Abstract
Coronavirus disease 2019 (COVID-19) has already raised serious concern globally as the number of confirmed or suspected cases have increased rapidly. Epidemiological studies reported that obesity is associated with a higher rate of mortality in patients with COVID-19. Yet, to our knowledge, there is no comprehensive systematic review and meta-analysis to assess the effects of obesity and mortality among patients with COVID-19. We, therefore, aimed to evaluate the effect of obesity, associated comorbidities, and other factors on the risk of death due to COVID-19. We did a systematic search on PubMed, EMBASE, Google Scholar, Web of Science, and Scopus between January 1, 2020, and August 30, 2020. We followed Cochrane Guidelines to find relevant articles, and two reviewers extracted data from retrieved articles. Disagreement during those stages was resolved by discussion with the main investigator. The random-effects model was used to calculate effect sizes. We included 17 articles with a total of 543,399 patients. Obesity was significantly associated with an increased risk of mortality among patients with COVID-19 (RRadjust: 1.42 (95%CI: 1.24–1.63, p < 0.001). The pooled risk ratio for class I, class II, and class III obesity were 1.27 (95%CI: 1.05–1.54, p = 0.01), 1.56 (95%CI: 1.11–2.19, p < 0.01), and 1.92 (95%CI: 1.50–2.47, p < 0.001), respectively). In subgroup analysis, the pooled risk ratio for the patients with stroke, CPOD, CKD, and diabetes were 1.80 (95%CI: 0.89–3.64, p = 0.10), 1.57 (95%CI: 1.57–1.91, p < 0.001), 1.34 (95%CI: 1.18–1.52, p < 0.001), and 1.19 (1.07–1.32, p = 0.001), respectively. However, patients with obesity who were more than 65 years had a higher risk of mortality (RR: 2.54; 95%CI: 1.62–3.67, p < 0.001). Our study showed that obesity was associated with an increased risk of death from COVID-19, particularly in patients aged more than 65 years. Physicians should aware of these risk factors when dealing with patients with COVID-19 and take early treatment intervention to reduce the mortality of COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, obesity, body mass index (BMI), mortality
Introduction
Rationale
The outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), has spread globally and created mounting concern (1). Healthcare organizations and providers are trying to find solutions to reduce the spread of disease and fatality rates. The rapid increase in the number of cases has created an unbearable burden on the healthcare system, especially in developing counties where healthcare systems are more fragile (2, 3). Early diagnosis of severe patients is essential to improve patient conditions and reduce mortality. Earlier classification of mild and severe COVID-19 patients could facilitate the proper utilization of limited resources (4). Multiple studies reported changes in several laboratory parameters [(e.g., the number of lymphocytes, C-reactive protein (CRP), interleukin-6 (IL-6), and erythrocyte sedimentation rates (ESR)] in the COVID-19 patients, but data are not sufficient to show their correlation according to severity and mortality (5, 6). Therefore, finding an appropriate risk factor is essential to classify mild and severe patients at an early stage.
Obesity is defined as abnormal fat accumulation and is a common, costly condition (7). According to the WHO report, obesity is classified into three groups based on body mass index (BMI). The prevalence of obesity has tripled since 1975 and is an established risk factor of other diseases such as diabetes, hypertension, heart disease, and cancers (8–10). Paradoxically, obesity has been shown to decrease mortality among patients diagnosed with pneumonia and ARDS (11–14). Recent epidemiological studies have shown obesity to be associated with both neutral and increased risk of mortality among patients diagnosed with COVID-19 (15, 16), but mechanisms underlying this are still unclear. Previous studies noted that the number of hospitalization and mechanical ventilation cases is higher in patients with obesity that can be associated with an increased rate of mortality (17, 18). Moreover, obesity is related to the downregulation of the inflammatory pathway, which leads to increase expression of inflammatory molecules, including interleukin-6 (IL-6). Age and elevated IL-6 were proclaimed as significant predictors of in-hospital mortality (19, 20).
Goal of Investigation
The objectives of the current comprehensive and rigorous meta-analysis were to investigate relevant epidemiological studies for evaluating the association between obesity and mortality of COVID-19 patients. The findings of this study could help healthcare providers to take preventive actions and use early treatment strategies for these high-risk groups.
Research Aims
- To determine whether obesity is associated with an increased rate of mortality among the patients diagnosed with COVID-19.
- To calculate the strength of association between class I, class II, and class III obesity and mortality of COVID-19 patients.
- To elucidate the association between associated factors (e.g., diabetes, CKD, COPD, smoking) and mortality of COVID-19 patients.
Methods
Meta-Analysis Guidelines
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to select potential study inclusion. Moreover, the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines were also considered for this study (21) (Supplementary Table 1).
