Abstract
Partial mycoheterotrophy, the ability of plants to obtain carbon from fungi throughout their life cycle in combination with photosynthesis, appears to be more common within the Plant Kingdom than previously anticipated. Recent studies using stable isotope analyses have indicated that isotope signatures in partially mycoheterotrophic plants vary widely among species, but the relative contributions of family- or species-specific characteristics and the identity of the fungal symbionts to the observed differences remain unclear. Here, we investigated in detail mycorrhizal communities and isotopic signatures in four co-occurring terrestrial orchids (Platanthera chlorantha, Epipactis helleborine, E. neglecta and the mycoheterotrophic Neottia nidus-avis). All investigated species were mycorrhizal generalists (i.e., associated with a large number of fungi simultaneously), but mycorrhizal communities differed significantly between species. Mycorrhizal communities associating with the two Epipactis species consisted of a wide range of fungi belonging to different families, whereas P. chlorantha and N. nidus-avis associated mainly with Ceratobasidiaceae and Sebacinaceae species, respectively. Isotopic signatures differed significantly between both Epipactis species, with E. helleborine showing near autotrophic behavior and E. neglecta showing significant enrichment in both carbon and nitrogen. No significant differences in photosynthesis and stomatal conductance were observed between the two partially mycoheterotrophic orchids, despite significant differences in isotopic signatures. Our results demonstrate that partially mycoheterotrophic orchids of the genus Epipactis formed mycorrhizas with a wide diversity of fungi from different fungal families, but variation in mycorrhizal community composition was not related to isotope signatures and thus transfer of C and N to the plant. We conclude that the observed differences in isotope signatures between E. helleborine and E. neglecta cannot solely be explained by differences in mycorrhizal communities, but most likely reflect a combination of inherent physiological differences and differences in mycorrhizal communities.
Keywords: mixotrophy, mycorrhiza, Orchidaceae, photosynthesis, stable isotopes, trophic modes
Introduction
Partial mycoheterotrophy (PMH), a form of mixotrophy that enables plants to obtain carbon from fungi (i.e., a net flow of C from a fungus to a plant) throughout their life cycle in combination with photosynthesis (Gebauer and Meyer, 2003), appears to be common within the Plant Kingdom (Selosse and Roy, 2009; Merckx, 2013; Jacquemyn and Merckx, 2019), but has only recently received increased attention (e.g., Těšitel et al., 2018). PMH has been considered to be an intermediate evolutionary stage from which full mycoheterotrophy has evolved (Julou et al., 2005; Selosse et al., 2017; Jacquemyn and Merckx, 2019). However, recent studies have indicated that the evolution from partial mycoheterotrophy to full mycoheterotrophy may be costly and involves specific adaptations (Roy et al., 2013; Gonneau et al., 2014) and a switch toward fungal partners that are able to provide more carbon and/or nitrogen to the plants (Ogura-Tsujita et al., 2012; Yagame et al., 2016; Jacquemyn and Merckx, 2019). As a result, PMH is considered to be evolutionary metastable (Těšitel et al., 2018) and displays an almost continuous gradient between autotrophy and full mycoheterotrophy (Gebauer et al., 2016; Jacquemyn et al., 2017; Schiebold et al., 2017; Jacquemyn and Merckx, 2019). However, what ecological factors determine the extent of dependency on fungal carbon and nitrogen and whether this is affected by the identity of the fungi involved in the mycorrhizal symbiosis, remains to some extent unclear (Hynson et al., 2013).
Previous research has shown that differences in stable isotope composition and N concentrations were related to plant family-specific physiological interactions with fungi and their environments (Hynson et al., 2016) as well as with environmental and developmental aspects in a given species (e.g., Matsuda et al., 2012; Roy et al., 2013; Gonneau et al., 2014). In orchids, light availability and fungal identity have been identified as important drivers of differences in carbon and nitrogen isotope abundance patterns. Partial mycoheterotrophy appears to be common in species associated with ectomycorrhizal fungi (e.g., Bidartondo et al., 2004; Selosse et al., 2004; Schiebold et al., 2017), and these species tend to be more flexible in responding to low-light conditions by increasing the proportional carbon gain from the fungal source than rhizoctonia-associated orchid species (Preiss et al., 2010; Liebel et al., 2015; Schweiger et al., 2019). Orchids associating with typical rhizoctonia fungi are usually not, or only weakly, enriched in heavy carbon and nitrogen isotopes (e.g., Stöckel et al., 2011; Jacquemyn et al., 2017; Schweiger et al., 2018). However, recent analyses using hydrogen stable isotope abundance have shown that rhizoctonia-associated orchids receive fungal organic matter as well (Gebauer et al., 2016; Schiebold et al., 2018; Schweiger et al., 2018, 2019), but precise characterization of the net flow remains to be established.
Further evidence for interactions with fungi affecting nitrogen and carbon uptake from fungi was given by Schiebold et al. (2017), who investigated carbon and nitrogen isotope abundance in fourteen Epipactis species. They showed that the type of mycorrhizal fungi found in the roots was related to variation in 15N enrichment of leaf tissue, suggesting that variation in mycorrhizal communities drives variation in isotope signatures. Because ectomycorrhizal fungi are generally involved in trophic interactions with surrounding trees, it was suggested that they provide larger and more stable supplies of fungal carbon and nitrogen than the more typical rhizoctonia fungi (Bruns et al., 2002; Selosse and Martos, 2014). However, recent research using high-throughput sequencing has shown that the mycorrhizal communities associating with orchid roots often consist of complex assemblages of multiple fungi from different fungal families and genera (Jacquemyn et al., 2015, 2016, 2017; Waud et al., 2017), all of which can potentially contribute to the resource budget of the plant. For example, adult plants of Liparis loeselii associated with various fungi of Thelephoraceae, Sebacinaceae, Russulaceae, Tulasnellaceae, Psathyrellaceae and Inocybaceae (Waud et al., 2017), thus combining rhizoctonia and ectomycorrhizal fungi. Roots of Epipactis palustris showed associations with a large number of fungal strains of different families, including Tulasnellaceae, Ceratobasidiaceae, Sebacinaceae and Thelephoraceae, and to a lesser extent Inocybaceae, Cortinariaceae, and Herpotrichiellaceae (Jacquemyn et al., 2016; Jacquemyn and Merckx, 2019). Similarly, Epipactis helleborine harbors ectomycorrhizal and rhizoctonia fungi (Jacquemyn et al., 2016; Xing et al., 2020), and in culture conditions the latter can be the sole associates (May et al., 2020). These results indicate that the mycorrhizal communities associating with orchids can be diverse and that a strict distinction in fungal syndromes cannot be easily made. They also question the previous finding that isotope signatures can be directly related to variation in mycorrhizal communities (Schiebold et al., 2017).
