Abstract
Background
Heart rate variability (HRV) and heart rate (HR) dynamics are used to predict the survival probability of patients after acute myocardial infarction (AMI), but the association has been established in patients with mixed levels of left ventricular ejection fraction (LVEF).
Objective
We investigated whether the survival predictors of HRV and HR dynamics depend on LVEF after AMI.
Methods
We studied 687 post-AMI patients including 147 with LVEF ≤35% and 540 with LVEF >35%, of which 23 (16%) and 22 (4%) died during the 25 month follow-up period, respectively. None had an implanted cardioverter-defibrillator. From baseline 24 h ECG, the standard deviation (SDNN), root mean square of successive difference (rMSSD), percentage of successive difference >50 ms (pNN50) of normal-to-normal R-R interval, ultra-low (ULF), very-low (VLF), low (LF), and high (HF) frequency power, deceleration capacity (DC), short-term scaling exponent (α1), non-Gaussianity index (λ25s), and the amplitude of cyclic variation of HR (Acv) were calculated.
Results
The predictors were categorized into three clusters; DC, SDNN, α1, ULF, VLF, LF, and Acv as Cluster 1, λ25s independently as Cluster 2, and rMSSD, pNN50, and HF as Cluster 3. In univariate analyses, mortality was best predicted by indices belonging to Cluster 1 regardless of LVEF. In multivariate analyses, however, mortality in patients with low LVEF was best predicted by the combinations of Cluster 1 predictors or Cluster 1 and 3 predictors, whereas in patients without low LVEF, it was best predicted by the combinations of Cluster 1 and 2 predictors.
Conclusion
The mortality risk in post-AMI patients with low LVEF is predicted by indices reflecting decreased HRV or HR responsiveness and cardiac parasympathetic dysfunction, whereas in patients without low LVEF, the risk is predicted by a combination of indices that reflect decreased HRV or HR responsiveness and indicator that reflects abrupt large HR changes suggesting sympathetic involvement.
Keywords: heart rate dynamics, heart rate variability, myocardial Infarction, mortality, redundancy, risk stratification, survival, left ventricular ejection fraction
Introduction
Despite significant achievements in its clinical management (Antman et al., 2004), acute myocardial infarction (AMI) remains a leading cause of death (Virani et al., 2020). AMIs occur in the United States at a rate of 1 person every 40 s, with an associated mortality of approximately 110,000 per year (Virani et al., 2020). Sudden cardiac death (SCD) is the most common cause of death after AMI (Zaman and Kovoor, 2014), and patients with a low left ventricular ejection fraction (LVEF) are at the highest risk of SCD during the early months to years after AMI (Solomon et al., 2005; Adabag et al., 2008). To prevent SCD, prophylactic implantation of cardioverter-defibrillators has been recommended for post-AMI patients with LVEF ≤35% (Moss et al., 2002; Goldberger and Lampert, 2006). However, the generalization of reperfusion therapy early after AMI onset (Aversano et al., 2002) has reduced the proportion of post-AMI patients with low LVEF, and consequently, the majority of SCDs occur in patients with LVEF >35%. It has become more important to find clinical markers to predict an increased risk of death in patients without low LVEF (Arevalo et al., 2016). In this study, we analyzed heart rate variability (HRV) and heart rate (HR) dynamics in post-AMI patients to determine the useful markers and combinations to predict mortality risk separately between patients with LVEF ≤35% and those with LVEF >35%.
The analysis of HRV and HR dynamics are widely used for survival risk stratification in cardiovascular diseases (Camm et al., 1996), particularly after AMI (Kleiger et al., 1987; Bigger et al., 1992; Peng et al., 1995; La Rovere et al., 1998; Huikuri et al., 2000; Bauer et al., 2006; Kantelhardt et al., 2007; Kiyono et al., 2007, 2008; Hayano et al., 2011a, 2017; Watanabe et al., 2016). The R-R interval time series data obtained from the 24 h Holter ECG are mainly used for these analyses and many indices have been proposed. The HRV indices are classified into time-domain and frequency-domain indices (Camm et al., 1996). The time-domain indices include the statistical measures of normal-to-normal (N-N) interval (R-R interval of consecutive sinus rhythms) variation, such as the standard deviation of 24 h N-N interval (SDNN) (Kleiger et al., 1987), root mean square of successive N-N interval difference (rMSSD), percentage of successive N-N intervals differing >50 ms (pNN50), deceleration capacity (DC) (Kantelhardt et al., 2007), and the amplitude of cyclic variation of HR (Acv) (Hayano et al., 2011b). Among these, rMSSD and pNN50 that quantify high-frequency N-N interval fluctuations reflect the tonic or sustained level of cardiac parasympathetic control (Berntson et al., 1997; Laborde et al., 2017). Due to a low-pass filter-like-transfer function, the sympathetic HR control cannot involve the modulation of these high-frequency fluctuations (Berger et al., 1989), and thus, these fluctuations are mediated purely by the vagus. In contrast, Acv reflects the HR responsiveness to apneic episodes during sleep. It quantifies the shortening in cardiac cycles caused by sleep-apnea-induced transient arousals. Because this HR response is abolished by atropine (Zwillich et al., 1982; Guilleminault et al., 1984), Acv is thought to reflect a reflex parasympathetic function. The tonic and reflex parasympathetic dysfunction is believed to be a risk for post-AMI mortality (Camm et al., 1996; Bauer et al., 2006; Hayano et al., 2017) because parasympathetic antagonism against sympathetic activation is important to maintain ventricular myocardial electric stability and to prevent the development of fatal ventricular arrhythmias (Hull et al., 1990, 1994; La Rovere et al., 1998).
The frequency-domain indices of HRV are calculated by the power spectral analysis of N-N interval time series and are quantified as the power of frequency components. Among such components, ultra-low frequency (<0.0033 Hz; ULF) and very-low-frequency (0.0033–0.04 Hz; VLF) components reflect fractal-like HR fluctuation that accounts for most of the power of 24 h HRV (Saul et al., 1988). A reduction in the VLF power is one of the most powerful predictors of post-AMI mortality (Bigger et al., 1992). In contrast, a reduction in the high-frequency component (HF, 0.15–0.40 Hz), which is thought to reflect cardiac parasympathetic dysfunction, paradoxically shows the lowest predictive power (Bigger et al., 1992). This paradox may be explained at least partly by the contamination of non-autonomic high-frequency R-R interval fluctuations caused by heart rate fragmentation (Costa et al., 2017; Hayano et al., 2020), which is a type of pacemaker dysfunction more likely to appear in high-risk patients (Costa et al., 2018).