Search Strategy
We did a compressive systematic search to collect all relevant articles that evaluated the effect of COVID-19 on patients in terms of obesity and mortality. Any article published in English was considered for inclusion; an article search strategy was developed to retrieve all articles between January 1 and August 30, 2020. Articles search were conducted in the most popular electronic databases such as Scopus, PubMed, EMBASE, and Web of Science. The following search terms were used to retrieve articles: “Obesity” OR “BMI,” OR “Overweight” AND “mortality related to COVID-19” OR “death related to COVID-19” (Supplementary Table 2). We removed all duplicate articles, and a final search for relevant articles was performed on the reference list of retrieved articles.
Selection Criteria
We developed a priori inclusion criteria and included articles of COVID-19 patients' mortality due to obesity, associated risk factors such as demographic factors, and comorbidities. Articles were included if they (a) were peer-reviewed, (b) were published in English, (c) were cohort or comparison design, (d) included patients with more than 20, (e) and reported effect size as odds ratio (OR), risk ratio (RR) or hazard ratio (HR). We excluded studies if they were published as a review or case series.
Study Selection
Two authors (MdI and TP) screened titles and abstracts from the search results. Predefined selection criteria were used to select relevant full-text articles during the screening process. Afterward, all full-text articles were evaluated carefully for inclusion and data extraction. The same two authors (MdI and TP), however, independently evaluated each potential article for inclusion. Any disagreement during those screening process was resolved by the main investigator (Y-CL).
Data Extraction
All the selected studies were then finally reviewed to extract potential information regarding obesity and mortality of patients with COVID-19 and associated risk factors. One author collected information about author name, publication years, study design, number of patients with COVID-19, number of alive patients and number of dead patients, percentage of mortality, risk factors, inclusion and exclusion criteria, and effect sizes.
Quality Assessment
The Quality In Prognosis Studies (QUIPS) tool was utilized to examine the risk of bias (RoB), which is recommended by the Cochrane Prognosis Methods group (22). The QUIPS tool has six evaluation domains that are used to evaluate validity and bias in studies of prognostic factors: (a) study participation, (b) study attrition, (c) prognostic factor measurement, (d) outcome measurement, (e) study confounding, and (f) statistical analysis and reporting (23). RoB is classified into three groups: low, moderate, and high risk. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was considered to assess the confidence in the estimate of effect. GRADE was utilized to determine the quality of evidence based on several factors such as risk of bias, inconsistency, imprecision, indirectness, and publication bias.
Statistical Analysis
The primary outcome was the mortality of patients with COVID-19 due to obesity, and secondary outcomes were increased risk of mortality among class I, class II, and class III obesity. In subgroup analyses, we also evaluated the risk of mortality associated with comorbidities, age, gender, and other factors. Risk ratios with 95%CIs were calculated from the HRs or ORs. We used a random-effects model to calculate the heterogeneity between studies. However, studies heterogeneity was assessed using Cochran Q statistics and inconsistency statistics (I2). We followed previous studies that considered heterogeneity as very low, low, medium, and high if I2 value 0~25%, 25–50%, 50~75%, and >75% (24, 25). The Forest plot was drawn to present effect size, and the funnel plot was drawn to present publication bias.
Results
Study Selection
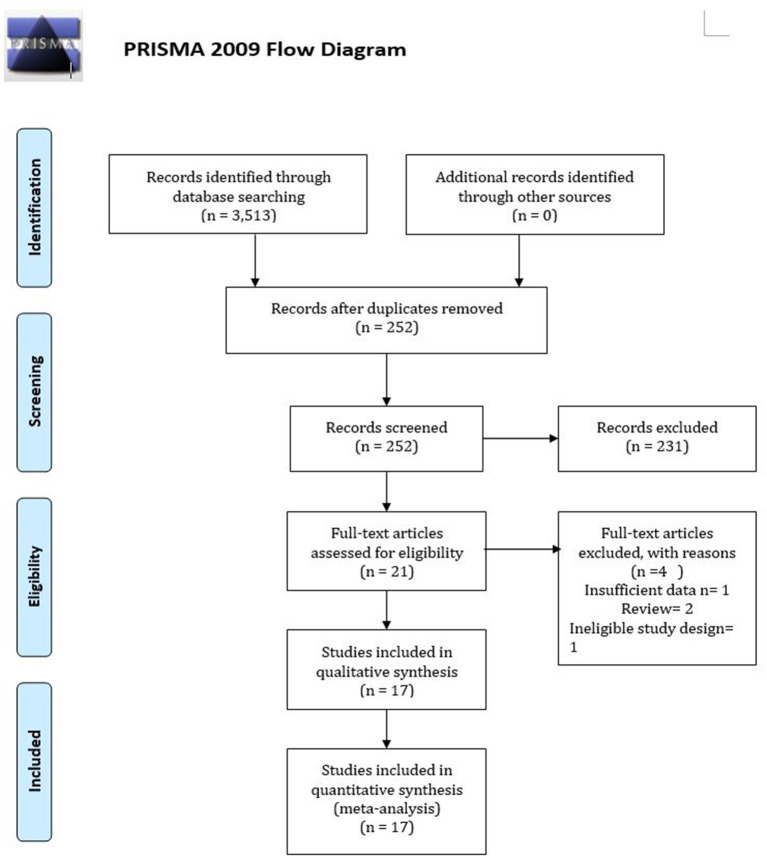
A total of 3,513 unique articles were retrieved after searching for electronic databases. Of those, 3,492 articles were excluded after reviewing their titles and abstracts because they have not fulfilled pre-specified selection criteria. Overall, 21 articles went to full-text review and were scrutinized for final inclusion. However, four studies were further excluded because they have been published in the form of a review. Finally, 17 articles met the inclusion criteria for meta-analysis (15, 16, 26–40). Figure 1 shows the overall study selection process.
