Abstract
Coronary microvascular disease (CMD) is present in 30% of patients with angina and is associated with increased morbidity and mortality. We now have an improved understanding of the pathophysiology of CMD and the invasive and noninvasive tests that can be used to make the diagnosis. Recent studies have shown that management of CMD guided by physiological testing yields better results than empirical treatment. Despite major advances in diagnosing and stratifying this condition, therapeutic strategies remain limited and poorly defined. This review article discusses recent advances in understanding the pathophysiology of CMD, the modalities that are available to diagnose it clinically, current management options and a look at what is in store for the future.
Keywords: coronary microvascular disease, coronary blood flow, coronary flow reserve, microvascular resistance, nitric oxide, physiology-stratified disease endotypes
Introduction
Angina is chest pain as a result of myocardial ischaemia, and it affects up to 2 million people in the UK [1]. This has historically been viewed as the manifestation of obstructive coronary artery disease (CAD). However, up to 50% of patients with angina, who undergo elective coronary angiography, are found to have nonobstructive coronary artery disease (NOCAD) [2]. The term NOCAD encompasses a broad range of pathologies, including coronary microvascular disease (CMD), epicardial coronary artery vasospasm and enhanced cardiac nociception with disparate prognostic outlooks and therapeutic implications. Over half of all patients with NOCAD have CMD [3], which is associated with a greater risk of major adverse cardiovascular events (MACE). Several studies have demonstrated that women, presenting with angina, are more likely to suffer from NOCAD and CMD [3]. The term CMD was coined in 1988 to describe the abnormality in the microcirculation leading to an inadequate vasodilatory response, or a pathological vasoconstrictive response, to physiological or pharmacological stress [4]. The main parameter used to diagnose CMD is diminished coronary flow reserve (CFR), or impaired ability of the microvasculature to augment its blood flow in response to stress [5,6]. CFR is defined as the ratio of flow at maximal hyperaemia (usually in response to adenosine) to the flow at rest. In the context of NOCAD, CFR informs about prognosis, the presence of ischaemia and likely response to therapy [5,6]. The focus of this review article will be on the pathophysiology, diagnosis and contemporary management of CMD.
Coronary microvasculature in health
The coronary vasculature comprises of epicardial arteries (>400 µm), pre-arterioles (100–400 µm), arterioles (<100 µm) and capillaries (<10 µm). The epicardial arteries function as capacitance vessels and respond to shear forces by endothelium-mediated dilatation. Epicardial arteries are visible on coronary angiography but represent only 5–10% of the coronary vasculature. The pre-arterioles, arterioles and capillaries form the coronary microvasculature. The pre-arterioles are characterised by a measurable pressure drop along their length. The arterioles have a high resting tone and are responsible for most of the coronary vascular resistance and dilate in response to changes in myocardial oxygen demand. The capillary bed delivers oxygen and substrates to the myocytes. The coronary circulation matches myocardial oxygen demand with supply via a complex interplay between myogenic tone, metabolic signals and circulating hormones [7]. The endothelium plays an important role in the modulation of vascular tone by synthesising and releasing several vasodilator substances, such as nitric oxide (NO) [7]. Increased endothelial wall shear stress and acetylcholine are determinants of coronary blood flow (CBF) in health. Both lead to the biosynthesis of NO, which acts on the neighbouring smooth muscle cells to induce vasodilation via the NO pathway [7].
Pathophysiology of coronary microvascular disease
Traditionally, the pathophysiology of CMD was thought to be a combination of microvascular architectural changes and endothelial dysfunction. Microvascular architectural changes include microvascular obstruction, with luminal narrowing of the arterioles and capillaries, and capillary rarefaction [8]. Alternatively, or concurrently, endothelial or vascular smooth muscle (VSM) dysfunction may lead to an attenuated vasodilatory response or a pathological vasoconstrictive response to stimuli, leading to a blunted augmentation of, or reduction of, CBF in response to stress [9]. These cellular mechanisms are described in Fig. 1. This can lead to a supply-demand mismatch in CBF, therefore leading to ischaemia and symptoms of angina.
Fig. 1.
