Abstract
Objective
Chronic pain is common in military veterans with traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD). Neurofeedback, or electroencephalograph (EEG) biofeedback, has been associated with lower pain but requires frequent travel to a clinic. The current study examined feasibility and explored effectiveness of neurofeedback delivered with a portable EEG headset linked to an application on a mobile device.
Design
Open-label, single-arm clinical trial.
Setting
Home, outside of clinic.
Subjects
N = 41 veterans with chronic pain, TBI, and PTSD.
Method
Veterans were instructed to perform “mobile neurofeedback” on their own for three months. Clinical research staff conducted two home visits and two phone calls to provide technical assistance and troubleshoot difficulties.
Results
N = 36 veterans returned for follow-up at three months (88% retention). During this time, subjects completed a mean of 33.09 neurofeedback sessions (10 minutes each). Analyses revealed that veterans reported lower pain intensity, pain interference, depression, PTSD symptoms, anger, sleep disturbance, and suicidal ideation after the three-month intervention compared with baseline. Comparing pain ratings before and after individual neurofeedback sessions, veterans reported reduced pain intensity 67% of the time immediately following mobile neurofeedback. There were no serious adverse events reported.
Conclusions
This preliminary study found that veterans with chronic pain, TBI, and PTSD were able to use neurofeedback with mobile devices independently after modest training and support. While a double-blind randomized controlled trial is needed for confirmation, the results show promise of a portable, technology-based neuromodulatory approach for pain management with minimal side effects.
Keywords: Chronic Pain, Neurofeedback, Traumatic Brain Injury, Post-traumatic Stress Disorder, Military Veterans
Introduction
Chronic pain is one of the most common physical problems in military veterans [1], especially among veterans who have served in the military since September 11, 2001 [2,3]. Between 44% and >50% of the active duty and veteran population report chronic pain, compared with 26–30% of the general US population [3]. Chronic pain adversely affects veterans’ physical, emotional, and social well-being [4] and is associated with worse community integration [5,6]. Traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD) [7,8] elevate the incidence of chronic pain [9]. In combination, chronic pain, TBI, and PTSD form a “polytrauma clinical triad” [10] that significantly affects veterans’ physical, emotional, and social well-being [1,6] and has been associated with increased risk of suicidality in post-9/11 veterans [11].
Effectively treating chronic pain in veterans is critical, but existing treatments pose barriers. Pharmacological approaches like opioids and other narcotic treatments elevate risk of abuse and harmful side effects [3,12]. Nonpharmacological treatments such as Cognitive Behavioral Therapy can be safe and effective in treating pain [13], but veterans do not always optimally utilize this type of treatment [14]. In a nationally representative sample of post-9/11 military veterans, two-thirds endorsed “It’s up to me to work out my own problems” [15], consistently treating chronic pain with self-management tools [16].
There is an urgent need for pain management that is both effective and viable for veterans with chronic pain, PTSD, and TBI. “Neurofeedback,” or electroencephalograph (EEG) biofeedback, trains people using operant conditioning principles [17] to gain more control over brain activity by balancing sympathetic and parasympathetic activity in the autonomic nervous system to achieve levels of target brain activity for calmness and mental relaxation [18]. Training that rewards select EEG frequencies increases or decreases of the amplitudes (power) of those EEG frequencies, resulting in enduring modifications of brain activity and associated cognitive, emotional, and behavioral functions [19]. EEG studies have shown that activity in pain perception pathways is linked to oscillations in alpha (8–13 Hz) waves [20], specifically suppressed alpha power and resting state peak alpha frequency [21]. Suppression of alpha rhythm (alpha event–related desynchronization) can “open the gates” to increased pain input from the periphery. The stronger the magnitude of alpha event–related desynchronization in anticipation of pain, the greater the subjectively rated experience of pain [22].
Alpha neurofeedback training involves a person learning to identify and improve alpha synchrony, often in conjunction with adjunctive techniques, that is, meditation, music, or games. When a person’s alpha synchrony closes “the gates,” increasing power in that band, the experience of pain decreases. According to the Association for Applied Psychophysiology’s clinical efficacy classification system [20], neurofeedback is efficacious or probably efficacious in ameliorating symptoms of pain from various physical and psychological origins as well as when pain is comorbid with conditions such as PTSD [18–20]. Changes in brainwave bandwidth activity, specifically increases in alpha frequency, have been demonstrated among individuals with chronic pain who undergo neurofeedback [23]. Neurofeedback has successfully treated pain in complex regional pain syndrome (CRPS) type 1, fibromyalgia, spinal cord injuries, and trigeminal neuralgia [23–26]. Neurofeedback has been associated with decreased pain and fatigue [23], improvements in worst pain [25], and decreased pain intensity [23].