Figure 1.
Flow diagram of the study selection process.
Study Characteristics
Table 1 shows the clinical characteristics of the included studies. Of those, 16 studies had a retrospective cohort study design, and one study had a prospective design. Eleven studies used data from North American patients, four studies used data from European countries (France, Italy, and the UK), and one study used data from 47 countries. The range of male patients was 43.3–80.2%. All of the studies used demographics, clinical variables.
Table 1.
Characteristics of the included studies assessing the risk of mortality of COVID-19 patients with obesity.
| Author | Country | Design |
Total sample |
Male (%) |
Age (mean/median) | Variable | Findings |
|---|---|---|---|---|---|---|---|
| Bello-Chavolla et al. (33) | Mexico | Retrospective | 177,133 | 57.70 | 63.5 | Demographics, clinical, smoking | 1.25 (1.17–1.34) |
| Klang et al. (35) | USA | Retrospective | 3,406 | 56 | 76 | Demographics, clinical, smoking | 1.31 (0.91–1.89) |
| Nakeshbandi et al. (36) | USA | Retrospective | 504 | 52 | 68 | Demographics, clinical, smoking | 1.30 (0.99–1.93) |
| Pettit et al. (37) | USA | Retrospective | 238 | 47.5 | 58.5 | Demographic, clinical, smoking | 1.70 (1.10–2.61) |
| Palaiodimos et al. (29) | USA | Retrospective | 200 | 49 | 64 | Demographics, clinical, smoking, symptoms | 3.78 (1.45–9.84) |
| Czernichow et al. (34) | France | Prospective | 5,795 | 65.41 | 59.6 | Demographics, and clinical | 2.30 (1.77–2.98) |
| Hamer et al. (15) | UK | Retrospective | 334,329 | 45.5 | 56.4 | Demographics, biomarker, smoking | 1.52 (0.94–2.44) |
| Rottoli et al. (38) | Italy | Retrospective | 482 | N/A | N/A | Demographic, clinical | 12.10 (3.24–45.07) |
| Zhang et al. (40) | China | Retrospective | 3,201 | N/A | N/A | laboratory | 1.35 (1.07–1.70) |
| Tartof et al. (31) | USA | Retrospective | 6,916 | 44.98 | 49.1 | Demographics, smoking, clinical characteristics | 1.95 (1.09–3.49) |
| Price-Haywood et al. (16) | USA | Retrospective | 3,481 | 43.3 | 55.5 | Demographics, clinical, locations | 0.99 (0.77–1.27) |
| Goyal et al. (39) | USA | Retrospective | 1,687 | 60 | 66.5 | Demographics, clinical, laboratory, smoking, in-hospital events | 1.41 (0.73–2.69) |
| Halasz et al. (27) | Italy | Retrospective | 242 | 80.2 | 64 | Demographics, clinical, laboratory | 1.99 (0.90–4.38) |
| Wang et al. (30) | USA | Retrospective | 58 | 52 | 67 | Demographics, laboratory, clinical | 2.04 (0.49–8.36) |
| Hajifathalian et al. (28) | USA | Retrospective | 770 | 61 | 63.5 | Demographics, clinical, laboratory data, clinical outcomes | 1.15 (0.61–2.13) |
| Kim et al. (26) | Multiple countries | Retrospective | 2,491 | 53.2 | 62 | Ethnicity, time of hospitalization, smoking status, clinical characteristics | 1.09 (0.91–1.29) |
| Anderson et al. (32) | USA | Retrospective | 2,466 | 58 | 67 | Demographics, clinical | 1.26 (1.03–1.54) |
Quality of Evidence
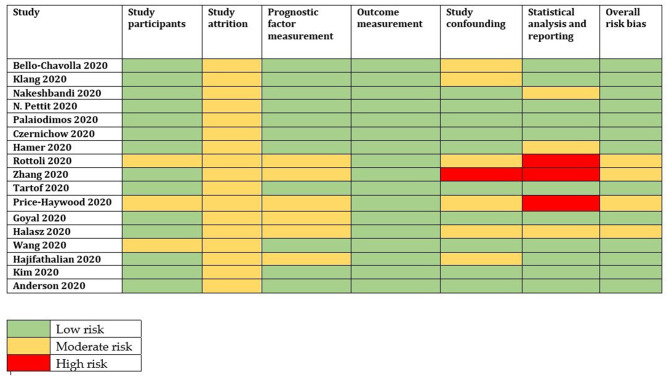
The QUIPS tool was used to evaluate the risk bias among the included studies. Thirteen studies were of low risk bias, and four studies were of moderate risk bias (Figure 2). The overall quality of evidence is strong in the meta-analysis. Supplementary Table 3 presents a summary of the GRADE evidence profile for our meta-analysis. For the increased rate of mortality among obese patients, the certainty was “low.”