The HR dynamics reflect the non-linear properties of HR fluctuation. Detrended fluctuation analysis (Peng et al., 1995) quantifies the scaling exponents of fractal-like HR dynamics and a reduction in the short-term (4–11 beats) scaling exponent (α1) is increased risk for post-AMI mortality (Huikuri et al., 2000). The non-Gaussianity index (λ) quantifies the probability density function for abrupt large HR changes suggesting sympathetic involvement (Kiyono et al., 2007). The λ is increased in patients with heart failure, known as the state of increased sympathetic activity, while other HRV indices are decreased (Kiyono et al., 2007). Additionally, λ is lower in these patients taking beta-blocker than in those without taking beta-blocker (Kiyono et al., 2007). An increase in λ calculated at a time scale of 25 s (λ25s) predicts increased risk for post-AMI cardiac mortality (Hayano et al., 2011a).
In the present study, we hypothesized that the HRV and HR dynamics indices and their combinations to predict post-AMI mortality risk differ between patients with and without low LVEF (≤35%). Most of earlier studies reporting predictive power of HRV and HR dynamics were conducted in post-AMI patients with mixed levels of LVEF (Kleiger et al., 1987; Bigger et al., 1992; Zuanetti et al., 1996; Lanza et al., 1998; Huikuri et al., 2000; Hayano et al., 2011a). The risk stratification models developed by the earlier studies may need to be reappraised separately depending on LVEF. The prophylactic ICD in post-AMI patients with low LVEF could also modify the risk structures. Considering these factors, we chose 24 h ECG data from the post-AMI cohort collected before ICD became clinically widespread and we compared the HRV and HR dynamics indices associated with mortality risk between patients with and without low LVEF. Furthermore, considering the possible redundancy existing among the indices of HRV and HR dynamics (Yuda et al., 2020), we categorized the indices into classes by cluster analysis and analyzed the class-relationships with mortality risk.
Materials and Methods
Study Cohort
We examined retrospective cohort data from a subset of the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study (Berkman et al., 2003) consisting of 687 patients who had an AMI and were admitted to the coronary care units of 4 of the 8 ENRICHD clinical trial sites (Washington University, St. Louis, Missouri; Duke University, Durham, North Carolina; Harvard University, Boston, Massachusetts; Yale University, New Haven, Connecticut) between October 1997 and January 2000. The sample included 327 participants of the ENRICHD clinical trial who scored 10 or higher on the Beck Depression Inventory (Beck, 1972) and 360 AMI control participants who were not randomized in the ENRICHD trial because they were not depressed, but were otherwise medically eligible for the trial. Patients were included if they had analyzable Holter ECG data >20.4 h (85% of 24 h) including >3 h of sleep period (time in bed). Patients were excluded if they: (1) had other life-threatening illnesses; (2) were too ill or logistically unable to participate; (3) had ECG data in sinus rhythm <80% of total recorded beats, or (4) had atrial fibrillation, atrial flutter, or an implanted pacemaker or defibrillator. The collection and analysis of Holter ECG recordings were approved by the ethics committees of the corresponding clinical sites. All participants provided written informed consent to participate in the study.
The end-point of the present study was all-cause mortality. Patients underwent follow-up assessments 6 months after study enrollment and annually thereafter for up to 30 months. The end-points were identified from follow-up visits, telephone calls, routine hospital surveillance, and contacts with patients’ physicians. The records of every identified hospitalization were obtained for review and confirmation by a panel of physicians. Death certificates were obtained for all reported deaths. The mortality endpoints used for the present study were either cardiac deaths (AMI, cardiac failure, and sudden cardiac death) or non-cardiac deaths.
Measurements
Holter ECGs were recorded for 24 h within 28 [median (IQR), 13 (6–19)] days after the index AMI. The ECG recordings were scanned at the Heart Rate Variability Core laboratory at Washington University on a Marquette SXP Laser scanner with software version 5.8 (Marquette Electronics) using standard procedures. The annotated beat file was exported to a workstation for analysis of HRV and HR dynamics indices.
Data Analysis
The time-domain and frequency-domain indices of HRV and the non-linear indices of HR dynamics that are known as major predictors of post-AMI mortality were calculated by the methods according to the recommended standard (Camm et al., 1996) and to previously published studies (Peng et al., 1995; Iyengar et al., 1996; Kantelhardt et al., 2007; Kiyono et al., 2007; Hayano et al., 2017).
Briefly, the time series of N-N intervals were derived from 24 h ECG data. For the time domain HRV indices, SDNN was computed as the 24 h standard deviation of N-N intervals, rMSSD was calculated as the square root of the mean square of 24 h successive N-N interval differences, pNN50 was obtained as the percentage of successive N-N intervals differing >50 ms, and DC was computed by the phase rectified signal averaging of the 24 h N-N interval time series (Kantelhardt et al., 2007). Acv was calculated by signal-averaging the amplitude of cyclic variation of HR detected by the method of auto-correlated wave detection with adaptive threshold algorithm (Hayano et al., 2011b).
For the frequency domain index, the N-N interval power spectrum was computed by a Fast Fourier transform with a Hanning window after interpolating with a horizontal step function and resampling at 2 Hz. The power spectral density was integrated for the power within the ULF (<0.0033 Hz), VLF (0.0033–0.04 Hz), LF (0.04–0.15 Hz), and HF (0.15–0.4 Hz) bands, respectively, and transformed into natural logarithmic values.
For the non-linear indices, the fractal correlation properties of HR dynamics were computed using the detrended fluctuation analysis and measured as the short-term (4–11 beat) scaling exponents (α1) (Peng et al., 1995; Iyengar et al., 1996). Also, the non-Gaussianity index of λ was calculated at a time scale of 25 s (λ25s) according to our previous work (Hayano et al., 2011a). This analysis detects the intermittency of HR increment. The intermittent behavior of HRV is related to non-Gaussian probability distribution with marked fat tails and a peak around the mean value, indicative of a higher probability of the interspersed appearance of large and small increments than the Gaussian fluctuations. The λ quantifies the fatness of the tails of the non-Gaussian probability distribution. The mathematical description of the non-Gaussianity index has been reported elsewhere (Kiyono et al., 2004, 2007).
Cluster Analysis of HRV and HR Dynamics Indices
To categorize HRV and HR dynamics indices, a cluster analysis was performed based on the correlation matrix between the indices. We used a divisive type cluster analysis. The analysis started with the assumption that all indices belong to a single cluster and continued to divide clusters until the eigenvalue of the second principal component of all clusters becomes less than 1. The cluster to which the index belongs was determined from the factor structure of the oblique principal component so that the index was classified into the clusters where the first principal component gives the highest correlation coefficient with the index.
Evaluation of Predictive Performance
The predictive performance of the discriminant models, including those consisting of a single index and those of the combinations of multiple indices, was analyzed by logistic regression and evaluated by Somers’ D and c-statistic. The logistic regression model provided prediction scores for individual participants and compared the scores between all possible pairs of survivors and non-survivors. Pairs with a survivor score higher than non-survivors were considered concordant, otherwise, they were considered discordant. Somers’ D was calculated as the difference between the number of concordant and discordant pairs divided by the number of all possible pairs, taking a value from -1 (all pairs disagree) to 1 (all pairs agree). The c-statistic reflected the area under the receiver-operating characteristic curve for the predictive performance of the regression models.