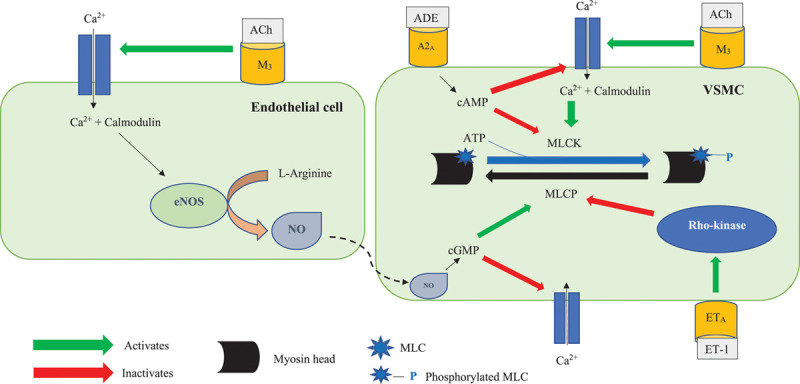
The endothelial and vascular smooth muscle cellular pathways (adapted from Lanza et al. [14]). Acetylcholine has dual effects on coronary microvasculature. It binds to the muscarinic 3 (M3) receptor on endothelial cells and leads to an influx of intracellular calcium (Ca2+) via the L-type calcium channels. Intracellular Ca2+ binds to the protein calmodulin, and the calcium-calmodulin complex activates the endothelial nitric oxide synthase (eNOS) enzyme, which catalyses the conversion of L-arginine into nitric oxide (NO). NO then diffuses into the neighbouring vascular smooth muscle cell (VSMC) and activates guanylate cyclase (GC) enzyme to catalyse the conversion of guanosine triphosphate (GTP) into cyclic GMP (cGMP). cGMP activates the protein kinase G (PKG), which, via a series of intracellular events, inactivates the calcium channels on the VSMC. This reduces the intracellular influx of Ca2+ into the VSMC, therefore leading to vasodilation. Acetylcholine also binds to the M3 receptor on the surface of VSMCs and, in the presence of endothelial dysfunction, leads to unopposed vasoconstriction. Adenosine (ADE) binds to its receptor (A2a) on the surface of VSMCs; this activates adenylate cyclase (AC) enzyme, which catalyses the conversion of ATP (ATP) to cyclic AMP (cAMP). cAMP activates the protein kinase A (PKA). PKA inactivates the calcium channels and prevents influx of Ca2+, therefore preventing vasoconstriction. Ca2+ enters VSMCs via the L-type calcium channels and binds to the protein calmodulin. The calcium-calmodulin complex activates myosin light chain kinase (MLCK), which phosphorylates myosin light chains (MLCs). MLCs are found on the myosin heads. MLC phosphorylation leads to cross-bridge formation between the myosin heads and the actin filaments, leading to vascular smooth muscle (VSM) contraction. cAMP inhibits MLCK, therefore promoting vasodilation. MLC phosphatase (MLCP) dephosphorylates MLC and promotes unbinding of the myosin-actin filaments, therefore leading to vasodilation. cGMP promotes MLCP activity. Endothelin-1 (ET-1) binds to its receptor (ETA) and activates Rho-kinase, which inhibits MLCP and leads to vasoconstriction. VSM relaxation, and therefore vasodilation, occurs when there is reduced phosphorylation of MLC. This can result from reduced intracellular Ca2+ concentration, inhibition of MLCK by increased intracellular concentration of cAMP and MLCP-activated MLC dephosphorylation.
This has remained the dogma of CMD pathogenesis for the past few decades. However, recent animal models and clinical physiology evaluations suggest that CMD may be a heterogeneous condition comprising distinct endotypes [5,6]. Rahman et al. have described these endotypes as ‘structural CMD’ and ‘functional CMD’ [5,6]. Both endotypes have an impaired augmentation of CBF in response to intravenous adenosine (CFR < 2.5). However, patients with structural CMD have an elevated minimal microvascular resistance (which translates to reduced maximal CBF), whereas patients with functional CMD have a normal minimal microvascular resistance, but nevertheless have reduced vasodilatory reserve as they have reduced tone at rest [5,6].
These endotypes have a similar core phenotype, with both groups demonstrating high prevalence of stress perfusion defects on cardiac magnetic resonance (CMR) imaging and reduced coronary perfusion efficiency, on wave intensity analysis, during physical exercise [5,6]. However, they differ in their pathogenesis at the microvascular level. Patients with functional CMD were found to have a heightened resting CBF [5,6]. This is suggestive of a submaximal vasodilatory state at rest, leading to an attenuated vasodilatory capacity in response to physiological stress. The elevated resting CBF in these patients could be an appropriate response to an increased myocardial oxygen demand or it could represent disordered autoregulation of the neuronal nitric oxide synthase (nNOS) pathway, which has been shown to regulate the resting CBF in both health and disease states [10,11]. On the other hand, patients with structural CMD have a normal resting CBF [5,6], similar to patients with preserved CFR, but they have an impaired ability to augment their CBF in response to physiological stress, leading to ischaemia. Patients with structural CMD appear to have more established cardiovascular risk factors, including poorly controlled hypertension, type 2 diabetes mellitus (T2DM) and a higher prevalence of exercise-induced hypertension [5,6]. It has been hypothesised that the attenuated reduction in afterload with exercise would interrupt the usual synergistic response of the coronary and peripheral circulations and predispose to ischaemia in patients with structural CMD [5,6]. However, it remains unclear whether patients with structural CMD have an impaired ability to augment their CBF as a result of architectural changes, such as microvascular hypertrophy or fibrosis limiting their ability to vasodilate, or whether it is due to dysregulation of the endothelial NOS (eNOS) pathway, which has been shown to regulate CBF in response to exertion [11].
The findings of Sezer et al. [12] and Van de Wouw et al. [13] further corroborate those of Rahman et al. Sezer et al. reported a bimodal distribution of impaired CFR in patients with T2DM depending on the duration of diabetes. Diminished CFR was due to elevated resting flow in the early stages of diabetes (<10 years duration) and due to heightened hyperaemic microvascular resistance and a reduction in hyperaemic coronary flow velocity in the latter stages of the disease (>10 years duration). The authors hypothesised that elevated resting flow in the early stages was due to either impaired coronary microvascular autoregulation or an adaptive response to altered myocardial energy metabolism. The authors further hypothesised that the increased resting CBF in the early stages of the disease may have led to structural changes in the coronary microvasculature, such as capillary rarefaction and fibrosis, ultimately leading to the attenuated hyperaemic CBF in the later stages of the disease [12]. Van de Wouw et al. reported that swine that were induced with hypertension, hyperlipidaemia and T2DM were found to have a heightened resting CBF, which led to reduced CFR. The swine demonstrated inefficient myocardial perfusion, requiring higher oxygen consumption for a given level of myocardial work. The abnormalities in myocardial oxygen delivery were accompanied by a reduction in lactate consumption, particularly during exercise, indicating an increased oxygen demand [13]. The authors suggested that the increased oxygen demand was either due to a myocardial substrate shift towards fatty acid oxidation leading to a reduced phosphate:oxygen ratio and increased oxygen consumption, or it was due to mitochondrial uncoupling leading to a reduction in phosphate:oxygen ratio, thereby increasing oxygen consumption at a given level of cardiac work [13].
