Abstract
Background:
Co-occurrence of tobacco use and heavy episodic drinking (HED; 5+ drinks for men and 4+ drinks for women per occasion) is common among young adults; both warrant attention and intervention. In a two-group randomized pilot trial, we investigated whether a Facebook-based smoking cessation intervention addressing both alcohol and tobacco use would increase smoking abstinence and reduce HED compared to a similar intervention addressing only tobacco.
Methods:
Participants were 179 young adults (age 18–25; 49.7% male; 80.4% non-Hispanic white) who we recruited from Facebook and Instagram who reported smoking 4+ days/week and past-month HED. The Smoking Tobacco and Drinking (STAND) intervention (N=84) and the Tobacco Status Project (TSP), a tobacco-only intervention (N=95), both included daily Facebook posts for 90 days and weekly live counseling sessions in private “secret” groups. We verified self-reported 7-day smoking abstinence via remote salivary cotinine tests at 3, 6, and 12 months (with retention at 83%, 66%, and 84%, respectively). Participants self- reported alcohol use.
Results:
At baseline, the participants averaged 10.4 cigarettes per day (SD=6.9) and 8.9 HED occasions in the past month (SD=8.1), with 27.4% in a preparation stage of change for quitting smoking cigarettes. Participants reported significant improvements in cigarette smoking and alcohol use outcomes over time, with no significant differences by condition. At 12 months, intent-to-treat smoking abstinence rates were 3.5% in STAND vs. 0% in TSP (biochemically verified) and 29.4% in STAND vs. 25.5% in TSP (self-reported). Compared to TSP, participants rated the STAND intervention more favorably for supporting health and providing useful information.
Conclusions:
Adding an alcohol treatment component to a tobacco cessation social media intervention was acceptable and engaging but did not result in significant differences by treatment condition in smoking or alcohol use outcomes. Participants in both conditions reported smoking and drinking less over time, suggesting covariation in behavioral changes.
Trial Registration:
Keywords: Smoking cessation, Tobacco, Alcohol, Young adults, Social media
1. Introduction
Cigarette smoking and alcohol use often co-occur in both general and young adult populations (McKee & Weinberger, 2013). Prevalence of cigarette smoking among individuals with an alcohol use disorder (AUD) is 80–90% (Bobo, McIlvain, Lando, Walker, & Leed-Kelly, 1998), and more people with AUD die of tobacco-related causes than die of alcohol-related causes (Hurt et al., 1996). Young adults aged 18–25 are more likely than older adults to report past-month use of cigarettes (23.5% in 2016) (Substance Abuse and Mental Health Services Administration, 2017). The Substance Abuse and Mental Health Services Administration (SAMHSA) defines heavy episodic drinking (HED), sometimes also referred to as “binge drinking”, as 5 or more standard drinks per occasion for males and 4 or more drinks per occasion for females (National Institute on Alcohol Abuse and Alcoholism, 2020a), and leads to increased risk for health problems and AUD. Additionally, HED is most common among young adults, age 18–25, with 57.1% reporting HED in the past month in 2016 (Substance Abuse and Mental Health Services Administration, 2017). In a national online survey of young adults who both drank alcohol and smoked cigarettes, those who reported occasional and frequent binge drinking smoked more cigarettes per day and were more likely to smoke cigarettes on the days with HED (Gubner, Delucchi, & Ramo, 2016). Co-use of cigarettes and alcohol is associated with greater likelihood of a dependence diagnosis on either substance in adulthood (Moss, Chen, & Yi, 2014), worse alcohol treatment outcomes (McKelvey, Thrul, & Ramo, 2017; Satre, Kohn, & Weisner, 2007), and increased risk of certain cancers (American Cancer Society, 2020; Lee et al., 2005; Pelucchi, Gallus, Garavello, Bosetti, & La Vecchia, 2006); and frequent HED is associated with fewer smoking quit attempts (Kahler et al., 2010).
Addressing tobacco use and HED simultaneously may lead to improved outcomes compared to targeting each substance separately. Previous research has found that integrating alcohol content into smoking cessation programs resulted in significantly greater smoking abstinence for adult quit line callers (Toll et al., 2015), greater short-term smoking abstinence and fewer drinks per week among adult heavy drinking smokers (Kahler et al., 2008), and fewer drinks consumed and HED occasions in a pilot study among young adults (Ames et al., 2010), when compared to interventions that only targeted cigarette smoking.
Extending interventions to digital environments can maximize reach and utility, particularly for young adults. For example, a 14-day mobile feedback intervention study targeting tobacco and alcohol use among college students led to a decrease in the number of cigarettes smoked (but no changes in HED) compared to a minimal assessment control condition (Witkiewitz et al., 2014). A longer 3-month mobile intervention study found that students who reported greater alcohol consumption and who were in an integrated tobacco and alcohol cessation arm had greater 7-day point prevalence of smoking abstinence than those in the smoking cessation only arm, though no beneficial intervention differences were found for the sample overall (Haug, Paz Castro, Kowatsch, Filler, & Schaub, 2017). Although this prior work is important, programs need strategies to expand available treatments to under-served young adults beyond college student populations and to harness the potential of integrated tobacco and alcohol interventions.
While previous digital interventions targeting tobacco and alcohol use have focused on mobile applications and assessments (Witkiewitz et al., 2014), social media platforms represent a promising strategy for reaching and delivering evidence-based interventions. Among individuals ages 18–29 in the United States who use the Internet, 88% have a Facebook account and 70% use Facebook daily (Pew Research Center, 2020). We developed the Tobacco Status Project (TSP), a 3-month smoking cessation intervention delivered through Facebook to young adults age 18–25, who smoke cigarettes with content individually tailored to participants’ readiness to quit smoking, drawn from the transtheoretical model of behavior change (Prochaska & DiClemente, 1983). We previously reported short-term efficacy, with intervention participants 2.5 times more likely to achieve biochemically verified abstinence from tobacco at the end of the intervention compared to those who received a referral to the smoking cessation website Smokefree.gov (Ramo, Thrul, et al., 2018). However, this effect did not persist through the 6- or 12-month follow-up. Integrating alcohol content into this smoking cessation intervention delivered through Facebook to young adults who also heavily drink alcohol may increase the likelihood of cigarette smoking abstinence compared to the tobacco-only intervention, with effects also on drinking.
The current pilot study, conducted in a sample of young adults who smoked cigarettes and reported recent HED, tested the primary hypothesis that participating in the Smoking Tobacco and Drinking (STAND) combined intervention would result in greater likelihood of biochemically verified abstinence from cigarette smoking when compared to participating in the TSP intervention, which addressed only tobacco. Thus, the primary hypothesis test was a comparison of these two interventions on a tobacco outcome. Secondary tobacco outcomes examined self-reported tobacco abstinence, reduction in the number of cigarettes smoked, quit attempts, being in a preparation stage of change, and severity of cigarette dependence. Secondary alcohol and co-use outcomes included past month days of HED, drinks per week, readiness to change alcohol use, problematic alcohol use, and past month days of alcohol and tobacco co-use.
2. Methods
2.1. Study design
A two-group, randomized controlled trial compared the STAND vs. TSP Facebook-based 90-day interventions, with follow-up assessments at 3, 6, and 12 months. Additional details about study protocol and intervention development can be found in previously published papers (Ramo, Kaur, et al., 2018; Ramo et al., 2019). We pre-registered the trial on ClinicalTrials.gov (NCT03163303).
2.2. Participants
We recruited participants from December 2017 to February 2018, through a Facebook and Instagram advertising campaign (Ramo, Rodriguez, Chavez, Sommer, & Prochaska, 2014). Briefly, we targeted ads by age (18–25), location (United States), and language (English). Images in ads themselves included a no smoking logo or broken cigarette. Ads included text reading “Seeking young adults looking to make changes to their smoking and drinking habits” or “Studies have shown that smoking and drinking work together to harm the brain. Are you worried?” as well as a statement that participants could earn up to $155. Ads included a link to a description of the study and an eligibility survey on the Qualtrics platform. Inclusion criteria included being English-literate and between 18 and 25 years old, using of Facebook at least 4 days per week, having smoked at least 100 cigarettes in their lifetime and currently smoking at least 1 cigarette per day on at least 4 days a week, and having had at least one HED day (4+ drinks for women, 5+ drinks for men in one occasion) in the past month. (This definition is broader than the National Institute on Alcohol Abuse and Alcoholism [NIAAA] definition that specifies a 2-hour time period for HED, but is consistent with NIAAA guidelines for reducing alcohol use risks [National Institute on Alcohol Abuse and Alcoholism, 2020b]).
