Abstract
Keshan disease (KD) is an endemic cardiomyopathy with myocardial lesions for which the precise etiology has not been determined. It was named after the location where it was first identified: Keshan County in China. KD is mainly distributed in a selenium-deficient zone from northeast to southwest China. In recent years, the incidence and prevalence of KD have significantly decreased. Meanwhile, more knowledge was accumulated about the etiology of KD and recent data indicated that genetic factor was involved. In this review, we will first update an overview of the epidemiological status and the environmental and genetic factors involved in this disease.
Keywords: epidemiology, genetics, Keshan disease
Introduction
Keshan disease (KD), an endemic cardiomyopathy found only in China, Korea and Japan, is characterized by multifocal myocardial necrosis and fibrosis, which are clinical symptoms similar to those of dilated cardiomyopathy [1]. It is named after the location where it was first identified in 1935: Keshan County, Heilongjiang Province, northeast China. Although the incidence and prevalence of KD were reduced significantly in recent years, the clinical prognosis of KD is still poor [2]. In our previous study, the five-year survival rate of 92 KD patients with a mean follow-up of 4.8 ± 1.9 years was only 77.1% [3]. Based on the duration of the disease, KD is clinically divided into four categories: acute, subacute, chronic and latent KD. Viral infection, selenium deficiency and malnutrition were once considered as factors in the pathogenesis of KD [1,2]. Recent studies also pointed out several genetic polymorphisms and mutations could be related to KD [4]. In this review, we will try to discuss the epidemiological status, environmental and genetic etiological factors that are related to KD.
The current epidemiological status of Keshan disease
Detection and incidence rates of Keshan disease in nationwide of China
The national surveillance of KD in China was conducted from 1990 to 2009. During the years, the results were reported in the annual conference of KD in China. This work was led by the Keshan Disease Research Institute, Centers for Disease Control of China. Later on, the national surveillance switched to a local surveillance since 2010, due to the low incidence rate of KD. In this review, we will try to have a whole picture of the epidemiology of KD from 2009 to 2018, especially focusing on the most recent updates of seven historically severe epidemic provinces in 2010–2018.
KD is classified into four categories as follows: (1) acute KD, characterized by cardiogenic shock, arrhythmia and acute heart failure, with sudden onset, rapid progression and acute circulatory dysfunction; (2) subacute KD, with a slower onset than acute KD and a more rapid one than chronic KD, with similar symptoms to acute KD, along with congestive heart failure; (3) chronic KD, characterized by cardiac dilatation, a slow onset, and a long course, with most patients having a cardiac function range of NYHA II–IV and (4) latent KD, which can develop in either previously unaffected individuals or those with the other three types of KD, featuring myocardial injury and usually arrhythmia, but with normal heart function [5]. The detection rate refers to the proportion of individuals diagnosed as KD among the total population during the survey. The incidence rate indicates the proportion of newly diagnosed individuals among the total population during the survey.
In total, 380 103 individuals were surveyed from 1990 to 2009, among whom 10 770 KD patients were identified, including 8827 latent KD patients and 1937 chronic KD patients being identified. The crude annual average detection rate of KD was 2.85%, ranging from 1.33 to 5.54%, the crude annual average detection rate of latent KD was 2.37%, ranging from 1.04 to 4.63%, and the equivalent value for chronic KD was 0.48%, ranging from 0.24 to 0.91%. As for the crude incidence rate of KD in this period, 1004 new KD patients were identified, including 834 latent KD patients and 164 chronic ones. The crude annual average incidence rates were 2.36‰, with a range of 0.49–9.15‰, 1.99‰ with a range of 0.28–8.60‰ and 0.33‰ with a range of 0.06–0.68‰, for KD, latent KD and chronic KD, respectively [6–20]. Overall, the findings show that detection rates of KD, latent KD and chronic KD decreased in 2007–2009, and the incidence rates of KD, latent KD and chronic KD remained stable.
From Fig. 1 and Table 1, it can be seen that, from 1990 to 2009, the lowest detection rates of KD, latent KD and chronic KD occurred in 1996, while the highest were in 2001. Regarding the trend of national crude KD detection rates, these were stable in 1995–1996 and 2003–2009, while there were relatively high KD detection rates in the period of 1996–2002. In addition, regarding the trend of latent KD detection rates, they were at a low level during 1995–1996, but high during the periods of 1996–2009; however, the average detection rate of latent KD in 2003–2009 was lower than those in the period of 1996–2002, and the detection rates of chronic KD had the same trend of latent KD.
Fig. 1.