Figure 2.
Risk of bias according to the QUIPS tool.
Primary Outcome
Obesity and Mortality of Patients With COVID-19
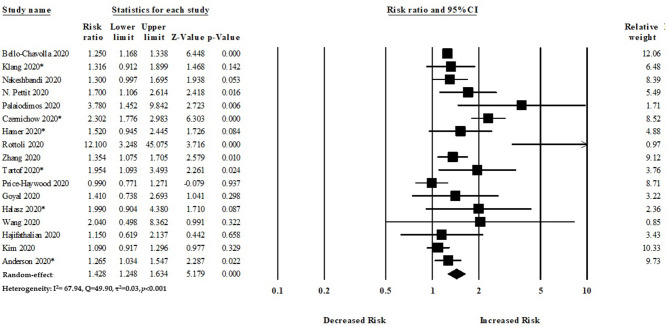
Of the 17 studies considered in our meta-analysis, 16 studies reported a significantly increased risk of mortality among patients with COVID-19, and one study reported a decreased risk of mortality. All the studies adjusted their effect size with potential variables to reduce potential bias. Collectively, our meta-analysis suggested a significantly increased risk of mortality with obesity, with a pooled RR of 1.42 (95%CI: 1.24–1.63, p < 0.001) (Figure 3). The I2 statistics among the studies was 67.94%, indicating a moderate risk of heterogeneity.
Figure 3.
Association between obesity and risk of mortality among COVID-19 patients.
Secondary Outcome
Types of Obesity and COVID-19 Mortality
We also pooled RR to evaluate the risk of mortality based on three types of obesity such as class I, class II, and class III obesity. Class III obesity was strongly associated with an increased risk of mortality, with a pooled RRadjust of 1.92 (95%CI: 1.50–2.47, p < 0.001). There was insignificant low heterogeneity among the studies (I2 = 31.99, Q = 5.88, tau2 = 0.02, p = 0.20) (Figure 4A). Class I and class II obesity also showed a strong association with an increased risk of mortality, with a pooled RRadjust of 1.27 (95%CI: 1.05–1.54, p = 0.01), and 1.56 (95%CI: 1.11–2.19, p < 0.01), respectively (Figures 4B,C). There was significant moderate heterogeneity among the studies for Class I (I2 = 60.00, Q = 5, tau2 = 0.03, p = 0.02) and higher heterogeneity among the studies were observed for class II (I2 = 80.34, Q = 25.43, tau2 = 0.12, p < 0.001).
Figure 4.
Association between (A) Class III, (B) class II, (C) class I obesity and risk of mortality among COVID-19 patients.
Subgroup Analysis
Subgroup analyses are presented in Table 2. The 7 studies reported the risk of mortality among patients with obesity who were more than 65 years old. The pooled RRadjust was 2.54 (95%CI: 1.62–3.97, p < 0.001) and heterogeneity among the studies was (I2 = 89.33, Q = 56.24, tau2 = 0.24, p < 0.001). Furthermore, six studies evaluated the risk of COVID-19 mortality among male patients and showed a significantly higher risk (RRadjust: 1.38, 95%CI: 1.25–1.51, p < 0.001). There was a lower risk of heterogeneity among the studies (I2 = 9.57, Q = 5.53, tau2 = 0.001, p = 0.35).
Table 2.