Neurofeedback has traditionally required travel to a clinic multiple times a week. For veterans, this is problematic given the large proportion who live in rural areas [16]. With advances in technology, portable, low-cost EEG headsets and software applications now exist [27] and potentially can be used to perform neurofeedback at home [28]. For example, the NeuroSky headset is a portable device that reads electroencephalographic (EEG) brain activity and power spectrum bands (alpha, beta, theta, delta, and gamma). When researchers simultaneously recorded data with a NeuroSky headset and a research grade EEG system, they found that the two devices yielded comparable data, demonstrating very good (r = 0.70) to excellent (r = 0.90) correlations [29]. Subsequent validation studies have found that the NeuroSky headset shows good test–retest reliability [30] and is correlated with medical-grade EEG in naturalistic settings, including amplitudes of EEG oscillations in the alpha band [31].
The NeuroSky headset safely measures EEG power spectrums and consists of a headset, a sensor arm, and an ear clip. The headset’s reference and ground electrodes are located on the ear clip, and the EEG electrode is on the sensor arm, which rests on the forehead just above the eye (FP1 position). It is powered by a single AAA battery. The FP1 position is a standard position in the 10–20 neurofeedback process [32], which has shown connectivity with bilateral medial prefrontal cortex (MPF) [33] that contains both dopamine pathways and serotonergic axons, important in functions including cognition, learning, reward, and emotion regulation.
There have been only a few studies of neurofeedback in veterans or trauma populations [34–36] examining effects on psychiatric symptoms, not chronic pain. None have involved mobile EEG headset devices. The current study examined the feasibility of “mobile neurofeedback” using a portable EEG headset in veterans with chronic pain, TBI, and PTSD for pain management.
Methods
Participants
After institutional review board (IRB) approval, participants were recruited primarily through veteran organizations and medical centers in the southeastern United States via flyers and social media advertisements. Specifically, we sent flyers and e-mails to state brain injury associations, military family organizations, universities and colleges with veteran student listservs, and local VA and non-VA medical centers serving veterans. Inclusion criteria were 1) served in the military after 9/11/01, verified by documentation; 2) chronic pain measured by reporting moderate to severe pain (>4) on a 0–10 rating scale in one or more body regions lasting for three months or more [37]; 3) met criteria for TBI reporting that one’s head was hurt/injured in a way that caused problems and led to at least one of the following: loss of consciousness or getting “knocked out” immediately after the injury or upon regaining consciousness, being dazed or “seeing stars” immediately after the injury or upon regaining consciousness, being unable to recall the event, a period greater than one hour after the injury before the veteran started remembering new things again, or needing brain surgery after the injury [38]; and 4) met criteria for Diagnostic and Statistical Manual of Mental Disorders–5th edition (DSM-5) definition of PTSD on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), reporting a traumatic event and experiencing intrusion, avoidance, negative alterations in cognitions and mood, and alterations in arousal and reactivity symptoms [39]. The CAPS was administered by staff under the direct supervision of a licensed clinical psychologist. Each rater was trained using CAPS standardized training (i.e., manual, videotapes, and co-rating training with a trained rater to a high level of interrater reliability (Fleiss’ kappa > 0.90) across five CAPS training tapes. Exclusion criteria included 1) history of epilepsy, seizure disorder, or any seizure or epileptic episode and 2) women who reported being pregnant or breastfeeding or plans to become pregnant or breastfeed within three months.
Procedure
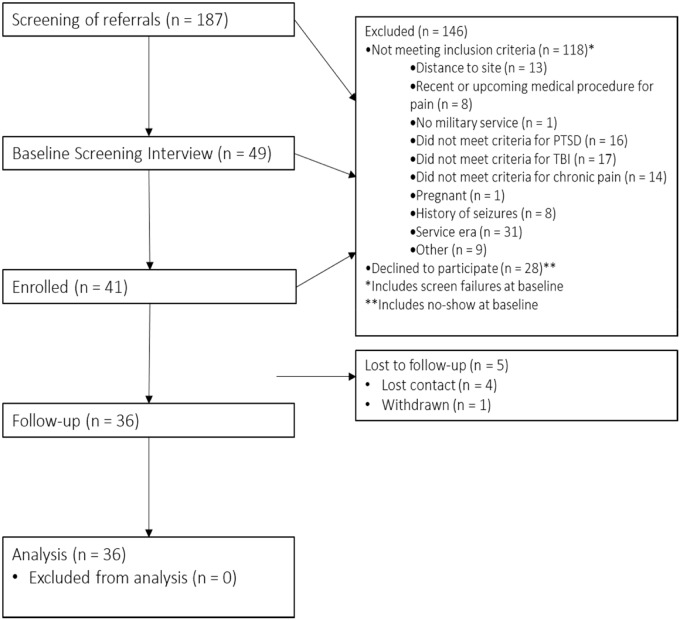
After an initial telephone screening to determine preliminary eligibility, 49 individuals completed the informed consent process during an in-office baseline evaluation. After an in-depth structured clinical assessment at our research office, N = 41 veterans who met the inclusion criteria for chronic pain, TBI, and PTSD were enrolled in the study (Figure 1). Veterans then completed baseline data collection and were compensated for participation.
Figure 1.
