Abstract
Members of the solute carrier (SLC) family of transporters are responsible for the cellular influx of a broad range of endogenous compounds and xenobiotics. These proteins are highly expressed in the gastrointestinal tract and eliminating organs such as the liver and kidney, and are considered to be of particular importance in governing drug absorption and elimination. Many of the same transporters are also expressed in a wide variety of organs targeted by clinically important anticancer drugs, directly affect cellular sensitivity to these agents, and indirectly influence treatment-related side effects. Furthermore, targeted intervention strategies involving the use of transport inhibitors have been recently developed, and have provided promising lead candidates for combinatorial therapies associated with decreased toxicity. Gaining a better understanding of the complex interplay between transporter-mediated on-target and off-target drug disposition will help guide the further development of these novel treatment strategies to prevent drug accumulation in toxicity-associated organs, and improve the safety of currently available treatment modalities. In this report, we provide an update on this rapidly emerging field with particular emphasis on anticancer drugs belonging to the classes of taxanes, platinum derivatives, nucleoside analogs, and anthracyclines.
Keywords: Adverse drug reactions, Anticancer, Solute carrier, Toxicity
1. Introduction
The expression and localization of transporters within specific tissues contributes to a dynamic interplay between intracellular substrate concentrations and the extracellular environment of various cell types. Disruption of this sensitive balance has the potential to modify intracellular accumulation of xenobiotics and may contribute to increases in the incidence and severity of tissue-specific organ damage. In this article, we will focus on the contribution of solute carrier (SLC) family members to the initial cellular influx of substrates and how these proteins can directly contribute to toxicities associated with small-molecule oncology drugs. Of note, transporters involved in cellular efflux, such as members of the ATP-binding cassette (ABC) family, might also play a role in toxicities associated with the same drugs. However, these proteins are predicted to mitigate the risk of toxicity rather than precipitating toxicity, and this area is beyond the scope of the present review. Rather than presenting a comprehensive overview of the field, we aimed in this article to highlight examples of well-established toxicities associated with commonly used anticancer drugs that are dependent on specific solute carriers in order to stimulate discussion within the transporter community and further advance the field.
1.1. Transporter Function
A selectively permeable plasma membrane is a ubiquitous feature of all life forms (Grecco et al. 2011; Singer and Nicolson 1972), and membrane transporters are the key regulators of this selective cellular permeability (Kaback et al. 2001). These proteins mediate the uptake and efflux of many endogenous metabolites such as amino acids, nucleosides, sugars, as well as many dietary compounds and therapeutic agents (Borst and Elferink 2002). Therefore, along with their essential contribution to normal physiology and pathophysiology, membrane transporters are also key determinants of therapeutic responses to drugs, including unwanted adverse events.
The human genome encodes more than 400 membrane-transporter genes belonging to two major super-families: ABC transporters and SLC transporters, which are involved in most essential biological processes (Borst and Elferink 2002; He et al. 2009; Hediger et al. 2013; Nigam 2015). Among these, about 20 “multi-specific” transporters belonging to either super-family have been widely implicated in drug transport (DeGorter et al. 2012; Giacomini et al. 2012; Nigam 2015). Tissue types that are involved in the absorption, distribution, metabolism, and excretion (ADME), such as the kidney, liver, intestine, and endothelial barriers, have high expression of transporters that accumulate substrates within these organ types (International Transporter Consortium et al. 2010). At the cellular level, transporter-mediated uptake or efflux can involve drug sensitive or resistant (Borst et al. 2000) phenotypes in target cells, thereby affecting therapeutic efficacy. Conversely, transporter-mediated drug accumulation in non-target cells can contribute to drug toxicity profiles (Sprowl and Sparreboom 2014). Consequently, drug transporters, in addition to drug-metabolizing enzymes, have emerged in recent years to be critical determinants of drug disposition, therapeutic efficacy, toxicity profiles, and drug–drug.
1.2. Role of SLCs in Toxicity
Increasing evidence has confirmed that SLC expression and localization at non-target tissues can play an important role in drug distribution and subsequent toxicity profiles (Sprowl and Sparreboom 2014; Yang and Han 2019). In addition, the ability of drugs to compete for the natural substrates of these transporters can potentially lead to altered cellular function and trigger unwanted adverse reactions. Since virtually all currently used oncology drugs can cause severe dose-limiting toxic side effects and, in some cases, cause life-threatening toxicities associated with organ damage, knowledge of specific SLCs that recognize such drugs can theoretically contribute to the development of improved and safer treatment strategies. Furthermore, the contribution of SLCs to observed differences in chemotherapeutic clinical response rates and toxicity profiles between genders remains poorly understood. The identification of pharmacodynamic biomarkers of SLC function that can potentially guide treatment decisions has led to the design of strategies involving the administration of concurrent medications that ameliorate the incidence and/or severity of these side effects. Besides a direct contribution of SLCs to the tissue-specific uptake of anticancer drugs, these proteins can also indirectly contribute to altered drug distribution patterns due to their involvement in clearance mechanisms in organs of elimination such as the liver and kidney. In the last few decades, technological advances in cloning have resulted in the identification of several important SLC families mediating the transport of organic cations and organic anions, the so-called organic cation transporters (OCTs), organic anion transporters (OATs), and organic anion transporting polypeptides (OATPs).
1.2.1. Organic Cation Transport
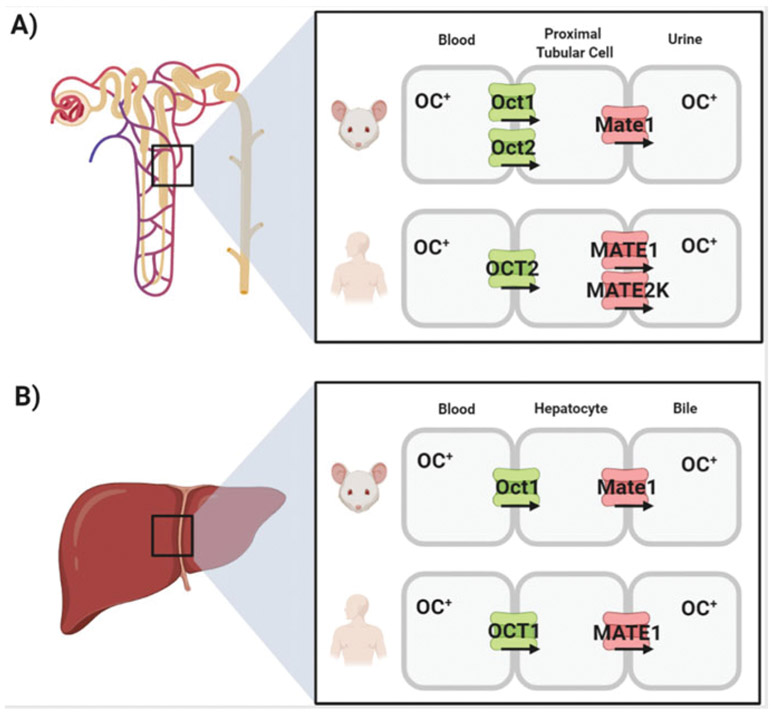
About 40% of approved prescription drugs are positively charged at neutral pH (“organic cations”), and the membrane transport of these agents depends on facilitated carriers. In recent years, considerable progress has been made in the study of transporters belonging to the OCT family. Subsequent studies in heterologous expression models have confirmed that members of this transporter family mediate the cellular uptake of many structurally diverse endogenous compounds and an increasingly large number of cationic anticancer drugs. The organic cation transporters OCT1 (SLC22A1) and OCT2 (SLC22A2) have particular relevance in this connection, since they are highly expressed at the basolateral membranes of hepatocytes and renal tubular cells, respectively, and these proteins are considered major transporters in the secretion of organic cations from the circulation into the liver and kidney. Consequently, OCT1 and OCT2 facilitate the hepatocellular and renal excretion of organic cationic compounds and play an important role in governing systemic elimination of many drugs. The contribution of OCTs to hepatic and renal organic cation secretion was first conclusively demonstrated from the clearance of the prototypic organic cation, tetraethylammonium (TEA), in mice from which Oct1, Oct2, or both were eliminated (Jonker et al. 2001, 2003). These studies demonstrated that the renal TEA clearance in the Oct1-null mouse [Oct1(−/−)] was actually increased, reflecting (1) reduced hepatic clearance, (2) elevated plasma concentrations of TEA, and (3) sufficient expression of Oct2 in the kidney to handle the increased plasma load. Renal TEA clearance in the Oct2-null mouse [Oct2(−/−)] was unchanged compared to control, reflecting sufficient expression of Oct1 in the murine kidney to efficiently clear TEA, even in the absence of Oct2. Most importantly, the elimination of both Oct1 and Oct2 in mice [Oct1/2(−/−)] completely eliminated active secretion of TEA. Although mice express substantial levels of both Oct1 and Oct2 in the kidney, in humans there is strong agreement that OCT2 dominates renal organic cation transport, whereas OCT1 dominates hepatic organic cation transport.
In the liver, a transporter belonging to the class of multidrug and toxin extrusion (MATE) proteins called MATE1 (SLC47A1) is localized at the bile-canalicular membrane of hepatocytes, and forms a functional unit with the basolaterally expressed OCT1 to mediate the secretion of many cationic drugs from the circulation across the hepatocyte into the bile (Fig. 1). In the luminal membrane of the proximal tubular epithelium, MATE1 cooperates with the basolaterally expressed OCT2 in the vectorial renal secretion of organic cations (Fig. 1). Previously reported experimental data indicate that the tubular secretion of several dual OCT2/MATE1 substrates, including cisplatin (Franke et al. 2010b) and oxaliplatin (Sprowl et al. 2013a), is decreased in Oct1/2(−/−) mice, but not in Oct1(−/−) and Oct2(−/−) mice (Filipski et al. 2009), as well as in Mate1 knockout [Mate1(−/−)] mice (Li et al. 2013; Nakamura et al. 2010). The contribution of these transporters to the hepatic and renal handling of xenobiotics in mammals has been most extensively studied for the biguanide analog metformin, a first-line medication for the treatment of type 2 diabetes that has intrinsic anticancer and chemo-preventative properties as well (Chae et al. 2016; Heckman-Stoddard et al. 2016). These studies have demonstrated that OCT1 mediates >75% of metformin uptake into hepatocytes, and that OCT2 (Oct1/2 in mice) mediates >60% of metformin uptake into renal tubular cells (Higgins et al. 2012). Consequently, the hepatic exposure of metformin is decreased ~4–8 fold in Oct1(−/−) mice (Shu et al. 2007; Wang et al. 2002), decreased 4.2-fold in Oct1/2 (−/−) mice (Higgins et al. 2012), and increased 8.4-fold in Mate1(−/−) mice (Li et al. 2013). In the case of Oct1/2- or Mate1-deficiency, this resulted in significantly altered systemic exposure to metformin (Higgins et al. 2012; Tsuda et al. 2009).
Fig. 1.
Schematic depiction of the vectorial transport of organic cations (OC+) in the kidney (a) and liver (b) of rodents and humans
Because hepatic uptake is required for inhibition of gluconeogenesis, the pharmacodynamic properties of metformin are markedly attenuated in Oct1(−/−) mice resulting in higher fasting plasma glucose levels (Shu et al. 2007). This is consistent with prior findings that several well-documented, relatively common loss-of-function variants in OCT1 are associated with a decreased glycemic response resulting in higher blood glucose levels in patients with type 2 diabetes (McCreight et al. 2016), and with altered efficacy in patients with cancer (Joerger et al. 2015), but not with the steady-state pharmacokinetics of metformin (Christensen et al. 2015; Stage et al. 2015a). Studies in dizygotic and monozygotic twin pairs have confirmed that the impact of reduced-function alleles in OCT1 on the pharmacokinetics of metformin in humans is of minor clinical importance (Stage et al. 2015b). In contrast, genetic variations in OCT2, MATE1, and MATE2-K, a related transporter expressed in human but not rodent kidney, have been shown to significantly affect the pharmacokinetics of metformin (Pawlyk et al. 2014; Wang and Weinshilboum 2014). These studies are consistent with the known physicochemical properties of metformin dictating that its elimination is predominantly by renal excretion (up to 90% of the dose), with a negligible contribution from liver metabolism or biliary secretion, despite the expression of MATE1 on the canalicular membrane of hepatocytes (Jensen et al. 2016). Moreover, both human and rodent OCT2 have about a 10- and 100-fold greater capacity to transport metformin as compared with OCT1 (Kimura et al. 2005). On this basis, metformin is recommended by regulatory agencies such as the FDA as a probe for determining OCT2-mediated transport when investigating possible drug interactions with new chemical entities, including anticancer drugs. As such, a sound mechanistic understanding of pharmacokinetic interactions with metformin is considered of high clinical importance. It should be pointed out that interactions involving altered levels within the kidney are not necessarily reflected in an equivalent change in systemic exposure (Sprowl and Sparreboom 2014). Nonetheless, published studies have demonstrated that many known OCT2 inhibitors, including cimetidine, dolutegravir (Song et al. 2016; Zong et al. 2014), ranitidine (Cho et al. 2014), and verapamil (Cho and Chung 2016) can significantly increase the area under the curve (AUC) of metformin (Koepsell 2015). Furthermore, it has been recommended that dose adjustments of metformin be considered to maintain optimal glycemic control when patients are starting/stopping these agents while taking metformin (Song et al. 2016). The clinical impact of these interactions is further supported by the recent finding in a population of >400,000 incidental metformin users that the risk of early therapy discontinuation, as a proxy for intolerance, is associated with the concomitant use of drugs that are known to inhibit OCT2 and/or MATE1 (Stage et al. 2016).
1.2.2. Organic Anion Transport
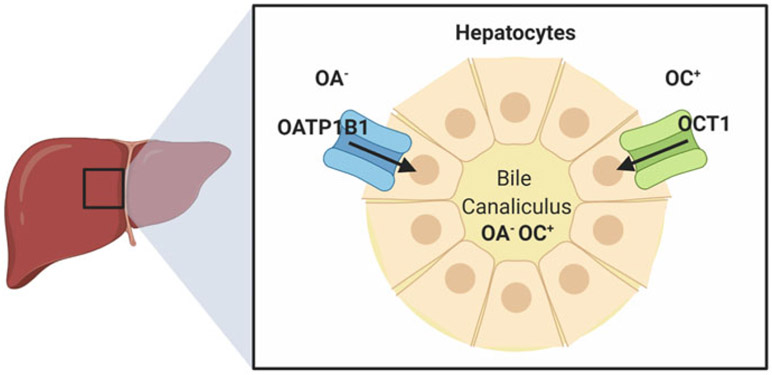
In the liver, two transporters belonging to the class of OATPs called OATP1B1 (SLCO1B1) and OATP1B3 (SLCO1B3; Fig. 2) are localized at basolateral membrane of hepatocytes and mediate the uptake of a remarkably broad range of substrates from the circulation across the outer membrane of hepatocytes. These agents include charged organic anions (e.g., methotrexate and statins), endogenous and xenobiotic glucuronides (e.g., of bilirubin and sorafenib), charged organic cations (e.g., tyrosine kinase inhibitors such as imatinib), polar zwitterions (e.g., fexofenadine), uncharged hydrophobic agents (e.g., taxanes such as paclitaxel), as well as chemically diverse platinum-based therapeutics (e.g., cisplatin) (Lancaster et al. 2013). The in vivo pharmacological characterization of this transport mechanism was first performed in mice knockout for the orthologue transporter Oatp1b2 [Oatp1b2(−/−)] (Zaher et al. 2008) with the prototypical substrate pravastatin, a member of the class of statins used for the treatment of dyslipidemia and the prevention of cardiovascular disease. This initial work demonstrated that the liver-to-plasma ratios of pravastatin were reduced by four to fivefold in Oatp1b2(−/−) mice, which was independently verified in another Oatp1b2-deficient mouse strain (Salphati et al. 2014). Subsequent investigation demonstrated that 95–99% of pravastatin uptake into the liver is mediated by OATP1B1 (Izumi et al. 2018), and that the altered liver-to-plasma ratios of pravastatin, as well as other substrates (Zimmerman et al. 2013), observed in Oatp1b2(−/−) mice can be at least partially restored in humanized transgenic animals with liver-specific expression of OATP1B1 (Salphati et al. 2014).