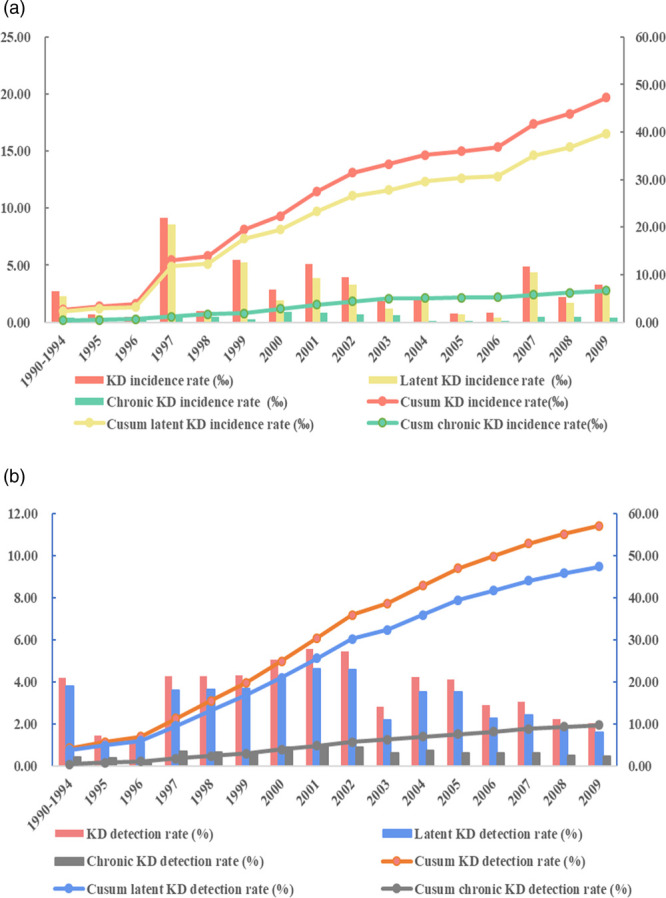
The national detection and incidence rate of Keshan disease (KD) in China from 1990 to 2009. (a) The plot showed the trends of the national detection rates of KD, latent KD and chronic KD during 1990–2009. (b) The plot showed the trends of the national incidence rates of KD, latent KD and chronic KD during 1990–2009.
Table 1.
The national (Cusum) detection and incidence rate of Keshan disease in China from 1990 to 2009
| Year | KD detection rate (%) | Cusum KD detection rate (%) | Latent KD detection rate (%) | Cusum latent KD detection rate (%) | Chronic KD detection rate (%) | Cusum chronic KD detection rate (%) | KD incidence rate (‰) | Cusum KD incidence rate (‰) | Latent KD incidence rate (‰) | Cusum latent KD incidence rate (‰) | Chronic KD incidence rate (‰) | Cusm chronic KD incidence rate (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990–1994 | 4.2 | 4.2 | 3.8 | 3.8 | 0.4 | 0.4 | 2.7 | 2.7 | 2.29 | 2.29 | 0.4 | 0.4 |
| 1995 | 1.43 | 5.63 | 1.04 | 4.84 | 0.39 | 0.79 | 0.69 | 3.39 | 0.63 | 2.92 | 0.06 | 0.46 |
| 1996 | 1.33 | 6.96 | 1.09 | 5.93 | 0.24 | 1.03 | 0.49 | 3.88 | 0.28 | 3.2 | 0.21 | 0.67 |
| 1997 | 4.26 | 11.22 | 3.58 | 9.51 | 0.68 | 1.71 | 9.15 | 13.03 | 8.6 | 11.8 | 0.55 | 1.22 |
| 1998 | 4.27 | 15.49 | 3.62 | 13.13 | 0.65 | 2.36 | 0.95 | 13.98 | 0.5 | 12.3 | 0.45 | 1.67 |
| 1999 | 4.29 | 19.78 | 3.69 | 16.82 | 0.59 | 2.95 | 5.5 | 19.48 | 5.25 | 17.55 | 0.25 | 1.92 |
| 2000 | 5.06 | 24.84 | 4.16 | 20.98 | 0.89 | 3.84 | 2.86 | 22.34 | 1.9 | 19.45 | 0.9 | 2.82 |
| 2001 | 5.54 | 30.38 | 4.63 | 25.61 | 0.91 | 4.75 | 5.13 | 27.47 | 3.87 | 23.32 | 0.87 | 3.69 |
| 2002 | 5.44 | 35.82 | 4.57 | 30.18 | 0.87 | 5.62 | 3.95 | 31.42 | 3.27 | 26.59 | 0.68 | 4.37 |
| 2003 | 2.79 | 38.61 | 2.19 | 32.37 | 0.6 | 6.22 | 1.84 | 33.26 | 1.23 | 27.82 | 0.61 | 4.98 |
| 2004 | 4.22 | 42.83 | 3.51 | 35.88 | 0.71 | 6.93 | 1.94 | 35.2 | 1.82 | 29.64 | 0.11 | 5.09 |
| 2005 | 4.11 | 46.94 | 3.51 | 39.39 | 0.6 | 7.53 | 0.76 | 35.96 | 0.67 | 30.31 | 0.09 | 5.18 |
| 2006 | 2.89 | 49.83 | 2.25 | 41.64 | 0.6 | 8.13 | 0.84 | 36.8 | 0.38 | 30.69 | 0.08 | 5.26 |
| 2007 | 3.03 | 52.86 | 2.41 | 44.05 | 0.62 | 8.75 | 4.88 | 41.68 | 4.41 | 35.1 | 0.49 | 5.75 |
| 2008 | 2.21 | 55.07 | 1.71 | 45.76 | 0.5 | 9.25 | 2.21 | 43.89 | 1.72 | 36.82 | 0.49 | 6.24 |
| 2009 | 2.02 | 57.09 | 1.58 | 47.34 | 0.44 | 9.69 | 3.33 | 47.22 | 2.89 | 39.71 | 0.44 | 6.68 |
Regarding the total incidence rate of KD in China during 1990–2009, the lowest incidence rates of KD and latent KD occurred in 1996, while the highest ones were in 1997. The lowest chronic KD incidence rate was in 1995 and the highest was in 2000. In addition, for the incidence rates of KD and latent KD, both of them reached high levels in 1996–1997, 1999–2002 and 2007–2009, but remained at low levels during 1994–1996, 1998 and 2003–2006, and there was a stable trend in the chronic KD incidence rate, as a basic control of chronic KD, during 1990–2009. The results of this analysis indicate that the status of KD exhibited relatively stable trends, in particular that the chronic KD detection and incidence rates were remained at lower levels.