CONSORT diagram of study recruitment flow.
Afterward, participants were trained to implement the mobile intervention for pain management. In the current study, the NeuroSky MindWave EEG headset (hereafter called “MindWave”) linked to a Mobile Neurofeedback app on an iPod Touch via Bluetooth. MindWave was determined to be a nonsignificant risk device by the institutional review board and was determined to have an approved Investigational Device Exemption (IDE). The study team complied with abbreviated IDE requirements, and devices were presented to participants as part of an investigational study.
The research described above guided the development of alpha training, in which operant conditioning is employed to influence alpha production in the FP1 area [40]. We configured the Mobile Neurofeedback app using a proprietary algorithm by NeuroSky to identify brainwave combinations reflecting alpha power indicative of relaxed, meditative mental states. We programmed the app to provide auditory feedback to users in the form of a relaxing sound to indicate brainwave patterns associated with a relaxed brain state.
Evidence-based guidelines for neurofeedback training range between 20 and 50 sessions [41], and in the current study, participants were asked to use MindWave with the Mobile Neurofeedback app to complete 10-minute neurofeedback sessions at least four times per week for three months. Before each session, participants were prompted to rate their pain, stress, and anger on a 0–10 scale. Then, they were instructed to close their eyes and listen to auditory feedback signaling a more physiologically relaxed state, as measured by MindWave. The goal was to reinforce alpha brain wave activity consistent with pain reduction. After each session, participants again rated their pain, anger, and stress, which for app utilization purposes also helped verify that the participant completed the neurofeedback session. Participants received a score from 0–100 indicating the average level of relaxation achieved during the session, which was recorded and graphed visually on the app to enable participants to track progress.
To support intervention use, collect data on intervention utilization and tolerability, and address any potential questions or technical difficulties, research coordinators visited participants in their homes one week and six weeks after baseline and called them by phone at three and nine weeks after baseline. Once the three-month intervention ended, veterans completed a follow-up evaluation at our research office and were again compensated for participation.
Measures
Demographic information including age, sex/gender, race/ethnicity, and education was collected at baseline. At both the baseline and three-month follow-up evaluations, average pain intensity for the past week and the past three months was measured using the 0–10 numeric rating scale [42]. Pain location was measured with the Regional Pain Scale [37]. The Patient-Reported Outcomes Measurement Information System (PROMIS) [43] pain interference and sleep disturbance subscales were also used, with item scores on a scale from 1 (“not at all”) to 5 (“very much”). Pain interference was assessed as the sum of eight items (Cronbach’s α = 0.96), and sleep disturbance as the sum of eight items (Cronbach’s α = 0.90). The PROMIS scale on anger was assessed as the sum of five item scores on a scale from 1 (“never”) to 5 (“always”; Cronbach’s α = 0.90).
For mental health symptoms, the 20-item self-report PTSD Checklist for DSM-5 (PCL-5) [39] was used to measure PTSD. The PCL items were scored on a five-point Likert scale asking, “In the past month, how much were you bothered by…” for each PTSD symptom, with responses ranging from 0 (“not at all”) to 4 (“extremely”). A total score was calculated by summing the responses (Cronbach’s α = 0.93). The 10-item Patient Health Questionnaire (PHQ-9) [44] was used to assess depressive symptoms in the past two weeks, with items scored on a scale from 0 (“not all all”) to 3 (“nearly every day”). All nine items were summed to quantify depression severity (Cronbach’s α = 0.90). A single PHQ-9 item, “Thoughts that you would be better off dead, or of hurting yourself in some way,” was used to measure suicidal ideation.
During the three-month intervention period, participants were prompted to rate their level of pain, anger, and stress on a 0–10 scale before and after each mobile neurofeedback session using a scale in the app interface. At each home visit and phone call, participants were asked an open-ended question to describe side effects and then presented a list of potential side effects. Participants rated severity (“mild,” “moderate,” “severe,” “life-threatening”) and association with the intervention (“not related,” “unlikely related,” “possibly related,” “probably related,” “definitely related”). We used a standard procedure for reporting all side effects or adverse events to the Data Safety Monitoring Board and IRB, specifically regarding any impact on the participant’s health, function, or well-being. At three-month follow-up, participants were asked about the mobile neurofeedback intervention: “What do you think helped with the pain?”
Data Analysis
Descriptive information on demographics, TBI characteristics, side effects, and clinical status was analyzed. To examine treatment effects, paired t tests were used, comparing baseline scores with three-month follow-up scores on the aforementioned measures of pain intensity, pain interference, depression, PTSD symptoms, anger, sleep disturbance, and suicidal ideation. A second set of repeated-measures analyses of covariance was conducted controlling for whether participants had received pharmacological or behavioral treatment for psychiatric problems during the study.
Given eight independent analyses used to examine primary outcomes, familywise error was controlled using the false discovery rate method proposed by Benjamini and Hochberg [45]. This methodology differentiates random findings from hypothesis-driven outcomes and is more powerful than Bonferroni-type adjustments that control the false positive rate. It entails ranking the P values for outcome measures from smallest to largest and then comparing these to the P values achieved by multiplying alpha by the rank order, divided by the number of tests. As such, only the largest P values from a given test are compared with the original alpha value.
