 21 Korean patients with T2DM, who took an SGLT2i relative to a control of 71 patients taking a sulfonylurea, the SGLT2i cohort experienced less members experiencing progression of diabetic retinopathy (14% versus 44%, P = 0.014) using the Early Treatment Diabetic Retinopathy Study (ETDRS) scale as a metric of diabetic retinopathy progression [113]. The ETDRS scale is a tool that uses a combination of imaging and anatomical markers associated with diabetic retinopathy to grade severity of disease [114]. Adjusting for age, duration of T2DM diagnosis, and HbA1c, they showed a significant lower risk for diabetic retinopathy progression (P = 0.013) [69,113]. While noteworthy, this review suffers from the same dilemma as the Mieno study, in which a low sample size hinders the credence of the findings and ultimately raises the necessity of a longitudinal large-scale study of a similar format. In addition, future studies should specify the type of diabetic retinopathy, NPDR, or PDR, and if the patient has developed DME.
Conclusion
SGLT2i have been well studied in terms of their efficacy in reducing cardiorenal adverse outcomes among multiple trials. Central to this is microvascular protection via regulating neurovascular physiology and controlling glucose metabolism in the patient with T2DM. These same effects imply a benefit in one of the most consequential outcomes in the hyperglycemic patient, diabetic retinopathy. This is supported by multiple experimental studies, basic science, translational biology, and mixed clinical evidence. However, a lack of comprehensive trials with clearly expressed primary outcomes and composites to correlate clinical outcomes limits the determination of the role of SGLT2i in treating the patient with diabetic retinopathy and one of its main complications of DME. This concept is accentuated by the notion that other systemic sequelae of T2DM that are well managed by SGLT2i have an intimate interplay with diabetic retinopathy, such as predicting the course of nephropathy, cardiovascular disease, psychosocial burden of T2DM, and mortality [115–120]. Finally, future studies need to be developed with standardized definitions of diabetic retinopathy with clear outcomes of the changes in visual acuity, OCT changes, and decreased treatment burden.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cox DJ, Kiernan BD, Schroeder DB, Cowley M. Psychosocial sequelae of visual loss in diabetes. Diabetes Educ. 1998; 24:481–484. [DOI] [PubMed] [Google Scholar]
- 2.Fenwick EK, Pesudovs K, Khadka J, Dirani M, Rees G, Wong TY, Lamoureux EL. The impact of diabetic retinopathy on quality of life: qualitative findings from an item bank development project. Qual Life Res. 2012; 21:1771–1782. [DOI] [PubMed] [Google Scholar]
- 3.Kuniss N, Freyer M, Müller N, Kielstein V, Müller UA. Expectations and fear of diabetes-related long-term complications in people with type 2 diabetes at primary care level. Acta Diabetol. 2019; 56:33–38. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012; 60:428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007; 298:902–916. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial Research Group. Progression of retinopathy with intensive versus conventional treatment in the diabetes control and complications trial. Ophthalmology. 1995; 102:647–661. [DOI] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853. [PubMed] [Google Scholar]
- 9.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016; 44:260–277. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson JK, Guzman SJ, Maryniuk MD, O’Brian CA, Kadohiro JK, Jackson RA, et al. The use of language in diabetes care and education. Diabetes Educ. 2017; 43:551–564. [DOI] [PubMed] [Google Scholar]
- 11.McGuire DK, Marx N, Johansen OE, Inzucchi SE, Rosenstock J, George JT. FDA guidance on antihyperglyacemic therapies for type 2 diabetes: one decade later. Diabetes Obes Metab. 2019; 21:1073–1078. [DOI] [PubMed] [Google Scholar]
- 12.Upadhyay J, Polyzos SA, Perakakis N, Thakkar B, Paschou SA, Katsiki N, et al. Pharmacotherapy of type 2 diabetes: an update. Metabolism. 2018; 78:13–42. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food & Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Vol. 2019 postmarket drug safety information for patients and providers. 2018.