Fig. 2.
Schematic depiction of the transport of organic anions (OA−) and organic cations (OC+) in human hepatocytes by OCT1 and OATP1B1
Historically, the two major transport families contributing the renal secretion of organic compounds were divided into two different groups named “organic cation system” and “organic anion system”; classical substrate for the former included TEA, which was inhibited by cimetidine, while the latter was shown to efficiently transport p-aminohippurate (PAH) and inhibited by probenecid (Hagenbuch 2010). It was not until the late 1990s that the transport systems responsible for the organic anion systems in the kidney were identified as OAT1 (SLC22A3) and OAT3 (SLC22A5) (VanWert et al. 2010). Even though early studies showed that organic anions such as pyrazionate and PAH did not impact the accumulation of organic cations such as cisplatin in renal cortex slices (Safirstein et al. 1984), recent in vivo investigation suggested an correlation between the organic anion system disruption and the development of cisplatin nephrotoxicity. Of note, a number of studies have reported that the classical organic anion inhibitor, probenecid, can reduce the tubular secretion of total platinum after cisplatin administration in rats (Osman and Litterst 1983), rabbits (Caterson et al. 1983), dogs (Klein et al. 1991), and humans (Jacobs et al. 1984), and can partially protect against cisplatin nephrotoxicity in mice (Ban et al. 1994; Ross and Gale 1979). Similar findings have been reported for furosemide (Daley-Yates and McBrien 1985), an agent now known, like probenecid, to be an inhibitor of OAT1/OAT3 (Hosoyamada et al. 1999; Kusuhara et al. 1999; Lu et al. 1999; Vallon et al. 2008).
2. Toxicity Induced by Chemotherapeutics
2.1. OATPs and Paclitaxel-Induced Peripheral Neuropathy
Paclitaxel is a taxane antineoplastic agent that elicits its antitumor effects by disrupting the microtubule dynamics and causing mitotic arrest and cell death in a variety of tumor types. Paclitaxel remains among the most widely used drugs in the treatment of a variety of solid tumors, including early-stage breast cancer, but its clinical use is associated with debilitating damage to peripheral nerves (neuropathy). This damage is a tremendous health problem worldwide and remains one of the most important complications of contemporary oncology regimens as it may limit further use of curative-intent treatment and/or may cause long-term quality of life concerns. Since the majority of patients with cancer receiving paclitaxel-based chemotherapy are at high risk of experiencing peripheral neuropathy, a large community of cancer survivors could potentially benefit from the SLC contributing to this chemotherapy-induced toxicity.
Several tubulin poisons, including paclitaxel, induce a chronic, dose-dependent sensory peripheral neuropathy that is characterized by tingling, numbness, increased sensitivity to cold and touch, and burning pain of the distal extremities. The incidence of this side effect is particularly high in the case of paclitaxel, as it occurs in up to 70–80% in patients with breast cancer (De Iuliis et al. 2015). With continued dosing, the painful symptoms increase in severity and can persist for years (Peters et al. 2007), or even cause a lifelong functional impairment that impacts quality of life (Mielke et al. 2006). The mechanistic basis of this side effect has remained uncertain until relatively recently (Carozzi et al. 2015). Previously, studies have reported that paclitaxel is able to induce injury to sensory neurons resulting in morphological and biochemical changes in the dorsal root ganglion (DRG) satellite glial cells, proliferation of macrophages within the peripheral nervous system, activation and increases within the microglial and astrocyte populations within the spinal cord (Brewer et al. 2016; Marmiroli and Cavaletti 2016). Additionally, paclitaxel also causes acute pain syndrome in patients that precipitates within 1–3 days of paclitaxel administration and resides within 1 week. This acute pain syndrome has been postulated to be caused by the activation of toll-like receptor 4 (TLR4) in the DRG and spinal dorsal horn (Yan et al. 2015), which has been shown to be the main site of paclitaxel accumulation within the nervous system (Cavaletti et al. 2000).
The notion that the DRG neurons play a central role in the elicit side effects associated with paclitaxel chemotherapy is supported by the rationale that paclitaxel has easy accessibility to the DRG and subsequent accumulation which is supported by reports of detectable levels of paclitaxel in the spinal cord and sciatic nerve, presumably mediated by the transport along the centripetal and centrifugal branches of the axon in DRGs (Cavaletti et al. 2000). Furthermore, previous cellular uptake studies have demonstrated that paclitaxel, along with other structurally related taxanes such as docetaxel, accumulates via a facilitated transport mechanism (Smith et al. 2005). Consequently, after administration of paclitaxel, tissue disposition patterns and resulting pathological changes are restricted to cell types that are capable of transporting the movement of paclitaxel from the extracellular environment. This is consistent with accumulating evidence that the transmembrane transport of paclitaxel is mediated by specific OATPs. In particular, it has been reported that both paclitaxel and docetaxel are transported substrates of human OATP1B1 and OATP1B3 (Baker et al. 2009; de Graan et al. 2012; Smith et al. 2005, 2007), as well as the single functional homologue Oatp1b2 in mice (Nieuweboer et al. 2014) and rats (Franke et al. 2010a; Nieuweboer et al. 2014). These findings have been independently verified (Iusuf et al. 2015; Marada et al. 2015; Sun et al. 2016; van de Steeg et al. 2011, 2013), and are consistent with in vitro studies that have identified paclitaxel as a potent inhibitor of OATP1B1- (Gui et al. 2008, 2009) and OATP1B3-mediated transport (Gui et al. 2008; Letschert et al. 2006; Yamaguchi et al. 2008). However, none of the other known nine human OATPs are capable of transporting paclitaxel (Svoboda et al. 2011), and similar results have been obtained with docetaxel (Lee et al. 2015). Thus, the reported differing affinities highlight the notion that OATPs capable of transporting paclitaxel need to be expressed in tissues such that the drug can cross the plasma membrane and then exert cellular injury. In this context, it is noteworthy that the Oatp1b2 protein is expressed in mouse DRG (Sprowl et al. 2013a), and an independent study evaluating all 15 mouse OATPs confirmed expression of Oatp1b2 in murine neurons (Feurstein et al. 2010).
Comparative pharmacokinetic analysis after a clinically relevant dose of paclitaxel in wild-type mice and Oatp1b2(−/−) produced a modest increase in systemic exposure (Durmus et al. 2015). The minor change in systemic exposure of Oatp1b2-deficient animals to paclitaxel suggests rodents recapitulate pharmacokinetic observations in human patients and serves as an appropriate preclinical model (Nieuweboer et al. 2014). The absence of significant changes in systemic exposure also demonstrates that the genotype-dependent differences are unlikely to influence the extent of paclitaxel-induced peripheral neuropathy. In line with docetaxel studies (de Graan et al. 2012), the genetic deficiency of Oatp1b2 contributed to significant decreases in liver and DRG uptake of paclitaxel (Nieuweboer et al. 2014). The diminished accumulation of paclitaxel in DRGs from Oatp1b2-deficient mice demonstrates the preclinical utility of evaluating peripheral neuropathy, namely the Von Frey Hairs test, to assess mechanical allodynia (Boehmerle et al. 2014; Peters et al. 2007; Yan et al. 2015). After paclitaxel treatment, wild-type mice experience a 50% decrease in sensitivity to mechanical stimulation. Although several reports have suggested that metabolites of paclitaxel may also contribute to peripheral neuropathy (Sparreboom et al. 1995), this phenotype is casually related to paclitaxel itself as direct administration of clinically relevant concentrations of three major paclitaxel liver metabolites: 6α-hydroxy-paclitaxel, 3′-p-hydroxy-paclitaxel, and 6α,3′-p-dihydroxy-paclitaxel did not produce neuropathic pain, when compared to the parent drug. In contrast to wild-type mice, Oatp1b2 deficiency recapitulated mechanical sensitivity that resembled baseline values or vehicle-treated group, and is protected from acute paclitaxel-induced peripheral neuropathy (Leblanc et al. 2018). Thermal sensitivity and electrical nerve conductance were also preserved in Oatp1b2(−/−) after chronic treatment. In support of Oatp1b2’s functional involvement in accumulation of paclitaxel in DRGs, cabazitaxel, a taxane derivative approved for the treatment of prostate cancer, rarely causes peripheral neuropathy (Omlin et al. 2015) and is not transported by OATP1B-type transporters (Nieuweboer et al. 2014).
Various approaches have been proposed to predict, prevent, and/or treat paclitaxel-induced peripheral neuropathy (Scripture et al. 2006). The predictive strategies have predominantly focused on the search for hereditary biomarkers that could identify patients at increased risk of toxicity through candidate gene (Boora et al. 2016; Green et al. 2009; Hertz et al. 2012, 2013; Leskela et al. 2011; Sissung et al. 2006; Tanabe et al. 2017; Abraham et al. 2014; de Graan et al. 2013) or genome-wide association studies (Baldwin et al. 2012; Bergmann et al. 2013; Lam et al. 2016; Sucheston et al. 2011). However, the findings from these studies done to date have identified non-overlapping single or pathway biomarker associations that preclude immediate clinical implementation (Brewer et al. 2016; Schneider et al. 2015a, b; Frederiks et al. 2015; Hertz 2013). In addition, the decision to act on a toxicity biomarker is hampered in many diseases by the lack of available alternative treatments to replace paclitaxel and/or the need for a patient-tailored reduction in the paclitaxel dose to prevent toxicity, which will have negative effects on the disease management.
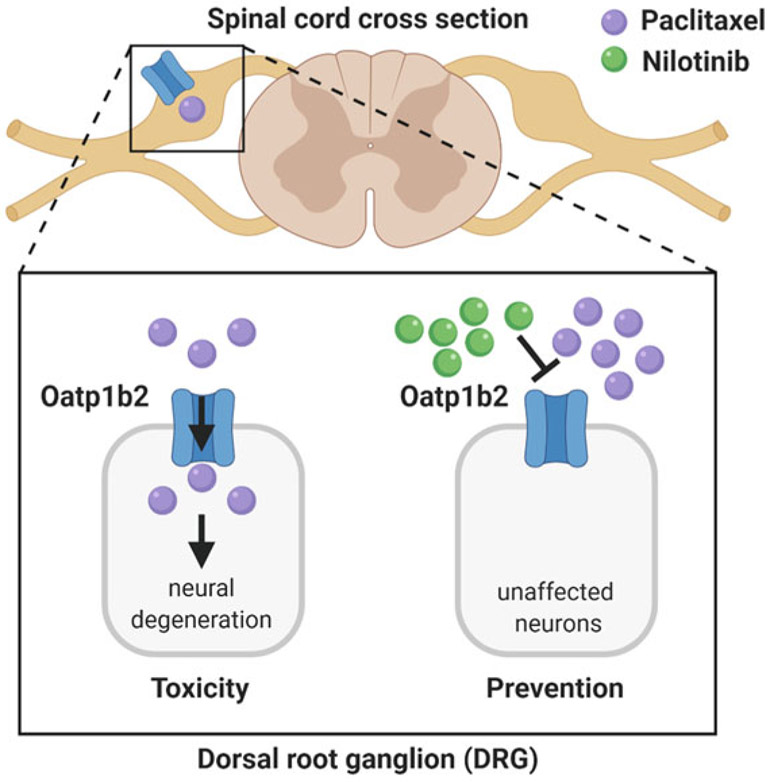
To date there have been more than 40 randomized controlled clinical trials of agents to prevent or treat peripheral neuropathy associated with paclitaxel, and these trials have not provided convincing evidence for a clinically beneficial agent (Hershman et al. 2014). One area of research currently being pursued is based on the hypothesis that agents with inhibitory properties toward OATP1B-type transporters could be exploited as neuro-protectants in conjunction with paclitaxel-based chemotherapy (Fig. 3). One of the candidate inhibitors is nilotinib, an inhibitor of the Bcr-Abl kinase used in the treatment of certain leukemias. Pretreatment with this agent protected against acute and chronic forms of paclitaxel-induced peripheral neuropathy in mice, without affecting the disposition properties of paclitaxel or its antitumor properties (Leblanc et al. 2018). The optimal pharmaceutical and pharmacological properties of nilotinib provide rationale for an excellent modulator of the off-target cellular disposition of paclitaxel and subsequent toxicities. Nilotinib is orally bioavailable and has a long half-life resulting from its relatively slow systemic clearance (Xia et al. 2012); this slow clearance will ensure sufficient nilotinib levels to modulate the cellular disposition of paclitaxel. Interestingly, high-dose TKI pulse-exposure, in contrast to a chronic low-dose daily exposure, is becoming a more well-received concept in the treatment of various cancer (Lipka et al. 2012). The increase of familiarity of clinicians with this high-dose pulse strategy of nilotinib will ultimately help facilitate the translation of our propose concept of utilizing nilotinib, as a transporter inhibitor, as adjunct therapy in paclitaxel-based chemotherapies to reduce toxicities. Interestingly, recent preclinical study of nilotinib-paclitaxel combination showed exquisite activity (Holbeck et al. 2017), resulting in tumor regressions in models of breast cancer xenografts with no tumor regrowth observed for more than 80 days following the end of therapy. Although confirmation of these initial findings is required, in patient-derived tumor models that more faithfully represent the characteristics of primary human breast cancer compared with xenografted cell lines (Hidalgo et al. 2014), these combined initial observations indicate that combining paclitaxel with inhibitors of OATP1B-type transporters such as nilotinib has the potential to simultaneously reduce toxicities and increase anticancer effects.
Fig. 3.
Proposed model of paclitaxel-induced injury to the peripheral nervous system. Murine Oatp1b2 mediates intracellular accumulation of paclitaxel, leading to peripheral neuropathy and neural degeneration (left). These effects can be blocked by the Oatp1b2 inhibitor nilotinib (right)
2.2. OCT2 and Oxaliplatin-Induced Peripheral Neurotoxicity
Oxaliplatin is a platinum-based chemotherapeutic that is widely used in the treatment of colorectal and gastric cancers, but its clinical use is associated with debilitating damage to peripheral nerves. This side effect remains one of the most important complications of contemporary oncology regimens as it may limit further treatment and/or may cause long-term quality of life concerns. The characteristic pattern of peripheral neurotoxicity associated with oxaliplatin affects up to 92% of patients (Argyriou et al. 2008), occurs immediately after infusion, and is characterized by cold-exacerbated paresthesia, muscle spasms, and fasciculations. Another distinctive feature of oxaliplatin-induced neurotoxicity is pharyngolaryngeal dysesthesia, an acute sensation of respiratory discomfort without any objective evidence of respiratory distress. Although these acute symptoms typically resolve within 1 week, at higher cumulative doses, oxaliplatin induces a dose-limiting sensory neurotoxicity that leads to functional impairment, which could last several months to even years following discontinuation of treatment or in more severe cases may display permanent, incomplete recovery (McWhinney et al. 2009).
The cellular and molecular mechanisms underlying oxaliplatin-induced neurotoxicity remain incompletely understood to this day. However, studies performed during the last decade have provided crucial insights into the pathophysiological events that contribute to the development of oxaliplatin-induced neurotoxicity. Unlike the central nervous system, the peripheral nervous system, consisting of nerves and ganglia outside of the brain and spinal cord, is not protected by the blood–brain barrier, and is particularly sensitive to oxaliplatin-induced injury. Within the peripheral nervous system, oxaliplatin accumulates and causes toxicity only in the sensory neurons, while the motor neurons are spared (Thompson et al. 1984). As with paclitaxel, the DRG has emerged as the major target of oxaliplatin-induced neurotoxicity (Cavaletti et al. 2001; McKeage et al. 2001), and both structural and functional alterations in the DRG are thought to be the major reason for the development of the observed toxicity (Jamieson et al. 2005). Morphological and anatomical examinations have determined key structural changes in the DRG during oxaliplatin treatment, including alterations in the nuclear/nucleolar morphology, selective atrophy of a subpopulation of cells, platinum-DNA adduct formation, and apoptosis (Cavaletti et al. 2001). The functional changes include sodium channel dysfunction, leading to altered peripheral-nerve excitability, as well as oxaliplatin-dependent changes in the function and expression of several ion channels in the DRG leading to increased nociception (Trevisan et al. 2013; Zhao et al. 2012). The ion channels are activated by mechanical, thermal, chemical, and noxious stimuli, and oxaliplatin-induced changes in their function and expression contribute to the sensory neuropathic symptoms observed in patients treated with oxaliplatin. Notably, oxaliplatin-induced neurotoxicity is not associated with axonal degeneration as observed in diabetic neuropathy, and damage to the DRG is believed to be causative in the development of neurotoxicity (Gregg et al. 1992).