With respect to power analysis, the aim of this pilot study was to produce an estimate of the intervention’s effect size for conducting future larger trials. In the present study, with a sample of 41 participants at baseline, 36 of whom returned for follow-up, we had 80% power to detect an effect size equivalent to Cohen’s d equal to 0.47. There was greater statistical power to examine effects of neurofeedback at the individual session level. Using self-reported ratings of pain, stress, and anger reported at the start and end of each neurofeedback session, we calculated change scores by subtracting presession scores from postsession scores. Given multiple sessions per person, multilevel modeling (MLM) was used to account for shared variance among observations nested within individuals and to model the change scores to determine if they differed significantly from zero [46]. Finally, qualitative data are presented regarding veterans’ perceptions of pain reduction in the context of the mobile neurofeedback intervention.
Results
Participant demographics and clinical characteristics are presented in Table 1. The average CAPS-5 score was 35.1, and veterans reported having sustained an average of 2.93 head injuries that met the criteria for TBI. Most veterans reported at least one TBI with loss of consciousness (LOC; N = 27, 69.23%) and amnesia lasting <24 hours (N = 30, 76.92%). On the Regional Pain Scale, 34 (82.93%) participants reported level ≥4 pain in the head, and 24 (58.54%) reported lower back pain at baseline. Of the 36 participants who completed the three-month follow-up, 11 (30.56%) reported receiving pharmacological treatment, two (5.56%) reported receiving psychosocial treatment, and nine (25.00%) reported receiving both during the course of the study.
Table 1.
Descriptive data in a sample of veterans with chronic pain, TBI, and PTSD
| Mean (SD) | No. (%) | |
|---|---|---|
| Age, y | 38.57 (10.04) | – |
| Gender | ||
| Female | – | 6 (14.63) |
| Male | – | 35 (85.37) |
| Race | ||
| White | – | 17 (41.46) |
| Black | – | 16 (39.02) |
| American Indian | – | 2 (4.88) |
| Other | – | 6 (14.63) |
| Education, y | 13.64 (4.43) | – |
| CAPS total score | 35.10 (7.58) | – |
| Total number of TBI | 2.93 (1.60) | – |
| 1 | – | 9 (21.95) |
| 2 | – | 13 (31.71) |
| 3 | – | 4 (9.76) |
| 4 | – | 2 (4.88) |
| 5 | – | 13 (31.71) |
| TBI with most severe LOC | ||
| No LOC | – | 12 (30.77) |
| <1 min | – | 8 (20.51) |
| 1–15 min | – | 13 (33.33) |
| 16–30 min | – | 3 (7.69) |
| >30 min | – | 3 (7.69) |
| TBI with most severe PTA | ||
| Less than 1 h | – | 14 (35.90) |
| 1–24 h | – | 16 (41.03) |
| >24 h–7 d | – | 6 (15.38) |
| >7 d | − | 3 (7.69) |
| Years since last TBI | 13.11 (9.86) | |
| Regional Pain Scale ≥4 (head) | – | 34 (82.95) |
| Regional Pain Scale ≥4 (lower back) | – | 24 (58.54) |
CAPS = Clinician-Administered PTSD Scale for DSM-5; LOC = loss of consciousness; PTA = post-traumatic amnesia; TBI = traumatic brain injury.
Participants completed a mean (SD; range) of 33.09 (30.73; 3–156) neurofeedback sessions. Although not correlated with the number of neurofeedback sessions, each outcome showed statistically significant reductions between baseline and three-month follow-up (Table 2). Compared with follow-up, veterans reported significantly reduced past-week pain intensity, from a mean of 6.41 to 5.39 (t = 3.64, P < 0.001). They also reported reduced mean scores on measures of past three-month pain intensity from 6.40 to 5.71 (t = 2.82, P = 0.008), pain interference from 27.27 to 23.81 (t = 2.41, P = 0.022), PTSD symptoms from 43.37 to 35.72 (t = 2.46, P = 0.019), and depression from 13.02 to 9.67 (t = 3.96, P < 0.001). Suicidal ideation also was lower at follow-up (t = 2.22, P = 0.033). At follow-up, over half the sample (56%, 20/36) reported reduced average three-month pain intensity, and of those, approximately one-third (30%, 6/20) reported a reduction of at least two points. Veterans reported significantly reduced means on PROMIS measures of anger from 16.63 to 14.94 (t = 2.54, P = 0.016) and sleep disturbance from 30.10 to 26.33 (t = 2.71, P = 0.010). All changes in outcome variables remained significant after controlling for any concurrent pharmacological or behavioral treatment.
Table 2.