- 14.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657. [DOI] [PubMed] [Google Scholar]
- 15.Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013; 1:140–151. [DOI] [PubMed] [Google Scholar]
- 16.Thewjitcharoen Y, Yenseung N, Malidaeng A, Nakasatien S, Chotwanvirat P, Krittiyawong S, et al. Effectiveness of long-term treatment with SGLT2 inhibitors: real-world evidence from a specialized diabetes center. Diabetol Metab Syndr. 2017; 9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarafidis PA, Tsapas A. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. J Med. 2015; 373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 18.Bilezikian JP, Watts NB, Usiskin K, Polidori D, Fung A, Sullivan D, Rosenthal N. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016; 101:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monica Reddy RP, Inzucchi SE. SGLT2 inhibitors in the management of type 2 diabetes. Endocrine. 2016; 53:364–372. [DOI] [PubMed] [Google Scholar]
- 20.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011; 22:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haneda M, Seino Y, Inagaki N, Kaku K, Sasaki T, Fukatsu A, et al. Influence of renal function on the 52-week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in japanese patients with type 2 diabetes mellitus. Clin Ther. 2016; 38:66–88.e20. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Konishi Y, Morikawa T, Zhang Y, Kitabayashi C, Kobara H, et al. Effect of a SGLT2 inhibitor on the systemic and intrarenal renin-angiotensin system in subtotally nephrectomized rats. J Pharmacol Sci. 2018; 137:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Li Y, Yang B, Li Z, Huang W, Qian H. C-aryl glucosides with substituents at the distal aryl ring as sodium-dependent glucose cotransporter inhibitors for the treatment of diabetes mellitus. Chem Biol Drug Des. 2015; 86:246–253. [DOI] [PubMed] [Google Scholar]
- 24.Rao K, Kumar KMP. Sodium glucose cotransporter-2 inhibitors in clinical practice: impact beyond glycemic control. Hypertension. 2016; 2:74–79. [Google Scholar]
- 25.Control, C.f.D. Common eye disorders. 2015.
- 26.Agarwal LT, Agarwal N. Prevalence of diabetic retinopathy among self-reported adult diabetics in districts of Eastern Nepal in a community based study. Nepal J Ophthalmol. 2017; 9:136–142. [DOI] [PubMed] [Google Scholar]
- 27.Le JT, Hutfless S, Li T, Bressler NM, Heyward J, Bittner AK, et al. Setting priorities for diabetic retinopathy clinical research and identifying evidence gaps. Ophthalmol Retina. 2017; 1:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Control, C.f.D. Watch out for diabetic retinopathy. 2018.
- 29.Holekamp NM, Campbell J, Almony A, Ingraham H, Marks S, Chandwani H, et al. Vision outcomes following anti–vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018; 191:83–91. [DOI] [PubMed] [Google Scholar]
- 30.Priščáková P, Minárik G, Inzucchi SE. Candidate gene studies of diabetic retinopathy in human. Mol Biol Rep. 2016; 43:1327–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016; 2016:2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina. 2012; 32:1821–1828. [DOI] [PubMed] [Google Scholar]
- 33.Kenworthy JA, Davis J, Chandra V, Clark JB, Desmond M. Worsening proteinuria following intravitreal anti-VEGF therapy for diabetic macular edema. J Vit Ret Dis. 2019; 3:54–56. [Google Scholar]
- 34.Hanna RM, Lopez EA, Hasnain H, Selamet U, Wilson J, Youssef PN, et al. Three patients with injection of intravitreal vascular endothelial growth factor inhibitors and subsequent exacerbation of chronic proteinuria and hypertension. Clin Kidney J. 2019; 12:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haruhara K, Tsuboi N, Nakao M, Koike K, Fukui A, Miyazaki Y, et al. A case of glomerulopathy associated with the vascular endothelial growth factor inhibitor bevacizumab. Nihon Jinzo Gakkai Shi. 2014; 56:600–605. [PubMed] [Google Scholar]
- 36.Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, et al. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010; 77:989–999. [DOI] [PubMed] [Google Scholar]
- 37.Stokes MB, Erazo MC, D’Agati VD. Glomerular disease related to anti-VEGF therapy. Kidney Int. 2008; 74:1487–1491. [DOI] [PubMed] [Google Scholar]