Statistical Analysis
We used SAS version 9.4 programs (SAS Institute, Cary, NC). Differences between survivors and non-survivors were evaluated by the Chi-square test for categorical data and by Wilcoxon two-sample test for continuous data. The SAS VARCLUS procedure with an oblique principal component cluster analysis was used to categorize the HRV and HR dynamics indices. The SAS LOGISTIC procedure was used for the logistic regression analysis for mortality risk stratification by HRV and HR dynamics indices and their combinations. All models included age as an independent predictor. For all statistical analyses, P < 0.05 was considered significant.
Results
Patients’ Characteristics
Patient characteristics are presented in Table 1. With baseline LVEF, the participants were divided into 147 patients with LVEF ≤35% (low LVEF) and 540 patients with LVEF >35%. During the follow-up period, 23 (16%) patients with low LVEF and 22 (4%) patients without low LVEF died from all-causes. Among patients with low LVEF, non-survivors were more often diabetic and mentally depressed, had lower LVEF, and had higher serum creatinine. Survivors were more likely to have had more frequent coronary angioplasty. Among patients without low LVEF, non-survivors were older and more often diabetic and smoker, had more frequent histories of coronary bypass surgery, had lower LVEF, had higher serum creatinine, and were more often Killip class III-IV after the index AMI. Survivors were more likely to have had an index AMI of the inferior wall and had more frequent acute reperfusion after the AMI.
TABLE 1.
Patients’ characteristics.
| LVEF ≤35% |
LVEF >35% |
|||||
| Survivor | Non-survivor | P* | Survivor | Non-survivor | P* | |
| Number of patients, n | 124 (84%) | 23 (16%) | 518 (96%) | 22 (4%) | ||
| Outcome | ||||||
| Follow-up (days), median (IQR) | 778 (590–1,024) | 499 (175–657) | <0.0001 | 769 (574–974) | 373 (203–696) | <0.0001 |
| Cardiac death | 0 (0%) | 18 (78%) | 0 (0%) | 14 (64%) | ||
| Demographic and clinical | ||||||
| Age (years), median (IQR) | 62 (53–71) | 63 (53–75) | 0.3 | 58 (49–67) | 69 (59–71) | 0.001 |
| Women | 37 (30%) | 10 (43%) | 0.1 | 215 (42%) | 10 (45%) | 0.7 |
| Body mass index (kg/m2), median (IQR) | 27.6 (24.5–31.2) | 28.3 (24.0–35.0) | 0.6 | 28.5 (25.4–32.1) | 28.4 (26.2–32.4) | 0.6 |
| Hypertension | 21 (17%) | 4 (17%) | 0.9 | 107 (21%) | 8 (36%) | 0.05 |
| Diabetes mellitus | 32 (26%) | 15 (65%) | 0.0002 | 128 (25%) | 17 (77%) | <0.0001 |
| Current smoker | 38 (31%) | 4 (17%) | 0.2 | 189 (36%) | 3 (14%) | 0.03 |
| BDI score ≥10 | 52 (42%) | 18 (78%) | 0.001 | 243 (47%) | 14 (64%) | 0.1 |
| History of myocardial infarction | 39 (31%) | 11 (48%) | 0.1 | 90 (17%) | 7 (32%) | 0.1 |
| History of coronary bypass surgery | 22 (18%) | 7 (30%) | 0.1 | 42 (8%) | 7 (32%) | <0.0001 |
| LVEF (%), median (IQR) | 30 (26–35) | 25 (20–30) | 0.0007 | 52 (45–55) | 45 (40–52) | 0.01 |
| Creatinine (mg/dL), median (IQR) | 1.0 (0.8–1.2) | 1.3 (1.1–2.3) | <0.0001 | 1.0(0.8–1.1) | 1.2 (0.9–2.1) | 0.01 |
| Index AMI | ||||||
| Killip class III-IV | 15 (12%) | 5 (22%) | 0.2 | 15 (3%) | 3 (14%) | 0.003 |
| Anterior wall AMI | 68 (55%) | 9 (39%) | 0.2 | 141 (27%) | 4 (18%) | 0.4 |
| Inferior wall AMI | 42 (34%) | 5 (22%) | 0.3 | 258 (50%) | 5 (23%) | 0.01 |
| Treatment | ||||||
| β-Blockers | 101 (81%) | 17 (74%) | 0.3 | 424 (82%) | 20 (91%) | 0.2 |
| Angiotensin converting enzyme inhibitors | 90 (73%) | 16 (70%) | 0.7 | 222 (43%) | 11 (50%) | 0.5 |
| Aspirin | 110 (89%) | 17 (74%) | 0.05 | 476 (92%) | 17 (77%) | 0.01 |
| Calcium channel blockers | 12 (10%) | 3 (13%) | 0.6 | 74 (14%) | 7 (32%) | 0.02 |
| Thrombolytic therapy after AMI | 38 (31%) | 6 (26%) | 0.7 | 173 (33%) | 2 (9%) | 0.02 |
| Coronary bypass after AMI | 27 (22%) | 1 (4%) | 0.05 | 73 (14%) | 2 (9%) | 0.5 |
| Coronary angioplasty<24 h after AMI | 66 (53%) | 4 (17%) | 0.004 | 334 (64%) | 12 (55%) | 0.2 |
| Acute reperfusion < = 12 h after AMI | 55 (44%) | 6 (26%) | 0.1 | 250 (48%) | 5 (23%) | 0.02 |
*Significance of difference by Wilcoxon two sample test for continuous variables and by chi-square test for categorical variables. AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction.
Cluster Analysis of HRV and HR Dynamics Indices
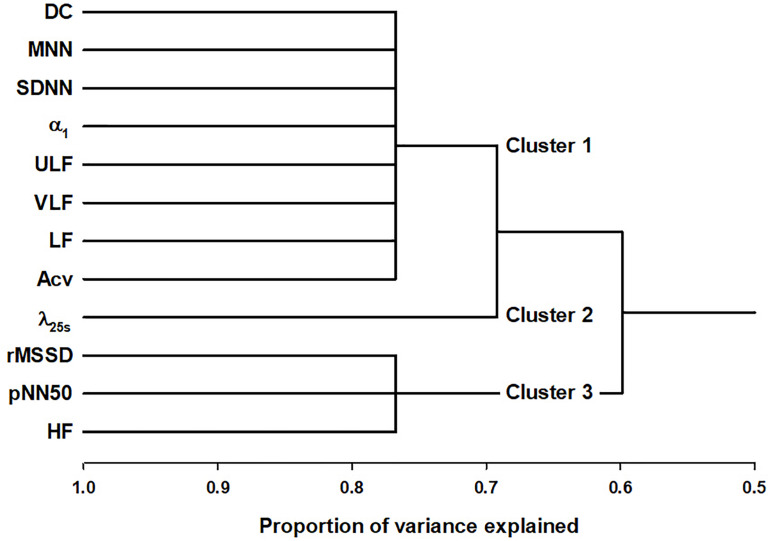
Figure 1 shows the tree diagram of the hierarchical cluster based on the principal component of the correlation matrix. The cluster analysis was performed in all 687 patients without separating with LVEF. The predictors were found to be categorized into three clusters; DC, SDNN, α1, ULF, VLF, LF, and Acv as Cluster 1, λ25s independently as Cluster 2, and rMSSD, pNN50, and HF as Cluster 3.