2.3. Study procedures
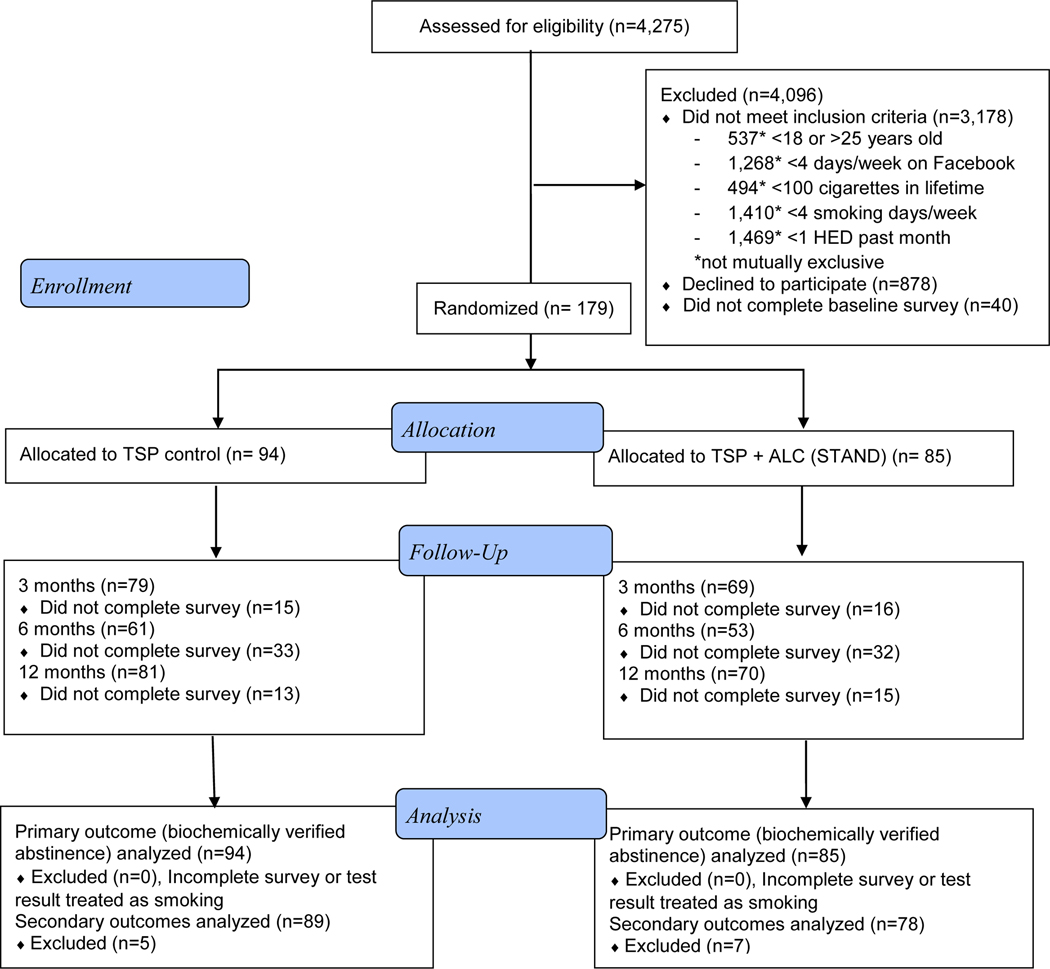
The University of California, San Francisco, Institutional Review Board (IRB) approved the study procedures. Of 10,954 visits to the study website, 4,275 individuals completed the online study screener. We present the CONSORT diagram in Figure 1. We invited eligible participants to review the study description and give informed consent for study enrollment with an electronic signature. The informed consent process included indicating understanding the risks and benefits of participating in the study, assessed through three multiple-choice questions. We asked those who consented to participate to verify their age by sending proof of age (usually a photo of their driver’s license) to study staff via email or Facebook message. After age-verification, we emailed participants a link to the baseline survey. We randomized participants who completed the baseline assessment to the STAND intervention (N=85) or TSP control (N=94) conditions 1:1 using a blocked random assignment sequence that the study biostatistician (K.L.D.) generated. We stratified randomization by two variables known to be related to outcomes and that the intervention addressed: daily smoking status (yes/no) and readiness to quit smoking (ready to quit in the next 30 days, “getting ready”; vs. not ready to quit in the next 30 days, “not ready”). The imbalance between study condition sizes was due to a slowing of recruitment and closing enrollment before we completed the final randomization blocks.
Figure 1:
Participant flow through Smoking Tobacco and Drinking (STAND) Facebook smoking cessation clinical trial, based on CONSORT guidelines.
STAND (Smoking Tobacco and Drinking) intervention: Tobacco + Alcohol content; TSP (Tobacco Status Project) intervention: Tobacco only content
The study coordinator (M.K.) assigned participants to “secret” Facebook groups that corresponded to their readiness to quit smoking. Secret groups are private and invisible to nonmembers. Groups began between December 2017 and March 2018 on a rolling basis to minimize wait time and with the aim of an average group size of 12. We created fourteen secret Facebook groups; each group was either STAND (n=7) or TSP (n=7) and also not ready (n=8) or getting ready (n=6) to quit smoking cigarettes. Group size ranged from 3 to 19 (average group size = 12.8, SD = 4.2) and average group size was similar between the two conditions (STAND average group size = 12.1, SD = 5.9; TSP average group size = 13.4, SD = 3.1). We kept groups open for the full 12 months of the trial, although the study team posted content for only the first 90 days.
Participants received $25 electronic gift cards for completion of each 20- to 30-minute online survey at baseline and 3- and 6-month follow-ups. We originally planned to offer $25 for the 12-month follow-up survey completion and an additional $25 for completing all assessments, but to increase retention at the final follow-up time point, we increased the 12-month survey completion incentive to $50 (for a possible total of $125), with IRB approval.
2.4. Study conditions
Both conditions consisted of two main features: daily Facebook posts for 90 days and weekly live counseling sessions with a PhD-level smoking cessation counselor for 12 weeks (“The Doctor Is In”). Posts contained a combination of images, text, and links to videos or articles designed to elicit a response from participants. Posts also assessed use patterns, provided strategies to support quitting such as use of nicotine replacement therapy and other cessation medication (i.e., bupropion and varenicline), and content to promote awareness of the tobacco industry’s efforts to target young adults. During the one-hour weekly “The Doctor Is In” sessions, the counselor posted content or a question for discussion in the group to which anyone could respond and was available for the rest of the hour to discuss this content or other issues. For each person who responded, the conversation between the counselor and participant would continue in threaded (i.e. nested) comments below their initial response comment. In the weekly “The Doctor Is In” live counseling sessions, we tailored the prompts and content to readiness to quit and based them on motivational interviewing (MI) and cognitive behavioral coping skills for smoking cessation.
2.4.1. Tobacco Status Project (TSP) control condition
We based content of TSP posts on the U.S. clinical practice guidelines for smoking cessation (Fiore & Jaen, 2008) and the transtheoretical model (TTM) of behavior change (Prochaska & DiClemente, 1983), and included the 5Rs (relevance, risks, rewards, roadblocks, and repetition). Online surveys and interviews with young adult smokers (Ramo et al., 2019) as well as analyses of commenting engagement in previous iterations of the TSP intervention (Thrul, Klein, & Ramo, 2015) informed format and content. We tailored posts to participants’ readiness to quit smoking (ready in the next 30 days vs. not ready in the next 30 days). For those not ready to quit, we based post content on TTM concepts of decisional balance and self-efficacy and elicited participants’ motivation and importance of changing tobacco use and problems associated with use. For those ready to quit, posts incorporated TTM processes of self-liberation, stimulus control, and counter-conditioning, as well as cognitive and behavioral coping skills. Posts encouraged setting a quit date and making a detailed quit plan. Screenshots of sample posts are available as supplementary material.