Change in treatment outcome variables from baseline to follow-up
| Baseline | Follow-up | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Min–Max | (N = 41) | (N = 36) | Paired t Test | Cohen’s d | ANCOVA t Test | Cohen’s d |
| Pain intensity (past wk) | 0–10 | 6.41 (1.24) | 5.39 (1.70) | t = 3.64, P < 0.001 | 0.61 | t = 3.85, P < 0.001 | 0.64 |
| Pain intensity (past 3 mo) | 0–10 | 6.40 (1.11) | 5.71 (1.60) | t = 2.82, P = 0.008 | 0.47 | t = 2.82, P = 0.008 | 0.47 |
| Pain interference (PROMIS) | 8–40 | 27.37 (6.02) | 23.81 (8.57) | t = 2.41, P = 0.022 | 0.40 | t = 2.72, P = 0.010 | 0.45 |
| PCL-5 | 0–80 | 43.37 (13.64) | 35.72 (16.51) | t = 2.46, P = 0.019 | 0.41 | t = 3.19, P = 0.003 | 0.53 |
| PHQ-9 | 0–27 | 13.02 (5.67) | 9.67 (5.42) | t = 3.96, P < 0.001 | 0.66 | t = 4.06, P < 0.001 | 0.68 |
| Suicidal ideation | 0–3 | 0.27 (0.55) | 0.11 (0.32) | t = 2.22, P = 0.033 | 0.37 | t = 2.22, P = 0.033 | 0.37 |
| Anger (PROMIS) | 5–25 | 16.63 (4.13) | 14.94 (4.73) | t = 2.54, P = 0.016 | 0.42 | t = 2.33, P = 0.026 | 0.39 |
| Sleep (PROMIS) | 8–40 | 30.10 (8.51) | 26.33 (8.48) | t = 2.71, P = 0.010 | 0.45 | t = 2.82, P = 0.008 | 0.47 |
ANCOVA t test and corresponding Cohen’s d reflect the effect of the intervention after controlling for whether participants received any pharmacological or behavioral treatment during the intervention period. All P values were significant after using the false discovery rate method to control for family-wise error.
ANCOVA = analysis of covariance; PCL-5 = PTSD Checklist for DSM-5; PHQ-9 = Patient Health Questionnaire-9; PROMIS = Patient-Reported Outcomes Measurement Information System.
Across N = 965 10-minute sessions for which before versus after individual session data were available, veterans reported reduced pain 67.05% (647/965) of the time, reduced stress 85.39% (824/965) of the time, and reduced anger 38.34% (370/965) of the time. Of note, 41.45% (400/965) of all sessions resulted in a reduction of pain by two points or more. Table 3 shows multilevel modeling analysis of session-specific change scores that revealed that, for pain, stress, and anger, interindividual differences accounted for most of the variance in change scores: 57.9%, 50.1%, and 61.0%, respectively. Analyses showed that the intercept for the anger model was not significant, suggesting that neurofeedback did not result in immediate decreased anger for the sample as a whole. The intercepts for the pain and stress change score models were significant, reflecting large decreases in those variables associated with neurofeedback for pain (Cohen’s d = 1.07) and for stress (Cohen’s d = 2.56).
Table 3.
Before vs after neurofeedback session changes in pain, anger, and stress
| Raw Scores |
Multilevel Modeling of Change |
|||||
|---|---|---|---|---|---|---|
| Presession |
Postsession |
Intercept | Presession Level | |||
| Variable | Mean (SD) | Min–Max | Mean (SD) | Min–Max | Coeff (SE) | Coeff (SE) |
| Pain | 4.85 (2.16) | 0–10 | 3.23 (2.17) | 0–10 | –1.41*** (0.25) | –0.57*** (0.02) |
| Stress | 5.02 (2.37) | 0–10 | 1.79 (1.94) | 0–10 | –3.22*** (0.24) | –0.81*** (0.03) |
| Anger | 2.94 (2.42) | 0–10 | 2.93 (2.22) | 0–10 | 0.15 (0.29) | –0.77*** (0.03) |
Change scores were derived by subtracting presession scores from postsession scores and from N = 965 sessions. Following 10-minute neurofeedback sessions, 67.05% (647/965) of the time veterans reported reduced pain, 85.39% (824/965) of the time veterans reported reduced stress, and 38.34% (370/965) of the time veterans reported reduced anger.
P < 0.001.
For the three-month intervention, veterans completed a mean (SD; range) of 3.46 (0.85; 1–4) home visits and/or phone calls with study staff; those not achieving all four were due in large part to difficulty with scheduling. Number of staff contacts was correlated with changes in three-month pain intensity (r = –0.34, P = 0.04), pain interference (r = –0.36, P = 0.03), and depressive symptoms (r = –0.37, P = 0.03) but not with changes in one-week pain intensity, anger, sleep problems, PTSD symptoms, or suicidal ideation (P > 0.05). Table 4 reports veterans’ perceptions about the mobile neurofeedback intervention. Of side effects that were probably or definitely related to mobile neurofeedback, headset discomfort and drowsiness were most common. With respect to opinions about mobile neurofeedback, veterans reported that 1) it was effective helping them to achieve a more relaxed state; 2) focusing on their pain helped them manage their symptoms; 3) it was favorable compared with pain management by narcotics or other medications; and 4) they appreciated the mindful or meditative state facilitated by the intervention.