FIGURE 1.
Cluster analysis of HRV and HR dynamics indices in post-AMI patients. DC, deceleration capacity; SDNN, standard deviation of normal-to-normal R-R interval during 24 h; ULF, power of ultra-low-frequency (<0.0033 Hz) component; VLF, power of very-low-frequency (0.0033–0.04 Hz) component; LF, power of low-frequency (0.04–0.15 Hz) component; Acv, amplitude of the cyclic variation of heart rate; λ25s, non-Gaussianity index for a segment length of 25 s; Fcv, frequency of the cyclic variation of heart rate; rMSSD, root mean square of successive R-R interval differences; pNN50, percentage of successive R-R intervals differing >50 ms, and HF, power of high-frequency (0.15–0.40 Hz) component.
Univariate Associations of HRV and HR Dynamics With Post-AMI Mortality
Table 2 shows the difference in HRV and HR dynamic indices between survivors and non-survivors. Regardless of LVEF, non-survivors had lower values for all indices in Cluster 1. Among patients with low LVEF, non-survivor has lower HF in Cluster 3, but λ25s (Cluster 2) did not differ significantly between survivors and non-survivors. Among patients without low LVEF, non-survivors had greater λ25s(Cluster 2), and lower values for all indices in Cluster 3.
TABLE 2.
Comparisons of baseline heart rate variability (HRV) and heart rate (HR) dynamics indices between survivors and non-survivors.
| Index, median (IQR) | LVEF ≤35% |
LVEF >35% |
||||
| Survivor | Non-survivor | P* | Survivor | Non-survivor | P* | |
| DC (ms) | 4.2(3.1−6.2) | 2.9(2.3−3.6) | 0.0002 | 5.3(3.7−6.7) | 2.7(2.2−3.8) | <0.0001 |
| SDNN (ms) | 87(63−109) | 56(47−73) | 0.0004 | 90(69−118) | 65(54−78) | 0.0008 |
| α1 | 1.2(0.9−1.3) | 0.8(0.6−1.1) | 0.002 | 1.2(1−1.3) | 0.8(0.7−1.2) | 0.001 |
| ULF [ln(ms2)] | 8.6(7.9−9.1) | 7.7(7.3−8.3) | 0.0005 | 8.7(8−9.2) | 8(7.6−8.4) | 0.001 |
| VLF [ln(ms2)] | 6.7(6−7.3) | 5.7(4.9−6.1) | 0.0002 | 6.8(6−7.5) | 5.4(4.6−6.3) | <0.0001 |
| LF[ln(ms2)] | 5.4(4.3−6.4) | 4.1(3.5−4.8) | 0.0005 | 5.6(4.6−6.3) | 4.7(2.8−5.3) | 0.0003 |
| Acv [ln(ms)] | 3.9(3.5−4.3) | 3.3(3−3.6) | <0.0001 | 4.2(3.7−4.5) | 3.2(2.9−3.7) | <0.0001 |
| λ25s | 0.5(0.5−0.7) | 0.6(0.4−0.7) | 0.7 | 0.5(0.5−0.6) | 0.6(0.5−0.6) | 0.002 |
| rMSSD (ms) | 20(14−34) | 16(12−24) | 0.1 | 23(16−33) | 15(11−29) | 0.01 |
| pNN50 (%) | 2.6(0.4−10.7) | 0.8(0.1−4.8) | 0.09 | 3.1(0.8−10.2) | 0.5(0.1−6.8) | 0.01 |
| HF [ln(ms2)] | 4.4(3.5−5.5) | 3.6(2.9−4.5) | 0.03 | 4.6(3.9−5.5) | 3.5(3−5.2) | 0.009 |
*Significance of difference by Wilcoxon two sample test. Abbreviations are explained in the legend of Figure 1.
Table 3 shows the results of the univariate logistic regression analysis. Regardless of LVEF, the top five predictors based on the c-statistic belonged to Cluster 1.
TABLE 3.
Predictive power of HRV and HR dynamics indices for post-AMI mortality (logistic regression analysis).
| Predictor | LVEF ≤35% |
LVEF >35% |
||||||
| Concordant,% | Discordant,% | Somers’ D | c-Statistic | Concordant,% | Discordant,% | Somers’ D | c-Statistic | |
| DC | 74.4 | 25.6 | 0.489 | 0.744 | 82.7 | 17.3 | 0.655 | 0.827 |
| SDNN | 75.6 | 24.4 | 0.512 | 0.756 | 77.1 | 22.9 | 0.542 | 0.771 |
| α1 | 70.0 | 30.0 | 0.399 | 0.700 | 74.7 | 25.3 | 0.493 | 0.747 |
| ULF | 74.1 | 25.9 | 0.481 | 0.741 | 77.7 | 22.3 | 0.553 | 0.777 |
| VLF | 75.4 | 24.6 | 0.508 | 0.754 | 80.9 | 19.1 | 0.618 | 0.809 |
| LF | 74.5 | 25.5 | 0.490 | 0.745 | 79.1 | 20.9 | 0.582 | 0.791 |
| Acv | 80.7 | 19.3 | 0.614 | 0.807 | 82.5 | 17.5 | 0.649 | 0.825 |
| λ25s | 53.5 | 46.5 | 0.070 | 0.535 | 74.9 | 25.1 | 0.497 | 0.749 |
| rMSSD | 56.5 | 43.5 | 0.130 | 0.565 | 73.3 | 26.7 | 0.465 | 0.733 |
| pNN50 | 56.6 | 43.4 | 0.133 | 0.566 | 73.0 | 27.0 | 0.461 | 0.730 |
| HF | 65.4 | 34.6 | 0.309 | 0.654 | 76.8 | 23.2 | 0.536 | 0.768 |
All classification models are adjusted for the effect of age. Boldface indicates the top five largest c-statistic values for LVEF ≥35% and LVEF >35%, respectively. Abbreviations are explained in the legend of Figure 1.
Multivariate Associations of HRV and HR Dynamics With Post-AMI Mortality
Table 4 shows the results of logistic regression analyses for all combinations between two predictors. Among patients with low LVEF, the top five performances were observed with the combinations between two predictors both in Cluster 1 and the combination between Cluster 1 and 3 predictors. In contrast, among patients without low LVEF, the top five performances were observed with the combinations between Cluster 1 and 2 predictors.