Subgroup analyses.
| Pooled estimate | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|
| N | RR with 95% CI | p-Value | τ2 | I2 | p-Value | |
| Demographic | ||||||
| Male | 6 | 1.38 (1.25–1.51) | <0.001 | 0.001 | 9.57 | 0.35 |
| ≥65 years | 7 | 2.54(1.62–3.97) | <0.001 | 0.24 | 89.33 | <0.001 |
| Comorbidity | ||||||
| Diabetes | 11 | 1.19 (1.07–1.32) | 0.001 | 0.012 | 54.15 | 0.016 |
| Hypertension | 9 | 1.07 (0.92–1.25) | 0.351 | 0.029 | 60.58 | 0.009 |
| CKD | 7 | 1.57 (1.29–1.91) | <0.001 | 0.036 | 69.18 | 0.003 |
| CPOD | 5 | 1.34 (1.18–1.52) | <0.001 | 0.004 | 16.12 | 0.312 |
| Stroke | 2 | 1.80 (0.89–3.64) | 0.100 | 0 | 0 | 0.37 |
| Others | ||||||
| Smoking | 6 | 1.13 (0.91–1.40) | 0.242 | 0.027 | 46.57 | 0.09 |
N, number of study; RR, risk ratio; CI, confidence Interval; CKD, chronic kidney disease; CPOD, chronic obstructive pulmonary disease. Bold value indicates subgroup and significant p-value.
We also evaluated the risk of mortality among obese COVID-19 patients with various comorbidity such as diabetes, hypertension, CKD, COPD, and stroke. The pooled RR for COVID-19 mortality among patients with diabetes was 1.19 (95%CI: 1.07–1.32, p = 0.001). However, the pooled RR among the patients with stroke, CKD, COPD, and hypertension were 1.80 (95%CI: 0.89–3.64, p = 0.10), 1.57 (95%CI: 1.57–1.91, p < 0.001), 1.34 (95%CI: 1.18–1.52, p < 0.001), and 1.07 (95%CI: 0.92–1.25, p = 0.35), respectively.
Risk of Bias
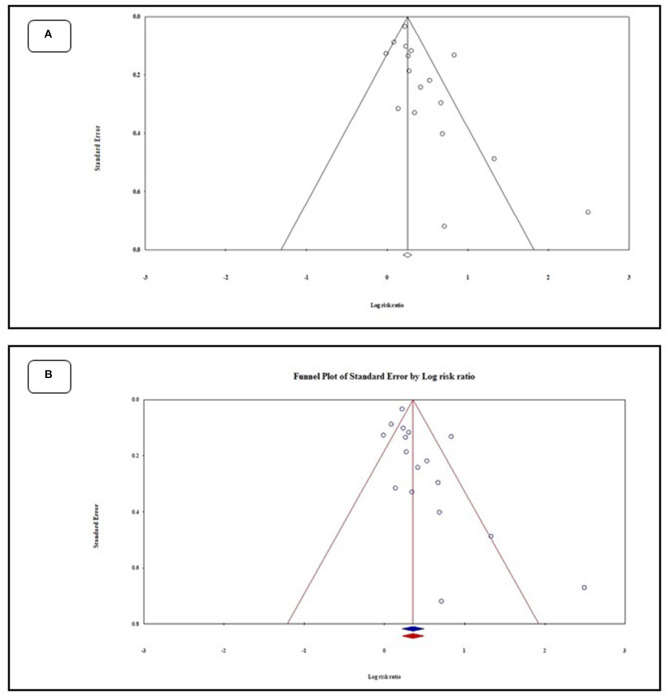
Figure 5A depicts the funnel plot that indicates no publication bias among the studies. Egger's regression test was used to evaluate the funnel asymmetry, which showed no publication bias (p < 0.05). Moreover, Figure 5B shows the funnel plot with missing studies imputed by the trim and fill method. There was no missing study to be filled in the plot, and the overall log risk ratio became 1.42 95%CI (1.24–1.63).
Figure 5.
Funnel plots of meta-analysis before (A) and after (B) applying the fill and trim method.
Discussion
Main Findings
This rigorous meta-analysis of 17 studies showed that obesity is associated with an increased risk of mortality among patients with COVID-19, especially patients aged more than 65 years. Furthermore, class III obesity patients observed a greater risk of mortality compared to class I and class II obesity. The risk of mortality was varied in COVID-19 patients with various comorbidities. Our findings may help to categorized patients at different risk groups and to make potential early prevention and treatment strategies possible.
Comparison With Previous Studies
Our study findings are similar to those of two previous studies (41, 42). Hussain et al. (41) included 14 studies, but only 6 studies were used to assess the relationship between obesity and mortality in COVID-19. This study lacks statistical power in subgroup analyses and included no consideration of different types of obesity and risk of mortality of patients with COVID-19. Another study showed a positive relation, though lack of evidence because they included preprints (non-peer-reviewed articles), and there were no subgroup analyses (42).