- 38.Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC. VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer. 2010; 46:439–448. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez VH, Campbell J, Holekamp NM, Kiss S, Loewenstein A, Augustin AJ, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol i data. Am J Ophthalmol. 2016; 172:72–79. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018; 19:1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honasoge A, Nudleman E, Smith M, Rajagopal R. Emerging insights and interventions for diabetic retinopathy. Curr Diab Rep. 2019; 19:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018; 6:691–704. [DOI] [PubMed] [Google Scholar]
- 43.Foote C, Perkovic V, Neal B. Effects of SGLT2 inhibitors on cardiovascular outcomes. Diab Vasc Dis Res. 2012; 9:117–123. [DOI] [PubMed] [Google Scholar]
- 44.Santer R, Calado J. Familial renal glucosuria and SGLT2: from a Mendelian trait to a therapeutic target. Clin J Am Soc Nephrol. 2010; 5:133–141. [DOI] [PubMed] [Google Scholar]
- 45.Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol. 2003; 14:2873–2882. [DOI] [PubMed] [Google Scholar]
- 46.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994; 93:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM; UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004; 122:1631–1640. [DOI] [PubMed] [Google Scholar]
- 48.Wakisaka M, Yoshinari M, Nakamura S, Asano T, Sonoki K, Shi Ah, et al. Suppression of sodium-dependent glucose uptake by captopril improves high-glucose-induced morphological and functional changes of cultured bovine retinal pericytes. Microvasc Res. 1999; 58:215–223. [DOI] [PubMed] [Google Scholar]
- 49.Wakisaka M, Kitazono T, Kato M, Nakamura U, Yoshioka M, Uchizono Y, Yoshinari M. Sodium-coupled glucose transporter as a functional glucose sensor of retinal microvascular circulation. Circ Res. 2001; 88:1183–1188. [DOI] [PubMed] [Google Scholar]
- 50.Wakisaka M, Nagao T. Sodium glucose cotransporter 2 in mesangial cells and retinal pericytes and its implications for diabetic nephropathy and retinopathy. Glycobiology. 2017; 27:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wakisaka M, Kamouchi M, Kitazono T. Lessons from the trials for the desirable effects of sodium glucose co-transporter 2 inhibitors on diabetic cardiovascular events and renal dysfunction. Int J Mol Sci. 2019; 20:5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995; 44:603–607. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Chen LJ, Yu J, Wang HJ, Zhang F, Liu Q, Wu J. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem. 2018; 48:705–717. [DOI] [PubMed] [Google Scholar]
- 54.Dubey K, Dubey R, Gupta RA, Gupta AK. Role of aldose reductase in secondary diabetes complications. Int J Pharm Life Sci. 2018; 9:20–20. [Google Scholar]
- 55.Newman A, Andrew N, Casson R. Review of the association between retinal microvascular characteristics and eye disease. Clin Exp Ophthalmol. 2018; 46:531–552. [DOI] [PubMed] [Google Scholar]
- 56.Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018; 9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54:1615–1625. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu F, Sano Y, Haruki H, Kanda T. Advanced glycation end-products induce basement membrane hypertrophy in endoneurial microvessels and disrupt the blood-nerve barrier by stimulating the release of TGF-β and vascular endothelial growth factor (VEGF) by pericytes. Diabetologia. 2011; 54:1517–1526. [DOI] [PubMed] [Google Scholar]
- 59.Buys AV, Van Rooy MJ, Soma P, Van Papendorp D, Lipinski B, Pretorius E. Changes in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc Diabetol. 2013; 12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blaslov K, Kruljac I, Mirošević G, Gaćina P, Kolonić SO, Vrkljan M. The prognostic value of red blood cell characteristics on diabetic retinopathy development and progression in type 2 diabetes mellitus. Clin Hemorheol Microcirc. 2019; 71:475–481. [DOI] [PubMed] [Google Scholar]