The key event that triggers the development of pathological changes in the structure and function of DRG is the initial accumulation of oxaliplatin. Multiple studies have shown that oxaliplatin preferentially accumulates in DRG (McKeage et al. 2001), accounting for its selective toxicity to the peripheral sensory system. Unlike sensory neurons, the motor neurons are spared due to lack of oxaliplatin accumulation, highlighting the role of oxaliplatin uptake in the development of neuropathic symptoms. Importantly, in patients, the severity of neuropathy correlates with the platinum concentration in peripheral nerves (Gregg et al. 1992), confirming that oxaliplatin uptake is the major determinant of neurotoxicity. Interestingly, oxaliplatin levels in DRG remain high for prolonged time periods, even after discontinuation of treatment (Holmes et al. 1998). Although efflux transporters (or lack thereof) may be playing a role in the highly prolonged levels of oxaliplatin in DRGs, the transporter-mediated initial influx of oxaliplatin is one of the earliest steps driving cellular accumulation and believed to be the major determinant for subsequent neurotoxicity.
Identification of the biological processes involved in oxaliplatin accumulation in DRG is critical to understanding the mechanisms responsible for oxaliplatin-induced neurotoxicity. Several recent studies have proposed candidate transporters of oxaliplatin and related platinum complexes in heterologous cell culture models. In particular, OCTs belonging to the SLC22A family that includes OCT2 (SLC22A2) have been implicated in the transport of oxaliplatin and other platinum-based drugs (Sprowl et al. 2013c). Using genetic and pharmacological strategies, in vivo evidence has been accumulating for the direct role of OCT2 in the development of oxaliplatin associated neurotoxicity (Sprowl et al. 2013a). More specifically, recent studies involving genetically engineered mouse models have documented that the acute and chronic forms of oxaliplatin-induced peripheral neurotoxicity are dependent on OCT2, a transporter that regulates the transfer of drug from the circulation into satellite glial cells (SGC) in the DRG. These studies also demonstrate that tyrosine phosphorylation of OCT2 by the protein kinase YES1 is essential for function and targeting this post-translational modification can be exploited to modulate transport activity (Hu and Sprowl 2018; Sprowl et al. 2013a, c, 2016).
In addition to OCT2, several other uptake transporters in rodents have been postulated to be of potential relevance to the pharmacokinetics and toxicity of oxaliplatin, including Oct1 (Li et al. 2011), Oct3 (Yokoo et al. 2008), Octn1 (Nishida et al. 2018), Mate1 (Jong et al. 2011), Ctr1 (Ip et al. 2013), and Oatp1b2 (Lancaster et al. 2013). Although these transporters are all expressed in isolated DRGs from wild-type mice, uptake studies in engineered HEK293 cells overexpressing individual transporters have suggested that oxaliplatin may not be a transported substrate of these OCTs and OATPs, and that transport by OCT2 was more efficient than that observed for MATE1 and OCT3 (Sprowl et al. 2013a; Zhang et al. 2006). Additional studies are required to define the individual and collective contributions of these alternative neuronal uptake mechanisms for oxaliplatin. Preliminary studies performed to resolve this question experimentally with the use of primary SGCs cultures from mouse DRGs have indicated that about 80% of oxaliplatin uptake in these cells is mediated by OCT2, suggesting that the collective contribution of alternative mechanisms, including passive diffusion, may be minimal.
Targeting OCT2 is particularly appealing as an approach in ameliorating oxaliplatin-induced toxicities because potent and specific inhibitors are unlikely to sacrifice treatment efficacy in view of the general lack of OCT2 expression in tumor cells (Franke et al. 2010b), including colorectal cancers (Sprowl et al. 2013a). Importantly, since OCT2 inhibition may reduce the initial accumulation of platinum-based agents, the neuroprotective effects of OCT2 inhibition observed in the acute toxicity models may also lead to reduction in chronic pain and neuropathic symptoms frequently observed in patients. This possibility is strongly supported by the recent finding that patients receiving oxaliplatin-based chemotherapy who manifest symptoms of the acute neurotoxicity syndrome are those who will also eventually develop the chronic toxicity (Argyriou et al. 2013).
2.3. OCT2 and Cisplatin-Induced Nephrotoxicity
Cisplatin is another DNA-crosslinking, platinum-based chemotherapeutic agent that is among the most widely used anticancer drugs in both adult and pediatric populations (Dasari and Bernard Tchounwou 2014). In the conventional treatment regimens in which the drug is administered once every 3 weeks, dose-limiting side effects include renal tubular dysfunction (nephrotoxicity), and to a lesser extent, hearing loss (ototoxicity) and damage to peripheral nerves. Severe and irreversible damage to the kidney remains the single most important complication of cisplatin treatment as it may limit further treatment or even threaten life. This side effect primarily affects the S3 segment of the renal proximal tubules and occurs in up to 40% of patients despite intensive prophylactic measures, including extensive pre- and post-hydration regimens with hypertonic saline (Arany and Safirstein 2003; de Jongh et al. 2003). Furthermore, about 20% of all acute renal failure cases among hospitalized patients are due to cisplatin-containing chemotherapy (Berns and Ford 1997). As previously observed with toxicities associated with paclitaxel and oxaliplatin, the exact pathogenesis of cisplatin-related chronic toxicities and identity of SLCs involved in these processes, in which quiescent cells are selectively damaged, has remained unclear until relatively recently (Waissbluth and Daniel 2013; Yao et al. 2007).
Using transfected HEK293 cells, it was previously reported that cisplatin is a substrate for OCT2, as indicated by saturable uptake with an estimated Michaelis-Menten constant of 11 μM (Filipski et al. 2008). The localization of OCT2 in the S3 segment of the renal proximal tubules (Leibbrandt et al. 1995) suggests that OCT2 may be a key regulator in the renal elimination of cisplatin and may indirectly regulate the extent to which the drug causes kidney damage. This hypothesis has been verified in several studies with the use of Oct1/2(−/−) mice (Filipski et al. 2009), which are partially protected against cisplatin nephrotoxicity (Ciarimboli et al. 2010). Additionally, higher expression of OCT2 in male rodents correlated with a greater propensity and susceptibility to proximal tubular injury compared to female rodents. Subsequent investigation demonstrated that OCT2 is also highly expressed in the cochlea, and that Oct1/2(−/−) mice are completely protected from platinum-induced ototoxicity (Ciarimboli et al. 2010; Lanvers-Kaminsky et al. 2015) as well as from cisplatin-mediated peripheral neurotoxicity (Sprowl et al. 2013 a; Hucke et al. 2019).
The demonstration that this solute carrier plays an important role in all clinically relevant platinum-related toxicities provides a rationale for the development of therapeutic interventions for cisplatin-containing regimens in patients involving the use of specific inhibitors of OCT2. Targeting OCT2 is particularly appealing as an approach in ameliorating cisplatin-induced toxicities because potent and specific inhibitors are unlikely to compromise treatment efficacy in view of the general lack of OCT2 expression in tumor cells (Franke et al. 2010b). However, the incomplete protection against cisplatin-associated renal tubular damage in Oct1/2(−/−) mice suggests the existence of a secondary pathway that contributes, independently of Oct1/Oct2-mediated renal tubular drug uptake, to cisplatin-induced renal damage (Sprowl et al. 2014). Recent rodent studies have suggested that the OCT2-independent pathway is regulated by the transporters OAT1 and OAT3, which mediate the accumulation of a nephrotoxic, mercapturic acid metabolite of cisplatin formed in the γ-glutamyltranspeptidase pathway (Hu et al. 2017).
Over the last three decades, various approaches have been proposed to afford tissue protection during cisplatin treatment, although most of these interventions have not been evaluated in animal models or humans. Indeed, there is still no known preventative treatment for cisplatin-induced renal dysfunction (dos Santos et al. 2012), ototoxicity (Langer et al. 2013; Travis et al. 2014), or neurotoxicity (Albers et al. 2014). Agents that have advanced to clinical testing, such as amifostine, are associated with intrinsic toxicity and, more importantly, do not appear to have a major impact on ameliorating the risk of developing severe nephrotoxicity (Gallegos-Castorena et al. 2007; Sastry and Kellie 2005), ototoxicity (Duval and Daniel 2012), or neurotoxicity (Hensley et al. 2009). Furthermore, the clinical application of many alternate strategies has been hampered by the recognition that (1) cisplatin has multiple intracellular targets and hence blocking a single injurious event will only have partial protective effects and (2) the protective approach may diminish the antitumor effects of cisplatin given the overlap in cell death signaling pathways between normal cells and tumor cells. Therefore, an ideal approach is to simultaneously protect the kidneys and other afflicted tissues such as cochlea and peripheral nerves without affecting the therapeutic effects in tumors. The development of such an approach would rely on the identification of the critical differences between normal and malignant cells during cisplatin treatment.
One of these agents, cimetidine, has shown some promise in ameliorating cisplatin-induced nephrotoxicity (Franke et al. 2010b) and ototoxicity (Ciarimboli et al. 2010), as well as oxaliplatin-induced neurotoxicity in experimental mouse models (Sprowl et al. 2013a). To obtain preliminary evidence for the usefulness of adding cimetidine to cisplatin-based therapy in cancer patients, randomized cross-over trials (Sprowl et al. 2013b) have been performed and demonstrated that inhibition of OCT2 function by cimetidine did not affect the antitumor or disposition properties of cisplatin. However, cimetidine did not completely eradicate renal tubular damage, in line with another recent clinical trial indicating that the renoprotective effects associated with cimetidine are only partial (Zhang and Zhou 2012). This confirms in vitro studies suggesting that cimetidine is an inefficient and non-specific inhibitor of OCT2 (Ito et al. 2012; Motohashi et al. 2004), and that identification of alternate OCT2 inhibitors is urgently needed. Such agents would ideally have (1) high potency, (2) high specificity, (3) low drug–drug interaction potential, (4) intrinsic antitumor properties, (5) favorable pharmaceutical properties, (6) non-overlapping toxicity profiles, and (7) potentially other renoprotective features, including inhibition of OAT1 and OAT3. Among possible candidates for further exploration, palbociclib, an FDA-approved inhibitor of CDK4/6 used in the treatment of breast cancer, is of particular interest because it has been previously shown that other inhibitors of cyclin dependent kinase are able to afford protection against cisplatin-induced kidney injury in experimental models (Price et al. 2009). Preliminary studies performed in mice have suggested that cisplatin-induced nephrotoxicity can be mitigated by pretreatment with palbociclib through a mechanism that is partially dependent on OCT2 (Pabla et al. 2015). Due to significant overlap of OCT/MATE inhibitors, it is important that the design of these intervention strategies aimed at selectively targeting OCT2-mediated uptake does not increase the residence time in proximal tubular cells due to unintended inhibition of MATE1-mediated efflux.
2.4. ENT1/OCTN1 and Cytarabine-Related Toxicities
Cytarabine is a nucleoside analog belonging to the family of antimetabolites due to its similarity in chemical structure to that of endogenous nucleosides. Cytarabine is utilized in a variety of leukemia subtypes but is a mainstay in the treatment of acute myeloid leukemia (AML) where it is an integral component of first-line therapy. All of the endogenous and xenobiotic nucleoside analogs are polar hydrophilic compounds that are poorly membrane permeable and require functional nucleoside transporter proteins to enter cells.
Nucleoside transporters facilitate the accumulation of both endogenous nucleosides and nucleoside-derived drugs that are utilized as anticancer and antiviral agents (Baldwin et al. 1999). Some cell types, including brain, enterocytes, and bone marrow cells, rely heavily on the nucleoside salvage pathway due to their inability to synthesis nucleosides de novo and thus rely heavily on the extracellular milieu for their primary source of nucleosides for use in RNA and DNA synthesis (Murray 1971). Nucleoside transporters play an integral role in the maintenance of extracellular concentrations of nucleosides, which are available to bind to receptors and modulate a variety of physiological processes. Transporter-mediated transport of nucleosides is thus a critical determinant in the salvage and consequently, nucleoside-mediated toxicity in many cell types (Griffith and Jarvis 1996).
The two major classes of nucleoside transporters in mammalian cells and tissues consist of equilibrative nucleoside transporters (ENTs) and concentrative nucleoside transporters (CNTs). Two proteins of the former class, ENT1 (SLC29A1) and ENT2 (SLC29A2), are known to mediate the transport of purine and pyrimidine nucleosides across biological membranes down their concentration gradients. These transporters exhibit broad substrate selectivity and are subdivided based on their sensitivity (ENT1) or resistance (ENT2) to inhibition by nanomolar concentrations of nitrobenzylmercaptopurine ribonucleoside (NBMPR) (Damaraju et al. 2003). In addition to ENT1, several non-canonical putative nucleoside transporters, including OCTN1, can potentially transport nucleoside analogs in a manner that is sensitive to nanomolar concentrations of NBMPR (Drenberg et al. 2017).
The clinical use of cytarabine is associated with dose-limiting damage to normal bone marrow (myelosuppression), which occurs in the majority of patients, as well as with damage to the skin (toxic erythema), and these complications may require dose-modification, limit further treatment, or even threaten life (Hwang et al. 2012; Zhang et al. 2014). Interestingly, OCTN1 is highly expressed in several organs of particular relevance to cytarabine-based chemotherapy regimens, including myeloid progenitor cells in the bone marrow, proximal tubular cells in the kidney (Kobayashi et al. 2004), and epidermal keratinocytes in the skin (Wu et al. 2000). Preliminary evidence pointing to potential causality of this connection has come from population-based genetic studies indicating that patients with a functional polymorphic germline variant of OCTN1 (L503F; rs1050152) experience an increased frequency of febrile neutropenia (Drenberg et al. 2015). Furthermore, overexpression of this genetic variant (L503F) in HEK293 cells is associated with increased transport function (Urban et al. 2007) and increased formation of Ara-CTP, the active triphosphorylated form of cytarabine (Drenberg et al. 2017).
Consistent with the thesis that OCTN1 may be contributing to cytarabine-related toxicities, it has been reported that this transport system may also be operational for related cytotoxic nucleoside analogs such as gemcitabine, an agent used in the treatment of pancreatic cancer with activity in certain subtypes of AML (Drenberg et al. 2019), that causes dose-limiting anemia and neutropenia. Although this site of toxicity directly aligns with the expression profile of OCTN1, further study is warranted to determine the contribution of individual SLCs to gemcitabine-related side effects. In this context, it is worth pointing out that the intracellular accumulation of nucleoside analogs such as cytarabine was recently reported to occur independently of OCTN1 (Tschirka et al. 2018). In this work, the authors used an LC-MS/MS-based method to measure the intracellular levels of unchanged cytarabine, whereas prior studies involved the use of an analysis based on the measurement of total radioactivity [i.e., the total of parent drug and metabolite(s)]. This is a potentially important methodological difference as cytarabine can undergo rapid enzyme-mediated metabolism once inside cells to form mono-, di-, and tri-phosphorylated forms (Owens et al. 1992), which may easily escape detection and result in underestimating the actual extent of uptake. This possibility is supported by the finding that cytarabine is rapidly and extensively phosphorylated in HEK293 cells (Drenberg et al. 2017), and by the demonstration that in a comparative analysis intracellular levels of total radioactivity originating from cytarabine in cells overexpressing OCTN1 are high, while levels of the unchanged parent drug, as measured by LC-MS/MS, remain undetectable (Anderson et al. 2019).
