ABSTRACT
Hepatocellular carcinoma (HCC) is a malignant tumor with poor prognosis, and is one of the leading causes of cancer-related deaths worldwide. Recently, the development of therapeutic drugs via novel mechanisms of action, involving molecular-targeted drugs and immune checkpoint inhibitors, has progressed in the field of HCC. However, the recurrence rate remains high, and further improvement of the prognosis of patients with HCC is urgently needed. Cancer stem cells (CSCs) are a promising target for further development of novel anti-cancer drugs because they are reportedly involved in tumor initiation, maintenance, recurrence, and resistance to conventional therapies. Although several studies have already been conducted, the functions and roles of CSCs in the development and progression of tumors remain to be elucidated. In this review article, we will clarify the fundamental knowledge of CSCs necessary for the understanding of CSCs and will outline so-far identified markers specific to liver CSCs and the pathological and therapeutic implications of CSCs in HCC.
Keywords: cancer stem cells, cell surface markers, hepatocellular carcinoma, lncRNA
Hepatocellular carcinoma (HCC), which is the most frequent primary liver cancer, is a poor-prognosis malignant tumor with a high recurrence rate. A study suggested that approximately 850,000 people have developed liver cancer in 2015, which is the sixth common cancer worldwide.1 In the same year, approximately 810,000 people have died of liver cancer globally, which is the fourth leading cause of cancer-related deaths.1 The etiologies accounting for HCC include hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol, and aflatoxin. In addition, non-alcoholic steatohepatitis (NASH) developed from a fatty liver without excessive alcohol consumption is becoming the major cause of HCC.2 In Japan, the numbers of patients with HCC and HCC-related deaths are apparently decreasing because of the establishment of preventive interventions, including HBV vaccination, direct acting antivirals for HCV, and decontamination of aflatoxin.3 However, globally, the prevalence and death rate of HCC tend to increase, and, interestingly, this increase was more profound in countries with high socio-demographic index, where the latest therapeutic options are believed to be more readily available to people.4 Although the research and development of drugs for NASH are currently underway, sufficient HCC prevention remains to be established.5, 6 Moreover, although several novel molecular-targeted drugs were recently approved for HCC treatment, these drugs have not attained a remarkable improvement in the prognosis of HCC patients.7 Thus, the identification of previously unrecognized molecular targets is keenly necessary to develop innovative drugs for HCC treatment.
We elaborated, in the present review article, the fundamental functions of cancer stem cells (CSCs) and summarized the molecular markers defining liver CSCs, all of which are suggested to have profound functions in HCC. Moreover, we explained the recent findings on the pathological and therapeutic significance of liver CSCs. This review emphasizes that CSCs will provide novel insights into the understanding of the development and progression of HCC and hold a promising potential as a therapeutic target for the diagnosis, treatment, and prevention of HCC.
TUMOR-INITIATING PROPERTY OF CSCS
CSCs are defined as cancer cells that are capable of self-renewal and differentiation into non-CSCs and were first determined as tumor-initiating cells.8, 9 That is, only a small subpopulation of cancer cells has been demonstrated to be able to initiate a tumor tissue in an immunocompromised mouse.8, 9 The stemness has been postulated to have conferred to cancer cells via (1) the transformation of tissue stem or progenitor cells with maintaining the self-renewal ability or (2) the reprogramming of cancer cells transformed from differentiated cells.10 The underlying molecular mechanisms remain unclear, although the above scenarios may occur depending on the context of tumor development.
In order to explain the process of cancer cell expansion, two major models have been proposed, namely, the stochastic (or clonal evolution) model and the hierarchy (or CSC) model.11 The former proposes that cancer cells with CSC-like properties are randomly produced via the gain and loss of a wide variety of cellular traits. Thus, all cancer cells could have the potential to be CSCs, and the proportion of CSCs would be varied. However, an autologous transplantation of leukemia cells demonstrated that the frequency of tumor-initiating cells was constant at approximately 1:100.12 A similar result was also observed by an in vitro colony formation assay.13 Hence, these observations better fit the hierarchy model that has been originally established in stem cell biology. In this model, it is proposed that a subpopulation of cells undergoing self-renewal and differentiation would exist at a certain proportion in a tumor tissue. However, following the long-term culture of isolated CSC marker-negative cancer cells, it was demonstrated that CSC marker-positive cells appeared at a proportion similar to that observed before the isolation.14 This is not the case for normal stem cells that, once differentiated, cannot revert to original cells without specific conditions and better suits the stochastic model rather than the hierarchy model. Since these observations are difficult to be elaborated by either one model, the heterogeneity of tumor tissues has been attempted to be expounded by constructing a hybrid model of both.15 The precise molecular mechanisms of tumor initiation by CSCs will provide novel insights for the understanding of the process of tumor development and will thus be potential therapeutic targets for the prevention and treatment of tumors.