These studies have improved our understanding of the pathophysiology of CMD. However, many questions remain unanswered. Is the increased resting CBF in patients with functional CMD due to a dysregulation of the neuronal NOS pathway or is it due to an increased oxygen demand at rest? Is the inability to augment CBF during exertion in patients with structural CMD due to architectural changes or due to dysregulation of the eNOS pathway? Are functional and structural microvascular dysfunction part of a disease continuum of the coronary microvasculature? Further studies are needed to answer these pertinent mechanistic questions.
Although a detailed review of epicardial coronary artery vasospasm, and consequent vasospastic angina (VSA), is beyond the scope of this focussed review, it is worth reviewing some of the features of this condition. Vascular smooth muscle cell (VSMC) contraction is regulated by a complex system of intracellular pathways. The central molecular mechanism leading to VSMC hypercontraction is Rho kinase-mediated enhancement of myosin light chain (MLC) phosphorylation [14] (Fig. 1). Other mechanisms contributing towards coronary vasospasm include endothelial dysfunction, low-grade inflammation and oxidative stress. However, these are unlikely to be the primary drivers of coronary vasospasm, and likely serve as additional pathophysiological risk factors.
Clinical presentation
Angina secondary to CMD is characterised by effort-induced symptoms often indistinguishable to those observed in patients with angina triggered by obstructive CAD. Patients with CMD tend to experience prolonged episodes of chest pain and a relatively poor response to oral nitrates [15]. On the other hand, patients suffering from epicardial coronary artery vasospasm, VSA, tend to suffer from angina at rest (especially at night and early morning). These symptoms can be precipitated by hyperventilation and respond to short-acting nitrates within seconds [16].
Recently, Ong et al. have proposed the Coronary Vasomotion Disorders International Study Group criteria to diagnose CMD [17]. This diagnostic criterion includes (1) presence of symptoms of myocardial ischaemia, (2) absence of obstructive epicardial CAD, (3) evidence of objective myocardial ischaemia on functional imaging and (4) evidence of impaired coronary microvascular function. Ong et al. have suggested that the presence of four of these criteria suggests a definite diagnosis of CMD, whilst the presence of three suggests probable CMD [17]. Whilst these criteria are logical and provide a useful framework for thinking about CMD, the need to perform both invasive and noninvasive characterisation may be one of the factors that have led to the poor uptake of this in routine clinical practice. Rahman et al. have shown that in patients with a high pretest probability of coronary vascular abnormality, that is, convincing history and presence of cardiovascular risk factors, CFR < 2.5 reliably identifies patients with NOCAD who will have myocardial ischaemia on CMR imaging (82% with CFR < 2.5 had stress perfusion defects vs. 22% with CFR ≥ 2.5) [5,6]. Therefore, we believe that a definite diagnosis of CMD can be achieved using a single invasive test that involves physiological assessment of the coronary vasculature. Equally, in patients who have a CT coronary angiogram as the initial investigation for angina, it may prove possible to make a diagnosis of CMD with a high degree of certainty on the basis of a high resolution stress perfusion CMR alone, without recourse to an invasive assessment. However, this assertion needs to be evaluated further and it should be noted that, at present, there is no noninvasive test for VSA.
Patient outcomes
Patients with angina and NOCAD have a poor quality of life, with 44% of patients continuing to suffer from chest pain after 12 months from their diagnosis of NOCAD [18]. These patients score similarly on the Seattle Angina Questionnaire (SAQ), for both physical limitations and quality of life, as patients with obstructive CAD [18]. They are also more likely to suffer from depression and anxiety either concurrently or as a result of their persistent symptoms [19], and they are more likely to utilise the healthcare system than patients with obstructive CAD [19].
There has been much debate over the long-term outcomes of patients with CMD. Earlier studies had shown that patients with cardiac syndrome X (CSX) had a favourable long-term prognosis [20]. CSX is an umbrella term that was previously used to describe patients with angina with NOCAD, and it comprised of several pathologically distinct diagnoses. Recent studies; however, have consistently reported adverse outcomes in patients with confirmed CMD. Of note, AlBadri et al. have reported that CFR < 2.3 independently predicted a higher risk of composite endpoint of death, myocardial infarction (MI), stroke and hospitalisation for heart failure in women with NOCAD and nonendothelium dependent microvascular dysfunction at a median follow-up of 9.7 years [21]. They also reported a trend towards higher rates of the composite endpoint in women with endothelium-dependent dysfunction [21]. Pepine et al., similarly, showed an increase in the composite outcome of death, nonfatal MI, nonfatal stroke, or hospitalisation for heart failure in women with NOCAD and CFR < 2.32 [22]. Murthy et al. reported an increased incidence of MACE, defined as cardiac death, nonfatal MI, late revascularisation and hospitalisation for heart failure, in patients with NOCAD and CFR < 2.0 on PET imaging after a median follow-up of 1.3 years [23]. CFR was a powerful predictor of MACE in their patient cohort [23]. Suwaidi et al. assessed endothelium-dependent microvascular function in 157 patients with angina and NOCAD [24]. Over a 28-month mean follow-up, none of their patients with normal endothelial function or mild endothelial dysfunction (0–50% increase in CBF in response to intracoronary acetylcholine) suffered from any adverse events. However, 14% of patients with severe endothelial dysfunction (<0% increase in CBF) suffered from MACE, which included MI, future revascularisation or cardiac death [24].