The status of Keshan disease in seven historically severe epidemic provinces in 2010–2018
Although the summary for the National Keshan Disease Surveillance conference was not produced after 2009, the survey was still continued in the focal monitoring area every year. The focal monitoring area included seven regions with a relatively higher disease incidence, namely, Heilongjiang, Jilin, Inner Mongolia, Sichuan, Yunnan Shaanxi and Shandong provinces, so we selected the data from these areas for analysis.
During 2011–2016, a total of 801 hospitals participated in surveying 27 046 people in Heilongjiang Province. Among these, there were 3051 cardiomyopathy patients and 143 patients diagnosed with KD, all of whom had chronic KD, and no new cases of KD were found, so the incidence rate was zero [21]. The annual average crude detection rate of KD, latent KD and chronic KD were 0.094%, 0 and 0.094% from 2011 to 2016, respectively.
In 2011, a total of 6746 people were surveyed in 16 villages in Jilin Province. A total of 55 KD patients were identified, giving a total crude detection rate of 0.815%. The latent and chronic KD cases numbered 46 (0.682%) and nine (0.133%) [22], respectively. In 2012, a total of 5110 residents were surveyed and there were four KD patients, whom were all diagnosed latent KD; thus, the detection rate of KD and latent KD were both 0.080% [23]. During 2014–2016, about 18 757, 15 931 and 14 936 people were surveyed in Jilin Province and 80 (0.427%), 61 (0.380%) and 60 (0.402%) KD patients were identified, respectively. The corresponding detection rates of chronic KD and latent KD were 0.064% (12/18 757) and 0.363% (68/18 757), 0.040% (6/15 931) and 0.350% (55/15 931), 0.134% (20/14 936) and 0.268% (40/14 936), respectively [24–26].
From 2006 to 2012, in Inner Mongolia Province, 31 463 residents were investigated. Among these, 1123 KD patients were detected, including 822 with latent KD and 301 with chronic KD. The detection rates of KD, latent KD and chronic KD were 3.57%, 2.61% and 0.96%, respectively [27]. The status of KD in Inner Mongolia was stable from 2006 to 2012.
During 2011–2013, we reported that the number of people surveyed in Sichuan Province was 14 295, among which 71 cases (0.50%) of KD were detected, including 40 cases of latent KD and 31 cases of chronic KD. The corresponding detection rates of chronic KD and latent KD were 0.28% and 0.22%, respectively [28,29]. During 2014–2015, a total of 62 793 people had been surveyed, and 64 (0.10%) KD patients were detected, including 14 (0.02%) chronic KD and 50 (0.08%) latent KD [30]. From the previous of our studies [28–30], it is shown that the status of KD in Sichuan Province was relatively stable from 2011 to 2013, while the detection rate dropped to a lower level during 2014–2015.
From 2010 to 2012, researchers monitored 21 300 people in Yunnan Province, among which 115 cases of KD were detected. The total detection rate was 0.54%. Overall, there were 96 cases of latent KD, with a detection rate of 0.45%, along with 19 cases of chronic KD, with a detection rate of 0.09%. No acute or subacute KD cases were identified [31–33].
In the period 2010–2018, only 2011 was the year in which KD monitoring was performed in Shaanxi Province. In that year, a total of 9145 people were surveyed, among whom 41 latent KD patients were detected, with a detection rate of 0.45% [34].
In Shandong Province, monitoring of 3031 people was carried out at seven survey sites during 2011–2015. A total of 53 KD patients were found, including 42 with latent KD and 11 with chronic KD; the detection rates were 1.75%, 1.39% and 0.36%, respectively [35].
These data indicate that the prevalence of KD in the main affected provinces has been consistently at a low level in recent years. As shown in Fig. 2, Inner Mongolia had the highest annual detection rate of chronic KD (0.137%), followed by Sichuan (0.131%), Heilongjiang (0.094%), Jilin (0.074%), Shandong (0.073%), Yunan (0.030%) and Shaanxi (0); the annual detection rates of two provinces were lower than 0.05%, while two of them were higher than 0.1%. Most provinces had reached a control level; however, KD has not been eliminated, further research should be done at the county and province levels.
Fig. 2.