Here we investigated in detail mycorrhizal communities and isotope signatures in four co-occurring orchid species (Platanthera chlorantha, Epipactis helleborine and E. neglecta and the fully mycoheterotrophic Neottia nidus-avis). Photosynthetic rates and stomatal conductance were also measured in the partial mycoheterotrophic Epipactis species. Previous research has shown that P. chlorantha mainly associates with Ceratobasidiaceae (Bidartondo et al., 2004; Esposito et al., 2016), while N. nidus-avis associates mainly with Sebacina species (McKendrick et al., 2002; Selosse et al., 2002; Bidartondo et al., 2004, Těšitelová et al., 2015; Yagame et al., 2016). Epipactis helleborine, on the other hand, is a mycorrhizal generalist that associates with a wide diversity of fungi from different families, including Tuberaceae, Thelephoraceae, Russulaceae, Inocybaceae and Sebacinaceae as well as some rhizoctonias (Bidartondo et al., 2004; Bidartondo and Read, 2008; Ogura-Tsujita and Yukawa, 2008; Jacquemyn et al., 2016; Suetsugu et al., 2017; May et al., 2020; Xing et al., 2020). Schiebold et al. (2017) have suggested that E. neglecta forms orchid mycorrhizas exclusively with the ectomycorrhizal species Tuber excavatum, but detailed investigations of the mycorrhizal communities associating with E. neglecta are currently lacking.
Materials and Methods
Study Species and Sites
We studied isotopic signatures and mycorrhizal communities in four orchid species (Platanthera chlorantha, Epipactis helleborine, E. neglecta and Neottia nidus-avis) co-occurring at two sites in the Belgian Ardennes. The first site (50°06'04”N; 5°09'24”E) comprised a beech forest bordering a spruce forest and contained the four species growing in close proximity. Platanthera chlorantha, E. neglecta, and N. nidus-avis were restricted to the beech forest, whereas E. helleborine mainly occurred in the spruce forest. The second site (50°06'09”N; 5°10'35”E) consisted of a mixed deciduous forest with oak (Quercus robur) and European hornbeam (Carpinus betulus) as dominant tree species and a mixed pine forest consisting of Scots pine (Pinus sylvestris) and oak. At this site, both N. nidus-avis and E. neglecta were found in the deciduous forest, while E. helleborine occurred in the mixed pine forest.
Sampling
Sampling took place in July 2018. To assess variation in orchid mycorrhizal communities among the different orchid species, 100 m2 plots were established at various places in the forest and within each plot young roots of five replicate individuals were collected for each species, making sure that no substantial damage was caused to the root systems. All sampled plants were in the flowering stage at the time of sampling. After roots had been carefully excavated from the soil, they were transported to the laboratory and immediately surface sterilized (30 s submergence in 1% sodium hypochlorite, followed by three 30 s rinse steps in sterile distilled water). Subsequently, DNA was extracted from 0.25 g of root fragments using the UltraClean Plant DNA Isolation Kit as described by the manufacturer (Mo Bio Laboratories Inc., Solana Beach, CA, USA).
At the same time of the root sampling, leaves were taken from the same five individual plants per orchid species for isotope analyses. In the case of N. nidus-avis, no leaves are available and flowering stalks were collected. Additionally, leaves were collected in the same 100 m2 plots from herbaceous autotrophic plants growing under the same light conditions and at the same distance from the soil as the orchids as a baseline for later analyses. Autotrophic reference plants included Fragaria vesca, Helleborus foetidus, Vincetoxicum hirundinaria, and Rubus fruticosus (site 1), and Geum urbanum, Lamium galeobdolon, Mercurialis perennis, Rubus fruticosus, Stachys sylvatica and the N-fixing Vicia sepium (site 2).
Molecular Analyses
DNA samples were subjected to PCR amplification using sample-specific barcode-labeled versions of the primers ITS86F (5′-GTGAATCATCGAATCTTTGAA-3′) (Turenne et al., 1999) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al., 1990), generating amplicons that cover the hypervariable ITS2 region (Waud et al., 2014). We used the dual-indexing strategy of Kozich et al. (2013) to amplify the samples. Two replicate PCR reactions were performed and combined for each sample in reaction volumes of 25 μl containing 1x Titanium Taq PCR buffer, 150 μM of each dNTP, 0.5 μM of each primer, 1 × Titanium Taq DNA polymerase (Clontech, Saint-Germain-en-Laye, France) and 2 μl 10-times diluted DNA. The reaction was initiated by denaturation at 94°C for 120 s, followed by 30 cycles of denaturation at 94°C for 45 s, annealing at 59°C for 45 s and elongation at 72°C for 45 s followed by a final elongation at 72°C for 10 min. Amplicons were then purified using Agencourt AMPure XP magnetic beads (Beckman Coulter Genomics GmbH, South Plainfield, UK) according to the manufacturer's instructions and duplicates were combined. After the purified products had been quantified using a Qubit High Sensitivity Fluorometer kit (Invitrogen, Carlsbad, CA, USA), amplicons from each sample were combined at equimolar concentrations into an amplicon library. Subsequently, the library was subjected to an ethanol precipitation and loaded on an agarose gel. The band of the expected size (c. 400 bp) was excised and the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) was used to purify the DNA again. Lastly, the DNA concentration was measured again and the library was diluted to 2 nM and sequenced at the Center for Medical Genetics (University of Antwerp, Antwerp, Belgium) using an Illumina MiSeq sequencer with v2 500 cycle reagent kit (Illumina, San Diego, CA, USA).
Sequences were received for each sample as de-multiplexed FASTQ files representing forward and reverse paired-end reads. Paired-end reads were merged and combined reads with a total expected error threshold above 0.5 were discarded using USEARCH (v10.0.240) (Edgar, 2013). The “classify.seqs” and “remove.lineage” commands in mothur (v. 1.36.1) were used to identify and remove potential mitochondrial, chloroplast, archaeal and eukaryote contaminants (Schloss et al., 2009). Remaining sequences were grouped into operational taxonomic units (OTUs) based on a 3% sequence dissimilarity cut-off using the UPARSE greedy algorithm in USEARCH, during which chimeric sequences and global singletons (i.e., OTUs with only one sequence represented in the entire data set) were removed (Edgar, 2013). Subsequently, the taxonomic origin of each OTU was determined with the “sintax” algorithm implemented in USEARCH (Edgar, 2016) in conjunction with the UNITE fungal database version 7.2 (22.08.2018) trained for the fungal ITS-2 region (Kõljalg et al., 2013). Taxonomic assignments were considered reliable when bootstrap confidence values exceeded 0.80.