2.5. OATPs and Irinotecan-Mediated Neutropenia and Diarrhea
Irinotecan is a prodrug of the topoisomerase I inhibitor, SN-38, and is utilized in a variety of chemotherapy containing regimens that are used to treat solid tumors such as colorectal cancers. Irinotecan is known to cause several debilitating adverse events such as myelosuppression and diarrhea. In contrast to the metabolism of irinotecan, which has been well documented and characterized (de Man et al. 2018), the pharmacokinetic processes of relevance to irinotecan disposition are less well characterized and are likely dependent on the interplay of drug transporters residing in organs such as the liver. In this context, the contribution of hepatocellular uptake transporters in the disposition of irinotecan remains poorly understood. Preclinical reports have shown that after irinotecan administration, the systemic exposure of SN-38, the active metabolite of irinotecan, is highly impacted by the deficiency of Oatp1a- and Oatp1b-type carriers in murine models and expression of human OATP1B1 or OATP1B3 (Iusuf et al. 2014). Furthermore, only a small fraction of the administered dose of irinotecan is excreted in the bile (0.9% for SN-38 and 3% for SN-38-glucuronide) (de Jong et al. 2006), which supports the notion that a large fraction of these metabolites formed within hepatocytes and being transported back into the system circulation, presumably by the hepatic efflux transporter ABCC3 (Kitamura et al. 2010). This increased SN-38 efflux can then be taken up again by adjacent hepatocytes in an OATP1B-mediated manner for further glucuronidation and/or biliary excretion termed “hepatocyte hopping” (Iusuf et al. 2012). This unusual mechanism is hypothesized as a physiological mechanism to overcome hepatocellular saturation and to efficiently facilitate detoxification through utilizing other elimination pathways such as Phase II conjugation and transporter-mediated excretion into the bile. This unusual mechanism for irinotecan elimination is supported by the observation of patients with functional variants of OATP1B1 that are associated with altered exposure to SN-38 and at risk for severe toxicity following irinotecan-based chemotherapies (Di Paolo et al. 2011).
Interestingly, in contrast to SN-38 and other known glucuronide metabolites (Ni et al. 2010; Zimmerman et al. 2013), SN-38-glucuronide accumulation is not mediated by OATP1B1 (Nozawa et al. 2005). Clinical observation of excessive SN-38-glucuronide buildup in the systemic circulation relative to unconjugated SN-38 could be explained by the lack of an efficient hepatocellular uptake mechanism for SN-38-glucuronide (Innocenti et al. 2014).
Since formation of SN-38 from irinotecan is essential to the therapy-related diarrhea (Fujita et al. 2016), recent studies have focused on connecting functional expression of intestinal transporters to the occurrence of this side effect. This work has resulted in the identification of OATP2B1 as a putative carrier of SN-38 on the basis of uptake studies performed in Xenopus oocytes (Fujita et al. 2016). This transporter is highly expressed in the small intestine (Tamai et al. 2000), and sensitive to inhibition by cyclosporine (Chen et al. 2020), an agent that has been exploited as a therapeutic to prevent the dose-limiting diarrhea associated with irinotecan treatment (Chester et al. 2003). The availability of a recently developed Oatp2b1-deficient mouse model will allow the unequivocal demonstration of a causal connection of OATP2B1-mediated transport of SN-38 with irinotecan-related diarrhea (Medwid et al. 2019).
2.6. SLCs and Doxorubicin-Related Cardiotoxicity
Doxorubicin, an anthracycline-derived DNA-intercalator, is widely used in the treatment of multiple tumor types, including breast cancers and soft tissue sarcomas. Common side effects associated with the use of doxorubicin include acute nausea and vomiting, mucositis, alopecia, and tissue extravasation. More serious, dose-limiting toxicities associated with doxorubicin include myelosuppression and cardiotoxicity, and these side effects are dependent on the cumulative dose administered. In particular, the risk for patients developing congestive heart failure is estimated at 5% (Von Hoff et al. 1979; Kremer et al. 2001), 18% (Kremer et al. 2001), and 36% (Swain et al. 2003) for cumulative doses of doxorubicin of <500 mg/m2, 500–600 mg/m2, and > 600 mg/m2, respectively. Manifestation of acute cardiotoxicity presents as arrhythmias or ventricular dysfunction. However, since the myocardium has limited regenerative capacity (Lionetti and Ventura 2013; Yamada et al. 2015), chronic cardiotoxicity induced by anthracyclines culminates into dilated cardiomyopathy and congestive heart failure (Boucek Jr. et al. 1997; Lipshultz et al. 2013), which can occur months or even years after cessation of therapy. The risk of developing chronic cardiotoxicity is particularly high in young adult and adolescent cancer survivors (Lipshultz et al. 2013). Clinical risk factors associated with doxorubicin-induced cardiotoxicity also include pre-existing cardiac dysfunction and age (Doyle et al. 2005) as well as prior therapy involving radiation or chemotherapy (Singal and Iliskovic 1998).
The mechanisms by which anthracyclines such as doxorubicin accumulate into cardiomyocytes remain largely unknown. At physiological pH 7.4, the hydrophobic weak base doxorubicin is slightly cationic (Raghunand et al. 1999), and this recognition has resulted in recent efforts to connect uptake of anthracyclines to OCTs that can explain cell-type specific toxicity profiles. In particular, studies have demonstrated that overexpression of OCTN1 (Okabe et al. 2008), OCT1 (Andreev et al. 2016), or OCT6 (Okabe et al. 2005; Ota et al. 2007) is associated with significantly increased drug uptake and sensitivity of leukemic and ovarian cancer cells following exposure to doxorubicin. Furthermore, studies in C. elegans and Danio rerio corroborate involvement of OCT1 and possibly OCT2 (Brosseau et al. 2015) in the cellular uptake of doxorubicin. It is likely that cardiac expression of OCTs capable of transporting doxorubicin in the myocardium contributes as an initiating event that ultimately leads to treatment-related cardiotoxicity.
Among the class of OCTs, studies have confirmed the presence of several members at both the mRNA and protein levels in the human heart, with OCTN2 having the highest expression, followed by OCT3, OCTN1, and OCT1, while OCT2 is undetectable (Grube et al. 2011). In view of the predominant expression of OCT2 in the kidney, it is possible that this transport mechanism contributes to urinary excretion of anthracyclines and to doxorubicin-induced nephrotoxicity (Filipski et al. 2009; Ayla et al. 2011). Additional investigation has indicated that expression of these transporters is confined to either the vasculature (OCTN1, OCTN2, and OCT3) or to cardiomyocytes (OCTN1 and OCT1) (Grube et al. 2011; Iwata et al. 2008) of the myocardium, although other studies have demonstrated ubiquitous localization in the heart (Nishimura and Naito 2008). It has been suggested that OCTN2 is involved in the uptake of certain cardiovascular drugs, and is spatially regulated in a rat cardiomyopathy model (Iwata et al. 2008). Indeed, supplementation with the high-affinity OCTN2 substrate, l-carnitine, can reduce doxorubicin-induced upregulation of heart fatty acid binding protein (H-FABP) (Sayed-Ahmed et al. 2010), suggesting the potential for an OCTN2-mediated mechanism for doxorubicin uptake. In addition, expression of the structurally related transporter OCTN1 is correlated with augmenting the blockage of HERG potassium channels and potentiating torsade de pointes (McBride et al. 2009), which can lead to serious cardiac arrhythmias.
OCT1 and OCT3 have also been linked to cardiovascular drug response, in particular to certain beta-blockers (Bachmakov et al. 2009; Kubo et al. 2013; Hassan et al. 2016), which are used principally as prophylactic treatment in managing cardiovascular function after anthracycline therapy. Additionally, OCT3 has been identified as an important regulator of neurological and cardiovascular response to endogenous substrates such as epinephrine and norepinephrine (Zwart et al. 2001; Hanafy et al. 2012; Ayala-Lopez et al. 2015; Zhu et al. 2012). Furthermore, case-control studies of the OCT3 gene locus have suggested its involvement in coronary vascular development, and multiples variants, including rs9381439, rs2048327, rs18190126, and rs9349379, were previously associated with decreased risk for coronary artery disease (Tregouet et al. 2009; Wang et al. 2016). Definitive demonstration of a direct contribution of any of the candidate transporters to doxorubicin-induced cardiotoxicity, for example, in mice with individual SLC deficiencies, remains warranted, and may provide opportunities for the future design of preventative intervention strategies with the use of selective transport inhibitors.
Since hepatobiliary excretion is the main elimination pathway of doxorubicin (Legha et al. 1982; Bronchud et al. 1990; Robert et al. 1985, 1987; Maniez-Devos et al. 1986), recent studies have attempted to connect the function of hepatic OATPs with the disposition of doxorubicin (Gong and Kim 2013). These investigations have shown that deficiency of Oatp1a- and Oatp1b-type transporters in mice is associated with increases in systemic exposure to doxorubicin. In addition, introduction of the human transporters OATP1B1, OATP1B3, or OATP1A2 in the livers of these knockout mice can partially recapitulate the pharmacokinetic profile of doxorubicin observed in wild-type mice (Durmus et al. 2014). Drug uptake studies in cell-based models engineered to overexpress human OATP1A2 variants have corroborated the results in mice, and are consistent with previous reports involving other substrates (Lee et al. 2017). Although several related transporters, including OATP2B1, OATP3A1, and OATP4A1, are known to be expressed in the heart (Hanafy et al. 2012; Grube et al. 2006; Atilano-Roque and Joy 2017), there is currently an apparent lack of documentation that establishes a correlation of these OATPs with the cardiac uptake of anthracyclines, and thus their direct relevance to cardiovascular function after doxorubicin treatment remains uncertain.
3. Toxicity Induced by Targeted Therapeutics
In oncology, the last two decades have seen a dramatic transition from the use of traditional cytotoxic chemotherapy to the emergence of a new paradigm in rational drug design coupled with an uprising in the development of targeted agents, including the tyrosine kinase inhibitors (TKIs). To date, >45 different TKIs have received approval by the FDA for the treatment of a variety of diseases that were previously essentially resistant to standard chemotherapy, and many more can be expected to become available in the future (Drenberg et al. 2013). However, while TKIs offer possibly a number of important theoretical advantages over conventional cytotoxic chemotherapy, they are still afflicted by some of the same problems, including an extensive inter-individual pharmacokinetic variability, the existence of a rather narrow therapeutic window, and the occurrence of multiple, debilitating adverse events (Drenberg et al. 2013).
Previous investigations on drug transporters and their contribution to pharmacological effects of TKIs have often exclusively focused on the effects on measures of systemic exposure, ignoring effects on local drug uptake and cellular retention in healthy tissues. Other investigations have tended to focus solely on the cellular target regulating pharmacological effects while ignoring the effects on systemic and/or local drug exposure. However, recently developed conceptual frameworks for an integrated approach have started to address questions related to the relevance of specific SLCs to the local tissue exposure of TKIs.
3.1. OAT6 and Sorafenib-Mediated Skin Toxicity
Sorafenib is a multi-kinase inhibitor (MKI) utilized in unresectable hepatocellular carcinoma, advanced renal cell carcinoma, and thyroid carcinoma. Common debilitating adverse events of sorafenib include fatigue, infection, alopecia, hand-foot skin reaction, and rash. Of the listed adverse events, cutaneous adverse effects are among the most frequently observed toxicities with many TKIs, and their intensity can significantly affect both quality of life and health care economics (Macdonald et al. 2015). In one study, 40% of renal cell carcinoma patients taking sorafenib had a dermatologic reaction (Kane et al. 2006).
A particularly painful complication seen most frequently during the early weeks of use with MKIs such as sorafenib and regorafenib is known as hand-foot skin reaction (HFSR), in which hyperkeratotic plaques develop predominantly over sites of pressure or friction (Inaba et al. 2011; Lipworth et al. 2009). These plaques may have significant inflammation and xerotic hyperkeratosis, often in a bilateral symmetric distribution, causing pain and debilitation that interfere with activities of daily living (Macdonald et al. 2015). Sequential biopsy specimens from patients receiving MKIs have revealed progressive accumulation of hyperkeratosis with focal parakeratosis. The clinical incidence of HFSR varies among MKIs with a particularly high incidence being observed with sorafenib (>60%) (Zimmerman et al. 2016), and this appears to be unrelated to increased excretion of MKIs through sweat (Jain et al. 2010).
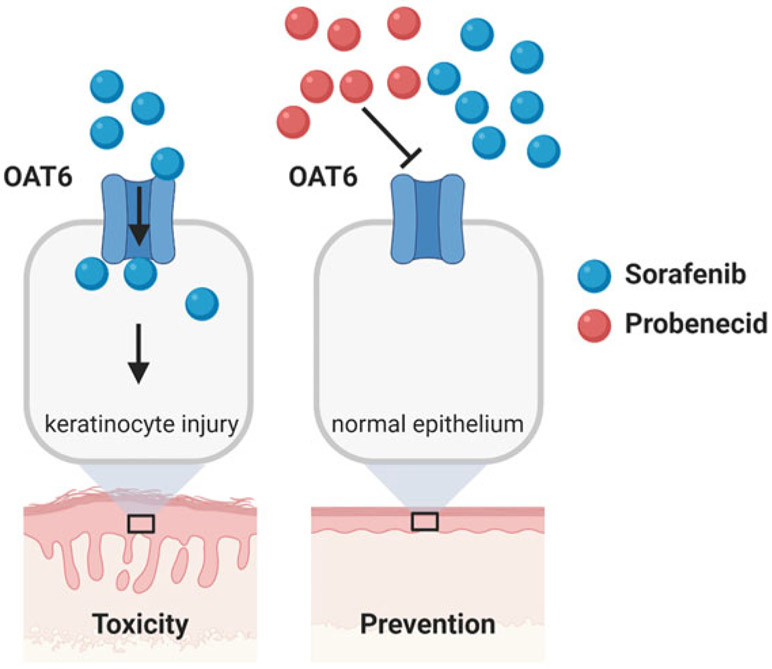
The hair follicle is a specialized mini-organ that is critically dependent on programmed keratinocyte differentiation (Botchkarev and Paus 2003; Cotsarelis 2006), and disruption of hair-follicle cycling or morphology is indicative of keratinocyte toxicity. Recent studies have indicated that sorafenib accumulates extensively in primary human epidermal keratinocytes compared to a panel of other TKIs, can decrease cell viability, and increase apoptosis (Zimmerman et al. 2016). The mechanism by which sorafenib is taken up into keratinocytes is concentration-, time-, and temperature-dependent. Since sorafenib is a poorly permeable compound, it is plausible that its uptake into keratinocytes is predominantly a transporter-mediated process. This hypothesis was verified by the recent demonstration that uptake of sorafenib in keratinocytes is dependent on OAT6, identified from a transportome-wide gene silencing screen (Tian et al. 2018), and that sorafenib-induced injury to keratinocytes in a mouse model of HFSR can be reversed by pretreatment with the OAT6 inhibitor, probenecid (Fig. 4) (Zimmerman et al. 2016). The translational significance of intervention strategies derived from the combinatorial use of probenecid and sorafenib or regorafenib (Belum et al. 2013) requires further investigation.
Fig. 4.
Proposed model of sorafenib-induced keratinocyte injury. Organic anionic transport 6 (OAT6) mediates intracellular concentrations of sorafenib, leading to cytotoxicity and keratinocyte injury (left). These effects can be blocked by the OAT6 inhibitor probenecid (right)
4. Conclusions
In order to better understand the contribution of SLCs to debilitating side effects associated with anticancer drugs, there is an urgent need to further characterize the role of these proteins in the transport of drugs in both target and off-target tissues. This research would require the (1) expansion of the SLC proteomics field in various tissue types associated with toxicities of importance, (2) metabolomics approaches to further understand the trafficking of endogenous substrates of each transporter, and (3) the availability of agnostic screening platforms that allow for more rapid identification of drug-transporter and inhibitor-transporter pairs. Understanding the delicate balance of on-target and off-target tissue accumulation could then be exploited as a basis for the development of predictive tools as well as for the discovery of novel intervention strategies with application in the clinic in order to improve the safety of currently available treatment modalities.
Acknowledgement
This work was supported in part by the National Institutes of Health Grants R01CA215802 (to A.S.), R01CA238946 (to S.H., M.B.L), and by the OSU Comprehensive Cancer Center using Pelotonia funds. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflicts of Interest The authors have no conflicts of interests.