TABLE 4.
Predictive performance (c-statistics) of combinations of two predictors among post-AMI patients grouped by LVEF.
| DC | SDNN | α1 | ULF | VLF | LF | Acv | λ25s | rMSSD | pNN50 | HF | ||
| DC | – | 0.824 | 0.823 | 0.825 | 0.824 | 0.823 | 0.830 | 0.840 | 0.828 | 0.830 | 0.824 | LVEF > 35% |
| SDNN | 0.773 | – | 0.775 | 0.778 | 0.808 | 0.792 | 0.824 | 0.803 | 0.771 | 0.772 | 0.780 | |
| α1 | 0.768 | 0.773 | – | 0.782 | 0.802 | 0.789 | 0.816 | 0.769 | 0.791 | 0.792 | 0.791 | |
| ULF | 0.743 | 0.745 | 0.726 | – | 0.810 | 0.800 | 0.830 | 0.815 | 0.782 | 0.781 | 0.792 | |
| VLF | 0.758 | 0.762 | 0.751 | 0.754 | – | 0.809 | 0.826 | 0.832 | 0.808 | 0.808 | 0.808 | |
| LF | 0.765 | 0.767 | 0.766 | 0.742 | 0.733 | – | 0.825 | 0.832 | 0.790 | 0.791 | 0.790 | |
| Acv | 0.806 | 0.810 | 0.811 | 0.806 | 0.807 | 0.816 | – | 0.836 | 0.824 | 0.823 | 0.826 | |
| λ25s | 0.728 | 0.728 | 0.672 | 0.739 | 0.746 | 0.740 | 0.807 | – | 0.801 | 0.787 | 0.8327 | |
| rMSSD | 0.738 | 0.769 | 0.730 | 0.732 | 0.761 | 0.773 | 0.816 | 0.551 | – | 0.728 | 0.769 | |
| pNN50 | 0.740 | 0.759 | 0.737 | 0.741 | 0.760 | 0.765 | 0.817 | 0.581 | 0.556 | – | 0.769 | |
| HF | 0.738 | 0.744 | 0.768 | 0.732 | 0.759 | 0.751 | 0.808 | 0.684 | 0.717 | 0.699 | – | |
| LVEF ≤ 35% | ||||||||||||
Data are c-statistics calculated by logistic regression analysis. Values in lower left half and upper right half represent those for patients with LVEF ≤35% and those with LVEF > 35%, respectively. Boldface indicates the top five largest values for LVEF ≤35% and LVEF >35%, respectively. All classification models are adjusted for the effect of age. Abbreviations are explained in the legend of Figure 1.
These features were also observed for the prediction models consisting of three predictors (Table 5). The mortality in patients with low LVEF was best predicted by the combinations of Cluster 1 and 3 predictors. In patients without low LVEF, the top four performances were observed with the combinations between Cluster 1 and 2, although the combinations of Cluster 1, 2, and 3 predictors also showed the 4th best performance.
TABLE 5.
Combinations of three predictors with the top five predictive performance.
| Best combination | Concordant,% | Discordant,% | Somers’ D | c-Statistic |
| LVEF ≤35% | ||||
| α1 + Acv + rMSSD | 82.1 | 17.9 | 0.641 | 0.821 |
| Acv + rMSSD + HF | 81.8 | 18.2 | 0.636 | 0.818 |
| Acv + rMSSD + pNN50 | 81.5 | 18.5 | 0.629 | 0.815 |
| SDNN + Acv + rMSSD | 81.5 | 18.5 | 0.63 | 0.815 |
| ULF + Acv + rMSSD | 81.5 | 18.5 | 0.63 | 0.815 |
| LVEF >35% | ||||
| ULF + Acv + λ25s | 84.4 | 15.6 | 0.689 | 0.844 |
| VLF + Acv + λ25s | 83.7 | 16.3 | 0.674 | 0.837 |
| ULF + VLF + λ25s | 83.2 | 16.8 | 0.665 | 0.832 |
| SDNN + VLF + λ25s | 83.1 | 16.9 | 0.661 | 0.831 |
| VLF + λ25s + pNN50 | 83.1 | 16.9 | 0.663 | 0.831 |
All prediction models are adjusted for the effect of age. Abbreviations are explained in the footnote in Table 2.
Discussion
In this study, we sought to determine if HRV and HR dynamics indices that predict mortality risk after AMI differ between patients with and without low LVEF (≤35%). Considering the possible redundancy existing among HRV and HR dynamics indices (Yuda et al., 2020), we first categorized the predictors into classes. The cluster analysis revealed that the predictors can be classified into 3 clusters thought to reflect the magnitude of HRV or HR responsiveness (Cluster 1: DC, SDNN, α1, ULF, VLF, LF, and Acv), the frequency of abrupt large HR changes (Cluster 2: λ25s), and cardiac parasympathetic function (Cluster 3: rMSSD, pNN50, and HF), respectively. Then, we examined the associations between clustered predictors and mortality risk in patients with and without low LVEF, separately. Univariate analyses showed that mortality was best predicted by indices belonging to Cluster 1 regardless of LVEF, but multivariate analyses showed that mortality in patients with low LVEF was best predicted by the combinations of two Cluster 1 predictors or Cluster 1 and 3 predictors, while in patients without low LVEF, it was best predicted by the combinations of Cluster 1 and 2 predictors. Our findings indicate that the mortality risk in post-AMI patients with low LVEF is predicted by decreased HRV or HR responsiveness and cardiac parasympathetic dysfunction, whereas in patients without low LVEF, the risk is predicted by a combination of decreased HRV or HR responsiveness and increased abrupt large HR changes suggesting sympathetic involvement.
To our knowledge, this is the first study to compare HRV and HR dynamics indices that predict mortality between post-AMI patients with and without low LVEF. Most of earlier studies reporting predictive power of HRV and HR dynamics were conducted in post-AMI patients with mixed levels of LVEF, although they reported the independence of the predictive power of the indices from LVEF (Kleiger et al., 1987; Bigger et al., 1992; Zuanetti et al., 1996; Lanza et al., 1998; Hayano et al., 2011a). Also, Huikuri et al. (2000) examined the predictive value of HRV and HR dynamics in post-AMI patients with LVEF ≤35% and reported that a decrease in α1 had greater predictive power of post-AMI mortality than conventional HRV indices. Bauer et al. (2006) demonstrated that a decrease in DC had greater predictive power than SDNN and LVEF and reported that the risk stratification by DC was more useful in patients with LVEF >30% than in those with LVEF ≤30%. Liu et al. (2020) recently reported that decreased SDNN, VLF, and DC were independently associated with increased risk of sudden arrhythmic death in post-AMI patients with LVEF ≤35% and that combination of SDNN, VLF, and DC may help identify a high-risk patient group. Lombardi et al. (1996) compared HRV and HR dynamics indices between post-AMI patients with and without low LVEF and they observed reduced HRV power in the entire frequency range in patients with low LVEF, suggesting diminished responsiveness of sinus node to autonomic modulatory inputs in these patients. None of these studies, however, reported the difference in predictors between post-AMI patients with and without low LVEF.