Stable Isotope Analyses
Samples were ground in 2 mL Eppendorf tubes in a ball mill (MM200, Retsch Gmbh, Haan, Germany) and analyzed for 13C/12C and 15N/14N ratios using a Thermo Flash 2000 elemental analyser coupled to a ThermoFinnigan DeltaV Advantage Continuous-Flow Isotope-ratio mass spectrometer. Relative abundances of the stable isotopes (δ values) were calculated as follows: δ13C or δ15N = (Rsample /Rstandard − 1) × 1,000 (%0), where Rsample is the 13C/12C or 15N/14N ratio of the sample, and Rstandard is the 13C/12C ratio of Vienna Pee Dee Belemnite or 15N/14N ratio of atmospheric N2, respectively. As internal standard we used alanine (δ13C = −22.16 ± 0.05%0; δ15N = 0.59 ± 0.05%0) and the average and standard deviation of the replicated measurements of this standard were −26.65 ± 0.05%0 for 13C and 0.81 ± 0.08%0 for 15N. As primary standards, we used caffeine IAEA-600 (δ13C = −27.77 ± 0.04%0) and ammonium sulfate IAEA-N-1 (δ15N = 0.40 ± 0.20%0). To compare isotope signatures between sites, normalized enrichment factors were calculated as ε = δS – δREF, where δS is a single δ13C or δ15N value of an orchid species or an autotrophic reference plant, and δREF is the mean value of all autotrophic reference plants occurring at the site (Preiss and Gebauer, 2008). Following Hynson et al. (2013), the percentage of mycoheterotrophic C gain in biomass was calculated for both partially mycoheterotrophic orchids as x = [(δCP – δCAT) / (δCNNA – δCAT)] × 100 %, where δCP, δCNNA, and δCAT are the mean δ13C values of the focal plant, N. nidus-avis (reference for mycoheterotrophic biomass), and surrounding autotrophic reference plants, respectively.
Photosynthetic Rates and Stomatal Conductance
Prior to the sampling of the roots and leaves, a Licor LI-6800 Portable Photosynthesis System (PPS) was used to assess photosynthetic rates and stomatal conductance in the two partial mycoheterotrophic orchids. For five plants of each species, we monitored one leaf using the PPS for 30 min and determined the natural variability in stomatal conductance and CO2 assimilation. Photosynthesis stabilization generally took place after 15 min. All measurements were done on bright, sunny days between 10:00 and 12:00 in July 2019, on the same day in each population.
Data Analysis
Based on read abundance of all detected fungi, variation in fungal community composition among sampled individuals of the different orchid species was visualized using non-metric multidimensional scaling (NMDS) in the R software package vegan (Oksanen et al., 2013) with the Bray-Curtis coefficient as distance measure. A Venn diagram using the R package VennDiagram was created to assess the overlap in mycorrhizal OTUs between the different orchid species. To test the hypothesis that mycorrhizal communities differed between species and sites, we performed permutational analysis of variance (PERMANOVA; Anderson, 2001) using the “adonis” function in the software package vegan (Oksanen et al., 2013). Both species and site were included as fixed factors in the analysis. Finally, species indicator analysis was used to investigate whether ectomycorrhizal fungi that were significantly associated with one of the investigated orchid species could be identified. The “multipatt” function in the R package indicspecies (De Cáceres et al., 2010) was used to define fungal OTUs that associate with a particular orchid species.
Normalized enrichment factors for 13C were plotted against those for 15N for each sampled orchid species and the autotrophic reference plants. For orchid species sampled at two different sites, a Student's t-test was used to test whether the mean δ13C or δ15N differed between sites. Analysis of variance (ANOVA) or its non-parametric alternative (Kruskal-Wallis test) was used to evaluate differences in mean δ13C and δ15N among species from a given site. If the null hypothesis (no difference between means) was rejected, Tukey's honestly significant difference (HSD) test was used to make pairwise multiple comparisons of the means. The alpha type I error threshold was set at 0.05. A Student's t-test was also used to see whether stomatal conductance and photosynthesis differed between the two partial mycoheterotrophic orchid species. All statistical analyses were performed using the R environment for statistical computing (R Development Core Team, 2013).
Results
Mycorrhizal Communities
In total, Illumina Miseq sequencing generated 2,069,182 sequences (2053 OTUs). After quality filtering, removal of unidentifiable OTUs, and rarefaction to 10,000 sequence per sample, the final data set comprised 1,706 fungal OTUs (345964 sequences), of which 70 (191026 sequences – 55.2%) were assigned to putatively orchid mycorrhizal OTUs according to Dearnaley et al. (2012) and information from previous studies that detected mycorrhizal fungi from the roots and protocorms of these and related orchid species (Supplementary Table 1). These belonged to various fungal genera, including Cenococcum (2 OTUs), Ceratobasidium (4 OTUs), Cortinarius (12 OTUs), Exophiala (3 OTUs), Hebeloma (1 OTU), Inocybe (6 OTUs), Lactarius (2 OTUs), Leptodontidium (1 OTU), Mycena (1 OTU), Peziza (1 OTU), Russula (6 OTUs), Sebacina (12 OTUs), Suillus (1 OTU) Tomentella (15 OTUs), Tricholoma (1 OTU), and Tuber (2 OTUs). Representative sequences for each mycorrhizal OTU found in this study were submitted to GenBank under the Accession Numbers MW364287 – MW364356.
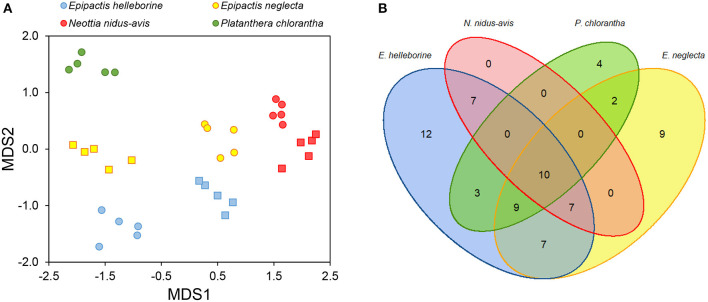
All individuals of the investigated orchid species associated with a large number of fungal OTUs [average number ± SD of OTUs detected per individual plant: 16.8 ± 1.6 (P. chlorantha); 24.1 ± 4.3 (E. helleborine); 21.6 ± 4.7 (E. neglecta); 9.0 ± 1.5 (N. nidus-avis)]. When only considering dominant OTUs (i.e., OTUs that represent more than 5% of the total number of reads), between 2.2 ± 0.8 (N. nidus-avis) and 4.6 ± 1.0 OTUs (E. helleborine) were observed per individual plant. Mycorrhizal community composition differed significantly among orchid species (pseudo-F = 11.18, p < 0.001) (Figure 1A), despite the fact there was considerable overlap in OTUs between species (Figure 1B). Eleven OTUs were found in all four species, but they all occurred with a low number of sequences. These belonged to the genera Exophiala, Sebacina, Cenococcum, Ceratobasidium, Tuber, Cortinarius, and Inocybe. For species that were sampled at the two sites, there was also a significant site effect (pseudo-F = 13.22, p < 0.001), but the magnitude of the site effect depended on the species (pseudo-F = 15.50, p < 0.001).