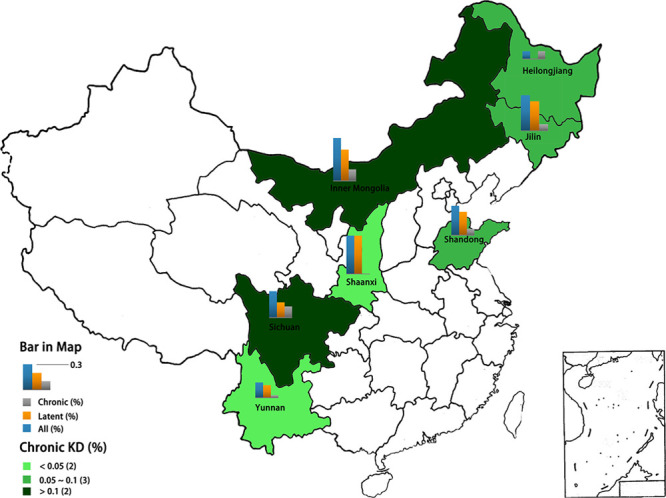
The detection rates of Keshan disease (KD) of the seven provinces in China during 2010–2016.
Selenium deficiency and virus infection
Although the etiological mechanism of KD remains largely unclear, selenium and Coxsackie virus infection have been suggested to be highly involved in the pathogenesis of KD [36,37]. KD and selenium deficiency were similar in geographical distribution, both located in the same belt region from northeast to southwest in China. Previous studies found that the selenium levels in whole-blood and hair samples in the Keshan endemic area residents were lower than those in the nonendemic areas [36,37]. Selenium supplementation significantly reduced the incidence of KD in a large number of population [36]. In addition, selenium deficiency has been linked to thyroiditis, Graves’ disease and goiter to low selenium status [38]. Some studies shown that the thyroid level might be different between KD patients and controls. Liu et al. reported that myocardial thyroxine (T4) 5´-monodeiodinase activity, endogenous triiodothyronine (T3) content in myocardial tissue and myocardial mitochondrial α-glycerol-phosphate dehydrogenase activity decreased in the endemic grain group, while the atrial natriuretic peptide content in myocardium was increased [39]. Wu et al. showed that abnormal thyroid hormone metabolism existed in the KD patients [40]. Subclinical or clinical thyroid dysfunction were correlated cardiac function and dilated cardiomyopathy [41,42].
It was accepted that selenium deficiency is the key etiological factor for KD; however, it may not be the only etiological factor, the role of virus infection in the KD could not be ruled out [3,43]. Coxsackie virus was detected in the myocardium of KD patients at different stages, and it was found that Coxsackie B virus can increase the virulence through the gene mutation in the selenium-deficient mice [44,45]. Li-Qun Ren et al. found the Coxsackievirus B3 RNA positive rate in KD patients; moreover, the area, range and the distribution of the positive signal of Coxsackie B3 virus were associated with the development of KD [44]. What’s more, the increases in serum soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecular-1 in KD patients may be related to Coxsackie B virus infection [45]. Interestingly, the selenium could have several effects, both direct and indirect, on CV [40,46,47]. Recent studies have shown that selenium could prevent genetic adaptions in the viral genomic RNA that lead to increased virulence and cardiac pathology [46]. Moreover, selenium deficiency could lead to the increase of T4 and the decrease of T3 in the peripheral largely due to the inactivation of type I deiodinases [40]. The decreased concentration of T3 could impair the activity of innate immune cells including natural killer cells and dendritic cells (DC), which may lower the efficacy of virus clearance [47].
Although selenium supplementation therapy is proved useful [36], the dose of selenium supplementation needs to be carefully calculated, because over-dose of selenium could lead to type 2 diabetes (T2D). In a recent systematic review of 13 observational studies, eight demonstrated a statistically significant positive association of Se concentration and T2D; however, among the randomized clinical trials, this association was not observed [48]. Therefore, further investigation is required to make a clear conclusion.
Progress in potential genetic etiology in Keshan disease
Family clustering of Keshan disease
KD is an endemic cardiomyopathy, which might be related to genetic factors. The tendency for KD to cluster within families has been reported in a few studies [4,49,50]. In 1983, Zhao et al. conducted a pedigree investigation in 130 children of Yi ethnicity with KD aged from 1 to 15 years old. These data showed that 29.23% of the patients had a family history of KD; the prevalence rate was significantly higher in their relatives than in the general population, and the prevalence rate of KD was higher in those who had married consanguineously, strongly suggesting a genetic component to KD [49]. Moreover, in 2003, Zhang et al. carried out an epidemiological survey in 52 families with KD in Huangling County, Shaanxi Province. The prevalence rate of KD in relatives of these patients was significantly higher than that in the general population locally [50]. The above studies suggested that KD was an inherited disease with clear familial clustering.