Contributor Information
Jason T. Anderson, Division of Pharmaceutics and Pharmacology, College of Pharmacy and Comprehensive Cancer Center, The Ohio State University, Columbus, OH, USA
Kevin M. Huang, Division of Pharmaceutics and Pharmacology, College of Pharmacy and Comprehensive Cancer Center, The Ohio State University, Columbus, OH, USA
Maryam B. Lustberg, Department of Medical Oncology, The Ohio State University, Comprehensive Cancer Center, Columbus, OH, USA
Alex Sparreboom, Division of Pharmaceutics and Pharmacology, College of Pharmacy and Comprehensive Cancer Center, The Ohio State University, Columbus, OH, USA.
Shuiying Hu, Division of Pharmaceutics and Pharmacology, College of Pharmacy and Comprehensive Cancer Center, The Ohio State University, Columbus, OH, USA.
References
- Abraham JE, Guo Q, Dorling L, Tyrer J, Ingle S, Hardy R, Vallier AL, Hiller L, Burns R, Jones L, Bowden SJ, Dunn JA, Poole CJ, Caldas C, Pharoah PP, Earl HM (2014) Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with paclitaxel. Clin Cancer Res 20(9):2466–2475. 10.1158/1078-0432.CCR-13-3232 [DOI] [PubMed] [Google Scholar]
- Albers JW, Chaudhry V, Cavaletti G, Donehower RC (2014) Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev 3(3):CD005228. 10.1002/14651858.CD005228.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Hu S, Fu Q, Baker SD, Sparreboom A (2019) Role of equilibrative nucleoside transporter 1 (ENT1) in the disposition of cytarabine in mice. Pharmacol Res Perspect 7(6):e00534. 10.1002/prp2.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev E, Brosseau N, Carmona E, Mes-Masson AM, Ramotar D (2016) The human organic cation transporter OCT1 mediates high affinity uptake of the anticancer drug daunorubicin. Sci Rep 6:20508. 10.1038/srep20508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany I, Safirstein RL (2003) Cisplatin nephrotoxicity. Semin Nephrol 23(5):460–464 [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP (2008) A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev 34(4):368–377. 10.1016/j.ctrv.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Cavaletti G, Briani C, Velasco R, Bruna J, Campagnolo M, Alberti P, Bergamo F, Cortinovis D, Cazzaniga M, Santos C, Papadimitriou K, Kalofonos HP (2013) Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer 119(2):438–444. 10.1002/cncr.27732 [DOI] [PubMed] [Google Scholar]
- Atilano-Roque A, Joy MS (2017) Characterization of simvastatin acid uptake by organic anion transporting polypeptide 3A1 (OATP3A1) and influence of drug-drug interaction. Toxicol In Vitro 45(Pt 1):158–165. 10.1016/j.tiv.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Ayala-Lopez N, Jackson WF, Burnett R, Wilson JN, Thompson JM, Watts SW (2015) Organic cation transporter 3 contributes to norepinephrine uptake into perivascular adipose tissue. Am J Physiol Heart Circ Physiol 309(11):H1904–H1914. 10.1152/ajpheart.00308.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayla S, Seckin I, Tanriverdi G, Cengiz M, Eser M, Soner BC, Oktem G (2011) Doxorubicin induced nephrotoxicity: protective effect of nicotinamide. Int J Cell Biol 2011:390238. 10.1155/2011/390238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmakov I, Glaeser H, Endress B, Morl F, Konig J, Fromm MF (2009) Interaction of beta-blockers with the renal uptake transporter OCT2. Diabetes Obes Metab 11(11):1080–1083. 10.1111/j.1463-1326.2009.01076.x [DOI] [PubMed] [Google Scholar]
- Baker SD, Verweij J, Cusatis GA, van Schaik RH, Marsh S, Orwick SJ, Franke RM, Hu S, Schuetz EG, Lamba V, Messersmith WA, Wolff AC, Carducci MA, Sparreboom A (2009) Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther 85(2):155–163. 10.1038/clpt.2008.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Mackey JR, Cass CE, Young JD (1999) Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today 5(5):216–224. 10.1016/S1357-4310(99)01459-8 [DOI] [PubMed] [Google Scholar]
- Baldwin RM, Owzar K, Zembutsu H, Chhibber A, Kubo M, Jiang C, Watson D, Eclov RJ, Mefford J, McLeod HL, Friedman PN, Hudis CA, Winer EP, Jorgenson EM, Witte JS, Shulman LN, Nakamura Y, Ratain MJ, Kroetz DL (2012) A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res 18(18):5099–5109. 10.1158/1078-0432.CCR-12-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban M, Hettich D, Huguet N (1994) Nephrotoxicity mechanism of cis-platinum (II) diamine dichloride in mice. Toxicol Lett 71(2):161–168 [DOI] [PubMed] [Google Scholar]
- Belum VR, Wu S, Lacouture ME (2013) Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Investig New Drugs 31(4):1078–1086. 10.1007/s10637-013-9977-0 [DOI] [PubMed] [Google Scholar]
- Bergmann TK, Vach W, Feddersen S, Eckhoff L, Green H, Herrstedt J, Brosen K (2013) GWAS-based association between RWDD3 and TECTA variants and paclitaxel induced neuropathy could not be confirmed in Scandinavian ovarian cancer patients. Acta Oncol 52(4):871–874. 10.3109/0284186X.2012.707787 [DOI] [PubMed] [Google Scholar]
- Berns JS, Ford PA (1997) Renal toxicities of antineoplastic drugs and bone marrow transplantation. Semin Nephrol 17(1):54–66 [PubMed] [Google Scholar]
- Boehmerle W, Huehnchen P, Peruzzaro S, Balkaya M, Endres M (2014) Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci Rep 4:6370. 10.1038/srep06370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boora GK, Kanwar R, Kulkarni AA, Abyzov A, Sloan J, Ruddy KJ, Banck MS, Loprinzi CL, Beutler AS (2016) Testing of candidate single nucleotide variants associated with paclitaxel neuropathy in the trial NCCTG N08C1 (Alliance). Cancer Med 5(4):631–639. 10.1002/cam4.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P, Elferink RO (2002) Mammalian ABC transporters in health and disease. Annu Rev Biochem 71:537–592. 10.1146/annurev.biochem.71.102301.093055 [DOI] [PubMed] [Google Scholar]
- Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 92(16):1295–1302 [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Paus R (2003) Molecular biology of hair morphogenesis: development and cycling. J Exp Zool B Mol Dev Evol 298(1):164–180. 10.1002/jez.b.33 [DOI] [PubMed] [Google Scholar]
- Boucek RJ Jr, Dodd DA, Atkinson JB, Oquist N, Olson RD (1997) Contractile failure in chronic doxorubicin-induced cardiomyopathy. J Mol Cell Cardiol 29(10):2631–2640 [DOI] [PubMed] [Google Scholar]
- Brewer JR, Morrison G, Dolan ME, Fleming GF (2016) Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol Oncol 140(1):176–183. 10.1016/j.ygyno.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronchud MH, Margison JM, Howell A, Lind M, Lucas SB, Wilkinson PM (1990) Comparative pharmacokinetics of escalating doses of doxorubicin in patients with metastatic breast cancer. Cancer Chemother Pharmacol 25(6):435–439. 10.1007/bf00686055 [DOI] [PubMed] [Google Scholar]
- Brosseau N, Andreev E, Ramotar D (2015) Complementation of the yeast model system reveals that Caenorhabditis elegans OCT-1 is a functional transporter of anthracyclines. PLoS One 10(7):e0133182. 10.1371/journal.pone.0133182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozzi VA, Canta A, Chiorazzi A (2015) Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett 596:90–107. 10.1016/j.neulet.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Caterson R, Etheredge S, Snitch P, Duggin G (1983) Mechanisms of renal excretion of cisdichlorodiamine platinum. Res Commun Chem Pathol Pharmacol 41(2):255–264 [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Zucchetti M, Marmiroli P, Tredici G (2000) Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology 21(3):389–393 [PubMed] [Google Scholar]
- Cavaletti G, Tredici G, Petruccioli MG, Donde E, Tredici P, Marmiroli P, Minoia C, Ronchi A, Bayssas M, Etienne GG (2001) Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur J Cancer 37(18):2457–2463. 10.1016/s0959-8049(01)00300-8 [DOI] [PubMed] [Google Scholar]
- Chae YK, Arya A, Malecek MK, Shin DS, Carneiro B, Chandra S, Kaplan J, Kalyan A, Altman JK, Platanias L, Giles F (2016) Repurposing metformin for cancer treatment: current clinical studies. Oncotarget 7(26):40767–40780. 10.18632/oncotarget.8194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Neul C, Schaeffeler E, Frisch F, Winter S, Schwab M, Koepsell H, Hu S, Laufer S, Baker SD, Sparreboom A, Nies AT (2020) Sorafenib activity and disposition in liver cancer does not depend on organic cation transporter 1. Clin Pharmacol Ther 107(1):227–237. 10.1002/cpt.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JD, Joel SP, Cheeseman SL, Hall GD, Braun MS, Perry J, Davis T, Button CJ, Seymour MT (2003) Phase I and pharmacokinetic study of intravenous irinotecan plus oral ciclosporin in patients with fuorouracil-refractory metastatic colon cancer. J Clin Oncol 21(6):1125–1132. 10.1200/JCO.2003.08.049 [DOI] [PubMed] [Google Scholar]
- Cho SK, Chung JY (2016) The MATE1 rs2289669 polymorphism affects the renal clearance of metformin following ranitidine treatment. Int J Clin Pharmacol Ther 54(4):253–262. 10.5414/CP202473 [DOI] [PubMed] [Google Scholar]
- Cho SK, Kim CO, Park ES, Chung JY (2014) Verapamil decreases the glucose-lowering effect of metformin in healthy volunteers. Br J Clin Pharmacol 78(6):1426–1432. 10.1111/bcp.12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MM, Hojlund K, Hother-Nielsen O, Stage TB, Damkier P, Beck-Nielsen H, Brosen K (2015) Steady-state pharmacokinetics of metformin is independent of the OCT1 genotype in healthy volunteers. Eur J Clin Pharmacol 71(6):691–697. 10.1007/s00228-015-1853-8 [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jurgens H, Schlatter E (2010) Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol 176(3):1169–1180. 10.2353/ajpath.2010.090610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G (2006) Epithelial stem cells: a folliculocentric view. J Invest Dermatol 126(7):1459–1468. 10.1038/sj.jid.5700376 [DOI] [PubMed] [Google Scholar]
- Daley-Yates PT, McBrien DC (1985) The renal fractional clearance of platinum antitumour compounds in relation to nephrotoxicity. Biochem Pharmacol 34(9):1423–1428 [DOI] [PubMed] [Google Scholar]
- Damaraju VL, Damaraju S, Young JD, Baldwin SA, Mackey J, Sawyer MB, Cass CE (2003) Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene 22(47):7524–7536. 10.1038/sj.onc.1206952 [DOI] [PubMed] [Google Scholar]
- Dasari S, Bernard Tchounwou P (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740C:364–378. 10.1016/j.ejphar.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graan AJ, Lancaster CS, Obaidat A, Hagenbuch B, Elens L, Friberg LE, de Bruijn P, Hu S, Gibson AA, Bruun GH, Corydon TJ, Mikkelsen TS, Walker AL, Du G, Loos WJ, van Schaik RH, Baker SD, Mathijssen RH, Sparreboom A (2012) Influence of polymorphic OATP1B-type carriers on the disposition of docetaxel. Clin Cancer Res 18(16):4433–4440. 10.1158/1078-0432.CCR-12-0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graan AJ, Elens L, Sprowl JA, Sparreboom A, Friberg LE, van der Holt B, de Raaf PJ, de Bruijn P, Engels FK, Eskens FA, Wiemer EA, Verweij J, Mathijssen RH, van Schaik RH (2013) CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin Cancer Res 19(12):3316–3324. 10.1158/1078-0432.CCR-12-3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Iuliis F, Taglieri L, Salerno G, Lanza R, Scarpa S (2015) Taxane induced neuropathy in patients affected by breast cancer: literature review. Crit Rev Oncol Hematol 96(1):34–45. 10.1016/j.critrevonc.2015.04.011 [DOI] [PubMed] [Google Scholar]
- de Jong FA, Kitzen JJ, de Bruijn P, Verweij J, Loos WJ (2006) Hepatic transport, metabolism and biliary excretion of irinotecan in a cancer patient with an external bile drain. Cancer Biol Ther 5(9):1105–1110 [DOI] [PubMed] [Google Scholar]
- de Jongh FE, van Veen RN, Veltman SJ, de Wit R, van der Burg ME, van den Bent MJ, Planting AS, Graveland WJ, Stoter G, Verweij J (2003) Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer 88(8):1199–1206. 10.1038/sj.bjc.6600884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S (2018) Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet 57(10):1229–1254. 10.1007/s40262-018-0644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGorter MK, Xia CQ, Yang JJ, Kim RB (2012) Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxicol 52:249–273. 10.1146/annurev-pharmtox-010611-134529 [DOI] [PubMed] [Google Scholar]
- Di Paolo A, Bocci G, Polillo M, Del Re M, Di Desidero T, Lastella M, Danesi R (2011) Pharmacokinetic and pharmacogenetic predictive markers of irinotecan activity and toxicity. Curr Drug Metab 12(10):932–943. 10.2174/138920011798062283 [DOI] [PubMed] [Google Scholar]
- dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC (2012) Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol 86(8):1233–1250. 10.1007/s00204-012-0821-7 [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL (2005) Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol 23(34):8597–8605. 10.1200/JCO.2005.02.5841 [DOI] [PubMed] [Google Scholar]
- Drenberg CD, Baker SD, Sparreboom A (2013) Integrating clinical pharmacology concepts in individualized therapy with tyrosine kinase inhibitors. Clin Pharmacol Ther 93(3):215–219. 10.1038/clpt.2012.247 [DOI] [PubMed] [Google Scholar]
- Drenberg CD, Paugh SW, Pounds SB, Shi L, Orwick SJ, Li L, Hu S, Gibson AA, Ribeiro RC, Rubnitz JE, Evans WE, Sparreboom A, Baker SD (2015) Inherited variation in OATP1B1 is associated with treatment outcome in acute myeloid leukemia. Clin Pharmacol Ther. 10.1002/cpt.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenberg CD, Gibson AA, Pounds SB, Shi L, Rhinehart DP, Li L, Hu S, Du G, Nies AT, Schwab M, Pabla N, Blum W, Gruber TA, Baker SD, Sparreboom A (2017) OCTN1 is a high-affinity carrier of nucleoside analogues. Cancer Res 77(8):2102–2111. 10.1158/0008-5472.CAN-16-2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenberg CD, Shelat A, Dang J, Cotton A, Orwick SJ, Li M, Jeon JY, Fu Q, Buelow DR, Pioso M, Hu S, Inaba H, Ribeiro RC, Rubnitz JE, Gruber TA, Guy RK, Baker SD (2019) A high-throughput screen indicates gemcitabine and JAK inhibitors may be useful for treating pediatric AML. Nat Commun 10(1):2189. 10.1038/s41467-019-09917-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmus S, Naik J, Buil L, Wagenaar E, van Tellingen O, Schinkel AH (2014) In vivo disposition of doxorubicin is affected by mouse Oatp1a/1b and human OATP1A/1B transporters. Int J Cancer 135(7):1700–1710. 10.1002/ijc.28797 [DOI] [PubMed] [Google Scholar]
- Durmus S, Lozano-Mena G, van Esch A, Wagenaar E, van Tellingen O, Schinkel AH (2015) Preclinical mouse models to study human OATP1B1- and OATP1B3-mediated drug-drug interactions in vivo. Mol Pharm 12(12):4259–4269. 10.1021/acs.molpharmaceut.5b00453 [DOI] [PubMed] [Google Scholar]
- Duval M, Daniel SJ (2012) Meta-analysis of the efficacy of amifostine in the prevention of cisplatin ototoxicity. J Otolaryngol Head Neck Surg 41(5):309–315 [PubMed] [Google Scholar]