Figure 1.
Variation in mycorrhizal communities associating with four different orchid species (Epipactis helleborine, E. neglecta, Neottia nidus-avis and Platanthera chlorantha). (A) NMDS graph displaying variation in fungal community composition between individuals of E. helleborine (blue), E. neglecta (yellow), P. chlorantha (green) and N. nidus-avis (red) sampled at two sites (site 1: dots, site 2: squares). (B) Venn diagram showing overlap in mycorrhizal OTUs between the sampled orchid species.
Species Indicator Analysis indicated that eighteen OTUs were significantly (P < 0.05) associated with E. helleborine, seventeen OTUs with E. neglecta, five with N. nidus-avis and eight with P. chlorantha. Mycorrhizal communities associating with P. chlorantha were dominated (75.8% of reads) by members of the genus Ceratobasidium, while those of N. nidus-avis were dominated (88.3% of reads) by members of the genus Sebacina (Figure 2). Additional fungi that were sporadically observed belonged to various genera of ectomycorrhizal fungi. Both E. neglecta and E. helleborine had more diversified fungal communities and no single fungal genus dominated their fungal community composition (Figure 2).
Figure 2.
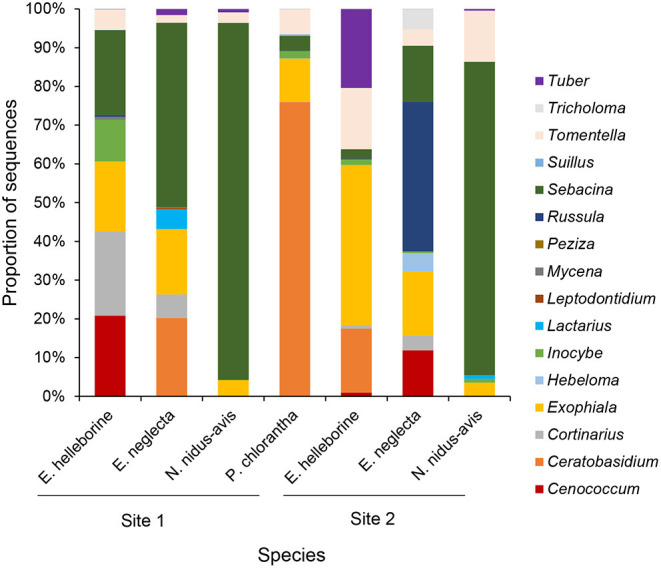
Bar charts representing the cumulative proportions of sequences belonging to different fungal genera observed in the sampled populations of each orchid species.
Isotope Signatures
Isotope signatures clearly differed between the sampled species (Figure 3). The non-photosynthetic N. nidus-avis was significantly enriched in both 13C and 15N, whereas P. chlorantha did not show any signs of enrichment in 13C and 15N. Epipactis neglecta was significantly enriched in 15N at both sites and at one site in 13C, while Epipactis helleborine was significantly enriched in 15N, but not in 13C (Supplementary Figures 1, 2. The linear two-source mixing model approach (Hynson et al., 2013) indicated that E. neglecta showed 49.5 and 55.0% heterotrophy at sites 1 and 2, respectively, while this was only 4.2 and 13.2% for E. helleborine. These values only take into account the organic matter derived from ectomycorrhizal fungi, since gain from any rhizoctonia partner is undetectable on the basis of 15N or 13C enrichment (see Introduction).
Figure 3.
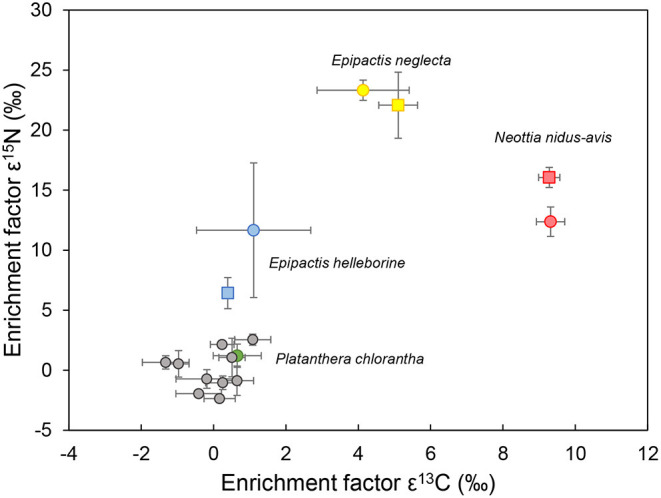
Enrichment factors ε13C and ε15N of four orchid species (blue: Epipactis helleborine, yellow: E. neglecta, red: Neottia nidus-avis and green: Platanthera bifolia) sampled at two different locations (site 1: dots; site 2: squares). Gray dots represent enrichment factors of autotrophic plants sampled at both locations. Values represent the mean of five individuals per species population, while bars represent standard deviations.
Photosynthetic Rates and Stomatal Conductance
Instantaneous measurements of leaf CO2 exchanges revealed that photosynthetic rates were low (mean ± SD: 2.42 ± 0.66 and 2.13 ± 0.86 μmol CO2·m−2·s−1 for E. helleborine and E. neglecta, respectively) and did not differ significantly (t = 0.60, p > 0.05) between individuals of both species (Figure 4A). Similarly, water vapor stomatal conductance values did not differ significantly (t = −0.06, p > 0.05) between the two species (Figure 4B).
Figure 4.
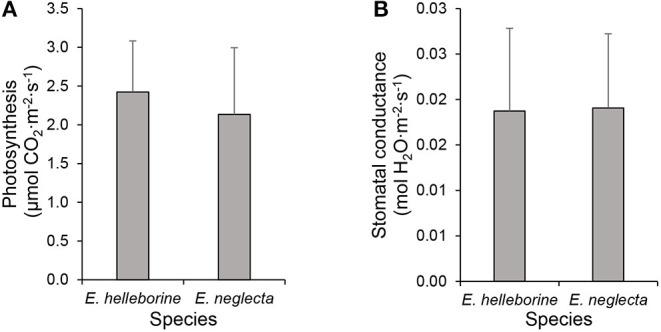
Instantaneous measurements of leaf gas exchanges on leaves of Epipactis helleborine and E. neglecta under natural growth conditions: (A) net CO2 assimilation and (B) water vapor stomatal conductance. Values represent the mean of five individuals per species population, while bars represent standard deviations.