Although the overall survival is reported to be good in patients with VSA, Takagi et al. reported a MACE rate of nearly 6%, comprising death, nonfatal MI, unstable angina, heart failure or appropriate implantable cardioverter defibrillator shocks in their cohort of 1429 patients (median follow-up of 32 months) [25]. Sato et al., similarly, reported a MACE rate of approximately 5% during a mean follow-up of 49 months in 873 patients with VSA [26].
The impact of metabolic and chronic inflammatory disorders on coronary microvascular function
Diabetes mellitus has long been linked with coronary microvascular dysfunction. A recent study has reported that 72% of patients with angina, NOCAD and diabetes mellitus have CMD [27]. This study also reported an association between glycated haemoglobin (HbA1c) levels and endothelial-independent and endothelial-dependent coronary microvascular function in patients with diabetes mellitus. These findings suggest a link between glycaemic control and coronary microvascular dysfunction, and may implicate CMD as a potential mediator of ischaemia in patients with diabetes mellitus [27]. Coronary microvascular dysfunction has been associated with the severity of microalbuminuria in patients with diabetes mellitus [28]. Animal and human studies have demonstrated endothelial and VSM dysfunction in diabetic models, which then progress on to irreversible architectural changes in the microvasculature. The exact mechanisms leading to the development of CMD in patients with diabetes mellitus are presently unknown; however, hyperglycaemia induced oxidative stress, insulin resistance and inflammation are the likely drivers. Endothelium-dependent vasodilation correlates with insulin sensitivity in healthy subjects, suggesting that insulin plays an important role in the early processes of endothelial dysfunction [29].
Along with diabetes mellitus, CMD has also been shown to be prevalent in patients with chronic inflammation, such as in inflammatory rheumatological disorders [30]. In the early stages of inflammatory rheumatological disorders pro-inflammatory mediators, such as tumour necrosis factor-alpha, promote endothelial dysfunction by reducing the synthesis and bioavailability of NO through a reduction in the expression of eNOS. Inflammatory cytokines are also responsible for the increased production of reactive oxygen species, which contribute to limiting NO availability [30].
Assessment of the coronary microvasculature
The coronary microvasculature cannot be directly visualised but there are several techniques to assess coronary microvascular function. In patients with NOCAD, CFR is the most commonly used test of microvascular function. Coronary microvascular function can also be assessed noninvasively, primarily using techniques that estimate myocardial perfusion at rest and during stress. In the following section, the practical aspects of making these assessments are discussed as well as their advantages and disadvantages.
Invasive intracoronary assessment in the catheter laboratory
Invasive intracoronary microvascular assessment is well tolerated and provides an accurate and reproducible evaluation of microvascular function [31]. The 2019 ESC guidelines recommend intracoronary microvascular assessment in patients with angina and NOCAD (class IIa recommendation) [32]. Invasive CFR requires measurement of coronary flow. While absolute coronary flow is difficult to measure in a clinical setting, it can currently be estimated by one of two techniques: Doppler to measure coronary flow velocity or thermodilution to measure cold bolus transit time, each requiring the use of different sensor-tipped ultra-thin intracoronary guidewires. CBF is estimated at rest and in response to adenosine (to test nonendothelium dependent function) or acetylcholine (to test endothelium-dependent function). In the absence of epicardial obstructive CAD, an impaired CFR (defined as <2.5 or <2.0 depending on the outcome measure) diagnoses the presence of CMD [33].
If intracoronary pressure is simultaneously measured, it is also possible to calculate coronary microvascular resistance, as the ratio between myocardial perfusion pressure (which approximates to distal coronary pressure (Pd) when venous pressure is negligible) and flow. When flow is estimated by Doppler flow velocity, the resulting index is called hyperaemic microvascular resistance (hMR); hMR = Pd/APV, where APV is average peak velocity. When assessed using thermodilution techniques it is defined as the index of microvascular resistance (IMR), calculated as the product of Pd and mean transit time (the time it takes for a bolus of saline to transit through a defined distance during maximal hyperaemia). There is a reasonable correlation between hMR and IMR [34], and for practical purposes, these indices are considered interchangeable at present, the technique used depending on availability and expertise in each cardiac unit.
Endothelium-dependent microvascular dysfunction is diagnosed when there is <50% increase in CBF, and ischaemic ECG changes and angina, in the absence of epicardial vasoconstriction, in response to acetylcholine. It is important to remember that patients with CMD may have either endothelium-dependent dysfunction, nonendothelium dependent dysfunction or both [3], and although CMD can be diagnosed using adenosine alone, the diagnostic accuracy is improved by using acetylcholine. This allows a complete microvascular functional assessment and informs about important clinical outcomes and may influence management strategies [33]. The 2019 ESC guidelines have given the use of acetylcholine provocation, to diagnose endothelium-dependent microvascular dysfunction, a class IIb recommendation [32].
In patients with symptoms consistent with VSA, it is diagnosed by demonstration of either transient ischaemic ECG changes during a spontaneous episode or epicardial coronary vasospasm (>90% reduction in epicardial coronary artery diameter) associated with ECG ischaemic changes and chest pain in response to a provocative agent [35]. However, the test only has modest sensitivity (approximately 70%), especially in younger patients. Furthermore, female patients are more likely to be hypersensitive to acetylcholine provocation, giving rise to false positives [36]. However, it should be noted that there are several logistical difficulties with carrying out acetylcholine-based endothelial function testing and its availability is currently restricted to specialist centres, although this is likely to increase following recent demonstrations of its utility. In view of this, the authors recently published a stepwise diagnostic algorithm that can be used in any patient with angina who presents to the cardiac catheter laboratory [37].