Table 4.
Veteran perceptions of mobile neurofeedback intervention
| Reported Side Effect* No. out of N = 41 | Did Not Report Side Effect No. out of N = 41 | |
|---|---|---|
| Headset discomfort | 14 | 27 |
| Drowsiness following neurofeedback | 13 | 28 |
| Irritability | 6 | 35 |
| Headache | 3 | 38 |
| Dizziness | 1 | 40 |
| Vibrating/buzzing | 1 | 40 |
| Muscle twitching | 1 | 40 |
| Responses to the question “What do you think helped with the pain?” | ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
All side effects listed were assessed to be probably or definitely related to neurofeedback. Severity levels for each were set at mild, moderate, severe, or life-threatening. None were severe or life-threatening. One instance of a moderate side effect of irritability occurred when a participant practiced four times in a row; this side effect ceased once the participant no longer did this. The remaining side effects were in the mild range.
Discussion
Overall, this preliminary study showed that veterans with chronic pain, PTSD, and TBI were able to use neurofeedback at home using mobile devices. In total, 88% of veterans returned for the three-month follow-up and averaged approximately three days a week of engaging in mobile neurofeedback. At three-month follow-up, veterans reported lower pain intensity, pain interference, depressive symptoms, PTSD symptoms, sleep disturbance, anger, and suicidal ideation. The intervention was well tolerated, and there were no significant adverse events reported. Across nearly 1,000 sessions, veterans reported reduced pain 67% of the time immediately following 10 minutes of mobile neurofeedback. These findings are consistent with other research showing that neurofeedback alleviates psychiatric symptoms in veterans or trauma populations [26–28].
Use of neurofeedback technology at home is consistent with veterans’ preference for self-management of symptoms [15]. Overall, the results suggested few problems with implementation or practice. The most common issue voiced by participants, discomfort from the headset, has already been addressed by Neurosky, as newer EEG headsets are more comfortable and adjustable. Veterans paradoxically perceived drowsiness, the other common side effect, as a benefit to help them get to sleep. Isolated and minor irritability was outweighed by reports of reduced stress the vast majority of the time (>85%) following neurofeedback sessions.
Findings of associations with reduced depression, PTSD, anger, sleep problems, and suicidal ideation support the potential of mobile neurofeedback for pain management. Ultimately, health care professionals may be able to include mobile neurofeedback as an adjunct treatment for chronic pain. However, research is still needed: What was the underlying mechanism leading to these benefits? Did neurofeedback lead to increased alpha power [20–22]? Was engaging in mindfulness alone, or relaxing for 10 minutes, the catalyst for improved outcomes? Could therapeutic alliance have contributed to results? Were findings the result of a placebo effect? Although the study yielded encouraging results, these questions point to the need for further experiments to examine if neurofeedback significantly contributed to the improvements seen in this study. In particular, a larger double-blind, randomized control trial is necessary to investigate potential mechanisms (e.g., less stress, greater pain tolerance, taking a time-out, placebo) underlying the current findings of reduced pain intensity.
Given that this study involved only an open-label, single-arm clinical trial, a randomized clinical trial would be critical to determine the efficacy of mobile neurofeedback for chronic pain. Further, it is critical for future research to examine how many sessions of neurofeedback are optimal for what level of pain intensity; for example, lack of a correlation between number of sessions and outcomes may be due to limited statistical power from a small study sample, but it also could be the case that different quantities of neurofeedback may be therapeutic for different pain intensities or different individuals. Although all of the primary outcomes were statistically significant, more research is needed to examine the clinical significance of the magnitude of these changes given that most of the changes were relatively small compared with the scales range. We found a wide range of app usage; future research should examine facilitators and barriers to utilization. Finally, the current study primarily measured symptoms, whereas measures of functionality and quality of life would enhance research on mobile neurofeedback.
The current study was limited by self-report measures, and future studies need to look at neurophysiological measures such as EEG brainwave activity [22]. Because our sample of women mirrored the proportion in the post-9/11 veteran population [15], future research would need to oversample women veterans to have the statistical power necessary to examine sex as a biological variable. Finally, we are unaware of any other study to use neurofeedback at home for pain management and thus relied on the number of sessions typical in traditional neurofeedback [40]. It is unknown what an “optimal” dose should be for mobile neurofeedback or whether the dose should vary according to level of pain. Our primary aim was to determine if neurofeedback for pain management could be implemented independently; given that that appears to be the case, future research should investigate optimal intervention dosage.
To our knowledge, this study is the first to test the feasibility of mobile neurofeedback in veterans with chronic pain, PTSD, and TBI. This pilot study shows promise of a portable, technology-based neuromodulatory approach to pain management that uses neurofeedback and overcomes treatment barriers by permitting veterans to manage pain in a self-directed manner outside a clinic [16,47]. More work is needed to better understand if and how mobile neurofeedback led to improved outcomes in this at-risk group of military veterans. Chronic pain is a prevalent problem among veterans and the general population. The current study takes a step toward exploring a low-risk, innovative approach to pain management.