2.4.2. Smoking Tobacco and Drinking (STAND) treatment condition
Findings from online focus groups with the target population (i.e., young adults who smoked cigarettes and reported recent HED) informed the STAND intervention design and content. Focus group participants revealed a preference for changing one substance at a time and greater receptivity to quitting smoking than reducing drinking (Ramo et al., 2019). Tobacco content was similar to content in the TSP control condition, but was a smaller proportion of overall material (55% for getting ready and 31% for not ready). Many posts (20% in getting ready and 24% in not ready) directly addressed alcohol and cigarette co-use and elicited thoughts about how alcohol use may have affected smoking behaviors or quit attempts, but we did not aim alcohol-related posts at eliminating alcohol use. We based content targeting HED (24% in getting ready and 45% in not ready) on the NIAAA Rethinking Drinking guide (Zakletskaia, Wilson, & Fleming, 2011) and the Guide to Alcohol Screening and Brief Intervention for Youth (National Institute on Alcohol Abuse and Alcoholism, 2011 revised October 2015), which recommends using the 5As to address alcohol use (ask, advise, assess, assist, and arrange). Content addressing alcohol use was similar, though not identical, in groups tailored to those ready to quit and not ready to quit smoking, based on focus group feedback that most young adults who engaged in HED were not ready to change their alcohol use.
Earlier posts during the 90-day intervention included questions to assess alcohol use patterns and provide normative feedback, and incorporated motivational interviewing (MI) strategies to address ambivalence about changing substance use. Later posts focused on combining MI with cognitive behavioral therapy (CBT) skills including coping with cravings, refusal skills, planning for emergencies, and managing negative moods. We also based the weekly “The Dr. Is In” live counseling session content on MI and CBT skills, but with the orientation toward changing either cigarette smoking or HED. We tailored tobacco content to readiness to quit cigarette smoking, with alcohol content mainly focused on motivating people who were not ready to quit or to change their drinking habits.
2.4.3. Monetary incentive for comment engagement
Previous trials of the TSP found that monetary incentives increased engagement in the Facebook intervention (Ramo, Thrul, et al., 2018). We tallied comments to posts in the current intervention and we sent participants who commented on at least 20 posts per month a $10 gift card at the end of each month (for a total possible $30).
2.4.4. Nicotine replacement therapy
Regardless of intervention condition, we offered participants 14-day starter packs of nicotine patches if they smoked on average 5 or more cigarettes per day in the past 30 days and did not have a medical contraindication (pregnancy or uncontrolled hypertension). We mailed patches to participants who accepted the offer at the start of the trial. Nicotine levels of the patches corresponded to the reported number of cigarettes smoked per day in the past 30 days (either 14 mg for 5–9 cigarettes/day or 21 mg patches for 10 or more cigarettes/day).
2.5. Measures
2.5.1. Sociodemographic characteristics and thoughts about abstinence at baseline
Sociodemographic characteristics assessed at baseline included age, gender, race, Latino ethnicity, income ($10,000 a year or less), geographic region, and education and employment status. Race options included white, Asian, Black, Hispanic, American Indian/Alaskan Native, Pacific Islander/Hawaiian Native, and other; and participants could select more than one option. Gender options included male, female, trans-male, trans-female, genderqueer/gender non-conformation, and different; and participants could select more than one option. We used residential zip codes to categorize participants as residing in one of four U.S. census regions: Northeast, Midwest, South, and West, using zip code classifications from the 2010 Census. At baseline, participants also reported their desire, expected success, and expected difficulty for quitting cigarette smoking on a scale from 1 to 10, and the importance and confidence in reducing or stopping alcohol use, also on a scale from 1 to 10.
2.5.2. Primary outcome
The primary outcome was salivary cotinine-based biochemically verified 7-day point prevalence abstinence (Hughes, Carpenter, & Naud, 2010) from tobacco products at 3, 6, and 12 months. While the Society for Research on Nicotine and Tobacco Workgroup does not require biochemically verified abstinence for Internet-based samples on abstinence measures for smoking cessation studies (SRNT Subcommittee on Biochemical Verification, 2002), the SRNT workgroup recommends collecting and reporting both biochemically verified and self-report data. Participants reported how many cigarettes they had smoked in the past week. We considered those who reported zero cigarettes smoked in the past 7 days to have self-reported abstinence. We mailed these participants a saliva cotinine test kit and asked them to send back results to study staff via email or Facebook messenger with two pictures: one of the participant spitting into the plastic test tube and another of the test strip result (Thrul, Meacham, & Ramo, 2018). Participants did not receive their survey incentive until they completed the test or they explicitly declined the test, and they were reminded that they would still receive the incentive even if the test came back indicating nicotine exposure. There was no additional incentive provided for completing a saliva test. Participants with salivary cotinine level < 11 ng/ml, indicating nonsmoking, were considered biochemically confirmed abstinent.
2.5.3. Secondary outcomes
Secondary outcomes included 7-day reported abstinence from cigarette smoking (yes/no) (Fanshawe et al., 2017), reduction in the number of cigarettes smoked in the past week by 50% or more when compared to baseline (yes/no), report of at least one 24-hour quit attempt during treatment (yes/no), in a preparation or higher stage of change (yes/no), and cigarette dependence level as measured by the 6-item Fagerstrom Test of Cigarette Dependence (Fagerstrom, 2012; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). We drew both reported abstinence and reduction in number of cigarettes smoked from a question about number of cigarettes smoked in the past week (where 0 was considered reported abstinence). To be in a preparation stage of change, participants had to report having quit or to be planning to quit in the next 30 days, and had to have reported a 24-hour quit attempt in the past 12 months at baseline, or since the last survey assessment at follow up (Prochaska & DiClemente, 1983). Given concerns about the increase in youth vaping and questions about its role in smoking cessation, we also assessed e-cigarette/vaping use, defined as use of an e-cigarette or e-hookah/vape pen with nicotine in the past month. Post content infrequently mentioned addressing e-cigarettes/vaping (0–4 of 90 posts) and primarily questioned the intentions of e-cigarette makers or referenced the limited evidence for the efficacy of e-cigarettes in promoting smoking cessation.
Secondary alcohol outcomes included days of HED, number of drinks in the past week (timeline follow back), readiness to limit alcohol use in the next 30 days, and problematic use of alcohol use using the Alcohol Use Disorder Identification Test – Concise (AUDIT-C) (Rubinsky, Dawson, Williams, Kivlahan, & Bradley, 2013).
Secondary outcomes related to cigarette and alcohol co-use included: proportion of days in the past month reporting both cigarette smoking and HED (timeline follow back), estimated percent of cigarette smoking that occurred while drinking, and combined cigarette smoking and drinking during last drinking occasion (yes/no).
2.5.4. Intervention acceptability and engagement
We measured engagement by number of comments each participant contributed in response to automated posts. We measured acceptability with eight questions asking about agreement on a 1 to 4 scale with statements about the ease, comprehensibility, helpfulness, and likeability of the intervention. We dichotomized results into strongly disagree/disagree vs. agree/strongly agree. We also recorded eligibility for and any reported use of NRT.
2.6. Analyses
Attrition analyses examined whether age, gender, cigarettes per week, and number of past-month HED occasions at baseline were related to completion of follow-up assessments. We compared smoking and drinking outcomes between STAND and TSP conditions using chi-square and t-tests at each time point. We conducted all comparisons as complete case analyses, except for biochemically verified abstinence and self-reported abstinence, which we analyzed as both complete case and modified intent to treat (ITT), where we analyzed data as randomized even though we were unable to include measurements from those who dropped out of the study. In ITT analyses, we classified participants who did not complete follow up surveys as smoking. We compared post-treatment (3-month) outcomes between STAND and TSP conditions using logistic regression for dichotomous outcomes and linear regression for continuous outcomes. Due to baseline differences between the STAND and TSP conditions in FTCD scores and several alcohol-use factors (likely due lack of randomization on drinking levels), 3-month outcome regression models also adjusted for corresponding baseline differences. At 3-month follow-up, we compared acceptability ratings and use of NRT patches that the study provided between conditions using chi-square and t-tests. We compared commenting engagement between study conditions and between self-reported abstinence status using a Mann-Whitney test.