Noninvasive assessment of the coronary microvasculature
Numerous noninvasive modalities have been used to assess the coronary microvasculature over the past few decades. Each has characteristic advantages and disadvantages that have affected their application in clinical practice.
PET imaging has been the most studied noninvasive imaging modality to assess coronary microvasculature in the research setting and there is evidence that links the presence of CMD detected on PET imaging to an increased risk of MACE [38]. However, the uptake of PET imaging in routine clinical practice has been limited due to the associated ionising radiation. Similarly, despite recent advances, the uptake of single-photon emission computerised tomography for quantitative perfusion imaging in CMD has been limited by technical issues [39], which has led to it being superseded by CMR imaging.
Echocardiography, using pulsed waved Doppler at the proximal coronary arteries, can be used to calculate the coronary flow velocity reserve at rest and peak hyperaemia [40]. This technique, however, is limited by interoperator measurement discrepancies and reduced resolution in obese patients.
CMR imaging is now the noninvasive modality of choice to diagnose patients with CMD. The imaging protocol consists of rest and vasodilator-stress first-pass myocardial perfusion study, each following the injection of a gadolinium-based contrast agent. Post-processing of the rest and stress images allows for the quantification of regional and global myocardial perfusion using semiquantitative (myocardial perfusion reserve index) and fully quantitative (myocardial blood flow) models. CMR imaging offers high spatial resolution due to temporal under-sampling techniques, improved signal-to-noise ratio using 3-Tesla scanning, lack of ionising radiation, reproducibility and the ability to perform a comprehensive assessment of the cardiovascular structure and function. CMR imaging interrogates the early stages of the ischaemic cascade and is well placed to identify subendocardial ischaemia that is characteristic of coronary microvascular dysfunction. A reduced myocardial perfusion reserve index has also been shown to predict prognosis in patients with CMD [41]. The limiting factor for the uptake of CMR has been the associated cost.
In summary, invasive assessment of the coronary microvascular function is accurate, reproducible, validated, well tolerated and endorsed by the most recent ESC Guidelines [32]. CMD can be diagnosed during a single cardiac catheterisation procedure, overcoming issues of specificity and sensitivity associated with noninvasive ischaemia tests. We recommend the use of intracoronary physiology assessment during the index procedure, if such facilities are available, in patients who have been referred for coronary angiography for angina and are found to have NOCAD. In circumstances where intracoronary physiology assessment is not possible during the index coronary angiography, we recommend that patients undergo functional imaging, such as stress perfusion CMR, to confirm subendocardial ischaemia consistent with CMD. The advantages of offering intracoronary physiology assessment during the index coronary angiography include a shorter time to diagnosis that may facilitate earlier targeted management for patients, characterisation of CMD endotype, reduced number of investigations that a patient must undergo and reduced costs to the healthcare system.
Management
Currently, there is a limited evidence base to guide therapeutic options in patients with CMD. For the most part, therapies used for angina due to obstructive CAD do not distinguish the underlying pathophysiological process. Ford et al. have recently shown that therapy based on invasive microvascular testing leads to improved symptom control in patients with angina and NOCAD [42], compared to an entirely empirical strategy. Management of CMD should encompass controlling cardiovascular risk factors, improving endothelial and VSM function (disease-modifying therapy), and achieving symptomatic relief. Appropriate management of hypertension, diabetes mellitus and hyperlipidaemia, as well as smoking cessation advice, is imperative as these contribute towards impaired microvascular function [43,44]. Obesity is highly prevalent in patients with CMD, and exercise programmes and cardiac rehabilitation may help facilitate important long-term lifestyle changes in these patients [45]. The current ESC guidelines recommend treatment with aspirin and statins (class I indication), and consideration of angiotensin-converting enzyme (ACE) inhibitors (class IIb indication) for patients with NOCAD [32]. Aspirin is recommended by extrapolation of epicardial CAD studies. Statin therapy improves vascular inflammation and endothelial function. Angiotensin II is a potent vasoconstrictor and may modulate coronary microvascular tone directly, therefore blocking it using an ACE inhibitor may prove beneficial in patients with CMD. These three agents may lead to improved coronary microvascular function but may have no immediate effect on symptom relief. Recently, there have been several studies investigating agents for symptomatic relief and microvascular function improvement in patients with CMD; some of these are discussed below and are summarised in Table 1.
Table 1.