In this study, we used retrospective cohort data of the ENRICHD study. The patients of this cohort had an AMI and admitted hospital between October 1977 and January 2000. Therefore, the fraction of patients who received a primary percutaneous coronary intervention was low and none of them had an ICD. We chose this cohort to allow comparison of post-AMI patients without low LVEF with a sufficiently sized group of patients with low LVEF whose survival risk is not affected by a prophylactic ICD. Additionally, the sample of this study included a subset of patients enrolled in the ENRICHD trial who had elevated symptoms of depression, which could affect the generalizability of our results. However, the proportion of the depressed patients with BDI scores ≥10 was 47.5%, which is comparable to the reported prevalence of depression (45–47%) in general post-AMI populations (Schleifer et al., 1989; Steeds et al., 2004).
We performed a cluster analysis of HRV and HR dynamics indices in the entire cohort of post-AMI patients. The indices were classified into three clusters and we observed that the associations between the HRV and HR dynamics indices and mortality risk were well explained as class-dependent relationships. These findings provide several insights into the underlying mechanisms.
First, the formation of clusters indicates that there are significant inter-correlations between these indices by the eigenvalue criteria of principal component analysis, supporting our previous finding of a big-data study reporting the substantial redundancy among HRV and HR dynamics indices (Yuda et al., 2020).
Second, the observation that all of the top five univariate predictors of post-AMI mortality belonged to Cluster 1 regardless of LVEF indicates the prognostic significance of the feature(s) common to the indices of this cluster. Although Cluster 1 includes a variety of indices, they commonly reflect the magnitude of HRV, such as SDNN, ULF, VLF, and LF, which are thought to be mediated by interactions between sympathetic and parasympathetic nerve activities, although parasympathetic dysfunction has been thought to be a primary cause of decreased HRV at rest and during sleep (Camm et al., 1996). Earlier studies reported that 92% of VLF power was suppressed by high dose atropine (0.04 mg/kg) (Taylor et al., 1998). DC has been developed to measure the rapid increase in R-R intervals caused only by parasympathetic control (Kantelhardt et al., 2007). The α1 increases with atropine and decreases with parasympathetic activation (Tulppo et al., 2001, 2005), although it decreases with increased levels of circulating noradrenaline in healthy men (Tulppo et al., 2001) and increases with β-blocker therapy in patients with heart failure (Lin et al., 2001; Ridha et al., 2002). Acv is thought to reflect a reflex parasympathetic function and its decrease indicates blunted parasympathetic responses to sleep apnea episodes (transient hypoxia, arousal, etc.) (Hayano et al., 2017). Acv is almost completely abolished by 2 mg of intravenous atropine but is unchanged by 5 mg of intravenous propranolol (Guilleminault et al., 1984). These indicate that decreased HRV or HR responsiveness mediated primarily by parasympathetic dysfunction is the most important single feature associated with mortality risk in post-AMI patients with and without low LVEF.
Third, the observation that mortality risk in patients with low LVEF was best predicted by the combinations of indices both in Cluster 1 or those in Cluster 1 and 3 indicates an increased risk of the coexistence of tonic/sustained and reflex parasympathetic disfunction. All of the top five combinations included Acv that reflects a reflex parasympathetic function. The other indices including those in Cluster 3 are thought to reflect the tonic or sustained level of parasympathetic function.
Fourth, the observation that mortality risk in patients without low LVEF was best predicted by the combinations of indices in Cluster 1 and 2 indicates an increased risk of the coexistence of decreased HRV or HR responsiveness and increased abrupt large HR changes. The λ25s reflects the fatness of tails of the probability density function of the magnitude of abrupt large HR changes. Its increase can occur when the relative frequency of large abrupt HR changes to smaller HR changes increases, suggesting the involvement of transient strong sympathetic activations (Kiyono et al., 2008, 2012). The λ is increased in patients with heart failure and the level of increase is associated with mortality risk, while no other HRV or HR dynamics indices predict it (Kiyono et al., 2007). The λ reflects the relative frequency of large abrupt HR changes but does not depend on the magnitude of HR change itself. Thus, this index could detect relative sympathetic overactivity even under the situation of reduces autonomic responsiveness.
Finally, the different predictive values of Cluster 2 predictor (λ25s) between patients with and without low LVEF may be explained by the presence of overt or subclinical heart failure. In patients with low LVEF, the prognostic value of the indices of sympathetic overactivity could be less because sympathetic nerve activity is increased by heart failure, which most of these patients may have. In patients without low LVEF, the indices of sympathetic overactivity could have greater predictive value because it may reflect the presence or development of heart failure in a part of these patients.
Limitations
Among Cluster 1 predictors, Acv was the best univariate predictor of post-AMI mortality, but this measure requires a cyclic variation of HR associated with sleep apnea episodes. Nevertheless, Acv was able to be calculated in all post-AMI patients. This is because the Holter ECG data having sleep period (time in bed) <3 h were not included in this study and because Acv can be calculated even in patients with subclinical sleep apnea if at least one episode of cyclic variation of HR is detected during sleep. Assuming cases in which Acv cannot be calculated, we examined logistic regression models excluding Acv, but the results for the relationships between clusters and mortality risk did not change (data not shown). Additionally, although study participants were recruited from four different clinical sites in diverse regions of the US, this study was performed using only one cohort of post-AMI patients. To confirm the present findings, future studies using different cohorts should be performed.
Conclusion
We investigated whether the survival predictors of HRV and HR dynamics depend on LVEF after AMI. The mortality risk in post-AMI patients with low LVEF is predicted by indices that reflect decreased HRV or HR responsiveness and cardiac parasympathetic dysfunction, whereas in patients without low LVEF, the risk is predicted by a combination of predictors reflecting decreased HRV or HR responsiveness and increased abrupt large HR changes suggesting sympathetic involvement.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data from this study are available upon request to the corresponding author. As the data contain potentially identifying or sensitive patient information, the use of the data is limited to the purpose and method of research approved by the ethics committees of the corresponding clinical sites. Requests to access these datasets should be directed to Junichiro Hayano, hayano@acm.org.
Ethics Statement
The studies involving human participants were reviewed and approved by the research ethics committees of Washington University, St. Louis, Missouri; Duke University, Durham, North Carolina; Harvard University, Boston, Massachusetts; and Yale University, New Haven, Connecticut. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JH and EY: conceptualization. JH: methodology, software, and writing—original draft preparation and visualization. EY, NU, and MK: validation. EY: formal analysis. MK and NU: investigation. RC and JB: resources, supervision, and funding acquisition. NU: data curation. EY and JB: writing—review and editing. JH and JB: project administration. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Lisa Berkman at the Harvard T. H. Chan School of Public Health for providing data from the Yale University clinical site for our analysis.