Acknowledgments
We would like to extend our sincere thanks to the participants who volunteered for this study. We would like to thank Kendra Corning, Chelsea Greenburg, Cynthia Strickland, and James Wolfe for coordinating data collection and intervention facilitation throughout the study.
Funding sources: This work was supported by the National Center for Complementary and Integrative Health (Award#R34 AT008399-0), Department of Defense (Award#W81XWH-17–1-0591), a Clinical Sciences Research and Development Senior Research Career Scientist Award #IK6CX001494 (JCB) from the US Department of Veterans Affairs, and the Veterans Affairs Mid-Atlantic Mental Illness Research, Education and Clinical Center (MIRECC).
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Center for Complementary and Integrative Health, Department of Defense, or the Department of Veterans Affairs.
Conflicts of interest: The authors declare there are no actual or potential conflicts of interest including any financial, personal, or other relationships with other people or organizations since the beginning of the submitted work that could inappropriately influence, or be perceived to influence, this work.
Trial registration: ClinicalTrials.gov ID: NCT02237885.
References
- 1. Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med 2006;7(4):339–43. [DOI] [PubMed] [Google Scholar]
- 2. Higgins D, Kerns R, Brandt C, Haskell S, Bathulapalli H, Gilliam W. Persistent pain and comorbidity among Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans. Pain Med 2014;15(5):782–90. [DOI] [PubMed] [Google Scholar]
- 3. Toblin RL, Quartana PJ, Riviere LA, Walper K, Hoge CW. Chronic pain and opioid use in us soldiers after combat deployment. JAMA Intern Med 2014;174(8):1400–1. [DOI] [PubMed] [Google Scholar]
- 4. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin 2016;34(2):357–78. [DOI] [PubMed] [Google Scholar]
- 5. Clark ME, Scholten JD, Walker RL, Gironda RJ. Assessment and treatment of pain associated with combat-related polytrauma. Pain Med 2009;10(3):456–69. [DOI] [PubMed] [Google Scholar]
- 6. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: Polytrauma clinical triad. J Rehabil Res Dev 2009;46(6):697–702. [DOI] [PubMed] [Google Scholar]
- 7. Dobscha SK, Clark ME, Morasco BJ, Freeman M, Campbell R, Helfand M. Systematic review of the literature on pain in patients with polytrauma including traumatic brain injury. Pain Med 2009;10(7):1200–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: A systematic review. JAMA 2008;300(6):711–9. [DOI] [PubMed] [Google Scholar]
- 9. Stojanovic MP, Fonda J, Fortier CB, et al. Influence of mild traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) on pain intensity levels in OEF/OIF/OND veterans. Pain Med 2016;17(11):2017–25. [DOI] [PubMed] [Google Scholar]
- 10. Buckenmaier CC, Galloway KT, Polomano RC, McDuffie M, Kwon N, Gallagher RM. Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med 2013;14(1):110–23. [DOI] [PubMed] [Google Scholar]
- 11. Blakey SM, Wagner HR, Naylor J, et al. Chronic pain, TBI, and PTSD in military veterans: A link to suicidal ideation and violent impulses? J Pain 2018;19(7):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seal KH, Bertenthal D, Barnes DE, et al. Traumatic brain injury and receipt of prescription opioid therapy for chronic pain in Iraq and Afghanistan veterans: Do clinical practice guidelines matter? J Pain 2018;19(8):931–41. [DOI] [PubMed] [Google Scholar]
- 13. Wetherell JL, Afari N, Rutledge T, et al. A randomized, controlled trial of Acceptance and Commitment Therapy and Cognitive-Behavioral Therapy for chronic pain. Pain 2011;152(9):2098–107. [DOI] [PubMed] [Google Scholar]
- 14. Cohen B, Gima K, Bertenthal D, Kim S, Marmar C, Seal K. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med 2010;25(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elbogen EB, Wagner HR, Johnson SC, et al. Are Iraq and Afghanistan veterans using mental health services? Psychiatr Serv 2013;64(2):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bair MJ, Matthias MS, Nyland KA, et al. Barriers and facilitators to chronic pain self-management: A qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med 2009;10(7):1280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skinner BF. Operant behavior. Am Psychol 1963;18(8):503–15. [Google Scholar]
- 18. Jensen MP, Day MA, Miro J. Neuromodulatory treatments for chronic pain: Efficacy and mechanisms. Nat Rev Neurol 2014;10(3):167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ros T, J Baars B, Lanius RA, Vuilleumier P. Tuning pathological brain oscillations with neurofeedback: A systems neuroscience framework. Front Hum Neurosci 2014;8:1008. doi:10.3389/fnhum.2014.01008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz M, Andrasik F. Biofeedback: A Practitioner's Guide. 4th ed New York: Guilford Press; 2017. [Google Scholar]