- 61.Agrawal R, Smart T, Nobre-Cardoso J, Richards C, Bhatnagar R, Tufail A, et al. Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci Rep. 2016; 6:15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H. Early detection of diabetic retinopathy. Surv Ophthalmol. 2018; 63:601–608. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, Chen S, Liu ML. Pathogenic roles of microvesicles in diabetic retinopathy. Acta Pharmacol Sin. 2018; 39:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin WJ, Kuang HY. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy. 2014; 10:1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trick GL, Burde RM, Gordon MO, Santiago JV, Kilo C. The relationship between hue discrimination and contrast sensitivity deficits in patients with diabetes mellitus. Ophthalmology. 1988; 95:693–698. [DOI] [PubMed] [Google Scholar]
- 66.Tregear SJ, Knowles PJ, Ripley LG, Casswell AG. Chromatic-contrast threshold impairment in diabetes. Eye (Lond). 1997; 11 (Pt 4):537–546. [DOI] [PubMed] [Google Scholar]
- 67.Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005; 7:1581–1587. [DOI] [PubMed] [Google Scholar]
- 68.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008; 30:65–84. [DOI] [PubMed] [Google Scholar]
- 69.Kowluru RA. Mitochondrial stability in diabetic retinopathy: lessons learned from epigenetics. Diabetes. 2019; 68:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kowluru RA, Mohammad G, Sahajpal N. Faulty homocysteine recycling in diabetic retinopathy. Eye Vis (Lond). 2020; 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayreh SS. Cotton-wool spots (inner retinal ischemic spots). Marshall S. editor. In: Ocular Vascular Occlusive Disorders. Switzerland: Springer; 2015. pp. 365–377. [Google Scholar]
- 72.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, Bazan NG. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis Sci. 2003; 44:4163–4170. [DOI] [PubMed] [Google Scholar]
- 73.Miwa Y, Hoshino Y, Shoda C, Jiang X, Tsubota K, Kurihara T. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem Int. 2019; 128:21–31. [DOI] [PubMed] [Google Scholar]
- 74.Crawford TN, Alfaro DV, III, Kerrison JB, Jablon EP. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009; 5:8–13. [DOI] [PubMed] [Google Scholar]
- 75.Usman Akram M, Khalid S, Tariq A, Younus Javed M. Detection of neovascularization in retinal images using multivariate m-Mediods based classifier. Comput Med Imaging Graph. 2013; 37:346–357. [DOI] [PubMed] [Google Scholar]
- 76.Wu MY, Yiang GT, Lai TT, Li CJ. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid Med Cell Longev. 2018; 2018:3420187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishibazawa A, Nagaoka T, Takahashi T, Yamamoto K, Kamiya A, Ando J, Yoshida A. Effects of shear stress on the gene expressions of endothelial nitric oxide synthase, endothelin-1, and thrombomodulin in human retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2011; 52:8496–8504. [DOI] [PubMed] [Google Scholar]
- 78.Burg MB, Kador PF. Sorbitol, osmoregulation, and the complications of diabetes. J Clin Invest. 1988; 81:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willermain F, Scifo L, Weber C, Caspers L, Perret J, Delporte C. Potential interplay between hyperosmolarity and inflammation on retinal pigmented epithelium in pathogenesis of diabetic retinopathy. Int J Mol Sci. 2018; 19:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amin R, Ali RS, Hidayat R, Permana H, Kartasasmita AS, Hilmanto D. The relationships between sorbitol dehydrogenase (SDH) level and diabetic rethinopathy in diabetes melitus type-2 patients. Int J Biol Med Res. 2017; 8:6020–6022. [Google Scholar]
- 81.Coucha M, Elshaer SL, Eldahshan WS, Mysona BA, El-Remessy AB. Molecular mechanisms of diabetic retinopathy: potential therapeutic targets. Middle East Afr J Ophthalmol. 2015; 22:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li W, Chen S, Mei Z, Zhao F, Xiang Y. Polymorphisms in sorbitol-aldose reductase (polyol) pathway genes and their influence on risk of diabetic retinopathy among han Chinese. Med Sci Monit. 2019; 25:7073–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaur N, Vanita V. Association of aldose reductase gene (AKR1B1) polymorphism with diabetic retinopathy. Diabetes Res Clin Pract. 2016; 121:41–48. [DOI] [PubMed] [Google Scholar]