Abbreviations
- Acv
amplitude of cyclic variation of heart rate
- ALLSTAR
Allostatic State Mapping by Ambulatory ECG Repository
- AMI
acute myocardial infarction
- DC
deceleration capacity
- ENRICHD
Enhancing Recovery in Coronary Heart Disease
- Fcv
frequency of cyclic variation of heart rate
- HR
heart rate
- HRF
heart rate fragmentation
- HRV
heart rate variability
- SDNN
standard deviation of normal-to-normal R-R interval
- VLF
very low frequency.
Footnotes
Funding. This research was supported by the Grants HL 093374, HL080664, and HL58946 from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, Maryland.
References
- Adabag A. S., Therneau T. M., Gersh B. J., Weston S. A., Roger V. L. (2008). Sudden death after myocardial infarction. JAMA 300 2022–2029. 10.1001/jama.2008.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antman E. M., Anbe D. T., Armstrong P. W., Bates E. R., Green L. A., Hand M., et al. (2004). ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J. Am. Coll. Cardiol. 44 671–719. 10.1016/j.jacc.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Arevalo H. J., Vadakkumpadan F., Guallar E., Jebb A., Malamas P., Wu K. C., et al. (2016). Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat. Commun. 7:11437. 10.1038/ncomms11437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversano T., Aversano L. T., Passamani E., Knatterud G. L., Terrin M. L., Williams D. O., et al. (2002). Thrombolytic therapy vs primary percutaneous coronary intervention for myocardial infarction in patients presenting to hospitals without on-site cardiac surgery: a randomized controlled trial. JAMA 287 1943–1951. 10.1001/jama.287.15.1943 [DOI] [PubMed] [Google Scholar]
- Bauer A., Kantelhardt J. W., Barthel P., Schneider R., Makikallio T., Ulm K., et al. (2006). Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet 367 1674–1681. [DOI] [PubMed] [Google Scholar]
- Beck A. T. (1972). Depression: Causes and Treatment. Philadelphia, PA: University of Pennsylvania Press. [Google Scholar]
- Berger R. D., Saul J. P., Cohen R. J. (1989). Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am. J. Physiol. 256 H142–H152. [DOI] [PubMed] [Google Scholar]
- Berkman L. F., Blumenthal J., Burg M., Carney R. M., Catellier D., Cowan M. J., et al. (2003). Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 289 3106–3116. 10.1001/jama.289.23.3106 [DOI] [PubMed] [Google Scholar]
- Berntson G. G., Bigger J. T., Jr., Eckberg D. L., Grossman P., Kaufmann P. G., Malik M., et al. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 34 623–648. [DOI] [PubMed] [Google Scholar]
- Bigger J. T., Jr., Fleiss J. L., Steinman R. C., Rolnitzky L. M., Kleiger R. E., Rottman J. N. (1992). Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 85 164–171. [DOI] [PubMed] [Google Scholar]
- Camm A. J., Malik M., Bigger J. T., Jr., Breithardt G., Cerutti S., Cohen R. J., et al. (1996). Task Force of the european society of cardiology and the north american society of pacing and electrophysiology. heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93 1043–1065. [PubMed] [Google Scholar]
- Costa M. D., Davis R. B., Goldberger A. L. (2017). Heart rate fragmentation: a new approach to the analysis of cardiac interbeat interval dynamics. Front. Physiol. 8:255. 10.3389/fphys.2017.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. D., Redline S., Davis R. B., Heckbert S. R., Soliman E. Z., Goldberger A. L. (2018). Heart rate fragmentation as a novel biomarker of adverse cardiovascular events: the multi-ethnic study of atherosclerosis. Front. Physiol. 9:1117. 10.3389/fphys.2018.01117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger Z., Lampert R. (2006). Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA 295 809–818. 10.1001/jama.295.7.809 [DOI] [PubMed] [Google Scholar]
- Guilleminault C., Connolly S., Winkle R., Melvin K., Tilkian A. (1984). Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet 1 126–131. [DOI] [PubMed] [Google Scholar]
- Hayano J., Kisohara M., Ueda N., Yuda E. (2020). Impact of Heart Rate Fragmentation on the Assessment of Heart Rate Variability. Appl. Sci. 10:3314. [Google Scholar]
- Hayano J., Kiyono K., Struzik Z. R., Yamamoto Y., Watanabe E., Stein P. K., et al. (2011a). Increased non-gaussianity of heart rate variability predicts cardiac mortality after an acute myocardial infarction. Front. Physiol. 2:65. 10.3389/fphys.2011.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano J., Watanabe E., Saito Y., Sasaki F., Fujimoto K., Nomiyama T., et al. (2011b). Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ. Arrhythm. Electrophysiol. 4 64–72. 10.1161/CIRCEP.110.958009 [DOI] [PubMed] [Google Scholar]
- Hayano J., Yasuma F., Watanabe E., Carney R. M., Stein P. K., Blumenthal J. A., et al. (2017). Blunted cyclic variation of heart rate predicts mortality risk in post-myocardial infarction, end-stage renal disease, and chronic heart failure patients. Europace 19 1392–1400. 10.1093/europace/euw222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huikuri H. V., Makikallio T. H., Peng C. K., Goldberger A. L., Hintze U., Moller M. (2000). Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 101 47–53. [DOI] [PubMed] [Google Scholar]
- Hull S. S., Jr., Evans A. R., Vanoli E., Adamson P. B., Stramba-Badiale M., Albert D. E., et al. (1990). Heart rate variability before and after myocardial infarction in conscious dogs at high and low risk of sudden death. J. Am. Coll. Cardiol. 16 978–985. [DOI] [PubMed] [Google Scholar]
- Hull S. S., Jr., Vanoli E., Adamson P. B., Verrier R. L., Foreman R. D., Schwartz P. J. (1994). Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation 89 548–552. [DOI] [PubMed] [Google Scholar]
- Iyengar N., Peng C. K., Morin R., Goldberger A. L., Lipsitz L. A. (1996). Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am. J. Physiol. 271 R1078–R1084. [DOI] [PubMed] [Google Scholar]
- Kantelhardt J. W., Bauer A., Schumann A. Y., Barthel P., Schneider R., Malik M., et al. (2007). Phase-rectified signal averaging for the detection of quasi-periodicities and the prediction of cardiovascular risk. CHAOS 17:015112 10.1063/1.2430636 [DOI] [PubMed] [Google Scholar]
- Kiyono K., Hayano J., Kwak S., Watanabe E., Yamamoto Y. (2012). Non-gaussianity of low frequency heart rate variability and sympathetic activation: lack of increases in multiple system atrophy and Parkinson disease. Front. Physiol. 3:34. 10.3389/fphys.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono K., Hayano J., Watanabe E., Struzik Z. R., Yamamoto Y. (2008). Non-Gaussian heart rate as an independent predictor of mortality in patients with chronic heart failure. Heart Rhythm. 5 261–268. [DOI] [PubMed] [Google Scholar]