Biological Plausibility
Several biological mechanisms have been reported to explain the association between obesity and increase the risk of mortality. First, it is reported that both ectopic fat and COVID-19 are responsible for upregulation of pro-inflammatory, angiotensin II (ATII), and prothrombotics. Patients with obesity observe a decreased level of inflammatory adipokines, adiponectin, which is liked to an increased rate of ATII (43, 44). Similarly, coronavirus reduces the activity of ACE2 inhibitor, which leads to increase ATII level (45, 46). Higher levels of ATII might contribute to the progression of lung injury among patients diagnosed with COVID-19 by triggering NADH/NADPH oxidase system (47) and promoting contraction and vasoconstriction (48). Furthermore, an increased expression of inflammatory molecules enhances the production of cytokines [e.g., tumor necrosis factor alpha (TNF-α) and IL-6] (49), which are associate with alveolar damage and an increased rate of mortality (50).
Public Health Implication
COVID-19 pandemic has created serious concern globally, and people are eagerly waiting for potential vaccines. There is no exact and effective treatment for this virus so far, and global morbidity and mortality thus increase day by day (51). COVID-19 shows a wide spectrum of symptoms; some patients recovered without complications. However, some patients affected by serious illness have needed to transfer to the ICU, require a prolonged hospital stay, and may even die (52). Elderly patients were more vulnerable in this disease because they have multiple diseases. A significant number of studies reported that elderly patients and patients with diabetes, stroke, CKD, and COPD are associated with bad outcomes (53, 54). Obesity, especially class 3 obesity, was associated with an increased rate of mortality among patients diagnosed with COVID-19. It is, however, not surprising because patients with obesity who had the H1N1 influenza virus also observed prolong hospitalization, mechanical ventilation, and death when it was calculated as an independent risk factor (55, 56).
Several population-based cohort studies reported that obesity is linked to increased comorbidity like diabetes, hypertension, and heart disease. However, the mortality rate among patients with obesity proportionally increased with BMI (57, 58). Moreover, obesity makes patients' conditions worse if patients develop infections by downregulating the inflammatory cascade. Hyperactivation of inflammatory pathways surge the level of cytokines, adiponectin, and leptin and distort both macro- and micro-vascular responses (59). Obesity is also associated with lung function impairment, which involves altering mechanics and airway resistance and decreasing gas exchange (60, 61). The findings of our study suggest that physicians should focus more on COVID-19 patients with obesity because this group of patients is at high risk of worse consequences. Moreover, our results highlight the need for vigilance, priority on testing, and an earlier start to treatment in obese patients with COVID-19. Previous studies also mentioned that obesity increased the rate of hospitalization, worsened patient conditions, and increased invasive mechanical ventilation in those diagnosed with COVID-19 (15, 62).
Strengths and Limitations
Our study has several strengths. First, this is the first comprehensive and rigorous meta-analysis that assessed the association between obesity and the risk of mortality among patients with COVID-19. This meta-analysis included 17 studies, and only peer-reviewed articles were included for calculating the magnitude of risk because preprint articles have lots of room for improvement. Second, the risk of bias is low, and heterogeneity among the study is moderate. However, several factors showed higher heterogeneity among the studies. Third, this meta-analysis also evaluated associated factors that can contribute to a high risk of mortality. Finally, only adjusted effect size was considered to calculate pooled RR, which indicates strong evidence with a low risk of bias. Our study has several limitations that need to be addressed. First, we could not compare risk among patients aged <65 due to data constraints. Second, pneumonia is a high-risk factor for mortality among COVID-19 patients because only one study reported about this issue. This study showed the association between COPD and the risk of mortality among patients with COVID-19. Third, our study did not show the difference in the rate of mortality among various races and locations; this could have added value to the present evidence.
Conclusion
We conducted a comprehensive systematic review and rigorous meta-analysis of studies reporting the risk of mortality among COVID-19 patients with obesity. Our study findings showed that obesity is associated with an increased risk of mortality among patients with COVID-19. Importantly, the risk of mortality was higher among class III obesity than class I and II obesity. Physicians should be aware of these risk factors and make a quick decision for intervention. Future studies are urgently needed to clarify the pathophysiological relationship between obesity and the risk of mortality among COVID-19 patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TP and MI: conceptualization. TP and HY: methodology. MI: Software, resources, data curation and writing —original draft preparation. ML, W-SJ, and Y-CL: validation. TP: formal analysis and visualization. M-HH: investigation. Y-CL: writing —review and editing and supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thanks our colleague who is a Native English Speaker for editing this manuscript.