Summary of medications that have been studied in patients with coronary microvascular disease
| Agent | Mechanism of action | Evidence base |
|---|---|---|
| Vasodilatory beta blockers (for example, nebivolol, carvedilol and celiprolol) | Vasodilatory properties can be due to activation of the eNOS pathway (nebivolol) or preservation of NO bioactivity (carvedilol) | Nebivolol augmented hyperaemic CBF and attenuated resting CBF in patients with idiopathic dilated cardiomyopathy with unobstructed coronary arteries and coronary microvascular dysfunction [46] and improved exercise time in patients with CSX [47]. Four months’ therapy of carvedilol improved flow-mediated dilatation, a marker of endothelium-dependent function, in patients with epicardial coronary artery disease [48]. |
| Calcium channel blockers: amlodipine or diltiazem | Reduces influx of calcium in the VSMCs; therefore, leading to vasodilation (Fig. 1) | Treatment with calcium channel blockers improved symptom control in patients with endothelium-dependent CMD over 48 months [49] |
| Ranolazine | #1: Inhibits the sodium–calcium co-transporter and reduces influx of calcium in cardiomyocytes | Bairey-Merz et al. [50] and Rambarat et al. [51] have shown that ranolazine improves myocardial perfusion and angina frequency in patients with angina, NOCAD and CFR < 2.5. Tagliamonte et al. reported an improvement in SAQ domains and CFR in patients with angina, NOCAD and myocardial ischaemia after 8 weeks of ranolazine [52] |
| #2: shifts ATP metabolism from inefficient fatty-acid oxidation to oxygen-sparing glucose oxidation | ||
| Endothelin receptor antagonists (ERA) | ERAs antagonise endothelin-1 (ET-1). ET-1 increases peripheral and coronary vascular tone by activating the ETA receptor on VSMCs (Fig. 1) | Oral atrasentan (ERA) improved endothelial function in patients with angina and NOCAD with impaired endothelium-dependent microvascular function [53] |
| Rho-kinase inhibitors | Rho-kinase inhibitors prevent the inactivation of MLCP, therefore, lead to VSM vasodilatation (Fig. 1) | Intracoronary fasudil (rho-kinase inhibitor) ameliorated myocardial ischaemia (defined as ischaemic ECG changes, increased myocardial lactate or both) in patients with endothelium-dependent microvascular dysfunction [54] |
| Angiotensin-converting enzyme (ACE) inhibitors | Angiotensin II is a potent coronary vasoconstrictor; therefore, ACE inhibitors, by inhibiting the action of Angiotensin II, may promote coronary microvascular vasodilation | Treatment with 16 weeks of quinapril improved CFR in women with angina, NOCAD and CMD. There was also improvement in angina frequency, although the study was not adequately powered for the latter outcome [57] |
ATP, adenosine triphosphate; CMD, coronary microvascular disease; CBF, coronary blood flow; CSX, coronary syndrome X; CFR, coronary flow reserve; eNOS, endothelial nitric oxide synthase; MLCP, myosin light chain phosphatase; NOCAD, nonobstructive coronary artery disease; SAQ, Seattle Angina Questionnaire; VSMC, vascular smooth muscle cell.
Beta blockers
Although all groups of beta blockers may help patients with CMD, those with vasodilatory properties may provide the most benefits. Nebivolol is a highly selective beta-1 blocker with vasodilatory properties through activation of the eNOS pathway and inhibition of endothelin-1 (ET-1) synthesis. Erdogan et al. showed that 1 month of Nebivolol improved CFR (from 2.02 to 2.61, on average), by augmenting the hyperaemic CBF and attenuating the resting CBF, in 21 patients with idiopathic dilated cardiomyopathy, unobstructed coronary arteries and coronary microvascular dysfunction [46]. Erdamer et al. showed that 12 weeks of Nebivolol improved exercise time and time to 1 mm ST depression on treadmill in 15 patients with CSX (defined as angina, abnormal exercise treadmill test and unobstructed coronary arteries) [47]. Carvedilol is also a vasodilatory beta blocker and has been found to ameliorate endothelial-dependent microvascular function in peripheral arterial trees patients with epicardial CAD [48]. Beta blockers should; however, be avoided in patients with VSA as they can lead to an unopposed alpha-1 adrenergic stimulation, leading to worsening of coronary artery vasospasm.
Calcium channel blockers
Calcium channel blockers antagonise the L-type calcium channels on VSMCs; therefore, they inhibit the influx of calcium ions, leading to reduced VSM contraction (Fig. 1). Ohba et al. investigated 50 patients with endothelium-dependent CMD [49]. 70% of these patients showed improvement in their frequency of angina with calcium channel blockers during a 48 month follow up period [49].
Ranolazine
Ranolazine inhibits the late sodium current in cardiomyocytes, decreasing sodium and calcium overload. During ischaemia, excess intracellular calcium impairs VSM relaxation and leads to impaired coronary perfusion. Ranolazine also promotes the switch from an inefficient fatty acid metabolism to oxygen-sparing glucose oxidation, therefore reducing oxygen consumption. Both Bairey-Merz et al. [50] and Rambarat et al. [51] have shown that ranolazine improved myocardial perfusion (on CMR imaging) and angina frequency in patients with NOCAD and CFR<2.5. Tagliamonte et al. reported an improvement in SAQ domains and CFR in 58 patients with angina, NOCAD and myocardial ischaemia after 8 weeks of ranolazine; the authors reported this to be secondary to a reduction in baseline coronary flow velocity and an augmented hyperaemic coronary flow velocity [52].
Endothelin receptor antagonists
ET-1 contributes to the regulation of vascular tone through endothelin-A (ETA) and endothelin-B (ETB) receptors. ETA receptors are located on VSM and mediate vasoconstriction by activating Rho-kinase (Fig. 1) and by reducing NO bioavailability (by inhibiting eNOS activity and increasing NO degradation). Reriani et al. showed that 6 months of Atresantan (ETA antagonist) improved endothelial function, measured as CBF response to intracoronary acetylcholine, in patients with angina, NOCAD and endothelial dysfunction [53].
Rho-kinase inhibitors
ET-1 binds to ETA receptor and activates Rho-kinase, which inhibits myosin light chain phosphatase (MLCP) (Fig. 1). MLCP dephosphorylates MLC and promotes unbinding of the myosin-actin filaments, leading to VSM dilatation. Therefore, activation of Rho-kinase promotes VSM contraction. Mohri et al. reported an improvement in symptoms of angina and myocardial ischaemia (demonstrated by coronary sinus lactate measurement and/or ECG changes) after intracoronary fasudil (Rho-kinase inhibitor) in patients with endothelium-dependent CMD [54].