- 84.Katakami N, Kaneto H, Takahara M, Matsuoka TA, Imamura K, Ishibashi F, et al. Aldose reductase C-106T gene polymorphism is associated with diabetic retinopathy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2011; 92:e57–e60. [DOI] [PubMed] [Google Scholar]
- 85.Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol. 2010; 88:241–248. [DOI] [PubMed] [Google Scholar]
- 86.Tang WH, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012; 3:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal. 2011; 15:1271–1284. [DOI] [PubMed] [Google Scholar]
- 88.Biswas S, Chakrabarti S. Pathogenetic mechanisms in diabetic retinopathy: from molecules to cells to tissues. Springer, editor. In: Mechanisms of Vascular Defects in Diabetes Mellitus. Switzerland: Springer; 2017. pp. 209–247. [Google Scholar]
- 89.Leley SP, Luo Q, Bhatwadekar AD. 588-P: diabetes leads to sodium-glucose cotransporter 2 (SGLT2) increase in the retina. Diabetes. 2019; 68 (Suppl 1):1–1.30573673 [Google Scholar]
- 90.Taher MM. Protective effect of sodium-glucose co-transporter 2 inhibitor (dapagliflozin) on diabetic retinopathy in streptozotocin induced diabetes in rats. Med J Cairo Univ. 2019; 87:465–470. [Google Scholar]
- 91.Homme RP, Singh M, Majumder A, George AK, Nair K, Sandhu HS, et al. Remodeling of retinal architecture in diabetic retinopathy: disruption of ocular physiology and visual functions by inflammatory gene products and pyroptosis. Front Physiol. 2018; 9:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoshizumi H, Ejima T, Nagao T, Wakisaka M. Recovery from diabetic macular edema in a diabetic patient after minimal dose of a sodium glucose co-transporter 2 inhibitor. Am J Case Rep. 2018; 19:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Behnammanesh G, Durante ZE, Peyton KJ, Martinez-Lemus LA, Brown SM, Bender SB, Durante W. Canagliflozin inhibits human endothelial cell proliferation and tube formation. Front Pharmacol. 2019; 10:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Devineni D, Polidori D, Curtin CR, Murphy J, Wang SS, Stieltjes H, Wajs E. Pharmacokinetics and pharmacodynamics of once- and twice-daily multiple-doses of canagliflozin, a selective inhibitor of sodium glucose co-transporter 2, in healthy participants. Int J Clin Pharmacol Ther. 2015; 53:438–446. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Nakano D, Guan Y, Hitomi H, Uemura A, Masaki T, et al. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int. 2018; 94:524–535. [DOI] [PubMed] [Google Scholar]
- 96.Maekawa T, Tadaki H, Sasase T, Motohashi Y, Miyajima K, Ohta T, Kume S. Pathophysiological profiles of SDT fatty rats, a potential new diabetic peripheral neuropathy model. J Pharmacol Toxicol Methods. 2017; 88:160–166. [DOI] [PubMed] [Google Scholar]
- 97.Takakura S, Toyoshi T, Hayashizaki Y, Takasu T. Effect of ipragliflozin, an SGLT2 inhibitor, on progression of diabetic microvascular complications in spontaneously diabetic Torii fatty rats. Life Sci. 2016; 147:125–131. [DOI] [PubMed] [Google Scholar]
- 98.Klen J, Goričar K, Dolžan V. Genetic variability in sodium-glucose cotransporter 2 influences glycemic control and risk for diabetic retinopathy in type 2 diabetes patients. J Med Biochem. 2019; 1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silva-Cardoso J, Sheikh O, Nashawi M, Pham S, Gallegos KM, Dinkha LR, Chilton RJ. Cardiorenal protection with SGLT2: lessons from the cardiovascular outcome trials. J Diabetes. 2020; 12:279–293. [DOI] [PubMed] [Google Scholar]
- 100.Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019; 139:2516–2527. [DOI] [PubMed] [Google Scholar]
- 101.Dziuba J, Alperin P, Racketa J, Iloeje U, Goswami D, Hardy E, et al. Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obesity Meta. 2014; 16:628–635. [DOI] [PubMed] [Google Scholar]
- 102.Tang H, Li G, Zhao Y, Wang F, Gower EW, Shi L, Wang T. Comparisons of diabetic retinopathy events associated with glucose-lowering drugs in patients with type 2 diabetes mellitus: a network meta-analysis. Diabetes Obesity Metab. 2018; 20:1262–1279. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, et al. ; RESTORE Extension Study Group. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014; 121:1045–1053. [DOI] [PubMed] [Google Scholar]