We conducted longitudinal models for five exploratory secondary outcomes involving smoking abstinence, smoking and drinking quantity, and tobacco and alcohol dependence: self-reported 7-day point prevalence abstinence, reduction in the number of cigarettes smoked in the past week by 50% or more, FTCD scores, days of HED, and AUDIT-C scores. We examined self-reported abstinence and 50% reduction in the number of cigarettes smoked as dichotomous outcomes with logistic regression, and we examined the other outcomes as continuous outcomes with linear regression. We conducted all models using generalized estimating equations (GEE) to account for correlated within person measures over time, with a specified auto-regressive correlation matrix, and time points specified as ordered categorical intervals of 1, 2, and 4, corresponding to 3, 6, and 12 months, respectively. GEE models included terms for time, treatment, and their interactions while being insensitive to missingess. We included actors related to attrition in final models as covariates to account for missingness due to nonresponse. This pilot study was not sufficiently powered to analyze the primary outcome of biochemically verified smoking abstinence in GEE analysis. We ran a series of models for each of five outcomes examining the effects of condition alone, time alone, condition and time, and condition interacted with time. Finally, we examined the effect of condition and time again, adjusting for baseline difference and variables related to attrition (Table 4). We ran analyses in SPSS v.25.
Table 4:
Longitudinal models testing the effect of treatment (STAND) vs. control (TSP) intervention on reported abstinence, cigarette and alcohol quantity, cigarette dependence, and alcohol misuse over 12 months (N = 413 follow up assessments).
| Self-Reported Smoking Abstinence (yes/no) | Cigarette quantity: Reduction1 by 50%+ (yes/no) | Cigarette dependence: FTCD score | Alcohol quantity: days HED2 | Alcohol misuse: AUDIT-C score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | AOR | p-value | B | AOR | p-value | B | p-value | B | p-value | B | p-value | |
| Condition (STAND vs. TSP) | 0.28 | 1.33 | 0.331 | 0.32 | 1.38 | 0.218 | −0.19 | 0.448 | −0.79 | 0.322 | −0.18 | 0.416 |
| Time (12 months vs. 3 months) | 0.94 | 2.57 | <.001 | 0.68 | 1.98 | <.001 | −0.49 | <.001 | −1.98 | 0.001 | −0.76 | 0.003 |
| Time (6 months vs. 3 months) | 0.56 | 1.75 | 0.018 | 0.45 | 1.57 | 0.023 | −0.10 | 0.540 | −1.32 | 0.016 | −0.30 | 0.226 |
| Baseline level | -- | -- | -- | -- | -- | -- | 0.47 | <.001 | 0.44 | <.001 | 0.78 | <.001 |
| Gender (Ref: Male)3 | 0.20 | 1.23 | 0.473 | 0.22 | 1.25 | 0.402 | −0.18 | 0.475 | −2.17 | 0.004 | −0.44 | 0.037 |
| Age at BL3 | −0.08 | 0.92 | 0.274 | −0.06 | 0.95 | 0.416 | −0.03 | 0.603 | 0.43 | 0.012 | 0.12 | 0.016 |
| Cigarettes past week (BL)3 | 0.00 | 1.00 | 0.643 | 0.00 | 1.00 | 0.993 | 0.00 | 0.240 | −0.01 | 0.235 | 0.004 | 0.072 |
β = effect estimate, AOR = adjusted odds ratio, BL = baseline
STAND (Smoking Tobacco and Drinking) intervention: Tobacco + Alcohol content; TSP (Tobacco Status Project) intervention: Tobacco only content
FTCD: Fagerstrom Test of Cigarette Dependence, HED: heavy episodic drinking
Reduction in cigarettes smoked per week
N = 411 follow up assessments
Variables related to attrition
3. Results
3.1. Participant characteristics
We describe overall participant characteristics in Table 1. In summary, participants tended to be in their early 20s and non-Hispanic white. While about half were male, 1 in 10 identified as transgender or non-binary.
Table 1.
Sample characteristics of young adults who participated in the STAND study at baseline (N=179).
| Total (N=179) | STAND (N=85) | TSP (N=94) | ||||
|---|---|---|---|---|---|---|
| n / M | % / SD | n / M | % / SD | n / M | % / SD | |
| Gender: Female | 72 | 40.2% | 33 | 38.8% | 49 | 41.5% |
| Male | 89 | 49.7% | 40 | 47.1% | 39 | 52.1% |
| Transgender or non-binary | 18 | 10.1% | 12 | 14.1% | 6 | 6.4% |
| Age | 22.1 | 2.20 | 22.1 | 2.32 | 22.1 | 2.08 |
| Racial/ethnic background: Non-Hispanic White Employment status | 144 | 80.4% | 66 | 77.6% | 78 | 83.0% |
| Employed, full-time (≥20 hours/week) | 77 | 43.0% | 37 | 43.5% | 40 | 42.6% |
| Employed, part-time (<20 hours/week) | 36 | 20.1% | 20 | 23.5% | 16 | 17.0% |
| Unemployed | 66 | 36.9% | 28 | 32.9% | 38 | 40.4% |
| Education status: Not in school | 121 | 67.6% | 57 | 67.1% | 64 | 68.1% |
| Part-time | 8 | 4.5% | 1 | 1.2% | 7 | 7.4% |
| Full-time | 50 | 27.9% | 27 | 31.8% | 23 | 24.5% |
| Years of school completed | 13.2 | 2.00 | 13.4 | 2.00 | 13.1 | 2.02 |
| Income < $10,000/year | 79 | 44.1% | 38 | 44.7% | 41 | 43.6% |
| Geographic region: South | 56 | 31.3% | 28 | 32.9% | 28 | 29.8% |
| West | 46 | 25.7% | 21 | 24.7% | 25 | 26.6% |
| Midwest | 44 | 24.6% | 20 | 23.5% | 24 | 25.5% |
| Northeast | 33 | 18.4% | 16 | 18.8% | 17 | 18.1% |
| Cigarette smoking | ||||||
| Usual number of cigarettes/day | 10.4 | 6.9 | 10.1 | 6.23 | 10.8 | 7.35 |
| Cigarettes past 7 days | 72.6 | 53.8 | 68.6 | 44.7 | 76.2 | 60.9 |
| Fagerstrom Test of Cig. Dependence | 5.7 | 1.76 | 5.3 | 1.54 | 6.1 | 1.85 |
| First cigarette morning <30min | 87 | 48.6% | 39 | 45.9% | 48 | 51.1% |
| Quitting cigarette smoking | ||||||
| 24h quit attempt past 12 months (yes) | 118 | 65.9% | 63 | 74.1% | 55 | 58.5% |
| Desire to quit, 1–10 | 6.0 | 2.5 | 6.1 | 2.6 | 5.9 | 2.5 |
| Expected success, 1–10 | 4.9 | 2.5 | 4.6 | 2.3 | 5.1 | 2.6 |
| Expected difficulty, 1–10 | 7.1 | 2.4 | 7.2 | 2.4 | 7.1 | 2.3 |
| Preparation stage of change1 | 49 | 27.4% | 25 | 29.4% | 44 | 25.5% |
| E-cigarette/vaporizer use past 30 days2 | 95 | 53.1% | 44 | 51.8% | 51 | 54.3% |
| Alcohol use | ||||||
| Heavy episodic drinking occasions past 30 days | 8.9 | 8.1 | 7.4 | 7.2 | 10.3 | 8.5 |
| Alcohol Use Disorder Identification Test score | 12.7 | 7.8 | 11.3 | 7.3 | 13.9 | 8.1 |
| Reducing alcohol use | ||||||
| Plans to limit drinking in next 30 days | 99 | 55.3% | 51 | 60.0% | 48 | 51.1% |
| Importance to reduce or stop, 1–10 | 5.1 | 3.1 | 5.1 | 3.3 | 5.0 | 3.0 |
| Confidence to reduce or stop, 1–10 | 7.1 | 2.7 | 7.1 | 2.8 | 7.1 | 2.6 |
| Alcohol and cigarettes co-use | ||||||
| Est. % of smoking while drinking | 50.8 | 30.5 | 44.4 | 29.3 | 56.7 | 30.6 |
| Combined use at last drinking occasion | 173 | 96.6% | 82 | 96.5% | 91 | 96.8% |
Plans to quit smoking in the next 30 days & had prior year quit attempt.
With nicotine.
Or is already limiting drinking.