Footnotes
Funding. This research was funded in part by the Ministry of Education (MOE) under grants MOE 109-6604-001-400 and DP2-109-21121-01-A-01 and the Ministry of Science and Technology (MOST) under grant MOST 109-2823-8-038-004.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.620044/full#supplementary-material
References
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ornell F, Schuch JB, Sordi AO, Kessler FHP. “Pandemic fear” and COVID-19: mental health burden and strategies. Braz J Psychiatry. (2020) 42:232–5. 10.1590/1516-4446-2020-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKibbin Warwick, J,., Fernando, Roshen, The global macroeconomic impacts of COVID-19: seven scenarios (March 2, 2020). CAMA Working Paper No. 19/2020. 10.2139/ssrn.3547729 Available online at: https://ssrn.com/abstract=3547729 [DOI]
- 4.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. (2020) 39:405. 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. (2020) 80:388–93. 10.1016/j.jinf.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. (2014) 168:561–6. 10.1001/jamapediatrics.2014.21 [DOI] [PubMed] [Google Scholar]
- 8.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology. (2003) 14:479–87. 10.1097/01.EDE.0000071413.55296.c4 [DOI] [PubMed] [Google Scholar]
- 9.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. (2006) 17:1695–702. 10.1681/ASN.2005060638 [DOI] [PubMed] [Google Scholar]
- 10.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; (2020). [PubMed] [Google Scholar]
- 11.Zhi G, Xin W, Ying W, Guohong X, Shuying L. “Obesity paradox” in acute respiratory distress syndrome: asystematic review and meta-analysis. PLoS ONE. (2016) 11:e0163677. 10.1371/journal.pone.0163677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King P, Mortensen EM, Bollinger M, Restrepo MI, Copeland LA, Pugh MJV, et al. Impact of obesity on outcomes for patients hospitalised with pneumonia. Eur Respir J. (2013) 41:929–34. 10.1183/09031936.00185211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie W, Zhang Y, Jee SH, Jung KJ, Li B, Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med. (2014) 12:61. 10.1186/1741-7015-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrales-Medina VF, Valayam J, Serpa JA, Rueda AM, Musher DM. The obesity paradox in community-acquired bacterial pneumonia. Int J Infect Dis. (2011) 15:e54–7. 10.1016/j.ijid.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 15.Hamer M, Gale CR, Kivimäki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID-19: a community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci. (2020) 117:21011–3. 10.1073/pnas.2011086117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with COVID-19. N Engl J Med. (2020) 382:2534–43. 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. (2020) 369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clinical Infectious Diseases. (2020) 71:896–7. 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda T, Obata R, Dahlia Rizk DO, Kuno TJ. The association of interleukin-6 value, interleukin inhibitors, and outcomes of patients with COVID-19 in New York City. J Med Virol. (2020) 96:463–71. 10.1002/jmv.26365 [DOI] [PubMed] [Google Scholar]
- 20.Aziz M, Fatima R, Assaly RJ. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. (2020) 92:2283–5. 10.1002/jmv.25948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, med PGJP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 22.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 23.Hemingway H, Riley RD, Altman DG. Ten steps towards improving prognosis research. BMJ. (2009) 339:b4184. 10.1136/bmj.b4184 [DOI] [PubMed] [Google Scholar]
- 24.Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen P-A, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. (2016) 47:181–91. 10.1159/000454881 [DOI] [PubMed] [Google Scholar]
- 25.Poly T, Islam M, Yang H-C, Wu C. Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporosis International. (2019) 30:103–14. 10.1007/s00198-018-4788-y [DOI] [PubMed] [Google Scholar]
- 26.Kim L, Garg S, O'Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Interim analysis of risk factors for severe outcomes among a cohort of hospitalized adults identified through the Us coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). medRxiv. (2020) 28:1606–12. [Google Scholar]
- 27.Halasz G, Leoni ML, Villani GQ, Nolli M, Villani M. Obesity, overweight and survival in critically ill patients with SARS-CoV-2 pneumonia: is there an obesity paradox? Preliminary results from Italy. Euro J Prev. Cardiol. (2020). 10.1177/2047487320939675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajifathalian K, Kumar S, Newberry C, Shah S, Fortune B, Krisko T, et al. Obesity is associated with worse outcomes in COVID-19: analysis of Early Data From New York City. Obesity. (2020) 28:1606–12. 10.1002/oby.22923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. (2020) 108:154262. 10.1016/j.metabol.2020.154262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Van Oekelen O, Mouhieddine T, Del Valle DM, Richter J, Cho HJ, et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol. (2020) 13:94. 10.1186/s13045-020-00934-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Annals Intern Med. (2020) 173:773–81. 