CSC MARKERS IN HCC
Side-population cells
As described above, CSCs have been determined as a subpopulation of cancer cells that can initiate tumor tissues in immunocompromised mice.10 However, CSC and non-CSC should be separated from a mixed cellular population in order to understand their detailed functions. As such, side-population (SP) cells have been taken advantage of since before CSC markers have been sufficiently elucidated.16 CSCs and normal stem cells are known to strongly express ATP-binding cassette (ABC) transporters that are involved in drug efflux and resistance to chemotherapy. Following staining with Hoechst 33342, which is a substrate for ABC transporters, dim cells, that is, SP cells, were isolated by a flow cytometer. This method was originally adopted to isolate hematopoietic stem cells and also oval cells, which are considered as hepatic progenitor cells.17, 18 SP cells derived from HCC cell lines have been demonstrated to be enriched with cancer cells with stemness and a high tumor-initiating potential. Chiba et al., for instance, found that two of four HCC cell lines contain SP cells with a high expression level of ABCB1 but at a quite low frequency of less than one percent of total cells and that these SP cells exhibited both hepatocytic and cholangiocytic features as observed in hepatic progenitor cells.16 Furthermore, SP cells from HCC cell lines showed more malignant characteristics than did non-SP cells and efficiently initiated tumor tissues in immunocompromised mice.16 Further investigation by the same research group also revealed that the polycomb protein, BMI1, was upregulated in liver SP cells, compared with that in non-SP cells and played a critical role in self-renewal and tumor-initiating property of SP cells.19 Another group also demonstrated the presence of SP cells with a high metastatic potential in HCC cell lines.20 It was suggested through a comparative proteomics approach that the AKT and nuclear factor kappa B (NFκB) pathways are involved in the regulation of liver SP cells.20
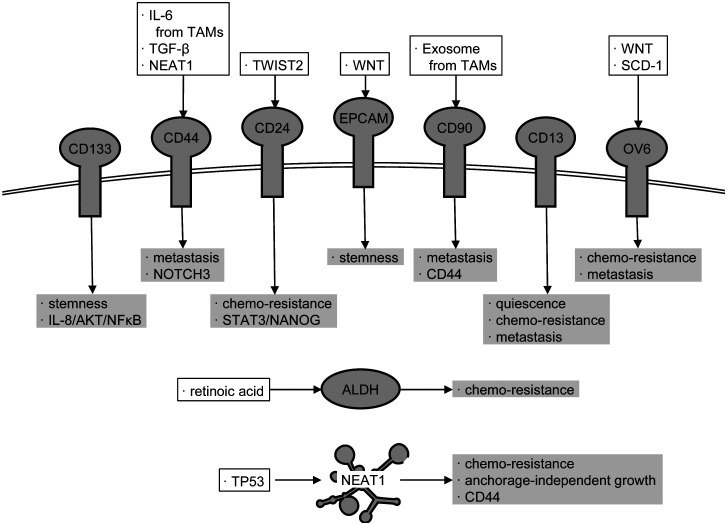
Further studies have thus far elucidated that CSCs and SP cells express several specific cell surface proteins that are not expressed in non-CSCs and non-SP cells and that there exist cells that express different CSC markers even in the same tumor tissue. Intriguingly, the functions of CSCs are slightly different from each other depending on the cell surface markers, although they share common properties, such as the tumor-initiating property. In HCC, as described below, several CSC markers, including CD133,21 CD44,22, 23 CD24,24 epithelial cell adhesion molecule (EPCAM),25 CD90,26, 27 CD13,28 OV6,29 and aldehyde dehydrogenase (ALDH), have been reported (Fig. 1).30 In addition, a huge number of long non-coding RNAs (lncRNAs) are expressed in cells and involved in a variety of cellular functions, including carcinogenesis. Recently, we demonstrated that nuclear paraspeckle assembly transcript 1 (NEAT1), a lncRNA, is involved in the enhancement and maintenance of CSC-like properties and is required for CD44 expression in HCC.31
Fig. 1.