STAND (Smoking Tobacco and Drinking) intervention: Tobacco + Alcohol content; TSP (Tobacco Status Project) intervention: Tobacco only content
3.2. Follow-up retention
At 3 months, the overall retention was 82.6% (81.1% in STAND, 84.0% in TSP). Males and participants who smoked fewer cigarettes per week at baseline were less likely to complete the 3-month assessment. At 6 months, the overall retention fell to 66.0% (62.4% in STAND, 64.9% in TSP). Again, males were less likely to complete the 6-month assessment. At 12 months, with the increased incentive, the overall retention rate from baseline was 84.0% (83.3% in STAND, 86.2% in TSP), and males and younger participants were less likely to complete the 12-month assessment.
3.3. Primary outcome
Biochemically verified abstinence from smoking did not significantly differ between STAND and TSP at 3, 6, or 12 months in complete case or intent to treat analyses (all p > .05). Biochemically verified abstinence (ITT) was 3.5% in STAND and 4.3% in TSP at 3 months, 0% in STAND and 1.2% in TSP at 6 months, and 3.5% in STAND and 0% at TSP at 12 months (Table 2). We obtained biochemical cotinine exposure for 14 of 24 participants (58.3%) self-reporting abstinence at 3 months; 11 of 29 (37.9%) self-reporting abstinence at 6 months, and 35 of 49 (71.4%) self-reporting abstinence at 12 months. At every follow-up assessment, most test kit results (50.0% at 3 months, 81.8% at 6 months, 91.4% at 12 months) indicated that participants had been exposed to nicotine; of those with exposure to nicotine at 12 months, 34.4% stated that they had been vaping e-cigarettes with nicotine.
Table 2:
Tobacco and alcohol outcomes among young adults in STAND treatment vs. TSP control conditions over 12 months (N=179).
| 3 months | 6 months | 12 months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STAND (N=69) | TSP (N=79) | STAND (N=53) | TSP (N=61) | STAND (N=70) | TSP (N=81) | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Tobacco Outcomes | ||||||||||||
| Verified abstinence (complete case) | 3 | 4.3% | 4 | 5.1% | 0 | 0% | 1 | 1.9% | 3 | 4.3% | 0 | 0% |
| Verified abstinence (ITT: missing = smoking) | 3 | 3.5% | 4 | 4.3% | 0 | 0% | 1 | 1.2% | 3 | 3.5% | 0 | 0% |
| Self-reported abstinence (complete case) | 15 | 21.7% | 9 | 11.4% | 14 | 26.4% | 14 | 23.0% | 25 | 35.7% | 24 | 29.6% |
| Self-reported abstinence (ITT: missing = smoking) | 15 | 17.6% | 9 | 9.6% | 14 | 16.5% | 14 | 14.9% | 25 | 29.4% | 24 | 25.5% |
| Reduction in cigarettes smoked by 50% or more | 32 | 47.1% | 35 | 44.3% | 35 | 68.6% | 30 | 49.2% | 46 | 66.7% | 46 | 57.5% |
| Quit attempt during treatment | 49 | 71% | 45 | 57.7% | -- | -- | -- | -- | -- | -- | -- | -- |
| Preparation or higher stage of change1 | 37 | 53.6% | 27 | 35.1% | 20 | 37.7% | 23 | 37.7% | 34 | 49.3% | 41 | 50.6% |
| FTCD (mean, SD) | 1.9 | 1.9 | 2.8 | 2.1 | 1.6 | 1.9 | 2.7 | 2.2 | 1.5 | 1.9 | 1.9 | 2.0 |
| Alcohol Outcomes | ||||||||||||
| Days HED in past month (mean, SD) | 4.8 | 7 | 7.6 | 8.2 | 3. 8 | 5.5 | 6 | 7.8 | 3.2 | 5.2 | 5.6 | 7.6 |
| Drinks in past week (median/IQR) | 4 | (0,12) | 6 | (0,17) | 5 | (0,11) | 7 | (0,15) | 1 | (0,7.8) | 4 | (0,16) |
| Plans to limit drinking in next 30 days (or is already) | 40 | 58% | 42 | 54.5% | 28 | 52.8% | 38 | 62.3% | 42 | 60.0% | 54 | 66.7% |
| AUDIT-C (mean, SD) | 3.9 | 2.7 | 5 | 2.9 | 3.7 | 2.7 | 4.8 | 3.0 | 3.5 | 2.4 | 4.1 | 3.1 |
| Tobacco & Alcohol Co-Use Outcomes | ||||||||||||
| Est. % of smoking while drinking (mean, SD) | 33.8 | 33.5 | 47.7 | 36.2 | 35.2 | 36.0 | 43.9 | 37.2 | 26.3 | 33.1 | 35.7 | 36.9 |
| Prop. of past 30 days HED + cigarettes (mean, SD) | 6.9 | 13.2 | 12.7 | 19.0 | 6 | 11 | 11.4 | 21.5 | 4.5 | 9.1 | 9.4 | 17.9 |
| Combined use last drinking occasion | 48 | 69.6% | 63 | 81.8% | 36 | 67.9% | 49 | 80.3% | 43 | 61.4% | 52 | 64.2% |
| Other Outcomes | ||||||||||||
| E-cigarette/vaping in past 30 days | 28 | 40.6% | 49 | 62.0% | 22 | 41.5% | 26 | 42.6% | 32 | 45.7% | 44 | 54.3% |
| Cigarettes in past week (mean, SD) | 40 | 45.7 | 54.7 | 53.6 | 27.5 | 34.3 | 51.3 | 54.3 | 33.8 | 39.5 | 41.5 | 51.2 |
STAND (Smoking Tobacco and Drinking) intervention: Tobacco + Alcohol content; TSP (Tobacco Status Project) intervention: Tobacco only content ITT (intent to treat) sample size: STAND N=85, TSP=94
FTCD: Fagerstrom Test of Cigarette Dependence, HED: heavy episodic drinking, AUDIT-C: Alcohol Use Disorder Identification Test - Concise SD: standard deviation, IQR: interquartile range
Has quit smoking or plans to quit smoking in the next 30 days & had quit attempt since the last since survey assessment
3.4. Secondary outcomes (tobacco)
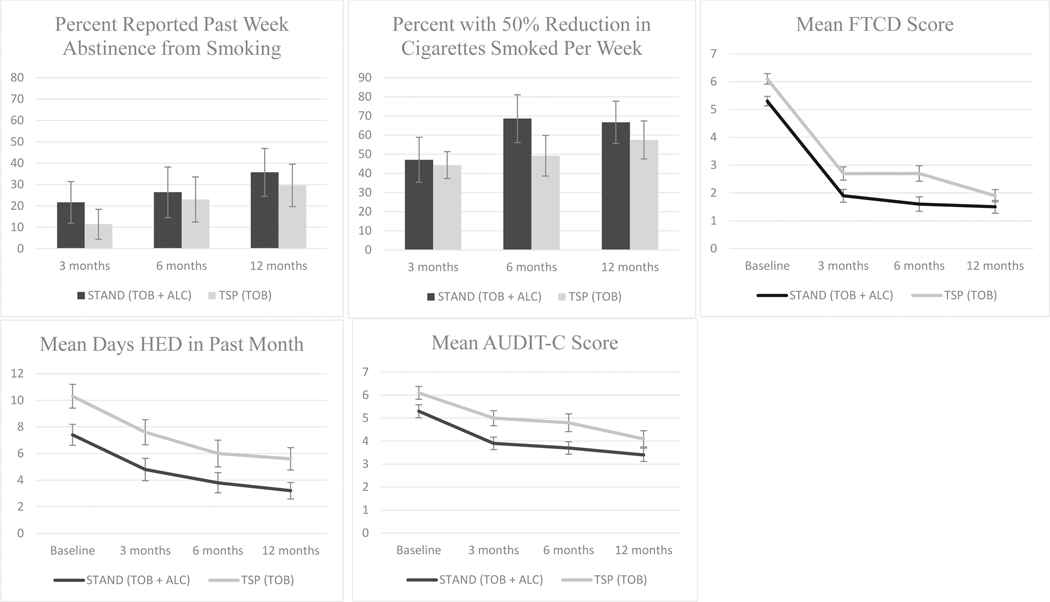
In longitudinal analyses, there were no significant differences by condition over time with respect to reported abstinence, reduction in the number of cigarettes smoked in the past week by 50% or more, or FTCD scores between STAND and TSP (Table 4; Figure 2). There was, however, a significant increase in the odds of reported abstinence and 50% reduction or more in cigarettes smoked and decreased FTCD scores for all study participants over 12 months. Self-reported 7-day point prevalence abstinence (ITT) was 17.6% in STAND and 9.6% in TSP at 3 months, 16.5% in STAND and 14.9% in TSP at 6 months, and 29.4% in STAND and 25.5% in TSP at 12 months (Table 2). Neither self-reported abstinence from smoking nor 50% reduction in cigarettes smoked in the past week significantly differed between STAND and TSP participants at any follow up assessment or over time (Table 3). There were also no significant differences in FTCD scores after adjusting for baseline differences in scores between the two conditions. Of note, STAND participants were significantly more likely to report being in the preparation or action stage (p=.025) and less likely to report vaping/e-cigarette use with nicotine at 3 months than TSP participants were (p = .01), although these differences no longer persisted at 6 or 12 months.