- 21. de Vries M, Wilder-Smith OH, Jongsma ML, et al. Altered resting state EEG in chronic pancreatitis patients. J Pain Res 2013;6:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Babiloni C, Brancucci A, Babiloni F, et al. Anticipatory cortico responses during the expectancy of a predictable painful stimulation. A high resolution eletroncephlography study. Eur J Neurosci 2003;18(6):1692–700. [DOI] [PubMed] [Google Scholar]
- 23. Jensen M, Gertz K, Kupper A, et al. Steps toward developing an EEG biofeedback treatment for chronic pain. Appl Psychophysiol Biofeedback 2013;38(2):101–8. [DOI] [PubMed] [Google Scholar]
- 24. Caro XJ, Winter EF. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: A pilot study. Appl Psychophysiol Biofeedback 2011;36(3):193–200. [DOI] [PubMed] [Google Scholar]
- 25. Sime A. Case study of trigeminal neuralgia using neurofeedback and peripheral biofeedback. J Neurother 2004;8(1):59–71. [Google Scholar]
- 26. Prinsloo S, Novy D, Driver L, et al. The long-term impact of neurofeedback on symptom burden and interference in patients with chronic chemotherapy-induced neuropathy. J Pain Symptom Manage 2018;55(5):1276–85. [DOI] [PubMed] [Google Scholar]
- 27. Stopczynski A, Stahlhut C, Petersen MK, et al. Smartphones as pocketable labs: Visions for mobile brain imaging and neurofeedback. Int J Psychophysiol 2014;91(1):54–66. [DOI] [PubMed] [Google Scholar]
- 28. Peake JM, Kerr G, Sullivan JP. A critical review of consumer wearables, mobile applications, and equipment for providing biofeedback, monitoring stress, and sleep in physically active populations. Front Physiol 2016;9:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnstone SJ, Blackman R, Bruggemann JM. EEG from a single-channel dry-sensor recording device. Clin EEG Neurosci 2012;43(2):112–20. [DOI] [PubMed] [Google Scholar]
- 30. Rogers JM, Johnstone SJ, Aminov A, Donnelly J, Wilson PH. Test-retest reliability of a single-channel, wireless EEG system. Int J Psychophysiol 2016;106:87–96. [DOI] [PubMed] [Google Scholar]
- 31. Rieiro H, Diaz-Piedra C, Morales JM, et al. Validation of electroencephalographic recordings obtained with a consumer-grade, single dry electrode, low-cost device: A comparative study. Sensors 2019;19(12):2808. doi:10.3390/s19122808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 1958;10:370–5. [PubMed] [Google Scholar]
- 33. Rojas GM, Alvarez C, Montoya CE, de la Iglesia-Vayá M, Cisternas JE, Gálvez M. Study of resting-state functional connectivity networks using EEG electrodes position as seed. Front Neurosci 2018;12:235. doi: 10.3389/fnins.2018.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peniston EG, Kulkosky PJ. Alpha-theta brainwave neurofeedback for Vietnam veterans with combat-related post-traumatic stress disorder. Med Psychother 1991;4(1):47–60. [Google Scholar]
- 35. van der Kolk BA, Hodgdon H, Gapen M, et al. A randomized controlled study of neurofeedback for chronic PTSD. PLoS One 2016;11(12):e0166752.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reiter K, Andersen SB, Carlsson J. Neurofeedback treatment and posttraumatic stress disorder: Effectiveness of neurofeedback on posttraumatic stress disorder and the optimal choice of protocol. J Nerv Ment Dis 2016;204(2):69–77. [DOI] [PubMed] [Google Scholar]
- 37. Wolfe F. Pain extent and diagnosis: Development and validation of the Regional Pain Scale in 12,799 patients with rheumatic disease. J Rheumatol 2003;30(2):369–78. [PubMed] [Google Scholar]
- 38. Kay T, Harrington DE, Adams R, et al. Definition of mild traumatic brain injury: Report from the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. J Head Trauma Rehabil 1993;8(3):86–7. [Google Scholar]
- 39. Weathers FW, Bovin MJ, Lee DJ, et al. The clinician-administered PTSD scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess 2018;30(3):383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thompson M, Thompson L. The Neurofeedback Book: An Introduction to Basic Concepts in Applied Psychophysiology. Wheat Ridge, CO: Association for Applied Psychophysiology and Biofeedback; 2003. [Google Scholar]
- 41. Hammond DC. What is neurofeedback: An update. J Neurother 2011;15(4):305–36. [Google Scholar]
- 42. Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med 2007;22(10):1453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B: Stat Methodol 1995;57(1):289–300. [Google Scholar]
- 46. Searle SR, Casella G, McCulloch C. Variance Components. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 47. Hendrick P, Bhattacharjee P, Blake H. A systematic review of outcome measures utilised to assess self-management in clinical trials in patients with chronic pain. Patient Educ Couns 2018;101(5):767–78. [DOI] [PubMed] [Google Scholar]