- Feurstein D, Kleinteich J, Heussner AH, Stemmer K, Dietrich DR (2010) Investigation of microcystin congener-dependent uptake into primary murine neurons. Environ Health Perspect 118(10):1370–1375. 10.1289/ehp.0901289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipski KK, Loos WJ, Verweij J, Sparreboom A (2008) Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res 14(12):3875–3880. 10.1158/1078-0432.CCR-07-4793 [DOI] [PubMed] [Google Scholar]
- Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A (2009) Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 86(4):396–402. 10.1038/clpt.2009.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke RM, Carducci MA, Rudek MA, Baker SD, Sparreboom A (2010a) Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J Clin Oncol 28(30):4562–4567. 10.1200/JCO.2010.30.7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke RM, Kosloske AM, Lancaster CS, Filipski KK, Hu C, Zolk O, Mathijssen RH, Sparreboom A (2010b) Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-D-glucosaminidase. Clin Cancer Res 16(16):4198–4206. 10.1158/1078-0432.CCR-10-0949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiks CN, Lam SW, Guchelaar HJ, Boven E (2015) Genetic polymorphisms and paclitaxel- or docetaxel-induced toxicities: a systematic review. Cancer Treat Rev 41(10):935–950. 10.1016/j.ctrv.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Fujita D, Saito Y, Nakanishi T, Tamai I (2016) Organic anion transporting polypeptide (OATP)2B1 contributes to gastrointestinal toxicity of anticancer drug SN-38, active metabolite of Irinotecan hydrochloride. Drug Metab Dispos 44(1):1–7. 10.1124/dmd.115.066712 [DOI] [PubMed] [Google Scholar]
- Gallegos-Castorena S, Martinez-Avalos A, Mohar-Betancourt A, Guerrero-Avendano G, Zapata-Tarres M, Medina-Sanson A (2007) Toxicity prevention with amifostine in pediatric osteosar-coma patients treated with cisplatin and doxorubicin. Pediatr Hematol Oncol 24(6):403–408. 10.1080/08880010701451244 [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Yee SW, Ratain MJ, Weinshilboum RM, Kamatani N, Nakamura Y (2012) Pharmacogenomics and patient care: one size does not fit all. Sci Transl Med 4(153):153ps118. 10.1126/scitranslmed.3003471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong IY, Kim RB (2013) Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab Pharmacokinet 28(1):4–18. 10.2133/dmpk.dmpk-12-rv-099 [DOI] [PubMed] [Google Scholar]
- Grecco HE, Schmick M, Bastiaens PI (2011) Signaling from the living plasma membrane. Cell 144(6):897–909. 10.1016/j.cell.2011.01.029 [DOI] [PubMed] [Google Scholar]
- Green H, Soderkvist P, Rosenberg P, Mirghani RA, Rymark P, Lundqvist EA, Peterson C (2009) Pharmacogenetic studies of paclitaxel in the treatment of ovarian cancer. Basic Clin Pharmacol Toxicol 104(2):130–137. 10.1111/j.1742-7843.2008.00351.x [DOI] [PubMed] [Google Scholar]
- Gregg RW, Molepo JM, Monpetit VJ, Mikael NZ, Redmond D, Gadia M, Stewart DJ (1992) Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol 10(5):795–803. 10.1200/JCO.1992.10.5.795 [DOI] [PubMed] [Google Scholar]
- Griffith DA, Jarvis SM (1996) Nucleoside and nucleobase transport systems of mammalian cells. Biochim Biophys Acta 1286(3):153–181 [DOI] [PubMed] [Google Scholar]
- Grube M, Kock K, Oswald S, Draber K, Meissner K, Eckel L, Bohm M, Felix SB, Vogelgesang S, Jedlitschky G, Siegmund W, Warzok R, Kroemer HK (2006) Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther 80(6):607–620. 10.1016/j.clpt.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Grube M, Ameling S, Noutsias M, Kock K, Triebel I, Bonitz K, Meissner K, Jedlitschky G, Herda LR, Reinthaler M, Rohde M, Hoffmann W, Kuhl U, Schultheiss HP, Volker U, Felix SB, Klingel K, Kandolf R, Kroemer HK (2011) Selective regulation of cardiac organic cation transporter novel type 2 (OCTN2) in dilated cardiomyopathy. Am J Pathol 178(6):2547–2559. 10.1016/j.ajpath.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, Hagenbuch B (2008) Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol 584(1):57–65. 10.1016/j.ejphar.2008.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Wahlgren B, Lushington GH, Hagenbuch B (2009) Identification, Ki determination and CoMFA analysis of nuclear receptor ligands as competitive inhibitors of OATP1B1-mediated estradiol-17beta-glucuronide transport. Pharmacol Res 60(1):50–56. 10.1016/j.phrs.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B (2010) Drug uptake systems in liver and kidney: a historic perspective. Clin Pharmacol Ther 87(1):39–47. 10.1038/clpt.2009.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafy S, El-Kadi AO, Jamali F (2012) Effect of inflammation on molecular targets and drug transporters. J Pharm Pharm Sci 15(3):361–375 [DOI] [PubMed] [Google Scholar]
- Hassan OT, Hassan RT, Arora RR (2016) Organic cation transporter-mediated clearance of cardiovascular drugs: a pharmacological perspective. Am J Ther 23(3):e855–e861. 10.1097/MJT.0000000000000148 [DOI] [PubMed] [Google Scholar]
- He L, Vasiliou K, Nebert DW (2009) Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics 3(2):195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman-Stoddard BM, Gandini S, Puntoni M, Dunn BK, DeCensi A, Szabo E (2016) Repurposing old drugs to chemoprevention: the case of metformin. Semin Oncol 43(1):123–133. 10.1053/j.seminoncol.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger MA, Clemencon B, Burrier RE, Bruford EA (2013) The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Asp Med 34(2–3):95–107. 10.1016/j.mam.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A 3rd, von Hoff D, Schuchter LM (2009) American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 27(1):127–145. 10.1200/JCO.2008.17.2627 [DOI] [PubMed] [Google Scholar]
- Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL, American Society of Clinical Oncology (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32(18):1941–1967. 10.1200/JCO.2013.54.0914 [DOI] [PubMed] [Google Scholar]
- Hertz DL (2013) Germline pharmacogenetics of paclitaxel for cancer treatment. Pharmacogenomics 14(9):1065–1084. 10.2217/pgs.13.90 [DOI] [PubMed] [Google Scholar]
- Hertz DL, Motsinger-Reif AA, Drobish A, Winham SJ, McLeod HL, Carey LA, Dees EC (2012) CYP2C8*3 predicts benefit/risk profile in breast cancer patients receiving neoadjuvant paclitaxel. Breast Cancer Res Treat 134(1):401–410. 10.1007/s10549-012-2054-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz DL, Roy S, Motsinger-Reif AA, Drobish A, Clark LS, McLeod HL, Carey LA, Dees EC (2013) CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann Oncol 24(6):1472–1478. 10.1093/annonc/mdt018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A (2014) Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 4(9):998–1013. 10.1158/2159-8290.CD-14-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JW, Bedwell DW, Zamek-Gliszczynski MJ (2012) Ablation of both organic cation transporter (OCT)1 and OCT2 alters metformin pharmacokinetics but has no effect on tissue drug exposure and pharmacodynamics. Drug Metab Dispos 40(6):1170–1177. 10.1124/dmd.112.044875 [DOI] [PubMed] [Google Scholar]
- Holbeck SL, Camalier R, Crowell JA, Govindharajulu JP, Hollingshead MG, Anderson LW, Polley EC, Rubinstein L, Srivastava AK, Wilsker DF, Collins JM, Doroshow JH (2017) The National Cancer Institute ALMANAC: a comprehensive screening resource for the detection of anticancer drug pairs with enhanced therapeutic activity. Cancer Res. 10.1158/0008-5472.CAN-17-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J, Stanko J, Varchenko M, Ding H, Madden VJ, Bagnell CR, Wyrick SD, Chaney SG (1998) Comparative neurotoxicity of oxaliplatin, cisplatin, and ormaplatin in a Wistar rat model. Toxicol Sci 46(2):342–351. 10.1006/toxs.1998.2558 [DOI] [PubMed] [Google Scholar]
- Hosoyamada M, Sekine T, Kanai Y, Endou H (1999) Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Phys 276(1):F122–F128. 10.1152/ajprenal.1999.276.1.F122 [DOI] [PubMed] [Google Scholar]
- Hu S, Sprowl JA (2018) Strategies to reduce solute carrier-mediated toxicity. Clin Pharmacol Ther. 10.1002/cpt.1185 [DOI] [PubMed] [Google Scholar]
- Hu S, Leblanc AF, Gibson AA, Hong KW, Kim JY, Janke LJ, Li L, Vasilyeva A, Finkelstein DB, Sprowl JA, Sweet DH, Schlatter E, Ciarimboli G, Schellens J, Baker SD, Pabla N, Sparreboom A (2017) Identification of OAT1/OAT3 as contributors to cisplatin toxicity. Clin Transl Sci 10(5):412–420. 10.1111/cts.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucke A, Rinschen MM, Bauer OB, Sperling M, Karst U, Koppen C, Sommer K, Schroter R, Ceresa C, Chiorazzi A, Canta A, Semperboni S, Marmiroli P, Cavaletti G, Schlatt S, Schlatter E, Pavenstadt H, Heitplatz B, Van Marck V, Sparreboom A, Barz V, Knief A, Deuster D, Zehnhoff-Dinnesen AA, Ciarimboli G (2019) An integrative approach to cisplatin chronic toxicities in mice reveals importance of organic cation-transporter-dependent protein networks for renoprotection. Arch Toxicol 93(10):2835–2848. 10.1007/s00204-019-02557-9 [DOI] [PubMed] [Google Scholar]
- Hwang YY, Trendell-Smith NJ, Yeung CK, Kwong YL (2012) Fatal palmar-plantar erythrodysesthesia after clofarabine and cytarabine chemotherapy. Acta Haematol 128(3):151–153. 10.1159/000338828 [DOI] [PubMed] [Google Scholar]
- Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, Heym KM, Christensen R, Onciu M, Shurtleff SA, Pounds SB, Pui CH, Ribeiro RC, Campana D, Baker SD (2011) Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol 29(24):3293–3300. 10.1200/JCO.2011.34.7427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti F, Schilsky RL, Ramirez J, Janisch L, Undevia S, House LK, Das S, Wu K, Turcich M, Marsh R, Karrison T, Maitland ML, Salgia R, Ratain MJ (2014) Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J Clin Oncol 32(22):2328–2334. 10.1200/JCO.2014.55.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9(3):215–236. 10.1038/nrd3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip V, Liu JJ, McKeage MJ (2013) Evaluation of effects of copper histidine on copper transporter 1-mediated accumulation of platinum and oxaliplatin-induced neurotoxicity in vitro and in vivo. Clin Exp Pharmacol Physiol 40(6):371–378. 10.1111/1440-1681.12088 [DOI] [PubMed] [Google Scholar]
- Ito S, Kusuhara H, Yokochi M, Toyoshima J, Inoue K, Yuasa H, Sugiyama Y (2012) Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther 340(2):393–403. 10.1124/jpet.111.184986 [DOI] [PubMed] [Google Scholar]
- Iusuf D, van de Steeg E, Schinkel AH (2012) Hepatocyte hopping of OATP1B substrates contributes to efficient hepatic detoxification. Clin Pharmacol Ther 92(5):559–562. 10.1038/clpt.2012.143 [DOI] [PubMed] [Google Scholar]
- Iusuf D, Ludwig M, Elbatsh A, van Esch A, van de Steeg E, Wagenaar E, van der Valk M, Lin F, van Tellingen O, Schinkel AH (2014) OATP1A/1B transporters affect irinotecan and SN-38 pharmacokinetics and carboxylesterase expression in knockout and humanized transgenic mice. Mol Cancer Ther 13(2):492–503. 10.1158/1535-7163.Mct-13-0541 [DOI] [PubMed] [Google Scholar]
- Iusuf D, Hendrikx JJ, van Esch A, van de Steeg E, Wagenaar E, Rosing H, Beijnen JH, Schinkel AH (2015) Human OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and clearance of docetaxel. Int J Cancer 136(1):225–233. 10.1002/ijc.28970 [DOI] [PubMed] [Google Scholar]
- Iwata D, Kato Y, Wakayama T, Sai Y, Kubo Y, Iseki S, Tsuji A (2008) Involvement of carnitine/organic cation transporter OCTN2 (SLC22A5) in distribution of its substrate carnitine to the heart. Drug Metab Pharmacokinet 23(3):207–215 [DOI] [PubMed] [Google Scholar]
- Izumi S, Nozaki Y, Kusuhara H, Hotta K, Mochizuki T, Komori T, Maeda K, Sugiyama Y (2018) Relative activity factor (RAF)-based scaling of uptake clearance mediated by organic anion transporting polypeptide (OATP) 1B1 and OATP1B3 in human hepatocytes. Mol Pharm. 10.1021/acs.molpharmaceut.8b00138 [DOI] [PubMed] [Google Scholar]
- Jacobs C, Coleman CN, Rich L, Hirst K, Weiner MW (1984) Inhibition of cis-diamminedichloroplatinum secretion by the human kidney with probenecid. Cancer Res 44(8):3632–3635 [PubMed] [Google Scholar]
- Jain L, Gardner ER, Figg WD, Chernick MS, Kong HH (2010) Lack of association between excretion of sorafenib in sweat and hand-foot skin reaction. Pharmacotherapy 30(1):52–56. 10.1592/phco.30.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson SM, Liu J, Connor B, McKeage MJ (2005) Oxaliplatin causes selective atrophy of a subpopulation of dorsal root ganglion neurons without inducing cell loss. Cancer Chemother Pharmacol 56(4):391–399. 10.1007/s00280-004-0953-4 [DOI] [PubMed] [Google Scholar]
- Jensen JB, Sundelin EI, Jakobsen S, Gormsen LC, Munk OL, Frokiaer J, Jessen N (2016) [11C]-metformin distribution in the liver and small intestine using dynamic PET in mice demonstrates tissue-specific transporter dependency. Diabetes. 10.2337/db16-0032 [DOI] [PubMed] [Google Scholar]
- Joerger M, van Schaik RH, Becker ML, Hayoz S, Pollak M, Cathomas R, Winterhalder R, Gillessen S, Rothermundt C (2015) Multidrug and toxin extrusion 1 and human organic cation transporter 1 polymorphisms in patients with castration-resistant prostate cancer receiving metformin (SAKK 08/09). Prostate Cancer Prostatic Dis 18(2):167–172. 10.1038/pcan.2015.8 [DOI] [PubMed] [Google Scholar]
- Jong NN, Nakanishi T, Liu JJ, Tamai I, McKeage MJ (2011) Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. J Pharmacol Exp Ther 338(2):537–547. 10.1124/jpet.111.181297 [DOI] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Mol CA, Buitelaar M, Koepsell H, Smit JW, Schinkel AH (2001) Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol 21(16):5471–5477. 10.1128/MCB.21.16.5471-5477.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH (2003) Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol 23(21):7902–7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback HR, Sahin-Toth M, Weinglass AB (2001) The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol 2(8):610–620. 10.1038/35085077 [DOI] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, Sridhara R, Garvey P, Justice R, Pazdur R (2006) Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res 12(24):7271–7278. 10.1158/1078-0432.CCR-06-1249 [DOI] [PubMed] [Google Scholar]
- Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, Inui K (2005) Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet 20(5):379–386 [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Kusuhara H, Sugiyama Y (2010) Functional characterization of multidrug resistance-associated protein 3 (mrp3/abcc3) in the basolateral efflux of glucuronide conjugates in the mouse small intestine. J Pharmacol Exp Ther 332(2):659–666. 10.1124/jpet.109.156943 [DOI] [PubMed] [Google Scholar]
- Klein J, Bentur Y, Cheung D, Moselhy G, Koren G (1991) Renal handling of cisplatin: interactions with organic anions and cations in the dog. Clin Invest Med 14(5):388–394 [PubMed] [Google Scholar]