Genetic polymorphisms of Keshan disease
Studies have shown that the pathogenesis of KD is related to immune disturbance, so immune function and genes encoding proteins related to oxidative stress have been a particular focus in KD research. Human leukocyte antigen (HLA), which plays an important role in immunomodulation, is one of the most complex and varied gene systems, which can be classified into HLA-I, II and III types. The HLA-II gene region includes at least the DR, DQ, DP, DOB and DM subregions. Moreover, the DR subregion contains one DRA gene and nine DRB genes, with HLA-DRB1 being the most polymorphic of all HLA-II genes [51–53]. Polymorphisms in the HLA-DRB1 gene have been found to be associated with the pathogenesis of dilated cardiomyopathy susceptibility in China and elsewhere [51–53]. Niu et al. were the first to report on the association between KD and genotyping of HLA-DRB1 gene in 118 KD patients. As a result, 10 alleles at the HLA-DRB1 locus of KD patients were identified, with DR15 (0.3438 vs. 0), DR4 (0.1563 vs. 0.139) and DR9 (0.1875 vs. 0.120) being found at higher frequencies in KD patients, suggesting that HLA-DRB1 gene polymorphism is associated with KD [52]. Moreover, Wei et al. reported a further study on the relationship between HLA-DRB1 gene polymorphism and KD. In this work, 13 related alleles were detected at the HLA-DRB1 locus. The frequencies of the DR7 allele of HLA-DRB1 were significantly lower in the KD group (0.0170 vs. 0.10000, P < 0.01) and chronic KD group (0.0091 vs. 0.10000, P < 0.01) than that in normal controls, indicating that this allele might be protective against KD. Meanwhile, the DR15 allele was found to be significantly associated with KD and linked to the susceptibility loci of KD (χ2 = 7.40, P < 0.01). Those findings indicate that KD might have a potential genetic etiology [53]. In another study, Wang et al. analyzed the association between HLA-DRB1 gene polymorphism and KD. The DR15 allele frequency of HLA-DRB1 in KD patients and their parents was 0.39 vs. 0.24. They found that KD is associated and linked with the DR15 allele of HLA-DRB1, (χ2 = 12.25, P < 0.01) [54]. The above studies show that DR15, DR4 and DR9 could increase the risk of KD while DR7 could reduce it. And the above analysis indicates that the up- or down-regulation of immune-related genes might increase the susceptibility to KD, and that polymorphism of HLA-II-type genes might be related to the pathogenesis of KD.
Several studies have shown that selenium and GPX-1 enzyme activity were also related to KD [3,54]. For example, a study by Lei et al. [54] showed that the blood selenium level and GPx-1 enzyme activity were significantly lower in KD patients than in normal controls, and a variant at the Pro198 site of the GPx-1 gene (Pro198Leu) was identified. The study showed that the frequency of Pro198Leu polymorphism in patients with KD (21.1%) was significantly higher than that in controls (10.7%), and the polymorphism frequency in the chronic KD group was the highest. Further study confirmed that there was a synergistic interaction between 198 allelic polymorphisms and selenium deficiency, and that overexpression of GPx-1 (198Leu) in neonatal rat cardiomyocytes decreased the activity of GPx-1 enzyme by 30%. Variation at residue 198 in GPx-1 gene might modify the function of the GPx-1 protein regulated by selenium, with the mutant requiring more selenium to achieve the same enzyme activity as the wild type. In 2014–2015, Jiang et al. [55] investigated blood selenium, GPx-1 enzyme activity and GPx gene polymorphism in 30 patients with KD and 30 healthy individuals. They found that the decrease of GPx enzyme activity was largely related to low selenium and GPx gene polymorphism. The blood selenium index and the GPx-1 enzyme activity in patients with Gpx-1 gene polymorphism were significantly lower than those in normal controls (t = 14.632, P < 0.05), suggesting that the interaction of GPx gene polymorphism and selenium deficiency could increase the risk of KD onset. Moreover, Wei et al. sequenced the GPx gene in KD patients and healthy controls. In that study, four and 24 mutated loci were detected in the promoter and exon regions of the Gpx1 gene, respectively, and a higher mutation frequency in exon 474 was detected in the KD group (7/36 vs. 2/41). Moreover, the blood selenium level and Gpx activity were lower in KD patients than in healthy controls in this KD-endemic region [56]. The above studies indicate that the synergistic interaction of low blood selenium and GPx gene polymorphism results in a decrease of GPx-1 enzyme activity, which increases the susceptibility to KD, and that Pro198Leu at GPx-1 is related to the risk of KD onset.
The human SCN5A gene is a member of the voltage-gated sodium channel family. It is expressed in human heart tissue, and has been shown to be mutated in many cardiomyopathy and arrhythmia cases. Jiang [57] studied the association between the H558R polymorphism of SCN5A and KD. For this, they recruited 71 KD patients and 80 region-matched control subjects from a KD-affected area in northeast China and found that the H558R polymorphism of exon 12 is significantly associated with KD, which might increase susceptibility to KD and QRS duration prolongation in Electrocardiograph. Moreover, Allele C carriers had a decreased risk for KSD with an odds ratio of 0.332 (95% CI, 0.160–0.692).