Figure 2:
Selected tobacco and alcohol outcomes plotted over 12 months for treatment (STAND) vs. control (TSP).
STAND (Smoking Tobacco and Drinking) intervention: Tobacco + Alcohol content; TSP (Tobacco Status Project) intervention: Tobacco only content FTCD: Fagerstrom Test of Cigarette Dependence, HED: heavy episodic drinking, AUDIT-C: Alcohol Use Disorder Identification Test – Concise All figures reflect complete case analyses
Table 3:
Regression estimates of tobacco and alcohol outcomes for treatment (STAND) vs. control (TSP) at end-of-intervention 3 month follow up (N=148).
| Effect estimate1 | p-value | |
|---|---|---|
| Tobacco Outcomes | ||
| Verified abstinence (ITT: missing = smoking) | .82 | .803 |
| Self-reported abstinence (ITT: missing=smoking) | 2.02 | .118 |
| Self-reported abstinence (complete case) | 2.16 | .093 |
| Reduction in cigarettes smoked by 50% or more | 1.11 | .738 |
| Quit attempt during treatment | 1.512 | .270 |
| Preparation or higher stage of change | 2.14 | .025 |
| FTCD (B) | −.302 | .310 |
| Alcohol Outcomes | ||
| Days HED in past month (B) | −.652 | .535 |
| Drinks in past week (B) | −2.233 | .288 |
| Plans to limit drinking in next 30 days | 1.04 | .934 |
| AUDIT-C (B) | −0.412 | .247 |
| Tobacco Alcohol Co-use Outcomes | ||
| Est. % of smoking while drinking (B) | −7.662 | .156 |
| Prop. of past 30 days HED + tobacco (B) | −.023 | .405 |
| Combined use last drinking episode | .51 | .086 |
| Other Outcomes | ||
| E-cigarette/vaping in past 30 days | .42 | .010 |
| Cigarettes in past week (B) | −14.79 | .075 |
STAND (Smoking Tobacco and Drinking) intervention: Tobacco + Alcohol content; TSP (Tobacco Status Project) intervention: Tobacco only content
ITT (intent to treat) sample size: STAND N=85, TSP=94
FTCD: Fagerstrom Test of Cigarette Dependence, HED: heavy episodic drinking, AUDIT-C: Alcohol Use Disorder Identification Test – Concise
SD: standard deviation, IQR: interquartile range
Effect estimates are odds ratios for dichotomous outcomes unless otherwise noted and B for continuous outcomes
Estimate adjusts for baseline differences in intervention arms
Adjusted for number of heavy episodic drinking days at baseline
3.5. Secondary outcomes (alcohol and co-use)
Once we adjusted for baseline differences, there were no significant differences at 3 months between STAND and TSP conditions with respect to days of HED in the past month, number of drinks in the past week, or AUDIT-C scores. With respect to co-use, there were similarly no differences in frequency of smoking while drinking or days reporting both HED and smoking after adjusting for baseline differences in HED occasions (Table 3). In longitudinal analyses, there were no significant differences over time with respect to days of HED or AUDIT-C scores, nor did the rate of change in alcohol use outcomes over time differ by condition (Table 4; Figure 2). As we found for tobacco outcomes, over 12 months there was a significant decrease in days of HED and AUDIT-C scores in both study conditions.
3.6. Acceptability and engagement
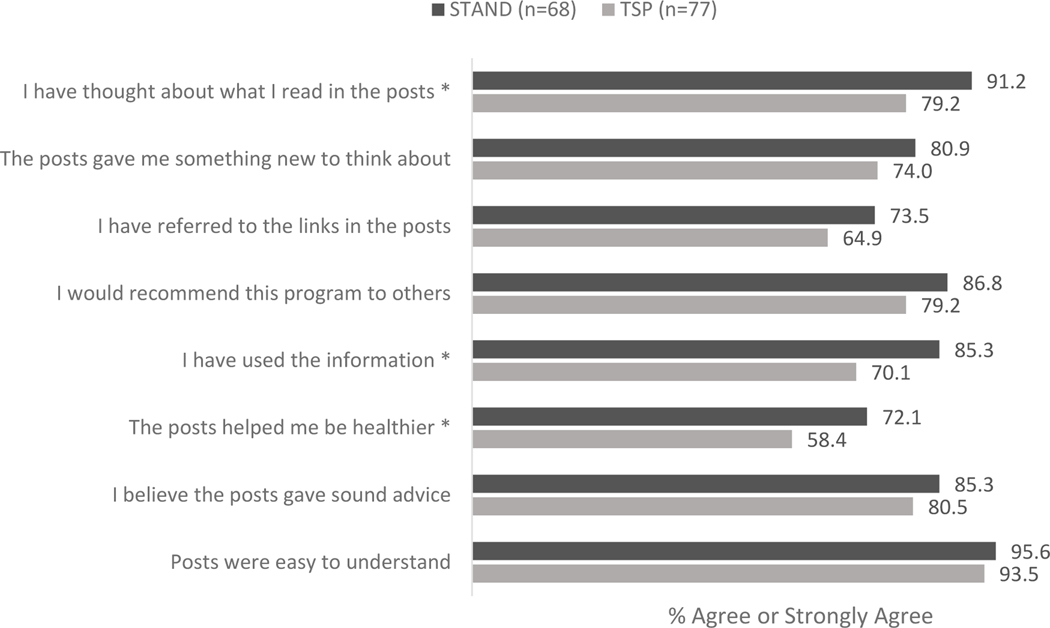
STAND participants were more likely than TSP participants to agree that the intervention helped them to be healthier, that they used the information in the posts, and that they thought about what they read in the posts (Figure 3). Agreement on other usability and acceptability measures did not significantly differ. Overall, 87% of participants (N=155) commented at least once and the median number of post and live counseling session comments per participant was 50.0 (interquartile range [IQR] = 6, 91). While there were no significant differences in number of comments that participants made between STAND and TSP, the 24 participants with self-reported smoking abstinence at 3 months contributed significantly more comments (median = 90.5, IQR =35.5, 101.8) than did the 155 participants without self-reported abstinence at 3 months (median = 38.0, IQR = 6, 89) (p = .039). Slightly more than 41% of participants (41.3%; N=74) attended The Doctor Is In live counseling sessions (N=74) and attendance was not significantly associated with self-reported abstinence (p = .355).
Figure 3:
Proportion of participants who ‘agree’ or ‘strongly agree’ with statements about content in the STAND (treatment) intervention vs. TSP (control) intervention.
STAND (Smoking Tobacco and Drinking) intervention: Tobacco + Alcohol content; TSP (Tobacco Status Project) intervention: Tobacco only content
* p <.05.
3.7. Use of nicotine patches
Of 179 participants, 150 were eligible for nicotine replacement therapy, and we messaged them to see if they were interested. Of these, 94 were interested and we mailed them nicotine patches. At the end of treatment/3-month follow-up, 81 reported receiving nicotine replacement therapy from the study. Of these, 54 participants reported trying nicotine replacement therapy, 14 participants used it for the full 2 weeks, and 8 participants reported continuing to use patches after the study supply ran out. There were no significant differences between STAND and TSP with respect to receiving the patch from the study and trying the patch. At the end of treatment/3-month follow up, 17.3% of those who received the nicotine patch reported past week abstinence vs. 15.2% of those who did not.