Discussion
Mycorrhizal Communities
The investigated orchid species associated with a wide diversity of fungal OTUs. Results for N. nidus-avis and P. chlorantha were largely consistent with previous reports that have shown that both species mainly form mycorrhizas with Sebacina and Ceratobasidium, respectively (McKendrick et al., 2002; Selosse et al., 2002; Bidartondo et al., 2004; Těšitelová et al., 2015; Esposito et al., 2016; Yagame et al., 2016). Apart from these dominant fungi, several other fungi belonging to various mycorrhizal groups (Exophiala, Inocybe and Tomentella) were also sporadically observed in these species, yet it remains to be seen whether these additional fungi are truly mycorrhizal (i.e., form intracellular pelotons in the orchid roots) or simply represent endophytic fungi. This loose and non-mycorrhizal type of fungal colonization has recently been reported for some ectomycorrhizal fungi in non-ectomycorrhizal hosts (Schneider-Maunoury et al., 2020), including Tuberaceae, Thelephoraceae and Inocybaceae that were found here in N. nidus-avis and P. chlorantha. Overall, these findings seem to conform to a form of association that has recently been called “apparent generalism,” in which the orchid species appears to be a generalist because it associates with a large number of fungal species, but it is in fact a specialist of a few key, dominant fungi (Shefferson et al., 2019). Apparent generalism would suggest that once some minimum number of key fungal species is reached, extra fungal species convey no or few additional benefits to the orchid.
The situation appears to be different in the two Epipactis species, where no dominant fungus was observed, and associations with a large number of fungi were found. Unlike previous claims that E. neglecta forms orchid mycorrhizas exclusively with Tuber sp. (Schiebold et al., 2017), our results showed that this species, similar to other species in this genus (Bidartondo et al., 2004; Bidartondo and Read, 2008; Jacquemyn et al., 2016, 2017; Suetsugu et al., 2017), associates with a wide range of fungi, including members of the genera Sebacina, Exophiala, and Cortinarius. Tuber was observed but not exclusively in E. neglecta, and the highest number of sequences was observed in the roots of E. helleborine. Besides, significant differences in mycorrhizal communities were observed between the two sites that were sampled. This pattern of interaction specificity was recently coined “true generalism,” in which an orchid species associates with multiple hosts that overlap functionally and are geographically interchangeable based on opportunity for encounter, which leads to frequent host switching (Shefferson et al., 2019). In this case, it is generally assumed that the orchid opportunistically interacts with the fungi that are locally available. Further evidence for this mode of association was given in Xing et al. (2020), who investigated mycorrhizal associations in E. helleborine populations in Europe and Asia, spanning a geographic distance of >7,000 km. Consistent with a pattern of true generalism, rapid decline in community similarity with increasing distance and strong spatial turnover in mycorrhizal partners were observed, indicating frequent host switching across large geographic distances.
Isotope Signatures
Isotope signatures of the investigated orchid species generally confirmed findings of previous studies (Bidartondo et al., 2004; Gonneau et al., 2014; Hynson et al., 2016; Schiebold et al., 2017; Suetsugu et al., 2017). Platanthera chlorantha was not significantly enriched in heavy carbon and nitrogen isotopes, while N. nidus-avis was significantly enriched in both isotopes. The two Epipactis species showed intermediate patterns of isotope enrichment. Although these results suggest that P. chlorantha is autotrophic, recent studies have indicated that orchids associated with typical rhizoctonia fungi of the families Ceratobasidiaceae, Serendipitaceae and Tulasnellaceae show hidden partial mycoheterotrophy, because these saprotrophic and endophytic fungi have relative abundances of 15N and 13C close to that of autotrophic plants (Gleixner et al., 1993; Selosse and Martos, 2014; Gebauer et al., 2016). Analyses of 2H and 18O content have shown that some rhizoctonia-associated orchids receive organic matter from their associated fungi (Gebauer et al., 2016; Schiebold et al., 2018; Schweiger et al., 2018), suggesting that rhizoctonia-mycorrhizal orchid species should be considered as partially mycoheterotrophic as well. However, characterization of the net flow has yet to be established and, up until now, no compelling evidence has been provided for the occurrence of achlorophyllous mutants or for individuals capable of living under the compensation point. Besides, it should be noted that so-called rhizoctonia-mycorrhizal orchids do not exclusively associate with rhizoctonia fungi, but often show associations with ectomycorrhizal fungi as well (Jacquemyn et al., 2017; Suetsugu et al., 2019), and even share some of the fungi associated with fully mycoheterotrophic orchids (Figures 1, 2).
Epipactis helleborine showed a near-autotrophic behavior, while E. neglecta was more mycoheterotrophic, particularly at site 2 where it was significantly enriched in both 13C and 15N. The linear two-source mixing model approach (Hynson et al., 2013) indicated that E. neglecta showed 49.5 and 55.0% heterotrophy at sites 1 and 2, respectively. The finding of near autotrophy in E. helleborine confirms recent results from pot cultures obtained by May et al. (2020). In this case, potted plants did not show higher N contents and higher isotopic (13C and 15N) abundances that are usually observed in partially mycoheterotrophic orchids. In contrast to what was observed in our study, the proportion of ectomycorrhizal fungi was low and a high percentage of rhizoctonias was found. Altogether, these findings suggest that the level of mycoheterotrophy in E. helleborine is variable, and to some extent depends on the prevailing environmental conditions.
The situation was different for E. neglecta. Consistent with previous research (Schiebold et al., 2017), this species was significantly enriched in 15N at both sites and in 13C at one site. Previous research has suggested that the high levels of 15N are due to the presence of Tuber or other ascomycetes (Schiebold et al., 2017). However, we did not find a higher prevalence of Tuber or ascomycetes in E. neglecta, suggesting that differences in mycorrhizal communities alone cannot explain the observed variation in isotope signatures in the two studied Epipactis species. Indeed, the mycorrhizal communities associated with E. helleborine and E. neglecta were more affected by site conditions than by the species themselves, whereas the isotope signatures were less affected by site conditions. This suggests that trophic modes in Epipactis are not necessarily driven by the mycorrhiza with which they associate, but may also reflect inherent differences in physiological traits of the species. Previous research has shown that developmental stage had a pronounced impact on the heterotrophy level in Epipactis (Roy et al., 2013; Gonneau et al., 2014), suggesting that physiological differences associated with the different developmental stages are more important than differences in mycorrhizal communities in determining trophic levels in Epipactis. Suetsugu et al. (2017) showed that achlorophyllous variants of E. helleborine var. sayekiana harbored similar mycobionts, mainly Wilcoxina, as green plants, but that the abundances of mycobionts were lower in the albino plants. Similar results have been found in other partially mycoheterotrophic orchids displaying albino plants such as Cephalanthera damasonium (Julou et al., 2005) and E. microphylla (Selosse et al., 2004). Nonetheless, albino plants were significantly more enriched in 13C and 15N than green plants due to the absence of photosynthesis.