- Kobayashi D, Aizawa S, Maeda T, Tsuboi I, Yabuuchi H, Nezu J, Tsuji A, Tamai I (2004) Expression of organic cation transporter OCTN1 in hematopoietic cells during erythroid differentiation. Exp Hematol 32(12):1156–1162. 10.1016/j.exphem.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Koepsell H (2015) Role of organic cation transporters in drug-drug interaction. Expert Opin Drug Metab Toxicol 11(10):1619–1633. 10.1517/17425255.2015.1069274 [DOI] [PubMed] [Google Scholar]
- Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA (2001) Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol 19(1):191–196. 10.1200/JCO.2001.19.1.191 [DOI] [PubMed] [Google Scholar]
- Kubo Y, Shimizu Y, Kusagawa Y, Akanuma S, Hosoya K (2013) Propranolol transport across the inner blood-retinal barrier: potential involvement of a novel organic cation transporter. J Pharm Sci 102(9):3332–3342. 10.1002/jps.23535 [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H (1999) Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem 274(19):13675–13680 [DOI] [PubMed] [Google Scholar]
- Lam SW, Frederiks CN, van der Straaten T, Honkoop AH, Guchelaar HJ, Boven E (2016) Genotypes of CYP2C8 and FGD4 and their association with peripheral neuropathy or early dose reduction in paclitaxel-treated breast cancer patients. Br J Cancer 115(11):1335–1342. 10.1038/bjc.2016.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster CS, Sprowl JA, Walker AL, Hu S, Gibson AA, Sparreboom A (2013) Modulation of OATP1B-type transporter function alters cellular uptake and disposition of platinum chemotherapeutics. Mol Cancer Ther 12(8):1537–1544. 10.1158/1535-7163.MCT-12-0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T, am Zehnhoff-Dinnesen A, Radtke S, Meitert J, Zolk O (2013) Understanding platinum-induced ototoxicity. Trends Pharmacol Sci 34(8):458–469. 10.1016/j.tips.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Lanvers-Kaminsky C, Sprowl JA, Malath I, Deuster D, Eveslage M, Schlatter E, Mathijssen RH, Boos J, Jurgens H, Am Zehnhoff-Dinnesen AG, Sparreboom A, Ciarimboli G (2015) Human OCT2 variant c.808G>T confers protection effect against cisplatin-induced ototoxicity. Pharmacogenomics 16(4):323–332. 10.2217/pgs.14.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc AF, Sprowl JA, Alberti P, Chiorazzi A, Arnold WD, Gibson AA, Hong KW, Pioso MS, Chen M, Huang KM, Chodisetty V, Costa O, Florea T, de Bruijn P, Mathijssen RH, Reinbolt RE, Lustberg MB, Sucheston-Campbell LE, Cavaletti G, Sparreboom A, Hu S (2018) OATP1B2 deficiency protects against paclitaxel-induced neurotoxicity. J Clin Invest 128(2):816–825. 10.1172/JCI96160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Leake BF, Teft W, Tirona RG, Kim RB, Ho RH (2015) Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance. Mol Cancer Ther 14(4):994–1003. 10.1158/1535-7163.MCT-14-0547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Leake BF, Kim RB, Ho RH (2017) Contribution of organic anion-transporting polypeptides 1A/1B to doxorubicin uptake and clearance. Mol Pharmacol 91(1):14–24. 10.1124/mol.116.105544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, Rasmussen SL, Blumenschein GR, Freireich EJ (1982) Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med 96(2):133–139 [DOI] [PubMed] [Google Scholar]
- Leibbrandt ME, Wolfgang GH, Metz AL, Ozobia AA, Haskins JR (1995) Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int 48(3):761–770 [DOI] [PubMed] [Google Scholar]
- Leskela S, Jara C, Leandro-Garcia LJ, Martinez A, Garcia-Donas J, Hernando S, Hurtado A, Vicario JC, Montero-Conde C, Landa I, Lopez-Jimenez E, Cascon A, Milne RL, Robledo M, Rodriguez-Antona C (2011) Polymorphisms in cytochromes P450 2C8 and 3A5 are associated with paclitaxel neurotoxicity. Pharmacogenomics J 11(2):121–129. 10.1038/tpj.2010.13 [DOI] [PubMed] [Google Scholar]
- Letschert K, Faulstich H, Keller D, Keppler D (2006) Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci 91(1):140–149. 10.1093/toxsci/kfj141 [DOI] [PubMed] [Google Scholar]
- Li S, Chen Y, Zhang S, More SS, Huang X, Giacomini KM (2011) Role of organic cation transporter 1, OCT1 in the pharmacokinetics and toxicity of cis-diammine(pyridine) chloroplatinum(II) and oxaliplatin in mice. Pharm Res 28(3):610–625. 10.1007/s11095-010-0312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, Polli JE, Shu Y (2013) Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol 273(1):100–109. 10.1016/j.taap.2013.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Ventura C (2013) Regenerative medicine approach to repair the failing heart. Vasc Pharmacol 58(3):159–163. 10.1016/j.vph.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Lipka DB, Wagner MC, Dziadosz M, Schnoder T, Heidel F, Schemionek M, Melo JV, Kindler T, Muller-Tidow C, Koschmieder S, Fischer T (2012) Intracellular retention of ABL kinase inhibitors determines commitment to apoptosis in CML cells. PLoS One 7(7):e40853. 10.1371/journal.pone.0040853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, Hudson MM, Kremer LC, Landy DC, Miller TL, Oeffinger KC, Rosenthal DN, Sable CA, Sallan SE, Singh GK, Steinberger J, Cochran TR, Wilkinson JD (2013) Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 128(17):1927–1995. 10.1161/CIR.0b013e3182a88099 [DOI] [PubMed] [Google Scholar]
- Lipworth AD, Robert C, Zhu AX (2009) Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology 77(5):257–271. 10.1159/000258880 [DOI] [PubMed] [Google Scholar]
- Lu R, Chan BS, Schuster VL (1999) Cloning of the human kidney PAH transporter: narrow substrate specificity and regulation by protein kinase C. Am J Phys 276(2):F295–F303. 10.1152/ajprenal.1999.276.2.F295 [DOI] [PubMed] [Google Scholar]
- Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A (2015) Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol 72(2):221–236. 10.1016/j.jaad.2014.07.033 [DOI] [PubMed] [Google Scholar]
- Maniez-Devos DM, Baurain R, Lesne M, Trouet A (1986) Doxorubicin and daunorubicin plasmatic, hepatic and renal disposition in the rabbit with or without enterohepatic circulation. J Pharmacol 17(1):1–13 [PubMed] [Google Scholar]
- Marada VV, Florl S, Kuhne A, Burckhardt G, Hagos Y (2015) Interaction of human organic anion transporter polypeptides 1B1 and 1B3 with antineoplastic compounds. Eur J Med Chem 92:723–731. 10.1016/j.ejmech.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Marmiroli P, Cavaletti G (2016) Drugs for the treatment of peripheral neuropathies. Expert Opin Pharmacother 17(3):381–394. 10.1517/14656566.2016.1120719 [DOI] [PubMed] [Google Scholar]
- McBride BF, Yang T, Liu K, Urban TJ, Giacomini KM, Kim RB, Roden DM (2009) The organic cation transporter, OCTN1, expressed in the human heart, potentiates antagonism of the HERG potassium channel. J Cardiovasc Pharmacol 54(1):63–71. 10.1097/FJC.0b013e3181abc288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreight LJ, Bailey CJ, Pearson ER (2016) Metformin and the gastrointestinal tract. Diabetologia 59(3):426–435. 10.1007/s00125-015-3844-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeage MJ, Hsu T, Screnci D, Haddad G, Baguley BC (2001) Nucleolar damage correlates with neurotoxicity induced by different platinum drugs. Br J Cancer 85(8):1219–1225. 10.1054/bjoc.2001.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney SR, Goldberg RM, McLeod HL (2009) Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 8(1):10–16. 10.1158/1535-7163.MCT-08-0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medwid S, Li MMJ, Knauer MJ, Lin K, Mansell SE, Schmerk CL, Zhu C, Griffin KE, Yousif MD, Dresser GK, Schwarz UI, Kim RB, Tirona RG (2019) Fexofenadine and rosuvastatin pharmacokinetics in mice with targeted disruption of organic anion transporting polypeptide 2B1. Drug Metab Dispos 47(8):832–842. 10.1124/dmd.119.087619 [DOI] [PubMed] [Google Scholar]
- Mielke S, Sparreboom A, Mross K (2006) Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer 42(1):24–30. 10.1016/j.ejca.2005.06.030 [DOI] [PubMed] [Google Scholar]
- Motohashi H, Uwai Y, Hiramoto K, Okuda M, Inui K (2004) Different transport properties between famotidine and cimetidine by human renal organic ion transporters (SLC22A). Eur J Pharmacol 503(1–3):25–30. 10.1016/j.ejphar.2004.09.032 [DOI] [PubMed] [Google Scholar]
- Murray AW (1971) The biological significance of purine salvage. Annu Rev Biochem 40:811–826. 10.1146/annurev.bi.40.070171.004115 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K (2010) Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol 80(11):1762–1767. 10.1016/j.bcp.2010.08.019 [DOI] [PubMed] [Google Scholar]
- Ni W, Ji J, Dai Z, Papp A, Johnson AJ, Ahn S, Farley KL, Lin TS, Dalton JT, Li X, Jarjoura D, Byrd JC, Sadee W, Grever MR, Phelps MA (2010) Flavopiridol pharmacogenetics: clinical and functional evidence for the role of SLCO1B1/OATP1B1 in flavopiridol disposition. PLoS One 5(11):e13792. 10.1371/journal.pone.0013792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuweboer AJ, Hu S, Gui C, Hagenbuch B, Ghobadi Moghaddam-Helmantel IM, Gibson AA, de Bruijn P, Mathijssen RH, Sparreboom A (2014) Influence of drug formulation on OATP1B-mediated transport of paclitaxel. Cancer Res 74(11):3137–3145. 10.1158/0008-5472.CAN-13-3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK (2015) What do drug transporters really do? Nat Rev Drug Discov 14(1):29–44. 10.1038/nrd4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Takeuchi K, Hosoda A, Sugano S, Morisaki E, Ohishi A, Nagasawa K (2018) Ergothioneine ameliorates oxaliplatin-induced peripheral neuropathy in rats. Life Sci 207:516–524. 10.1016/j.lfs.2018.07.006 [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S (2008) Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab Pharmacokinet 23(1):22–44 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I (2005) Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos 33(3):434–439. 10.1124/dmd.104.001909 [DOI] [PubMed] [Google Scholar]
- Okabe M, Unno M, Harigae H, Kaku M, Okitsu Y, Sasaki T, Mizoi T, Shiiba K, Takanaga H, Terasaki T, Matsuno S, Sasaki I, Ito S, Abe T (2005) Characterization of the organic cation transporter SLC22A16: a doxorubicin importer. Biochem Biophys Res Commun 333(3):754–762. 10.1016/j.bbrc.2005.05.174 [DOI] [PubMed] [Google Scholar]
- Okabe M, Szakacs G, Reimers MA, Suzuki T, Hall MD, Abe T, Weinstein JN, Gottesman MM (2008) Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol Cancer Ther 7(9):3081–3091. 10.1158/1535-7163.MCT-08-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlin A, Sartor O, Rothermundt C, Cathomas R, De Bono JS, Shen L, Su Z, Gillessen S (2015) Analysis of side effect profile of alopecia, nail changes, peripheral neuropathy, and dysgeusia in prostate cancer patients treated with docetaxel and cabazitaxel. Clin Genitourin Cancer 13(4):e205–e208. 10.1016/j.clgc.2015.01.010 [DOI] [PubMed] [Google Scholar]
- Osman NM, Litterst CL (1983) Effect of probenecid and N’-methylnicotinamide on renal handling of cis-dichlorodiammineplatinum-II in rats. Cancer Lett 19(1):107–111 [DOI] [PubMed] [Google Scholar]
- Ota K, Ito K, Akahira J, Sato N, Onogawa T, Moriya T, Unno M, Abe T, Niikura H, Takano T, Yaegashi N (2007) Expression of organic cation transporter SLC22A16 in human epithelial ovarian cancer: a possible role of the adriamycin importer. Int J Gynecol Pathol 26(3):334–340. 10.1097/01.pgp.0000236951.33914.1b [DOI] [PubMed] [Google Scholar]
- Owens JK, Shewach DS, Ullman B, Mitchell BS (1992) Resistance to 1-β-d-arabinofuranosylcytosine in human T-lymphoblasts mediated by mutations within the deoxycytidine kinase gene. Cancer Res 52(9):2389–2393 [PubMed] [Google Scholar]
- Pabla N, Gibson AA, Buege M, Ong SS, Li L, Hu S, Du G, Sprowl JA, Vasilyeva A, Janke LJ, Schlatter E, Chen T, Ciarimboli G, Sparreboom A (2015) Mitigation of acute kidney injury by cell-cycle inhibitors that suppress both CDK4/6 and OCT2 functions. Proc Natl Acad Sci U S A 112(16):5231–5236. 10.1073/pnas.1424313112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk AC, Giacomini KM, McKeon C, Shuldiner AR, Florez JC (2014) Metformin pharmacogenomics: current status and future directions. Diabetes 63(8):2590–2599. 10.2337/db13-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, Wong GY, Mantyh PW (2007) Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 203(1):42–54. 10.1016/j.expneurol.2006.07.022 [DOI] [PubMed] [Google Scholar]
- Price PM, Safirstein RL, Megyesi J (2009) The cell cycle and acute kidney injury. Kidney Int 76(6):604–613. 10.1038/ki.2009.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunand N, He X, van Sluis R, Mahoney B, Baggett B, Taylor CW, Paine-Murrieta G, Roe D, Bhujwalla ZM, Gillies RJ (1999) Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer 80(7):1005–1011. 10.1038/sj.bjc.6690455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Vrignaud P, Nguyen-Ngoc T, Iliadis A, Mauriac L, Hurteloup P (1985) Comparative pharmacokinetics and metabolism of doxorubicin and epirubicin in patients with metastatic breast cancer. Cancer Treat Rep 69(6):633–640 [PubMed] [Google Scholar]
- Robert J, Bui NB, Vrignaud P (1987) Pharmacokinetics of doxorubicin in sarcoma patients. Eur J Clin Pharmacol 31(6):695–699 [DOI] [PubMed] [Google Scholar]
- Ross DA, Gale GR (1979) Reduction of the renal toxicity of cis-dichlorodiammineplatinum(II) by probenecid. Cancer Treat Rep 63(5):781–787 [PubMed] [Google Scholar]
- Safirstein R, Miller P, Guttenplan JB (1984) Uptake and metabolism of cisplatin by rat kidney. Kidney Int 25(5):753–758 [DOI] [PubMed] [Google Scholar]
- Salphati L, Chu X, Chen L, Prasad B, Dallas S, Evers R, Mamaril-Fishman D, Geier EG, Kehler J, Kunta J, Mezler M, Laplanche L, Pang J, Rode A, Soars MG, Unadkat JD, van Waterschoot RA, Yabut J, Schinkel AH, Scheer N (2014) Evaluation of organic anion transporting polypeptide 1B1 and 1B3 humanized mice as a translational model to study the pharmacokinetics of statins. Drug Metab Dispos 42(8):1301–1313. 10.1124/dmd.114.057976 [DOI] [PubMed] [Google Scholar]
- Sastry J, Kellie SJ (2005) Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr Hematol Oncol 22(5):441–445. 10.1080/08880010590964381 [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmed MM, Al-Shabanah OA, Hafez MM, Aleisa AM, Al-Rejaie SS (2010) Inhibition of gene expression of heart fatty acid binding protein and organic cation/carnitine transporter in doxorubicin cardiomyopathic rat model. Eur J Pharmacol 640(1–3):143–149. 10.1016/j.ejphar.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Schneider BP, Hershman DL, Loprinzi C (2015a) Symptoms: chemotherapy-induced peripheral neuropathy. Adv Exp Med Biol 862:77–87. 10.1007/978-3-319-16366-6_6 [DOI] [PubMed] [Google Scholar]