Factors defining liver CSCs. To maintain their properties in HCC, CSCs express the factors including specific cell surface proteins as well as intracellular proteins and lncRNAs. The proposed regulators of the factors are shown in open squares. Their target signaling pathways and phenotypes are shown in gray squares. Details are discussed in the main text.
It has been demonstrated that the knockdown or knockout of these CSC markers in cancer cells result in the impairment of CSC-like properties, which suggests that these molecules and their downstream signaling pathways play crucial roles in the regulation of CSC-like properties. Accordingly, these CSC-specific molecules not only attract interest in terms of molecular cancer biology but also are expected as novel therapeutic targets for HCC treatment. This section will briefly describe the pathological implications of each CSC marker in HCC.
CD133
CD133 encoded by the PROM1 gene is expressed as a membrane glycoprotein mainly in neurons and bone marrow progenitor cells and is suggested to have functions required for the maintenance of undifferentiated status.32, 33 It was demonstrated that CD133-positive HCC cells exhibited strong expression of a liver progenitor marker, α-fetoprotein (AFP), and weak expression of mature hepatocyte markers, glutamine synthetase, and cytochrome P450 family 3 subfamily A member 4.21 These findings suggest that CD133 also plays a regulatory role in the maintenance of the undifferentiated status of CSCs in HCC. In addition, it was shown that CD133 induced the activation of the mitogen-activated protein kinase cascade through interleukin-8 (IL-8) and neurotrophine.34 Moreover, CD133 expression in HCC was correlated with the activation of AKT35 and NFκB,36 both of which are crucial regulators of liver CSCs and SP cells.20 Conversely, the suppression of CD133 attenuated CSC-like properties and tumorigenic and metastatic potential, which suggests that CD133 would be a promising target for HCC treatment in a study in which mice are used.35, 36
CD44
CD44 is expressed as a variant isoform (CD44v) incorporating variant exons, as well as standard isoform (CD44s).22, 23 Reportedly, CD44v is expressed in CSCs derived from a wide variety of tumor tissues and potentiates cellular antioxidant capacity by enhancing the synthesis of glutathione.37, 38 However, CSCs in HCC mainly express CD44s. This fact prompted us to examine the function of CD44s in HCC by constructing CD44-knocked out HCC cells.23 In our previous report, CD44s has been demonstrated to be involved in the induction of antioxidant enzyme genes expression and the maintenance of CSC-like properties in an HCC cell line, possibly via NOTCH3.23 Meanwhile, another research group reported that CD44 expression in HCC was induced by IL-6 secreted by tumor-associated macrophages (TAMs), which results in the enhancement of CSC-like properties.39 In addition, CD44s was suggested to enhance the metastatic potential of HCC cells rather than the maintenance of stemness, under the control of transforming growth factor-β (TGF-β).22
CD24
Lee et al. analyzed the gene expression profile of tumor tissues that survived after treatment with cisplatin in mice transplanted with HCC cells and found that CD24 was significantly upregulated in the survived cancer cells.24 CD24 is known as a tumor-related gene highly expressed in various tumor tissues and is suggested to regulate CSC-like properties by inducing NANOG expression via signal transducer and activator of transcription 3 (STAT3) in HCC.24 Moreover, it was proven that TWIST2, a transcription factor involved in epithelial–mesenchymal transition (EMT), enhances CSC-like properties by inducing CD24,40 suggesting the TSWIST2-CD24-STAT3-NANOG pathway as a novel regulatory mechanism for CSC regulation in HCC.
EPCAM
In a healthy liver, EPCAM is expressed in bile duct epithelial cells. Liver progenitor cells also express EPCAM, but its expression is diminished as hepatocyte differentiation progresses and thus is absent in mature hepatocytes. CSCs in HCC also exhibit the high expression levels of EPCAM under the control of the WNT signaling pathway.41 The expression of EPCAM and AFP has been demonstrated to be possibly used to discriminate between malignant HCC and bile duct epithelial cells.25, 42 In HCC cells double-positive for EPCAM and AFP, the expressions of CD133, liver progenitor markers, and WNT target genes were concomitantly upregulated, whereas those of mature hepatocyte markers were downregulated.42 Moreover, the knockdown of EPCAM in HCC cells impaired CSC-like properties.42 In addition to its regulatory function of stemness, the co-culture of HCC cells with major histocompatibility complex (MHC)-independent γδT cells in the presence of a bi-specific antibody recognizing EPCAM and CD3 resulted in the increased cell lysis of HCC cells,43 which suggests that EPCAM can be applied as a target for HCC immunotherapy. Nevertheless, to distinguish between HCC and bile duct epithelial cells, further studies are required for the development and clinical application of EPCAM-targeting drugs.