Other anti-anginals
Along with the agents described above, other anti-anginals have also been used in patients with CMD. These include nicorandil, ivabradine and trimetazidine. Whilst these medications lack robust evidence-base for efficacy in CMD, there are small studies/anecdotal evidence supporting their use. Ivabradine is an If channel blocker that selectively reduces sinus node activity. A study demonstrated improved anginal symptoms in patients with CMD taking ivabradine; however, there was no improvement in the patients’ CFR [55]. The effects of the vasodilator nicorandil in patients with CMD have only been assessed in small studies. One of these studies demonstrated improved anginal symptoms and ST-depression during exercise in 11 patients with angina and NOCAD; however, this study had no control groups [56].
Numerous recent articles have suggested treatment algorithms for patients with CMD [37,45]. These form a useful aid for clinicians. Given the accumulating evidence that CMD is a heterogeneous condition with distinct pathophysiology underlying each endotype, it is tempting to speculate that therapies stratified by physiology may yield better outcomes than historical one-size-fits all type studies. However, we still await proof of this concept.
Conclusion
CMD, in the absence of obstructive CAD, is common in patients presenting with angina and is associated with a poor quality of life and adverse cardiovascular outcomes. CMD can now be diagnosed both invasively, in the catheter laboratory, and noninvasively. At present, the management for patients remains empirical and based on extrapolation of epicardial CAD data. With an improved understanding of its underlying pathophysiology, the next frontier in the management of patients with CMD is the identification or development of physiologically stratified therapies to improve prognosis and symptom control in these patients.
Future directions
In the past decade, there has been an increased awareness of CMD as a disease entity, and we have seen an exponential rise in the research being carried out in this field. We now have a better understanding of the pathophysiology of CMD and robust techniques to detect it clinically. The main challenge for the field during the next decade will be to promote the uptake of invasive intracoronary assessment to fully evaluate the coronary vasculature in patients with angina and NOCAD. This needs to be followed by prospective follow-up studies detailing outcomes in patients with CMD. This will likely lead to the development of physiology-stratified therapy to improve the symptom control and quality of life in patients with CMD.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.National Institute for Health and Clinical Excellence. NICE CG 126. Management of Stable Angina. 2011. www.nice.org.uk/guidance/CG126. [PubMed]
- 2.Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012; 33:734–744. [DOI] [PubMed] [Google Scholar]
- 3.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015; 8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 4.Cannon RO, III, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988; 61:1338–1343. [DOI] [PubMed] [Google Scholar]
- 5.Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. 2019; 140:1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman H, Demir OM, Khan F, Ryan M, Ellis H, Mills MT, et al. Physiological stratification of patients with angina due to coronary microvascular dysfunction. J Am Coll Cardiol. 2020; 75:2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncker DJ, Koller A, Merkus D, Canty JM., Jr Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis. 2015; 57:409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosseri M, Yarom R, Gotsman MS, Hasin Y. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation. 1986; 74:964–972. [DOI] [PubMed] [Google Scholar]
- 9.Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. 2018; 72:2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, et al. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009; 119:2656–2662. [DOI] [PubMed] [Google Scholar]
- 11.Shabeeh H, Melikian N, Dworakowski R, Casadei B, Chowienczyk P, Shah AM. Differential role of endothelial versus neuronal nitric oxide synthase in the regulation of coronary blood flow during pacing-induced increases in cardiac workload. Am J Physiol Heart Circ Physiol. 2013; 304:H1277–H1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sezer M, Kocaaga M, Aslanger E, Atici A, Demirkiran A, Bugra Z, et al. Bimodal pattern of coronary microvascular involvement in diabetes mellitus. J Am Heart Assoc. 2016; 5:e003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van de Wouw J, Sorop O, van Drie R, Duin R, Nguyen I, Joles J, et al. Perturbations in myocardial perfusion and oxygen balance in swine with multiple risk factors: a novel model of ischemia and no obstructive coronary artery disease. Basic Res Cardiol. 2020; 115:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011; 124:1774–1782. [DOI] [PubMed] [Google Scholar]
- 15.Lanza GA, De Vita A, Kaski JC. ‘Primary’ microvascular angina: clinical characteristics, pathogenesis and management. Interv Cardiol. 2018; 13:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, et al. ; Coronary Vasomotion Disorders International Study Group (COVADIS). The who, what, why, when, how and where of vasospastic angina. Circ J. 2016; 80:289–298. [DOI] [PubMed] [Google Scholar]
- 17.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, et al. ; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018; 250:16–20. [DOI] [PubMed] [Google Scholar]
- 18.Tavella R, Cutri N, Tucker G, Adams R, Spertus J, Beltrame JF. Natural history of patients with insignificant coronary artery disease. Eur Heart J Qual Care Clin Outcomes. 2016; 2:117–124. [DOI] [PubMed] [Google Scholar]
- 19.Hanssen TA, Iqbal A, Forsdahl SH, Trovik T, Schirmer H. Changes in symptoms of anxiety and depression following diagnostic angiography: a prospective cohort study. Eur Heart J Qual Care Clin Outcomes. 2018; 4:106–112. [DOI] [PubMed] [Google Scholar]
- 20.Lichtlen PR, Bargheer K, Wenzlaff P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol. 1995; 25:1013–1018. [DOI] [PubMed] [Google Scholar]
- 21.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019; 73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010; 55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014; 129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000; 101:948–954. [DOI] [PubMed] [Google Scholar]