4. Discussion
Young adult participants in two Facebook-based tobacco cessation interventions—one targeting tobacco alone, the other with an added focus on alcohol—reported significant reductions over time in both cigarette smoking and alcohol use. Self-reported abstinence increased over time and the number of cigarettes smoked, days of HED, and FTCD and AUDIT-C scores decreased over time. However, outcomes did not differ significantly by treatment condition, and remote biochemical verification of smoking status proved challenging. Two secondary outcomes were significantly better for STAND participants than for TSP participants at the end of the intervention (i.e., they were less likely to use e-cigarettes and more likely to be in the preparation-action stages of change for quitting smoking); however, the differences were no longer significant at the 6- and 12-month follow-ups. Both STAND and TSP were generally engaging for young adults, and participants who reported smoking abstinence at the end of the intervention commented significantly more than those who did not report smoking abstinence. Participants in the STAND intervention rated several acceptability aspects more favorably than did participants in the TSP intervention.
Social perceptions regarding relative risk of different substances as well as alcohol use levels in the sample may have contributed to these outcome patterns. While the harms of tobacco use are well known, intervention development focus group participants reported greater interest in quitting cigarette smoking than changing their alcohol use (Ramo et al., 2019), and prior studies have found that young adults have more favorable social norms regarding alcohol use and are less likely to acknowledge health risks of alcohol use than smoking (Cohn et al., 2019). The inclusion criterion of only one recent HED occasion in the past month (versus consuming 4+/5+ drinks within a 2-hour window), while applicable to a broader potential population and consistent with national standards regarding unhealthy drinking, may have led to a heterogeneous sample without enough alcohol-related problems to see a treatment effect.
In comparison to the previous TSP randomized controlled trial with 500 participants, which found a significant post-treatment effect on cigarette smoking relative to an inactive referral control condition (Ramo, Thrul, et al., 2018), this pilot trial comparing two active conditions may have been underpowered to detect differences in cigarette smoking cessation. Rates of smoking cessation and other secondary outcomes (e.g., making a quit attempt, being in preparation) in STAND were consistently higher than those in TSP, and the effect sizes for reported smoking abstinence across 12 months, though not significant, were of similar magnitude (AOR = 1.33 for STAND vs. TSP; OR = 1.29 for TSP vs. control referral). The finding of comment engagement volume on posts predicting self-reported abstinence contrasts with the analyses in the TSP trial that found no relationship between self-reported abstinence and post and counselor engagement volume (Ramo, Thrul, et al., 2018), yet is consistent with a feasibility trial that did find a relationship (Ramo, Thrul, Chavez, Delucchi, & Prochaska, 2015).
In this and other trials of a Facebook smoking cessation intervention (Ramo, Thrul, et al., 2018), participants’ “compliance” rates with the biochemical verification were relatively low (38–70%), which may have contributed to low estimated abstinence prevalence and limited power to detect differences between conditions. While a previous analysis of the original TSP trial data found no systematic differences with respect to who completes the biochemical verification procedure in terms of sociodemographics, smoking cessation goal, or readiness to quit (Thrul et al., 2018), biochemical verification may be an additional burden for participants enrolled in a low touch digital trial. Additionally, in the current trial there were occasional issues with the quality of the test kits, resulting in unreadable test strips and the need to resend test kit packages and require participants to perform the test again.
A larger concern for both digital clinical trials and broader tobacco control efforts is the growing popularity of e-cigarette/vaping use (or electronic nicotine delivery systems; ENDS), the emergence of “dual users” of both e-cigarettes and combusted cigarettes (Owusu et al., 2019), and an inconclusive evidence base for the role of ENDS in promoting or hindering smoking cessation (Kalkhoran, Chang, & Rigotti, 2019; Romijnders, van Osch, de Vries, & Talhout, 2018; Zhu, Zhuang, Wong, Cummins, & Tedeschi, 2017). Many participants in this study who reported abstinence yet provided test results indicating exposure to nicotine also reported vaping when we followed-up with them individually. Furthermore, in the comment responses to study posts, participants often reported vaping as a personal tool for smoking cessation. Other potential reasons for discrepancies between self-report and biochemically verified abstinence besides misreporting include use of other smokeless tobacco products, heavy secondhand exposure, relapse between reporting and receiving the kit, and some foods (e.g., eggplant, tomatoes, and peppers).
Nevertheless, in supplemental analyses, people who vaped were no more or less likely to report abstinence. However, it is worth noting that at the 3-month follow-up (though not at 6- or 12-months), participants in STAND were less likely to report vaping nicotine than were participants in TSP, despite similar prevalence of past month use at baseline. We rarely mentioned content addressing e-cigarettes/vaping in either condition (0–1 post in STAND, 3–4 posts in TSP). It may be that the STAND intervention increased awareness of overall health or how social settings affect substance use and thus contributed to post-treatment differences in vaping nicotine.
There are a number of additional limitations to these findings. First, the sample was all U.S.-based and primarily non-Hispanic white, which may limit generalizability to other races and ethnic groups or other geographic locations. The aforementioned issues with test strips in the biochemical verification procedure (with respect to staff and participant burden and vaping detection) and the delay in receiving participation incentive while waiting for the test kit may have led to reduced verified abstinence rates. Future research may consider other forms of biochemical verification of combustible smoking abstinence such as personal carbon monoxide detectors (Herbec, Brown, Shahab, & West, 2019) or combinations of biomarkers (Benowitz et al., 2019). Response rates for the 6-month follow-up survey were substantially lower than 3 or 12 months, and the increase of survey incentive from $25 to $50 likely influenced the increase in response rate at 12 months. As noted above, we conducted this pilot trial to determine feasibility of the approach and it was not sufficiently powered to detect differences in a rare primary outcome over time or to provide meaningful effect size estimates. Additionally, by conducting multiple hypotheses testing some of the significant findings may have been due to chance (i.e., Type I error). Last, we randomized participants based on smoking level and intention to quit, yet any differences in cigarette dependence, alcohol use and misuse, and combined use were no longer significant after adjusting for pre-existing baseline differences in alcohol use. Future studies targeting multiple substances should endeavor to randomize to trial conditions based on all substances or behaviors targeted and may want to consider increasing the proportion of content addressing the interaction between substances.
The study had a number of strengths, including the effective recruitment of a geographically diverse sample with a high proportion of male and non-binary participants compared to other smoking cessation trials, and a high proportion of non-students compared to young adult substance use research studies typically conducted with college students. A further strength is that the study engaged a broad range of young adult smokers, including those who were not ready to quit smoking at the start of the trial. This is one of the first social media interventions to address both tobacco and alcohol use in a young adult population, with high ratings of acceptability and high study follow-up participation.
5.1. Conclusion
In conclusion, this pilot study found that a social media intervention addressing tobacco and alcohol use was no more effective at increasing smoking abstinence than an intervention addressing only tobacco. This trial nevertheless contributes to literature supporting this modality in reaching and engaging young adults who smoke cigarettes and drink, a traditionally challenging group to engage in smoking cessation interventions. We will conduct ongoing analyses of the study data to examine the patterns and content of comments posted in Facebook groups. Future work should examine how to incorporate evidence regarding the pros and cons of ENDS for smoking cessation, as well as how to address use of multiple substances among young adults with varying levels of risk and readiness to change.
Supplementary Material
Highlights.
We compared two Facebook-based smoking cessation interventions
One intervention addressed tobacco and alcohol, the other addressed only tobacco
Participants reported significant improvements in smoking and alcohol use over time
There were no significant differences between the two interventions
The tobacco + alcohol intervention was rated more favorably than the tobacco one
Acknowledgements
The authors would like to thank study participants and Mandy Chan and Juliet Yonek for their contributions to study and data management.
Sources of Support
Funding for this research and manuscript preparation comes from the National Institute of Health grants R34 DA041637, K01 DA046697, and K24 AA025703. LJM was funded by the Swiss National Science Foundation grant P2ZHP1_174812.
Funding: NIH R34 DA041637, K01 DA046697, K24 AA025703; Swiss National Science Foundation P2ZHP1_174812
Footnotes
Competing Interests
DER is an advisor to Carrot, Inc, which makes a smoking cessation device. JPP has provided consultation to pharmaceutical and technology companies that make medications and other treatments for quitting smoking and has served as an expert witness in lawsuits against tobacco companies. The other authors declare that they have no financial relationships with any organizations that might have an interest in the submitted work.