CD90
Using six human HCC cell lines and an immortalized hepatocyte line, Yang et al. investigated the relationship between several liver progenitor markers and tumor-initiating potential.26, 27 Consequently, they identified CD90 as an HCC-specific protein whose expression was correlated with tumor-initiating potential.26, 27 CD90 expression in HCC has been suggested to be regulated by exosomes secreted from TAMs.44 Intriguingly, most CD90-positive cells are also positive for CD44 whose expression is also regulated by TAMs.39 Consistently, the metastatic potential of CD90-positive cells has been shown to be suppressed by an anti-CD44 antibody.26 Moreover, it has been observed that, whereas EPCAM and CD133 are co-expressed with immature hepatocyte markers, CD44 and CD90 are co-expressed with mesenchymal markers that are indicative of high metastatic potential.45 These results suggest that CD90 mainly regulates the metastatic potential of HCC cells via CD44.
CD13
CD13, also known as aminopeptidase N, was identified via gene expression profiling, as a cell surface protein that is highly expressed in SP cells of HCC.28 Notably, unlike other CSC markers, cells highly expressing CD13 are mostly in the G0/G1 phase,28 and thus, CD13 is considered as a marker of quiescent CSCs. Cancer cells in a quiescent state are generally known to be relatively resistant against various anti-cancer drugs because most of those drugs target cellular machineries required for cell proliferation. However, ubenimex, a protease inhibitor, which also inhibits the aminopeptidase activity of CD13, has been shown to have potentiated the sensitivity of HCC cell lines to 5-fluorouracile.28 It has also been demonstrated that CD13 was co-expressed with N-cadherin, a mesenchymal marker, and moderately alleviated oxidative stress during the induction of mesenchymal phenotype in HCC cells.46 Thus, it is suggested that CD13 contributes to the efficient induction of EMT by protecting HCC cells from the oxidative stress associated with EMT and thereby promotes the acquisition of metastatic potential. However, how this aminopeptidase activity regulates CSC-like properties in HCC remains to be elucidated.
OV6
OV6 is a monoclonal antibody reacting with oval cells that appear in the canals of Hering after severe liver damage of rats. This antibody was produced by using neoplastic nodules in the rat liver as an antigen.47 The target protein has not been fully specified, although it was shown that a cytoskeletal protein with a molecular weight of 56 kDa was reacted with OV6.47 The oval cells are considered as hepatic progenitor cells found in the rat livers, as described above. However, it is known that oval-like cells that are reacted with OV6 also appear in damaged liver and HCC of humans. Consistently, it was demonstrated that OV6-positive cells isolated from HCC also expressed other CSC markers and had high tumor-initiating potential.29 WNT/β-catenin signaling increased the number of OV6-positive cells and contributed to cisplatin resistance in HCC.29 Moreover, the frequency of OV6-positive cells was significantly correlated with poor prognosis of patients with HCC.48 Intriguingly, OV6-positive cells were mainly found in the invasion front of HCC tumor tissues, and an in vitro assay revealed that these OV6-positive cells have a high-migrating and invasive potential.48 Additionally, the same group also demonstrated that stromal cell-derived factor 1 induced the expression of OV6 antigen through C-X-C motif chemokine receptor 4 and thereby promoted metastasis of HCC cells.48
ALDH
A proteome analysis of HCC cell lines identified ALDH as a protein highly expressed in CD133-positive cells.30 It has been shown that ALDH and CD133 double-positive cells had higher CSC-like properties than those of ALDH-negative and CD133-positive cells.30 Additionally, ALDH has been suggested to enhance the survival of cancer cells via the detoxification of endogenous and exogenous aldehydes, and the scavenging of reactive oxygen species.49 Conversely, ALDH is also known to be a critical enzyme for the synthesis of retinoic acid, which has an antitumor activity, in general. How ALDH activity in CSCs affects retinoid signaling remains to be elucidated. Notably, a CSC-targeted therapy using retinoic acid was proposed because the retinoic acid lowered the ALDH activity in lung cancer cell lines in a negative feedback manner.50, 51
LncRNA
The lncRNA, NEAT1, is expressed as short variant 1 (3.8 kb; NEAT1v1) and long variant 2 (22.7 kb; NEAT1v2). Although the expression of NEAT1 was induced by tumor protein p53 (TP53), it was demonstrated that NEAT1 promoted carcinogenesis by activating DNA repair and cell proliferation signals.52, 53 We recently elucidated that the knockout of the NEAT1 gene resulted in decreased CSC-like properties of HCC cell lines, which were concomitant with the abolishment of CD44 expression.31 Rescue experiments supported the notion that CD44 expression in HCC was highly dependent on NEAT1 expression. As described above, since CD44 regulates CSCs, it was postulated that NEAT1 might maintain CSC-like properties via CD44. However, we found that NEAT1 overexpression restored the CSC-like properties even in CD44-deficient HCC cells.31 These findings suggested that NEAT1 maintained the CSC-like properties of HCC cell lines in both CD44-independent and CD44-dependent manners.