In addition, gene expression analyses have shown that genes related to mycorrhizal symbiosis were upregulated in the albino variants (Suetsugu et al., 2017), together with genes involved in autolysis and amino-acid catabolism (Lallemand et al., 2019b). It is therefore not unlikely that differences in gene expression of genes related to carbon and nitrogen transport and use differ between Epipactis species and that these differences, rather than differences in mycorrhizal communities, explain the observed variation in isotopic signatures. Given that E. helleborine is a very widespread species that occurs in many different habitats and associates with a wide variety of fungi (Xing et al., 2020), selection on these genes is probably limited. Recent research has indeed shown that the plastid genome of E. helleborine retains a full set of photosynthetic genes without any evidence of selective relaxation (Lallemand et al., 2019a) and has intact photosynthetic abilities. In contrast, E. neglecta usually occurs in the understory of beech and oak forests, where very little light penetrates to the forest soil. Under these conditions, selection toward more efficient nutrient transport may be selected for, leading to more efficient carbon and nitrogen uptake from fungi.
Conclusion
Establishing a firm relationship between mycorrhizal partners and isotope signatures appeared to be more difficult than previously suggested (Schiebold et al., 2017). Although the dominant partners varied, all investigated orchid species associated with a wide variety of fungi, supporting previous calls for carefully reporting minor traces of fungi that may be meaningful (Selosse et al., 2010, 2018). Autotrophic and fully mycoheterotrophic species had more specialized fungal communities than partially mycoheterotrophic species, which associated with a wide range of fungal partners, none of which was dominant. In partially mycoheterotrophic species, isotope signatures differed between the two Epipactis species, with E. neglecta being more mycoheterotrophic than E. helleborine, but differences in isotope signatures could not be related to differences in mycorrhizal communities, suggesting that inherent physiological differences contribute to the observed variation. In order to fully show the effect of mycorrhizal communities on isotope signatures without the confounding effects of possible physiological differences between species, experiments should be ideally conducted with a single orchid species that shows large variation in mycorrhizal communities. In this context, E. helleborine could be a perfect study system, as previous research has shown that its mycorrhizal communities differ substantially between European and Asian populations, the latter being dominated by ectomycorrhizal ascomycetes (Tuber), while the former are dominated by basiodiomycetous ectomycorrhiza (Xing et al., 2020).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
HJ and RB conceived the study. HJ, AE, and MW collected the data. MW performed the molecular analyses. M-AS and TF were in charge of the stable isotope analyses. HJ wrote the first draft. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Daniel Tyteca for locating the study populations and David Eyland for help with the photosynthesis measurements. Three reviewers provided useful comments on previous drafts that substantially improved the quality of this study.
Footnotes
Funding. This research was financially supported by the Flemish Fund for Scientific Research (FWO – grant G093019N). M-AS laboratories are supported by the 2015/18/A/NZ8/00149 grant funded by National Science Centre (Poland) and the Fondation Ars Cuttoli & Paul Appell, under the umbrella of the Fondation de France.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.618140/full#supplementary-material
References
- Anderson M. J. (2001). A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 26, 32–46. 10.1046/j.1442-9993.2001.01070.x [DOI] [Google Scholar]
- Bidartondo M. I., Burghardt B., Gebauer G., Bruns T. D., Read D. J. (2004). Changing partners in the dark: Isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc. R. Soc. B Biol. Sci. 271, 1799–1806. 10.1098/rspb.2004.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidartondo M. I., Read D. J. (2008). Fungal specificity bottlenecks during orchid germination and development. Mol. Ecol. 17, 3707–3716. 10.1111/j.1365-294X.2008.03848.x [DOI] [PubMed] [Google Scholar]
- Bruns T. D., Bidartondo M. I., Taylor D. L. (2002). Host specificity in ectomycorrhizal communities: what do the exceptions tell us? Integr. Comp. Biol. 42, 352–359. 10.1093/icb/42.2.352 [DOI] [PubMed] [Google Scholar]
- De Cáceres M., Legendre P., Moretti M. (2010). Improving indicator species analysis by combining groups of sites. Oikos 119, 1674–1684. 10.1111/j.1600-0706.2010.18334.x [DOI] [Google Scholar]
- Dearnaley J. W. D., Martos F., Selosse M. A. (2012). Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects, in Fungal Associations, ed. Hock B. (Berlin: Springer-Verlag; ), 207–230. [Google Scholar]
- Edgar R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2016). SINTAX, a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv 74161. 10.1101/074161 [DOI] [Google Scholar]
- Esposito F., Jacquemyn H., Waud M., Tyteca D. (2016). Mycorrhizal fungal diversity and community composition in two closely related Platanthera (Orchidaceae) species. PLoS ONE 11:e0164108. 10.1371/journal.pone.0164108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer G., Meyer M. (2003). 15N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol. 160, 209–223. 10.1046/j.1469-8137.2003.00872.x [DOI] [PubMed] [Google Scholar]