- Schneider BP, Li L, Radovich M, Shen F, Miller KD, Flockhart DA, Jiang G, Vance G, Gardner L, Vatta M, Bai S, Lai D, Koller D, Zhao F, O’Neill A, Smith ML, Railey E, White C, Partridge A, Sparano J, Davidson NE, Foroud T, Sledge GW Jr (2015b) Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res 21(22):5082–5091. 10.1158/1078-0432.CCR-15-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scripture CD, Figg WD, Sparreboom A (2006) Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol 4(2):165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett CM, Giacomini KM (2007) Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 117(5):1422–1431. 10.1172/JCI30558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339(13):900–905. 10.1056/NEJM199809243391307 [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175(4023):720–731 [DOI] [PubMed] [Google Scholar]
- Sissung TM, Mross K, Steinberg SM, Behringer D, Figg WD, Sparreboom A, Mielke S (2006) Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer 42(17):2893–2896. 10.1016/j.ejca.2006.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A (2005) Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther 4(8):815–818 [DOI] [PubMed] [Google Scholar]
- Smith NF, Marsh S, Scott-Horton TJ, Hamada A, Mielke S, Mross K, Figg WD, Verweij J, McLeod HL, Sparreboom A (2007) Variants in the SLCO1B3 gene: interethnic distribution and association with paclitaxel pharmacokinetics. Clin Pharmacol Ther 81(1):76–82. 10.1038/sj.clpt.6100011 [DOI] [PubMed] [Google Scholar]
- Song IH, Zong J, Borland J, Jerva F, Wynne B, Zamek-Gliszczynski MJ, Humphreys JE, Bowers GD, Choukour M (2016) The effect of Dolutegravir on the pharmacokinetics of metformin in healthy subjects. J Acquir Immune Defic Syndr. 10.1097/QAI.0000000000000983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparreboom A, Huizing MT, Boesen JJ, Nooijen WJ, van Tellingen O, Beijnen JH (1995) Isolation, purification, and biological activity of mono- and dihydroxylated paclitaxel metabolites from human feces. Cancer Chemother Pharmacol 36(4):299–304. 10.1007/BF00689047 [DOI] [PubMed] [Google Scholar]
- Sprowl JA, Sparreboom A (2014) Uptake carriers and oncology drug safety. Drug Metab Dispos 42(4):611–622. 10.1124/dmd.113.055806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowl JA, Ciarimboli G, Lancaster CS, Giovinazzo H, Gibson AA, Du G, Janke LJ, Cavaletti G, Shields AF, Sparreboom A (2013a) Oxaliplatin-induced neurotoxicity is dependent on the organic cation transporter OCT2. Proc Natl Acad Sci USA 110(27):11199–11204. 10.1073/pnas.1305321110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowl JA, Mathijssen RH, Sparreboom A (2013b) Can erlotinib ameliorate cisplatin-induced toxicities? J Clin Oncol 31(27):3442–3443. 10.1200/JCO.2013.50.8184 [DOI] [PubMed] [Google Scholar]
- Sprowl JA, Ness RA, Sparreboom A (2013c) Polymorphic transporters and platinum pharmacodynamics. Drug Metab Pharmacokinet 28(1):19–27. 10.2133/dmpk.dmpk-12-rv-073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowl JA, Lancaster CS, Pabla N, Hermann E, Kosloske AM, Gibson AA, Li L, Zeeh D, Schlatter E, Janke LJ, Ciarimboli G, Sparreboom A (2014) Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res 20(15):4026–4035. 10.1158/1078-0432.CCR-14-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowl JA, Ong SS, Gibson AA, Hu S, Du G, Lin W, Li L, Bharill S, Ness RA, Stecula A, Offer SM, Diasio RB, Nies AT, Schwab M, Cavaletti G, Schlatter E, Ciarimboli G, Schellens JH, Isacoff EY, Sali A, Chen T, Baker SD, Sparreboom A, Pabla N (2016) A phosphotyrosine switch regulates organic cation transporters. Nat Commun 7:10880. 10.1038/ncomms10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stage TB, Brosen K, Christensen MM (2015a) A comprehensive review of drug-drug interactions with metformin. Clin Pharmacokinet 54(8):811–824. 10.1007/s40262-015-0270-6 [DOI] [PubMed] [Google Scholar]
- Stage TB, Damkier P, Pedersen RS, Christensen MM, Christiansen L, Christensen K, Brosen K (2015b) A twin study of the trough plasma steady-state concentration of metformin. Pharmacogenet Genomics 25(5):259–262. 10.1097/FPC.0000000000000133 [DOI] [PubMed] [Google Scholar]
- Stage TB, Lee MP, Hallas J, Christensen MM, Brosen K, Christensen K, Gagne JJ, Pottegard A (2016) Early discontinuation of metformin in individuals treated with inhibitors of transporters of metformin. Basic Clin Pharmacol Toxicol 118(6):487–495. 10.1111/bcpt.12579 [DOI] [PubMed] [Google Scholar]
- Sucheston LE, Zhao H, Yao S, Zirpoli G, Liu S, Barlow WE, Moore HC, Thomas Budd G, Hershman DL, Davis W, Ciupak GL, Stewart JA, Isaacs C, Hobday TJ, Salim M, Hortobagyi GN, Gralow JR, Livingston RB, Albain KS, Hayes DF, Ambrosone CB (2011) Genetic predictors of taxane-induced neurotoxicity in a SWOG phase III intergroup adjuvant breast cancer treatment trial (S0221). Breast Cancer Res Treat 130(3):993–1002. 10.1007/s10549-011-1671-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Li J, Guo C, Xing H, Xu J, Wen Y, Qiu Z, Zhang Q, Zheng Y, Chen X, Zhao D (2016) Pharmacokinetic effects of curcumin on docetaxel mediated by OATP1B1, OATP1B3 and CYP450s. Drug Metab Pharmacokinet 31(4):269–275. 10.1016/j.dmpk.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Svoboda M, Wlcek K, Tafemer B, Hering S, Stieger B, Tong D, Zeillinger R, Thalhammer T, Jager W (2011) Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport. Biomed Pharmacother 65(6):417–426. 10.1016/j.biopha.2011.04.031 [DOI] [PubMed] [Google Scholar]
- Swain SM, Whaley FS, Ewer MS (2003) Congestive heart failure in patients treated with doxoru-bicin: a retrospective analysis of three trials. Cancer 97(11):2869–2879. 10.1002/cncr.11407 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A (2000) Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun 273(1):251–260. 10.1006/bbrc.2000.2922 [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Shimizu C, Hamada A, Hashimoto K, Ikeda K, Nishizawa D, Hasegawa J, Shimomura A, Ozaki Y, Tamura N, Yamamoto H, Yunokawa M, Yonemori K, Takano T, Kawabata H, Tamura K, Fujiwara Y (2017) Paclitaxel-induced sensory peripheral neuropathy is associated with an ABCB1 single nucleotide polymorphism and older age in Japanese. Cancer Chemother Pharmacol 79(6):1179–1186. 10.1007/s00280-017-3314-9 [DOI] [PubMed] [Google Scholar]
- Thompson SW, Davis LE, Kornfeld M, Hilgers RD, Standefer JC (1984) Cisplatin neuropathy. Clinical, electrophysiologic, morphologic, and toxicologic studies. Cancer 54(7):1269–1275 [DOI] [PubMed] [Google Scholar]
- Tian A, Lu H, Zhang J, Fu S, Jiang Z, Lam W, Guan F, Chen L, Feng L, Cheng Y (2018) Multikinase inhibitor sorafenib induces skin toxicities in tumor-bearing mice. Cancer Chemother Pharmacol 81(6):1025–1033. 10.1007/s00280-018-3575-y [DOI] [PubMed] [Google Scholar]
- Travis LB, Fossa SD, Sesso HD, Frisina RD, Herrmann DN, Beard CJ, Feldman DR, Pagliaro LC, Miller RC, Vaughn DJ, Einhorn LH, Cox NJ, Dolan ME (2014) Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst 106(5). 10.1093/jnci/dju044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, Grosshennig A, Linsel-Nitschke P, Perret C, DeSuremain M, Meitinger T, Wright BJ, Preuss M, Balmforth AJ, Ball SG, Meisinger C, Germain C, Evans A, Arveiler D, Luc G, Ruidavets JB, Morrison C, van der Harst P, Schreiber S, Neureuther K, Schafer A, Bugert P, El Mokhtari NE, Schrezenmeir J, Stark K, Rubin D, Wichmann HE, Hengstenberg C, Ouwehand W, Wellcome Trust Case Control Consortium, Cardiogenics Consortium, Ziegler A, Tiret L, Thompson JR, Cambien F, Schunkert H, Samani NJ (2009) Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet 41(3):283–285. 10.1038/ng.314 [DOI] [PubMed] [Google Scholar]
- Trevisan G, Materazzi S, Fusi C, Altomare A, Aldini G, Lodovici M, Patacchini R, Geppetti P, Nassini R (2013) Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res 73(10):3120–3131. 10.1158/0008-5472.CAN-12-4370 [DOI] [PubMed] [Google Scholar]
- Tschirka J, Kreisor M, Betz J, Grundemann D (2018) Substrate selectivity check of the ergothioneine transporter. Drug Metab Dispos 46(6):779–785. 10.1124/dmd.118.080440 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Terada T, Mizuno T, Katsura T, Shimakura J, Inui K (2009) Targeted disruption of the multidrug and toxin extrusion 1 (MATE1) gene in mice reduces renal secretion of metformin. Mol Pharmacol 75(6):1280–1286. 10.1124/mol.109.056242 [DOI] [PubMed] [Google Scholar]
- Urban TJ, Yang C, Lagpacan LL, Brown C, Castro RA, Taylor TR, Huang CC, Stryke D, Johns SJ, Kawamoto M, Carlson EJ, Ferrin TE, Burchard EG, Giacomini KM (2007) Functional effects of protein sequence polymorphisms in the organic cation/ergothioneine transporter OCTN1 (SLC22A4). Pharmacogenet Genomics 17(9):773–782. 10.1097/FPC.0b013e3281c6d08e [DOI] [PubMed] [Google Scholar]
- Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK (2008) Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol 294(4):F867–F873. 10.1152/ajprenal.00528.2007 [DOI] [PubMed] [Google Scholar]
- van de Steeg E, van Esch A, Wagenaar E, van der Kruijssen CM, van Tellingen O, Kenworthy KE, Schinkel AH (2011) High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin Cancer Res 17(2):294–301. 10.1158/1078-0432.CCR-10-1980 [DOI] [PubMed] [Google Scholar]
- van de Steeg E, van Esch A, Wagenaar E, Kenworthy KE, Schinkel AH (2013) Influence of human OATP1B1, OATP1B3, and OATP1A2 on the pharmacokinetics of methotrexate and paclitaxel in humanized transgenic mice. Clin Cancer Res 19(4):821–832. 10.1158/1078-0432.CCR-12-2080 [DOI] [PubMed] [Google Scholar]
- VanWert AL, Gionfriddo MR, Sweet DH (2010) Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos 31(1):1–71. 10.1002/bdd.693 [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM (1979) Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91(5):710–717 [DOI] [PubMed] [Google Scholar]
- Waissbluth S, Daniel SJ (2013) Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hear Res 299:37–45. 10.1016/j.heares.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Wang L, Weinshilboum R (2014) Metformin pharmacogenomics: biomarkers to mechanisms. Diabetes 63(8):2609–2610. 10.2337/db14-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y (2002) Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther 302(2):510–515. 10.1124/jpet.102.034140 [DOI] [PubMed] [Google Scholar]
- Wang L, Chen J, Zeng Y, Wei J, Jing J, Li G, Su L, Tang X, Wu T, Zhou L (2016) Functional variant in the SLC22A3-LPAL2-LPA gene cluster contributes to the severity of coronary artery disease. Arterioscler Thromb Vasc Biol 36(9):1989–1996. 10.1161/ATVBAHA.116.307311 [DOI] [PubMed] [Google Scholar]
- Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, Ganapathy V (2000) Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta 1466(1–2):315–327 [DOI] [PubMed] [Google Scholar]
- Xia B, Heimbach T, He H, Lin TH (2012) Nilotinib preclinical pharmacokinetics and practical application toward clinical projections of oral absorption and systemic availability. Biopharm Drug Dispos 33(9):536–549. 10.1002/bdd.1821 [DOI] [PubMed] [Google Scholar]
- Yamada S, Arrell DK, Martinez-Fernandez A, Behfar A, Kane GC, Perez-Terzic CM, Crespo-Diaz RJ, McDonald RJ, Wyles SP, Zlatkovic-Lindor J, Nelson TJ, Terzic A (2015) Regenerative therapy prevents heart failure progression in dyssynchronous nonischemic narrow QRS cardiomyopathy. J Am Heart Assoc 4(5). 10.1161/JAHA.114.001614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Kobayashi M, Okada M, Takeuchi T, Unno M, Abe T, Goto J, Hishinuma T, Mano N (2008) Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer Lett 260(1–2):163–169. 10.1016/j.canlet.2007.10.040 [DOI] [PubMed] [Google Scholar]
- Yan X, Maixner DW, Yadav R, Gao M, Li P, Bartlett MG, Weng HR (2015) Paclitaxel induces acute pain via directly activating toll like receptor 4. Mol Pain 11:10. 10.1186/s12990-015-0005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Han L (2019) Roles of renal drug transporter in drug disposition and renal toxicity. Adv Exp Med Biol 1141:341–360. 10.1007/978-981-13-7647-4_7 [DOI] [PubMed] [Google Scholar]
- Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334(2):115–124. 10.1097/MAJ.0b013e31812dfe1e [DOI] [PubMed] [Google Scholar]
- Yokoo S, Masuda S, Yonezawa A, Terada T, Katsura T, Inui K (2008) Significance of organic cation transporter 3 (SLC22A3) expression for the cytotoxic effect of oxaliplatin in colorectal cancer. Drug Metab Dispos 36(11):2299–2306. 10.1124/dmd.108.023168 [DOI] [PubMed] [Google Scholar]
- Zaher H, Meyer zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, Palandra J, Stock JL, Kim RB, Ware JA (2008) Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol 74(2):320–329. 10.1124/mol.108.046458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou W (2012) Ameliorative effects of SLC22A2 gene polymorphism 808 G/T and cimetidine on cisplatin-induced nephrotoxicity in Chinese cancer patients. Food Chem Toxicol 50(7):2289–2293. 10.1016/j.fct.2012.03.077 [DOI] [PubMed] [Google Scholar]
- Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM (2006) Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res 66(17):8847–8857. 10.1158/0008-5472.CAN-06-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bolognia J, Marks P, Podoltsev N (2014) Enhanced skin toxicity associated with the combination of clofarabine plus cytarabine for the treatment of acute leukemia. Cancer Chemother Pharmacol 74(2):303–307. 10.1007/s00280-014-2504-y [DOI] [PubMed] [Google Scholar]
- Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S (2012) Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsive-ness of TRPA1 in mice. Mol Pain 8:55. 10.1186/1744-8069-8-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HJ, Appel DI, Grundemann D, Richelson E, Markowitz JS (2012) Evaluation of organic cation transporter 3 (SLC22A3) inhibition as a potential mechanism of antidepressant action. Pharmacol Res 65(4):491–496. 10.1016/j.phrs.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Zimmerman EI, Hu S, Roberts JL, Gibson AA, Orwick SJ, Li L, Sparreboom A, Baker SD (2013) Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res 19(6):1458–1466. 10.1158/1078-0432.CCR-12-3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman EI, Gibson AA, Hu S, Vasilyeva A, Orwick SJ, Du G, Mascara GP, Ong SS, Chen T, Vogel P, Inaba H, Maitland ML, Sparreboom A, Baker SD (2016) Multikinase inhibitors induce cutaneous toxicity through OAT6-mediated uptake and MAP 3K7-driven cell death. Cancer Res 76(1):117–126. 10.1158/0008-5472.CAN-15-0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong J, Borland J, Jerva F, Wynne B, Choukour M, Song I (2014) The effect of dolutegravir on the pharmacokinetics of metformin in healthy subjects. J Int AIDS Soc 17(4 Suppl 3):19584. 10.7448/IAS.17.4.19584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP (2001) Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol 21(13):4188–4196. 10.1128/MCB.21.13.4188-4196.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]