- Kiyono K., Struzik Z. R., Aoyagi N., Sakata S., Hayano J., Yamamoto Y. (2004). Critical scale invariance in a healthy human heart rate. Phys. Rev. Lett. 93:178103. [DOI] [PubMed] [Google Scholar]
- Kiyono K., Struzik Z. R., Yamamoto Y. (2007). Estimator of a non-Gaussian parameter in multiplicative log-normal models. Phys. Rev. E. 76:041113. [DOI] [PubMed] [Google Scholar]
- Kleiger R. E., Miller J. P., Bigger J. T., Jr., Moss A. J. (1987). Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 59 256–262. [DOI] [PubMed] [Google Scholar]
- La Rovere M. T., Bigger J. T., Jr., Marcus F. I., Mortara A., Schwartz P. J. (1998). Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351 478–484. [DOI] [PubMed] [Google Scholar]
- Laborde S., Mosley E., Thayer J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Frontiers in psychology. 8:213. 10.3389/fpsyg.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza G. A., Guido V., Galeazzi M. M., Mustilli M., Natali R., Ierardi C., et al. (1998). Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am. J. Cardiol. 82 1323–1328. 10.1016/s0002-9149(98)00635-3 [DOI] [PubMed] [Google Scholar]
- Lin L. Y., Lin J. L., Du C. C., Lai L. P., Tseng Y. Z., Huang S. K. (2001). Reversal of deteriorated fractal behavior of heart rate variability by beta-blocker therapy in patients with advanced congestive heart failure. J. Cardiovasc. Electrophysiol. 12 26–32. [DOI] [PubMed] [Google Scholar]
- Liu X., Xiang L., Tong G. (2020). Predictive values of heart rate variability, deceleration and acceleration capacity of heart rate in post-infarction patients with LVEF >/=35. Ann. Nonin. Electrocardiol. 25:e12771. 10.1111/anec.12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F., Sandrone G., Mortara A., Torzillo D., La Rovere M. T., Signorini M. G., et al. (1996). Linear and nonlinear dynamics of heart rate variability after acute myocardial infarction with normal and reduced left ventricular ejection fraction. Am. J. Cardiol. 77 1283–1288. 10.1016/s0002-9149(96)00193-2 [DOI] [PubMed] [Google Scholar]
- Moss A. J., Zareba W., Hall W. J., Klein H., Wilber D. J., Cannom D. S., et al. (2002). Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 346 877–883. 10.1056/NEJMoa013474 [DOI] [PubMed] [Google Scholar]
- Peng C. K., Havlin S., Stanley H. E., Goldberger A. L. (1995). Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. CHAOS 5 82–87. 10.1063/1.166141 [DOI] [PubMed] [Google Scholar]
- Ridha M., Makikallio T. H., Lopera G., Pastor J., de Marchena E., Chakko S., et al. (2002). Effects of carvedilol on heart rate dynamics in patients with congestive heart failure. Ann. Nonin. Electrocardiol. 7 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul J. P., Albrecht P., Berger R. D., Cohen R. J. (1988). Analysis of long term heart rate variability: methods, 1/f scaling and implications. Comput. Cardiol. 14 419–422. [PubMed] [Google Scholar]
- Schleifer S. J., Macari-Hinson M. M., Coyle D. A., Slater W. R., Kahn M., Gorlin R., et al. (1989). The nature and course of depression following myocardial infarction. Arch. Intern. Med. 149 1785–1789. [PubMed] [Google Scholar]
- Solomon S. D., Zelenkofske S., McMurray J. J., Finn P. V., Velazquez E., Ertl G., et al. (2005). Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 352 2581–2588. 10.1056/NEJMoa043938 [DOI] [PubMed] [Google Scholar]
- Steeds R. P., Bickerton D., Smith M. J., Muthusamy R. (2004). Assessment of depression following acute myocardial infarction using the Beck depression inventory. Heart 90 217–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. A., Carr D. L., Myers C. W., Eckberg D. L. (1998). Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 98 547–555. 10.1161/01.cir.98.6.547 [DOI] [PubMed] [Google Scholar]
- Tulppo M. P., Kiviniemi A. M., Hautala A. J., Kallio M., Seppanen T., Makikallio T. H., et al. (2005). Physiological background of the loss of fractal heart rate dynamics. Circulation 112 314–319. 10.1161/CIRCULATIONAHA.104.523712 [DOI] [PubMed] [Google Scholar]
- Tulppo M. P., Makikallio T. H., Seppanen T., Shoemaker K., Tutungi E., Hughson R. L., et al. (2001). Effects of pharmacological adrenergic and vagal modulation on fractal heart rate dynamics. Clin. Physiol. 21 515–523. [DOI] [PubMed] [Google Scholar]
- Virani S. S., Alonso A., Benjamin E. J., Bittencourt M. S., Callaway C. W., Carson A. P., et al. (2020). Heart Disease and stroke statistics-2020 update: a report from the american heart association. Circulation 141 e139–e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Watanabe E., Kiyono K., Yamamoto Y., Hayano J. (2016). “Heart rate variability and cardiac diseases,” in Clinical Assessment of the Autonomic Nervous System, eds Iwase S., Hayano J., Orimo S. (Berlin: Springer; ), 163–178. [Google Scholar]
- Yuda E., Ueda N., Kisohara M., Hayano J. (2020). Redundancy among risk predictors derived from heart rate variability and dynamics: ALLSTAR big data analysis. Ann. Nonin. Electrocardiol. 2:e12790. 10.1111/anec.12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S., Kovoor P. (2014). Sudden cardiac death early after myocardial infarction: pathogenesis, risk stratification, and primary prevention. Circulation 129 2426–2435. 10.1161/CIRCULATIONAHA.113.007497 [DOI] [PubMed] [Google Scholar]
- Zuanetti G., Neilson J. M., Latini R., Santoro E., Maggioni A. P., Ewing D. J. (1996). Prognostic significance of heart rate variability in post-myocardial infarction patients in the fibrinolytic era. The GISSI-2 results. Gruppo Italiano per lo Studio della Sopravvivenza nell’. Infarto Miocardico. Circulat. 94 432–436. 10.1161/01.cir.94.3.432 [DOI] [PubMed] [Google Scholar]
- Zwillich C., Devlin T., White D., Douglas N., Weil J., Martin R. (1982). Bradycardia during sleep apnea. Characteristics and mechanism. J. Clin. Invest. 69 1286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data from this study are available upon request to the corresponding author. As the data contain potentially identifying or sensitive patient information, the use of the data is limited to the purpose and method of research approved by the ethics committees of the corresponding clinical sites. Requests to access these datasets should be directed to Junichiro Hayano, hayano@acm.org.