- Gebauer G., Preiss K., Gebauer A. C. (2016). Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytol. 211, 11–15. 10.1111/nph.13865 [DOI] [PubMed] [Google Scholar]
- Gleixner G., Danier H.-J., Werner R. A., Schmidt H. L. (1993). Correlations between the 13C content of primary and secondary plant products in different cell compartments and that in decomposing basidiomycetes. Plant Physiol. 102, 1287–1290. 10.1104/pp.102.4.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonneau C., Jersáková J., de Tredern E., Till-Bottraud I., Saarinen K., Sauve M., et al. (2014). Photosynthesis in perennial mixotrophic Epipactis spp. (Orchidaceae) contributes more to shoot and fruit biomass than to hypogeous survival. J. Ecol. 102, 1183–1194. 10.1111/1365-2745.12274 [DOI] [Google Scholar]
- Hynson N. A., Madsen T. P., Selosse M. A., Adam I. K. U., Ogura-Tsujita Y., Roy M., et al. (2013). The physiological ecology of mycoheterotrophy, in Mycoheterotrophy: The Biology of Plants Living on Fungi, ed. Merckx V. S. F. T. (Berlin: Springer-Verlag; ), 297–342. [Google Scholar]
- Hynson N. A., Schiebold J. M.-I., Gebauer G. (2016). Plant family identity distinguishes patterns of carbon and nitrogen stable isotope abundance and nitrogen concentration in mycoheterotrophic plants associated with ectomycorrhizal fungi. Ann. Bot. 118, 467–479. 10.1093/aob/mcw119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H., Merckx V. S. F. T. (2019). Mycorrhizal symbioses and the evolution of trophic modes in plants. J. Ecol. 107, 1567–1581. 10.1111/1365-2745.13165 [DOI] [Google Scholar]
- Jacquemyn H., Waud M., Brys R., Lallemand F., Courty P.-E., Robionek A., et al. (2017). Mycorrhizal associations and trophic modes in coexisting orchids: an ecological continuum between auto- and mixotrophy. Front. Plant Sci. 8:1497. 10.3389/fpls.2017.01497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H., Waud M., Lievens B., Jacquemyn H. (2016). Differences in mycorrhizal communities between Epipactis palustris, E. helleborine and its presumed sister species E. neerlandica. Ann. Bot. 118, 105–114. 10.1093/aob/mcw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H., Waud M., Merckx V. S. F. T., Lievens B., Brys R. (2015). Mycorrhizal diversity, seed germination and long-term changes in population size across nine populations of the terrestrial orchid Neottia ovata. Mol. Ecol. 24, 3269–3280. 10.1111/mec.13236 [DOI] [PubMed] [Google Scholar]
- Julou T., Burghardt B., Gebauer G., Berveiller D., Damesin C., Selosse M.-A. (2005). Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol. 166, 639–653. 10.1111/j.1469-8137.2005.01364.x [DOI] [PubMed] [Google Scholar]
- Kõljalg U., Nilsson R. H., Abarenkov K., et al. (2013). Towards a unified paradigm for sequence-based identification of Fungi. Mol. Ecol. 22, 5271–5277. 10.1111/mec.12481 [DOI] [PubMed] [Google Scholar]
- Kozich J. J., Westcott S. L., Baxter N. T., Highlander S. K., Schloss P. D. (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120. 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F., Logacheva M., Le Clainche I., Berard A., Zheleznaia E., May M., et al. (2019a). Thirteen new plastid genomes from mixotrophic and autotrophic species provide insights into heterotrophy evolution in Neottieae orchids. Genome Biol. Evol. 11, 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F., Martin-Magniette M. L., Gilard F., Gakiere B., Launay-Avon A., Delannoy E., et al. (2019b) In situ transcriptomic metabolomic study of the loss of photosynthesis in the leaves of mixotrophic plants exploiting fungi. Plant J. 98, 826–841. [DOI] [PubMed] [Google Scholar]
- Liebel H. T., Bidartondo M. I., Gebauer G. (2015). Are carbon and nitrogen exchange between fungi and the orchid Goodyera repens affected by irradiance? Ann. Bot. 115, 251–261. 10.1093/aob/mcu240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Shimizu S., Mori M., Ito S.-I., Selosse M.-A. (2012). Seasonal and environmental changes of mycorrhizal associations and heterotrophy levels in mixotrophic Pyrola japonica (Ericaceae) growing under different light environments. Am. J. Bot. 99, 1177–1188. 10.3732/ajb.1100546 [DOI] [PubMed] [Google Scholar]
- May M., Jakalski M., Novotná A., Dietel J., Ayasse M., Lallemand F., et al. (2020). Three-year pot culture of Epipactis helleborine reveals autotrophic survival, without mycorrhizal networks, in a mixotrophic species. Mycorrhiza 30, 51–61. 10.1007/s00572-020-00932-4 [DOI] [PubMed] [Google Scholar]
- McKendrick S. L., Leake J. R., Taylor D. L., Read D. J. (2002). Symbiotic germination and development of the myco-heterotrophic orchid Neottia nidus-avis in nature and its requirement for locally distributed Sebacina spp. New Phytol. 154, 233–247. 10.1046/j.1469-8137.2002.00372.x [DOI] [Google Scholar]
- Merckx V. (2013). Mycoheterotrophy: The Biology of Plants Living on Fungi. New York, NY: Springer. [Google Scholar]
- Ogura-Tsujita Y., Yokoyama J., Miyoshi K., Yukawa T. (2012). Shifts in mycorrhizal fungi during the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae). Am. J. Bot. 99, 1158–1172. 10.3732/ajb.1100464 [DOI] [PubMed] [Google Scholar]
- Ogura-Tsujita Y., Yukawa T. (2008). Epipactis helleborine shows strong mycorrhizal preference towards ectomycorrhizal fungi with contrasting geographic distributions in Japan. Mycorrhiza 18, 331–338. 10.1007/s00572-008-0187-0 [DOI] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F. G., Kindt R., Stevens H., Legendre P., Wagner H., et al. (2013). VEGAN: Community Ecology Package. R Package Version 2.1-43/r2893. Available online at: http://R-Forge.R-project.org/projects/vegan/ (accessed October 15, 2020).