Gene mutations and Keshan disease
Mutation of the SCN5A gene, which encodes a myocardial sodium channel, leads to cardiac electrophysiological disorders, resulting in Brugada syndrome, hereditary long QT syndrome and cardiac conduction system dysfunction. Mutations in the coding region of the SCN5A gene can also cause familial and hereditary cardiomyopathy, leading to sudden cardiac death. Moreover, recent studies have shown that SCN5A gene mutations can also damage the contractile function of the myocardium, leading to the development of dilated cardiomyopathy into heart failure [57]. In 2007, Han Shuang et al. reported a study of SCN5A gene mutations in KD patients, as determined by PCR. Specifically, they determined the sequence of exon 28 of the SCN5A gene in KD patients, and the results showed that an abnormal single strand was found in KD patients, and point mutations were present in exon 28 of the SCN5A gene, indicating that the structure of the SCN5A gene-encoded myocardial sodium channel in KD patients differs from that in the normal population. This suggests that SCN5A gene mutation might be one of the factors leading to increased susceptibility to environmental factor-induced KD [58].
The above studies show that KD is associated with multiple HLA-DRB1 alleles. Meanwhile, a low selenium level and GPx-1 gene polymorphisms showed synergistic effects on increasing the risk of KD onset. Mutation of the SCN5A gene might be one of the factors leading to increased susceptibility to environmental factors that induce the onset of KD. The gene polymorphism and mutation in KD patients were shown in Table 2.
Table 2.
Gene polymorphism and mutation in Keshan disease patient
| Gene name | Site | Change | Gene ID | Description | Location | Author |
|---|---|---|---|---|---|---|
| Gene polymorphism | ||||||
| HLA-DRB1 | 3123 | Major histocompatibility complex, class II, DR beta 1 | Chromosome 6, NC_000006.12 | |||
| DR15, DR4, DR9 | Up-regulated | XiaolinNiu | ||||
| DR7 | Down-regulated | Jin Wei | ||||
| DR15 | Exist genetic susceptibility | Yaping Wang | ||||
| GPX-1 | 24 404 | Glutathione peroxidase 1 | Chromosome 8, NC_005107.4 | |||
| 198 | Pro198Leu | Cong Lei | ||||
| Exon 474 | A higher mutation frequency | H.L. Wei | ||||
| SCN5A | Exon 12 | H558R polymorphism | 6331 | Sodium voltage-gated channel alpha subunit 5 | Chromosome 3, NC_000003.12 | Jiang S |
| Gene mutation | ||||||
| SCN5A | Exon28 | Exist genetic mutation | 6331 | Sodium voltage-gated channel alpha subunit 5 | Chromosome 3, NC_000003.12 | Shaung Han |
Conclusion
KD is an endemic cardiomyopathy that is exclusively found in China, Korea and Japan. According to the data on KD reported from 1990 to the present, clear progress has been made in controlling KD. The KD with detection rates had significantly decreased between 2000–2004 and 2005–2009. In terms of these historically severely affected KD provinces in 2010–2018, the crude annual detection rates of two provinces had lower than 5‰. Studies shown that the DR15 allele of HLA-DRB1, the GPx-1 gene (Pro198Leu), exon 474 of GPX-1 gene and exon 28 mutation of the SCN5A gene were found at higher frequencies in KD patients; however, DR7 allele of HLA-DRB1 and Allele C of H558R polymorphism of SCN5A were lower frequencies in KD patients.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81470521 and No. 81770379).
Data availability: data are available from the authors upon request. The data that support the findings of this study are available from the corresponding author (Xiaoping Li; e-mail: lixiaoping0119@163.com) upon request.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Liu X, He S, Peng J, Guo X, Tan W. Expression profile analysis of selenium-related genes in peripheral blood mononuclear cells of patients with Keshan disease. Biomed Res Int. 2019; 2019:4352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao Y, Wei F, Xiaoping L, et al. Status and etiology progress of keshan disease. Adv Cardiovasc Dis. 2017, 38:225–229. [Google Scholar]
- 3.Chao Y, Rong L, Hongmei Z, et al. Clinical characteristics and prognosis of 92 patients with Keshan disease. J clinical cardiology (China). 2018: 34:773–777. [Google Scholar]
- 4.Lifei X, Xingyu Y, Rong L, Xiaoping L. Genetic progress of Keshan disease. Adv Cardiovasc Dis. 2018; 39:671–673. [Google Scholar]
- 5.Li Q, Liu M, Hou J, Jiang C, Li S, Wang T. The prevalence of Keshan disease in China. International J Cardiology. 2013; 168:1121–1126. [DOI] [PubMed] [Google Scholar]
- 6.Yuyao Z, Zhongbo W, Youzhang X. Summary of the data of national Keshan disease surveillance in 1990-1994. Chin J Control Endemic Dis. 1996; 11:53–56. [Google Scholar]