STAND main outcome paper
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Cancer Society. (2020). Alcohol use and cancer. Retrieved from: https://www.cancer.org/cancer/cancer-causes/diet-physical-activity/alcohol-use-and-cancer.html. (Accessed. [Google Scholar]
- Ames SC, Werch CE, Ames GE, Lange LJ, Schroeder DR, Hanson AC, & Patten CA (2010). Integrated smoking cessation and binge drinking intervention for young adults: a pilot investigation. Annals of Behavioral Medicine, 40(3), 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo JK, McIlvain HE, Lando HA, Walker RD, & Leed-Kelly A. (1998). Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction, 93(6), 877–887. [DOI] [PubMed] [Google Scholar]
- Cohn AM, Amato MS, Zhao K, Wang X, Cha S, Pearson JL, . . . Graham AL (2019). Discussions of alcohol use in an online social network for smoking cessation: Analysis of topics, sentiment, and social network centrality. Alcoholism: Clinical and Experimental Research, 43(1), 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K. (2012). Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research, 14(1), 75–78. [DOI] [PubMed] [Google Scholar]
- Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone-Banks J, & Hartmann-Boyce J. (2017). Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews, 11, CD003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, & Jaen CR (2008). A clinical blueprint to accelerate the elimination of tobacco use. JAMA, 299(17), 2083–2085. [DOI] [PubMed] [Google Scholar]
- Gubner NR, Delucchi KL, & Ramo DE (2016). Associations between binge drinking frequency and tobacco use among young adults. Addictive Behaviors, 60, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug S, Paz Castro R, Kowatsch T, Filler A, & Schaub MP (2017). Efficacy of a technology-based, integrated smoking cessation and alcohol intervention for smoking cessation in adolescents: Results of a cluster-randomised controlled trial. Journal of Substance Abuse Treatment, 82, 55–66. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Herbec A, Brown J, Shahab L, & West R. (2019). Lessons learned from unsuccessful use of personal carbon monoxide monitors to remotely assess abstinence in a pragmatic trial of a smartphone stop smoking app - A secondary analysis. Addictive Behavior Reports, 9, 100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ, & Naud S. (2010). Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic review. Nicotine & Tobacco Research, 12(7), 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, & Melton LJ 3rd. (1996). Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA, 275(14), 1097–1103. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Borland R, Hyland A, McKee SA, O’Connor RJ, Fong GT, & Cummings KM (2010). Quitting smoking and change in alcohol consumption in the International Tobacco Control (ITC) Four Country Survey. Drug and Alcohol Dependence, 110(1–2), 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, LaChance HR, Ramsey SE, Abrams DB, Monti PM, & Brown RA (2008). Addressing heavy drinking in smoking cessation treatment: A randomized clinical trial. Journal of Consulting and Clinical Psychology, 76(5), 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoran S, Chang Y, & Rigotti NA (2019). Electronic cigarette use and cigarette abstinence over two years among U.S. Smokers in the population assessment of tobacco and health study. Nicotine & Tobacco Research, 22(5), 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Lee JM, Wu DC, Hsu HK, Kao EL, Huang HL, . . . Wu, M. T. (2005). Independent and combined effects of alcohol intake, tobacco smoking and betel quid chewing on the risk of esophageal cancer in Taiwan. International Journal of Cancer, 113(3), 475–482. [DOI] [PubMed] [Google Scholar]
- McKee SA, & Weinberger AH (2013). How can we use our knowledge of alcohol-tobacco interactions to reduce alcohol use? Annual Review of Clinical Psychology, 9, 649–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey K, Thrul J, & Ramo D. (2017). Impact of quitting smoking and smoking cessation treatment on substance use outcomes: An updated and narrative review. Addictive Behaviors, 65, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, & Yi HY (2014). Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug and Alcohol Dependence, 136, 51–62. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (2011. revised October 2015). Alcohol screening and brief intervention for youth: A practitioner’s guide. Retrieved from: https://www.niaaa.nih.gov/sites/default/files/youth-guide.pdf. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (2020a). Rethinking drinking. Retrieved from: https://www.rethinkingdrinking.niaaa.nih.gov/. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (2020b). Rethinking drinking: What are the different drinking levels? Retrieved from: https://www.rethinkingdrinking.niaaa.nih.gov/how-much-is-too-much/is-your-drinking-pattern-risky/Drinking-Levels.aspx. [Google Scholar]
- Owusu D, Huang J, Weaver SR, Pechacek TF, Ashley DL, Nayak P, & Eriksen MP (2019). Patterns and trends of dual use of e-cigarettes and cigarettes among U.S. adults, 2015–2018. Preventive Medicine Reports, 16, 101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelucchi C, Gallus S, Garavello W, Bosetti C, & La Vecchia C. (2006). Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Research and Health, 29(3), 193–198. [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center. (2020). Social media fact sheet. Retrieved from: https://www.pewresearch.org/internet/fact-sheet/social-media/. [Google Scholar]
- Prochaska JO, & DiClemente CC (1983). Stages and processes of self-change of smoking: toward an integrative model of change. Journal of Consulting and Clinical Psychology, 51(3), 390–395. [DOI] [PubMed] [Google Scholar]
- Ramo DE, Kaur M, Corpuz ES, Satre DD, Delucchi K, Brown SA, & Prochaska JJ (2018). Using Facebook to address smoking and heavy drinking in young adults: Protocol for a randomized, controlled trial. Contemporary Clinical Trials, 68, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Meacham MC, Kaur M, Corpuz ES, Prochaska JJ, & Satre DD (2019). Development of a social media-based intervention targeting tobacco use and heavy episodic drinking in young adults. Addiction Science & Clinical Practice, 14(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Rodriguez TM, Chavez K, Sommer MJ, & Prochaska JJ (2014). Facebook recruitment of young adult smokers for a cessation trial: Methods, metrics, and lessons learned. Internet Interventions, 1(2), 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Thrul J, Chavez K, Delucchi KL, & Prochaska JJ (2015). Feasibility and quit rates of the tobacco status project: A Facebook smoking cessation intervention for young adults. Journal of Medical Internet Research, 17(12), e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Thrul J, Delucchi KL, Hall S, Ling PM, Belohlavek A, & Prochaska JJ (2018). A randomized controlled evaluation of the tobacco status project, a Facebook intervention for young adults. Addiction. doi: 10.1111/add.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijnders K, van Osch L, de Vries H, & Talhout R. (2018). Perceptions and reasons regarding e-cigarette use among users and non-users: A narrative literature review. International Journal of Environmental Research, 15(6), doi: 10.3390/ijerph15061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, & Bradley KA (2013). AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcoholism: Clinical and Experimental Research, 37(8), 1380–1390. [DOI] [PubMed] [Google Scholar]
- Satre DD, Kohn CS, & Weisner C. (2007). Cigarette smoking and long-term alcohol and drug treatment outcomes: a telephone follow-up at five years. American Journal on Addictions, 16(1), 32–37. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4(2), 149–159. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2017). Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17– 044, NSDUH Series H-52). Retrieved from: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. [Google Scholar]
- Thrul J, Klein AB, & Ramo DE (2015). Smoking cessation intervention on Facebook: Which content generates the best engagement? Journal of Medical Internet Research, 17(11), e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrul J, Meacham MC, & Ramo DE (2018). A novel and remote biochemical verification method of smoking abstinence: Predictors of participant compliance. Tobacco Prevention & Cessation, 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, Martino S, O’Malley SS, Fucito LM, McKee SA, Kahler CW, . . . Cummings, K. M. (2015). A randomized trial for hazardous drinking and smoking cessation for callers to a quitline. Journal of Consulting and Clinical Psychology, 83(3), 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Desai SA, Bowen S, Leigh BC, Kirouac M, & Larimer ME (2014). Development and evaluation of a mobile intervention for heavy drinking and smoking among college students. Psychology of Addictive Behaviors, 28(3), 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakletskaia L, Wilson E, & Fleming MF (2011). Alcohol use in students seeking primary care treatment at university health services. Journal of American College Health, 59(3), 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SH, Zhuang YL, Wong S, Cummins SE, & Tedeschi GJ (2017). E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ, 358, j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.