- 25.Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, et al. ; Japanese Coronary Spasm Association. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013; 62:1144–1153. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Kaikita K, Nakayama N, Horio E, Yoshimura H, Ono T, et al. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc. 2013; 2:e000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sara JD, Taher R, Kolluri N, Vella A, Lerman LO, Lerman A. Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non-obstructive coronary artery disease. Cardiovasc Diabetol. 2019; 18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potier L, Chequer R, Roussel R, Mohammedi K, Sismail S, Hartemann A, et al. Relationship between cardiac microvascular dysfunction measured with 82Rubidium-PET and albuminuria in patients with diabetes mellitus. Cardiovasc Diabetol. 2018; 17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fossum E, Høieggen A, Moan A, Rostrup M, Nordby G, Kjeldsen SE. Relationship between insulin sensitivity and maximal forearm blood flow in young men. Hypertension. 1998; 32:838–843. [DOI] [PubMed] [Google Scholar]
- 30.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009; 30:1837–1843. [DOI] [PubMed] [Google Scholar]
- 31.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012; 5:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020; 41:407–477. [DOI] [PubMed] [Google Scholar]
- 33.Rahman H, Demir OM, Ryan M, McConkey H, Scannell C, Ellis H, et al. Optimal use of vasodilators for diagnosis of microvascular angina in the cardiac catheterization laboratory. Circ Cardiovasc Interv. 2020; 13:e009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams RP, de Waard GA, De Silva K, Lumley M, Asrress K, Arri S, et al. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am J Cardiol. 2018; 121:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltrame J, Crea F, Kaski J, Ogawa H, Ong P, Sechtem U, et al. ; COVADIS group. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017; 38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 36.Sueda S, Kohno H, Ochi T, Uraoka T. Overview of the acetylcholine spasm provocation test. Clin Cardiol. 2015; 38:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman H, Corcoran D, Aetesam-Ur-Rahman M, Hoole SP, Berry C, Perera D. Diagnosis of patients with angina and non-obstructive coronary disease in the catheter laboratory. Heart. 2019; 105:1536–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campisi R, Marengo FD. Coronary microvascular dysfunction in women with nonobstructive ischemic heart disease as assessed by positron emission tomography. Cardiovasc Diagn Ther. 2017; 7:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feher A, Sinusas A. Quantitative assessment of coronary microvascular function: dynamic SPECT, PET, ultrasound, CT and MR imaging. Circ Cardiovasc Imaging. 2017; 10:e006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009; 103:626–631. [DOI] [PubMed] [Google Scholar]
- 41.Liu A, Wijesurendra RS, Liu JM, Forfar JC, Channon KM, Jerosch-Herold M, et al. Diagnosis of microvascular angina using cardiac magnetic resonance. J Am Coll Cardiol. 2018; 71:969–979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Ford TJ, Stanley B, Sidik N, Good R, Rocchiccioli P, McEntegart M, et al. 1-Year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Cardiovasc Interv. 2020; 13:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labazi H, Trask A. Coronary microvascular disease as an early culprit in the pathophysiology of diabetes and metabolic syndrome. Pharmacol Res. 2017; 123:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gullu H, Caliskan M, Ciftci O, Erdogan D, Topcu S, Yildirim E, et al. Light cigarette smoking impairs coronary microvascular functions as severely as smoking regular cigarettes. Heart. 2007; 93:1274–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ford TJ, Berry C. How to diagnose and manage angina without obstructive coronary artery disease: lessons from the British Heart Foundation CorMicA Trial. Interv Cardiol. 2019; 14:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erdogan D, Gullu H, Caliskan M, Ciftci O, Baycan S, Yildirir A, Muderrisoglu H. Nebivolol improves coronary flow reserve in patients with idiopathic dilated cardiomyopathy. Heart. 2007; 93:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erdamar H, Sen N, Tavil Y, Yazici HU, Turfan M, Poyraz F, et al. The effect of nebivolol treatment on oxidative stress and antioxidant status in patients with cardiac syndrome-X. Coron Artery Dis. 2009; 20:238–234. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda Y, Akita H, Terashima M, Shiga N, Kanazawa K, Yokoyama M. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000; 140:753–759. [DOI] [PubMed] [Google Scholar]
- 49.Ohba K, Sugiyama S, Sumida H, Nozaki T, Matsubara J, Matsuzawa Y, et al. Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as nonobstructive coronary artery disease. J Am Heart Assoc. 2012; 1:e002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016; 37:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rambarat CA, Elgendy IY, Handberg EM, Bairey Merz CN, Wei J, Minissian MB, et al. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction: Women’s Ischemia Syndrome Evaluation – Coronary Vascular Dysfunction ancillary study. Int J Cardiol. 2019; 276:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tagliamonte E, Rigo F, Cirillo T, Astarita C, Quaranta G, Marinelli U, et al. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography. 2015; 32:516–521. [DOI] [PubMed] [Google Scholar]
- 53.Reriani M, Raichlin E, Prasad A, Mathew V, Pumper GM, Nelson RE, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010; 122:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003; 41:15–19. [DOI] [PubMed] [Google Scholar]
- 55.Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013; 112:8–13. [DOI] [PubMed] [Google Scholar]
- 56.Yamabe H, Namura H, Yano T, Fujita H, Kim S, Iwahashi M, et al. Effect of nicorandil on abnormal coronary flow reserve assessed by exercise 201Tl scintigraphy in patients with angina pectoris and nearly normal coronary arteriograms. Cardiovasc Drugs Ther. 1995; 9:755–761. [DOI] [PubMed] [Google Scholar]
- 57.Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011; 162:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]