- 7.Zhongbo W. Summary of the national Keshan disease surveillance meeting in 1995. Chin J Endemiol. 1996; 15:256. [Google Scholar]
- 8.The national Surveillance Group for Keshan disease. Summary of the national Keshan disease surveillance meeting in 1996. Chin J Endemiol. 1997; 16:392. [Google Scholar]
- 9.Zhongbo W. Summary of the national Keshan disease surveillance meeting in 1997. Chin J Endemiol. 1998; 17:274. [Google Scholar]
- 10.The national Surveillance Group for Keshan disease. Summary of the national Keshan disease surveillance meeting in 1998. Chin J Endemiol. 1999; 6:473. [Google Scholar]
- 11.The national Surveillance Group for Keshan disease. Summary of the national Keshan disease surveillance meeting in 1999-2000. Chin J Endemiol. 2001; 20:01. [Google Scholar]
- 12.The national Surveillance Group for Keshan disease. Summary of the national keshan disease surveillance meeting in 2001. Chin J Endemiol. 2002; 21:130. [Google Scholar]
- 13.The national Surveillance Group for Keshan disease. Summary of the national keshan disease surveillance meeting in 2002. Chin J Endemiol. 2003; 18:30. [Google Scholar]
- 14.Wang T, Hou J, Li Q, Wang Z, Xu B, Dong G, et al. 2003 survey and analysis on Keshan disease in China. Chin J Endemiol. 2004; 23:444–447. [Google Scholar]
- 15.Wang T, Hou J, Li Q, Zhang L, Li X, Gao L, et al. Overall analysis of national Keshan disease surveillance in 2004. Chin J Endemiol. 2005; 24:401–403. [Google Scholar]
- 16.The national Surveillance Group for Keshan disease. Summary of the national Keshan disease surveillance meeting in 2005. Chin J Endemiol. 2006; 25:405–407. [Google Scholar]
- 17.The national Surveillance Group for Keshan disease. Analysis of the data of national Keshan disease surveillance in 2006 [J]. Chin J Endemiol. 2008; 27:296–299. [Google Scholar]
- 18.The national Surveillance Group for Keshan disease. Analysis of the data of national Keshan disease surveillance in 2007 [J]. Chin J Endemiol. 2008; 27:412–415. [Google Scholar]
- 19.The national Surveillance Group for Keshan disease. Analysis of the data of national Keshan disease surveillance in 2009. Chin J Endemiol. 2015; 34:425–429. [Google Scholar]
- 20.Wang T, Hou J, Li Q. Analysis of the data of the national Keshan disease surveillance in 2000-2004. Chin J Endemiol. 2005; 24:676–679. [Google Scholar]
- 21.Kang J, Zhang Z, Cui X. Monitoring and analysis of the Keshan disease, 2011-2016, Heilongjiang. Chin J of PHM. 2017; 33:520–522. [Google Scholar]
- 22.Zhan D, Wan X, Li D. The status of Keshan disease in jilin province. Chin J Ctrl Endem Dis. 2013; 28:372–373. [Google Scholar]
- 23.Wan X, Li D, Chen Z. Analysis of Keshan disease in jilin province in 2011. Chin J Ctrl Endem Dis. 2013; 28:133–135. [Google Scholar]
- 24.Liang H, Wan X, Wang J, Ge X, Li D, Miao D. Analysis of Keshan disease control and elimination self-examination project in jilin province in 2014. Chin J Ctrl Endem Dis. 2015; 30:380. [Google Scholar]
- 25.Wan X, Liang H, Wang J, Ge X. Analysis of the surveillance of Keshan disease in jilin in 2015. Chin J Ctrl Endem Dis. 2016; 31:726–729. [Google Scholar]
- 26.Liang H, Wan X, Wang S, Wang J. Analysis of the surveillance of Keshan disease in jilin in 2016. Chin J Ctrl Endem Dis. 2017; 32:1207–1210. [Google Scholar]
- 27.Fan J, Wang Z, Liu X, Li J, Guo F, Liu X, et al. Analysis of the surveillance of Keshan disease in inner mongolia from 2006 to 2012. Chin J Ctrl Endem Dis. 2014; 29:327–329. [Google Scholar]
- 28.Cai W, Li F, Yang F. The research of dilated cardiomyopathy and Keshan disease in sichuan province. Chin J Ctrl Endem Dis. 2015; 30:245–247. [Google Scholar]
- 29.Li F, Cai W, Ouyang B. Investigation of Keshan disease in Sichuan in2011-2013. Chin J Ctrl Endem Dis. 2014; 29:436–438. [Google Scholar]
- 30.Li F, Cai W, Li J. Survey on Keshan disease in Sichuan, 2014-2015. J Occupational Health Damage. 2016; 31:293–295. [Google Scholar]
- 31.Li Z, Yang L, Zhao S, Huang W, Wang YB, Yang P. Analysis of Keshan disease investigation result in Yunnan province in 2010. Foreign Medical Sci Section Med geography. 2012; 33:26–29. [Google Scholar]
- 32.Li Z, Yang L, Huang W, Ye F, Wang YB, Yang P. Analysis of the surveillance Keshan disease in Yunnan province in 2011. Foreign Medical Sci Section Med geography. 2013; 34:234–237. [Google Scholar]
- 33.Li Z, Huang W, Ye F, Wang A, Wang YB, Huang K. Analysis of Keshan disease investigation result in Yunnan province in 2012. Chin J Ctrl Endem Dis. 2015; 30:489–491. [Google Scholar]
- 34.Quan L, Hou J, Wang T, Xu B, Sun S. Prevalence of dilated cardiomyopathy and quasi-latent Keshan disease in six province of China. Chin J Endemiol. 2013; 32:431–435. [Google Scholar]