In addition to NEAT1, several lncRNAs have been reported to be involved in the maintenance of CSC-like properties, including highly upregulated in liver cancer (HULC),54 metastasis-associated lung adenocarcinoma transcript 1 (MALAT1),55 and LINC00324.56HULC is a target gene of a lncRNA, cancer upregulated drug resistant (CUDR), which induced hepatocytic differentiation of embryonic stem cells and promoted the malignant growth of the hepatocyte-like cells.57 In liver CSCs, HULC enhanced autophagy by inducing the expression of sirtuin 1 and thereby promoted the cell cycle in a cyclin D1-dependent manner.54 MALAT1 has been shown to be upregulated in HCC.55 The knockdown of MALAT1 led to a decrease in CD133 and CD90 double-positive CSC populations in association with the suppression of WNT/β-catenin signaling, although its detailed mechanism is unclear.55 LINC00324 was also identified as a lncRNA highly expressed in HCC, and, interestingly, its expression was significantly associated with FAS ligand (FASL).56 Mechanistically, LINC00324 has been shown to have an interaction with PU box binding protein, which is a transcription factor that directly regulates FASL expression.56 Moreover, the knockdown of LINC00324 or FASL decreased the expression of stemness-related genes.56 However, it is known that FASL induces apoptosis in cells that express FAS. How LINC00324 regulates CSC-like properties via FASL remains unclear. These lncRNAs provide more insights into the regulatory mechanisms of CSC by identifying their target genes, although, unlike surface protein markers, they cannot be used for the isolation of CSCs.
MALIGNANT FUNCTIONS OF CSCS IN HCC
As described above, CSCs exhibit not only tumor initiation but also other malignant features, including resistance to conventional therapies, EMT phenotype, and immunomodulatory property. Sorafenib is a standard molecular-targeted drug used for the treatment of advanced HCC. Liver CSCs are frequently endowed with sorafenib resistance through several pathways. The downregulation of angiopoietin-like protein 1 (ANGPTL1) in HCC has been shown to be associated with sorafenib resistance.58 Conversely, the overexpression of ANGPTL1 suppressed sorafenib resistance, and CSC-like properties, in association with decreased EMT phenotype through the downregulation of an EMT factor SLUG.58 Metformin, a type 2 diabetes drug, inhibited the EMT process and reduced CSC-like properties and sorafenib resistance in an HCC cell line.59 Conversely, microrchidia family CW-type zinc finger 2 (MORC2) also enhanced CSC-like properties and sorafenib resistance.60 Mechanistically, MORC2 induced DNA methylation of neurofibromatosis 2 and kidney and brain protein genes by DNA methyltransferase 3A, leading to the suppression of the anti-tumorigenic Hippo signaling pathway.