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, et al. Body mass index and risk for intubation or death in SARS-CoV-2 Infection: a retrospective cohort study. Ann Intern Med. (2020) 173:782–90. 10.7326/M20-3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bello-Chavolla OY, Bahena-Lopez JP, Antonio-Villa NE, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. (2020) 105:dgaa346 10.1210/clinem/dgaa346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czernichow S, Beeker N, Rives-Lange C, Guerot E, Diehl JL, Katsahian S, et al. Obesity doubles mortality in patients hospitalized for SARS-CoV-2 in Paris hospitals, France: a cohort study on 5795 patients. Obesity (Silver Spring). (2020) 12:2282–89. 10.1002/oby.23014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity. (2020) 28:1595–9. 10.1002/oby.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakeshbandi M, Maini R, Daniel P, Rosengarten S, Parmar P, Wilson C, et al. The impact of obesity on COVID-19 complications: a retrospective cohort study. Int J Obesy. (2020) 44:1832–7. 10.1038/s41366-020-0648-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettit NN, MacKenzie EL, Ridgway J, Pursell K, Ash D, Patel B, et al. Obesity is associated with increased risk for mortality among hospitalized patients with COVID-19. Obesity. (2020) 28:1806–10. 10.1002/oby.22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rottoli M, Bernante P, Belvedere A, Balsamo F, Garelli S, Giannella M, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian Centre. Eur J Endocrinol. (2020) 183:389–97. 10.1530/EJE-20-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goyal P, Ringel JB, Rajan M, Choi JJ, Pinheiro LC, Li HA, et al. Obesity and COVID-19 in New York City: a retrospective cohort study. Ann Intern Med. (2020) 173:855–8. 10.7326/M20-2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang F, Xiong Y, Wei Y, Hu Y, Wang F, Li G, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. (2020) 92:2536–42. 10.1002/jmv.26039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussain A, Mahawar K, Xia Z, Yang W, Shamsi E-H. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. (2020) 14:295–300. 10.1016/j.orcp.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. (2020) 21:e13128. 10.1111/obr.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-α. Arterioscler Thromb Vasc Biol. (2008) 28:863–70. 10.1161/ATVBAHA.107.156687 [DOI] [PubMed] [Google Scholar]
- 44.Ran J, Hirano T, Fukui T, Saito K, Kageyama H, Okada K, et al. Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metabolism. (2006) 55:478–88. 10.1016/j.metabol.2005.10.009 [DOI] [PubMed] [Google Scholar]
- 45.Cheng H, Wang Y, Wang GQJ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. (2020) 92:726–30. 10.1002/jmv.25785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong J-C, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. (2020) 126:1456–74. 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Murugesan P, Huang K, Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol. (2020) 17:170–94. 10.1038/s41569-019-0260-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhaun N, Webb DJ. Endothelins in cardiovascular biology and therapeutics. Nat Rev Cardiol. (2019) 16:491–502. 10.1038/s41569-019-0176-3 [DOI] [PubMed] [Google Scholar]
- 49.Um J-Y, Chung H-S, Song M-Y, Shin H-D, Kim H-M. Association of interleukin-1β gene polymorphism with body mass index in women. Clin Chem. (2004) 50:647–50. 10.1373/clinchem.2003.025858 [DOI] [PubMed] [Google Scholar]
- 50.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. (2020) 20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. (2020) 20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. (2020) 323:2052–9. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. (2020) 80:e1–6. 10.1016/j.jinf.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkata C, Sampathkumar P, Afessa B. editors. Hospitalized patients with 2009 H1N1 influenza infection: the Mayo Clinic experience. Mayo Clin Proc. (2010) 85:798–805. 10.4065/mcp.2010.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A (H1N1) disease. PLoS ONE. (2010) 5:e9694. 10.1371/journal.pone.0009694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lancet PSCJT. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. (2009) 373:1083–96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. (2011) 364:719–29. 10.1056/NEJMoa1010679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murugan A, Sharma G. Obesity and respiratory diseases. Chron Respir Dis. (2008) 5:233–42. 10.1177/1479972308096978 [DOI] [PubMed] [Google Scholar]
- 60.Steele RM, Finucane FM, Griffin SJ, Wareham NJ, Ekelund U. Obesity is associated with altered lung function independently of physical activity and fitness. Obesity. (2009) 17:578–84. 10.1038/oby.2008.584 [DOI] [PubMed] [Google Scholar]
- 61.Falagas M, Athanasoulia A, Peppas G, Karageorgopoulos DE. Effect of body mass index on the outcome of infections: a systematic review. Obes Rev. (2009) 10:280–9. 10.1111/j.1467-789X.2008.00546.x [DOI] [PubMed] [Google Scholar]
- 62.Chang TH, Chou CC, Chang LYJOR. Effect of obesity and body mass index on coronavirus disease 2019 severity: a systematic review and meta-analysis. Obes Rev. (2020) 21:e13089. 10.1111/obr.13089 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.