- Preiss K., Adam I. K. U., Gebauer G. (2010). Irradiance governs exploitation of fungi: fine-tuning of carbon gain by two partially mycoheterotrophic orchids. Proc. R. Soc. B Biol. Sci. 277, 1333–1336. 10.1098/rspb.2009.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss K., Gebauer G. (2008). A methodological approach to improve estimates of nutrient gains by partially myco-heterotrophic plants. Isot. Environ. Health Stud. 44, 393–401. 10.1080/10256010802507458 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Roy M., Gonneau C., Rocheteau A., Berveiller D., Thomas J.-C., Damesin C., et al. (2013). Why do mixotrophic plants stay green? A comparison between green and achlorophyllous orchid individuals in situ. Ecol. Monogr. 83, 95–117. 10.1890/11-2120.1 [DOI] [Google Scholar]
- Schiebold J. M.-I., Bidartondo M. I., Karasch P., Gravendeel B., Gebauer G. (2017). You are what you get from your fungi: nitrogen stable isotope patterns in Epipactis species. Ann. Bot. 119, 1085–1095. 10.1093/aob/mcw265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebold J. M.-I., Bidartondo M. I., Lenhard F., Makiola A., Gebauer G. (2018). Exploiting mycorrhizas in broad daylight: partial mycoheterotrophy is a common nutritional strategy in meadow orchids. J. Ecol. 106, 168–178. 10.1111/1365-2745.12831 [DOI] [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing MOTHUR: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Maunoury L., Deveau A., Moreno M., Todesco F., Belmondo S., Murat C., et al. (2020). Two ectomycorrhizal truffles, Tuber melanosporum and T. aestivum, colonize endophytically roots of non-ectomycorrhizal plant in natural environments. New Phytol. 225, 2542–2556. 10.1111/nph.16321 [DOI] [PubMed] [Google Scholar]
- Schweiger J. M. I., Bidartondo M. I., Gebauer G. (2018). Stable isotope signatures of underground seedlings reveal the organic matter gained by adult orchids from mycorrhizal fungi. Funct. Ecol. 32, 870–881. 10.1111/1365-2435.13042 [DOI] [Google Scholar]
- Schweiger J. M. I., Kemnade C., Bidartondo M. I., Gebauer G. (2019). Light limitation and partial mycoheterotrophy in rhizoctonia-associated orchids. Oecologia 189, 375–383. 10.1007/s00442-019-04340-0 [DOI] [PubMed] [Google Scholar]
- Selosse M.-A., Charpin M., Not F. (2017). Mixotrophy everywhere on land and in water: the grand ecart hypothesis. Ecol. Lett. 20, 246–263. 10.1111/ele.12714 [DOI] [PubMed] [Google Scholar]
- Selosse M.-A., Faccio A., Scappaticci G., Bonfante P. (2004). Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb. Ecol. 47, 416–426. 10.1007/s00248-003-2034-3 [DOI] [PubMed] [Google Scholar]
- Selosse M.-A., Martos F. (2014). Do chlorophyllous orchids heterotrophically use mycorrhizal fungal carbon? Trends Plant Sci. 19, 683–685. 10.1016/j.tplants.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Selosse M.-A., Martos F., Perry B. A., Padamsee M., Roy M., Pailler T. (2010). Saprotrophic fungal symbionts in tropical achlorophyllous orchids: Finding treasures among the ‘molecular scraps’? Plant Signal. Behav. 5, 349–353. 10.4161/psb.5.4.10791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selosse M.-A., Roy M. (2009). Green plants that feed on fungi: facts and questions about mixotrophy. Trends Plant Sci. 14, 64–70. 10.1016/j.tplants.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Selosse M.-A., Schneider-Maunoury L., Martos F. (2018). Time to re-think fungal ecological niches? New Phytol. 217, 968–972. 10.1111/nph.14983 [DOI] [PubMed] [Google Scholar]
- Selosse M.-A., Weiß M., Jany J. L., Tillier A. (2002). Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) L.C.M. Rich and neighbouring tree ectomycorrhizae. Mol. Ecol. 11, 1831–1844. 10.1046/j.1365-294X.2002.01553.x [DOI] [PubMed] [Google Scholar]
- Shefferson R. P., Bunch W., Cowden C. C., Lee Y.-I., Kartzinel T. R., Yukawa T., et al. (2019). Does evolutionary history determine specificity in broad ecological interactions? J. Ecol. 107, 1582–1593. 10.1111/1365-2745.13170 [DOI] [Google Scholar]
- Stöckel M., Meyer C., Gebauer G. (2011). The degree of mycoheterotrophic carbon gain in green, variegated and vegetative albino individuals of Cephalanthera damasonium is related to leaf chlorophyll concentrations. New Phytol. 189, 790–796. https// .org/10.1111/j.1469-8137.2010.03510.x [DOI] [PubMed] [Google Scholar]
- Suetsugu K., Yamato M., Matsubayashi J., Tayasu I. (2019). Comparative study of nutritional mode and mycorrhizal fungi in green and albino variants of Goodyera velutina, an orchid mainly utilizing saprotrophic rhizoctonia. Mol. Ecol. 28, 4290–4299. 10.1111/mec.15213 [DOI] [PubMed] [Google Scholar]
- Suetsugu K., Yamato M., Miura C., Yamaguchi K., Takahashi K., Ida Y., et al. (2017). Comparison of green and albino individuals of the partially mycoheterotrophic orchid Epipactis helleborine on molecular identities of mycorrhizal fungi, nutritional modes and gene expression in mycorrhizal roots. Mol. Ecol. 26, 1652–1669. 10.1111/mec.14021 [DOI] [PubMed] [Google Scholar]
- Těšitel J., Těšitelová T., Minasiewicz J., Selosse M.-A. (2018). Mixotrophy in land plants: why to stay green? Trends Plant Sci. 23, 656–659. 10.1016/j.tplants.2018.05.010 [DOI] [PubMed] [Google Scholar]
- Těšitelová T., Kotilínek M., Jersáková J., Joly F.-X., Košnar J., Tatarenko I., et al. (2015). Two widespread green Neottia species (Orchidaceae) show mycorrhizal preference for Sebacinales in various habitats and ontogenetic stages. Mol. Ecol. 24, 1122–1134. 10.1111/mec.13088 [DOI] [PubMed] [Google Scholar]
- Turenne C. Y., Sanche S. E., Hoban D. J., Karlowsky J. A., Kabani A. M. (1999). Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 37, 1846–1851. 10.1128/JCM.37.6.1846-1851.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud M., Brys R., Van Landuyt W., Lievens B., Jacquemyn H. (2017). Mycorrhizal specificity does not limit the distribution of an endangered orchid species. Mol. Ecol. 26, 1687–1701. 10.1111/mec.14014 [DOI] [PubMed] [Google Scholar]
- Waud M., Busschaert P., Ruyters S., Jacquemyn H., Lievens B. (2014). Impact of primer choice on characterization of orchid mycorrhizal communities using 454 pyrosequencing. Mol. Ecol. Resour. 14, 679–699. 10.1111/1755-0998.12229 [DOI] [PubMed] [Google Scholar]
- White T. J., Bruns T. D., Lee S., Taylor J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, in PCR Protocols: A Guide to Methods and Applications, eds Innis M. A., Gelfand D. H., Sninsky J. J., White T. J. (San Diego, CA: Academic Press; ), 315–322. [Google Scholar]
- Xing X., Gao Y., Zhao Z., Waud M., Duffy K. J., Selosse M.-A., et al. (2020). Similarity in mycorrhizal communities associating with two widespread terrestrial orchids decays with distance. J. Biogeogr. 47, 421–433. 10.1111/jbi.13728 [DOI] [Google Scholar]
- Yagame T., Ogura-Tsujita Y., Kinoshita A., Iwase K., Yukawa T. (2016). Fungal partner shifts during the evolution of mycoheterotrophy in Neottia. Am. J. Bot. 103, 1630–1641 10.3732/ajb.1600063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.