- 35.Yang X, Kang C, Wang W. Analysis of the surveillance Keshan disease in qi county, Shandong Province. Chin J Ctrl Endem Dis. 2017; 32:507–509. [Google Scholar]
- 36.Chen J. An original discovery: selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr. 2012; 21:320–326. [PubMed] [Google Scholar]
- 37.Zhang YS, Li RL, Liu Y, Yu H, Keke H. Research progress on the role of selenium in human health (author’s transl). Internal Medicine. 2018; 13:623–625. [Google Scholar]
- 38.Winther KH, Rayman MP, Bonnema SJ, Hegedüs L. Selenium in thyroid disorders - essential knowledge for clinicians. Nat Rev Endocrinol. 2020; 16:165–176. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Yue L, Wang F, et al. Studies on the relationship between the pathogenic factors of Keshan disease and changes of thyroid hormone metabolism in myocardial tissue and on the mechanism and meanings. Endemic Diseases Bulletin. 1995; 10:53–56. [Google Scholar]
- 40.Wu H, Xia Y, Chen X. Selenium deficiency and thyroid hormone metabolism and function. Advances in Physiology. 1995:12–16. [PubMed] [Google Scholar]
- 41.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007; 116:1725–1735. [DOI] [PubMed] [Google Scholar]
- 42.Khalife WI, Tang YD, Kuzman JA, Thomas TA, Anderson BE, Said S, et al. Treatment of subclinical hypothyroidism reverses ischemia and prevents myocyte loss and progressive LV dysfunction in hamsters with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005; 289:H2409–H2415. [DOI] [PubMed] [Google Scholar]
- 43.Lei C, Niu X, Ma X, Wei J. Is selenium deficiency really the cause of Keshan disease? Environ Geochem Health. 2011; 33:183–188. [DOI] [PubMed] [Google Scholar]
- 44.Ren LQ, Li XJ, Li GS, Zhao ZT, Sun B, Sun F. Coxsackievirus B3 infection and its mutation in Keshan disease. World J Gastroenterol. 2004; 10:3299–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Niu X, Lei C. Circulating adhesion molecules in patients with Keshan disease and their relationship with Coxsackie B virus infection. J Huazhong Univ Sci Technolog Med Sci. 2009; 29:173–176. [DOI] [PubMed] [Google Scholar]
- 46.Beck MA, Kolbeck PC, Rohr LH, Shi Q, Morris VC, Levander OA. Benign human enterovirus becomes virulent in selenium-deficient mice. J Med Virol. 1994; 43:166–170. [DOI] [PubMed] [Google Scholar]
- 47.Montesinos MDM. Thyroid hormone action on innate immunity. Front Endocrinol (Lausanne). 2019; 10:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohler LN, Foote J, Kelley CP, Florea A, Shelly C, Chow HS. Selenium and type 2 diabetes: systematic review. Nutrients. 2018; 10:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao C. The Genetic research in children with Keshan disease of Yi nationality. Hereditas (Beijing). 1983; 5:37–38. [Google Scholar]
- 50.Zhang J, Liu ZG. Epidemiological survey on families with Keshan disease in Huangling County in Shaanxi. Chin J Endemiol. 2007; 26:421–422. [Google Scholar]
- 51.Deng J, Luo R, Li X. HLA-DRB1 gene polymorphism is associated with idiopathic dilated cardiomyopathy: a meta-analysis. J Cardiovasc Med (Hagerstown). 2011; 12:648–652. [DOI] [PubMed] [Google Scholar]
- 52.Niu X, Wang Y, Zhang H, Zhu J, Wei J, Dong X. The study of genotyping of HLA-DRB1 by PCR-SSOP in Keshan disease in Shaanxi Province. Chin J Ctrl Endem Dis. 2003; 18:327–328. [Google Scholar]
- 53.Wei J, Niu XL, Dong X, Wang YP, Zhu JH. The study on relation of HLA-DRB1 gene polymorphism to Keshan disease and its association and linkage in the core families. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2007; 24:91–93. [PubMed] [Google Scholar]
- 54.Lei C, Niu XL, Wei J, Zhu JH, Zhu Y. Polymorphisms in the glutathione peroxidase-1 gene associated with increased risk of Keshan disease. Zhonghua Yu Fang Yi Xue Za Zhi. 2010; 44:617–621. [PubMed] [Google Scholar]
- 55.Jiang S. Analysis the relationship between GPx gene polymorphism, blood selenium and GPx-1 enzyme activity in patients with Keshan disease. Chin J Ctrl Endem Dis. 2016; 31:644–645. [Google Scholar]
- 56.Wei HL, Pei JR, Jiang CX, Zhou LW, Lan T, Liu M, et al. Analysis of glutathione peroxidase 1 gene polymorphism and Keshan disease in Heilongjiang province. ChinGenet Mol Res. 2011; 10:2996–3001. [DOI] [PubMed] [Google Scholar]
- 57.Jiang S, Li FL, Dong Q, Liu HW, Fang CF, Shu C, et al. H558R polymorphism in SCN5A is associated with Keshan disease and QRS prolongation in Keshan disease patients. Genet Mol Res. 2014; 13:6569–6576. [DOI] [PubMed] [Google Scholar]
- 58.Han S, Shu C, Li H. Initial study of gene mutation of SCN5A in Keshan disease. J Qiqihar Med College. 2007; 28:1155–1157. [Google Scholar]