- 104.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005; 438:960–966. [DOI] [PubMed] [Google Scholar]
- 105.Mieno H, Yoneda K, Yamazaki M, Sakai R, Sotozono C, Fukui M. The Efficacy of Sodium-Glucose Cotransporter 2 (SGLT2) inhibitors for the treatment of chronic diabetic macular oedema in vitrectomised eyes: a retrospective study. BMJ Open Ophthalmol. 2018; 3:e000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lobo CL, Bernardes RC, Cunha-Vaz JG. Alterations of the blood-retinal barrier and retinal thickness in preclinical retinopathy in subjects with type 2 diabetes. Arch Ophthalmol. 2000; 118:1364–1369. [DOI] [PubMed] [Google Scholar]
- 107.Sander B, Thornit DN, Colmorn L, Strøm C, Girach A, Hubbard LD, et al. Progression of diabetic macular edema: correlation with blood retinal barrier permeability, retinal thickness, and retinal vessel diameter. Invest Ophthalmol Vis Sci. 2007; 48:3983–3987. [DOI] [PubMed] [Google Scholar]
- 108.Deák GG, Schmidt-Erfurth UM, Jampol LM. Correlation of central retinal thickness and visual acuity in diabetic macular edema. JAMA Ophthalmol. 2018; 136:1215–1216. [DOI] [PubMed] [Google Scholar]
- 109.Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018; 41:e4–e5. [DOI] [PubMed] [Google Scholar]
- 110.Kaul S. Response to comment on Kaul. Mitigating cardiovascular risk in type 2 diabetes with antidiabetes drugs: a review of principal cardiovascular outcome results of EMPA-REG OUTCOME, LEADER, and SUSTAIN-6 Trials. Diabetes Care 2017; 40: 821–831. Diabetes Care. 2017; 40:e175–e176. [DOI] [PubMed] [Google Scholar]
- 111.Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, Bramlage P, Schmieder RE. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol. 2017; 16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ott C, Raff U, Schmidt S, Kistner I, Friedrich S, Bramlage P, et al. Effects of saxagliptin on early microvascular changes in patients with type 2 diabetes. Cardiovasc Diabetol. 2014; 13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cho EH, Park SJ, Han S, Song JH, Lee K, Chung YR. Potent oral hypoglycemic agents for microvascular complication: sodium-glucose cotransporter 2 inhibitors for diabetic retinopathy. J Diabetes Res. 2018; 2018:6807219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gangnon RE, Davis MD, Hubbard LD, Aiello LM, Chew EY, Ferris FL, 3rd, Fisher MR; Early Treatment Diabetic Retinopathy Study Research Group. A severity scale for diabetic macular edema developed from ETDRS data. Invest Ophthalmol Vis Sci. 2008; 49:5041–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu XR, Zhang YP, Bai L, Zhang XL, Zhou JB, Yang JK. Prediction of risk of diabetic retinopathy for all-cause mortality, stroke and heart failure: evidence from epidemiological observational studies. Medicine (Baltimore). 2017; 96:e5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Hecke MV, Dekker JM, Stehouwer CD, Polak BC, Fuller JH, Sjolie AK, et al. ; EURODIAB Prospective Complications Study. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care. 2005; 28:1383–1389. [DOI] [PubMed] [Google Scholar]
- 117.Serlin Y, Shafat T, Levy J, Winter A, Shneck M, Knyazer B, et al. Angiographic evidence of proliferative retinopathy predicts neuropsychiatric morbidity in diabetic patients. Psychoneuroendocrinology. 2016; 67:163–170. [DOI] [PubMed] [Google Scholar]
- 118.Hung CC, Lin HY, Hwang DY, Kuo IC, Chiu YW, Lim LM, et al. Diabetic retinopathy and clinical parameters favoring the presence of diabetic nephropathy could predict renal outcome in patients with diabetic kidney disease. Sci Rep. 2017; 7:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie J, Ikram MK, Cotch MF, Klein B, Varma R, Shaw JE, et al. Association of diabetic macular edema and proliferative diabetic retinopathy with cardiovascular disease: a systematic review and meta-analysis. JAMA Ophthalmol. 2017; 135:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic Retinopathy in Predicting Diabetic Nephropathy in Patients with Type 2 Diabetes and Renal Disease: A Meta-Analysis. Switzerland: Springer; 2013. [DOI] [PubMed] [Google Scholar]