The induction of EMT is suggested to be an important process to acquire CSC-like properties. The EMT factor TWIST2, which is overexpressed in HCC, induced liver CSCs by directly augmenting CD24 transcription as well as increased migration and invasion abilities.40 SLUG is also a well-known EMT factor and plays an essential role in the induction of EMT by hypoxia-inducing factor 1α (HIF1α).61 SLUG induced by HIF1α further enhanced the CSC-like properties in HCC through NOTCH1 signaling.61 The cancer stemness of leukemia cells has been demonstrated to be suppressed by the retinoic acid-inducible gene I (RIG-I).62 The knockdown of RIG-I in HCC induced CSCs and upregulated TGF-β expression in the HCC cells.63 TGF-β is a multi-functional cytokine that induces not only EMT phenotype but also CSC-like properties in HCC.64 Consistently, TGF-β secreted from the cells knocking down RIG-I expression directly suppressed the maturation of dendritic cells (DCs), which leads to the evasion of tumor immunity.63
In tumor tissues, vascular endothelial cells and immune cells also exist. Drugs that target these non-tumor cells have already been developed, for instance, vascular endothelial cell growth factor receptor inhibitors and programmed cell death-1 (PD-1)/PD-1 ligand-1 (PD-L1) inhibitors.7 In addition, non-tumor cells with tumor-specific functions, including cancer-associated fibroblasts (CAFs),65 tissue-associated myeloid cells,66 and pericytes,67 have been recently found in tumor tissues, and it is revealed that these cells promote the growth and metastasis by interacting with tumor cells.68 Moreover, these non-tumor cells resident in tumor tissues also provide a niche for the induction and maintenance of CSCs. For instance, TAMs induced CSCs in HCC by secreting TGF-β69 or IL-6.39 These factors also induced EMT, drug resistance, and further recruited myeloid-derived suppressor cells (MDSCs) that suppress tumor immunity.39, 69, 70 The stem cell factor (SCF)/c-KIT axis plays an essential role in maintaining CSCs in ovarian and lung cancers and leukemia.71,72,73 It was demonstrated that SCF produced by cancer cells, such as in HCC as well as breast, lung, ovarian, and gastrointestinal cancers, induced the tumor infiltration and activation of mast cells.74 The tumor-infiltrated mast cells suppressed tumor immunity by recruiting regulatory T (Treg) cells and releasing adenosine.74 The mast cells also produced C-C motif chemokine ligand 2, which further recruited MDSCs to HCC tissues, and promoted IL-17 production by MDSCs.74, 75 IL-17 sequentially activated the immunosuppressive function of Treg cells.75 These results suggest that SCF produced by HCC cells creates an immune-suppressive tumor microenvironment, possibly in association with the induction and maintenance of liver CSCs. CD206, a mannose receptor, is expressed in immature DCs and immunosuppressive M2-macrophages and is suggested to be involved in immunosuppression.76, 77 Moreover, CD206 expression was found in HCC and was correlated with tumor size, metastasis, and poor prognosis.78 CSCs in HCC cells exhibited a higher expression of CD206 than did non-CSCs, and the knockdown of CD206 suppressed their metastatic potential.78 This result suggests that CD206 regulates multiple aspects of liver CSCs.
CSC AS A THERAPEUTIC TARGET FOR HCC
Cyclooxygenase 2 (COX2) and its products, prostaglandins, are also essential regulatory factors for CSCs. It was demonstrated that the expression level of COX2 was correlated with the presence of CSCs in not only HCC but also in other types of cancers.79,80,81 Celecoxib, a COX2 inhibitor, decreased CD44+/CD133+ and SP cells in HCC cells.82 Mechanistically, celecoxib inhibited the production of prostaglandin E2 (PGE2) and suppressed the PGE2-induced activation of AKT signaling by the upregulation of PTEN.79, 82 Moreover, celecoxib sensitized liver CSCs to epirubicin by downregulating multi-drug resistance protein 1 (ABCB1) besides the downregulation of CD44 and CD133.83 Celecoxib, combined with epirubicin, increased tumor-infiltrating CD8+ T cells, decreased Treg cells, and downregulated PD-L1 expression in HCC tissues.83
Extracellular matrix provides stem cells with a niche, which maintains their stemness, and supports stable self-renewal and asymmetric cell division. Hyaluronan has been demonstrated to be an essential extracellular matrix for breast and liver CSCs.84, 85 Breast CSCs interacted with TAMs via hyaluronan produced by CSCs and thereby promoted the production of platelet-derived growth factor-BB by TAMs to activate CAFs.84 The activated CAFs, sequentially, secreted fibroblast growth factor (FGF) 7 and FGF9, which enhanced CSC-like properties in breast cancer.84 This vicious cycle was blocked by 4-methylumbeliferone (4Mu), an inhibitor of hyaluronan synthase 2, which is upregulated in breast CSCs.84 4Mu also suppressed HCC growth, concomitant with the downregulation of CSC markers, including CD133, CD90, EPCAM, CD44, and CD13 as well as CD47, which acts as a “don’t eat me” signal to protect cancer cells from phagocytosis by macrophages.85 Consistently, 4Mu combined with adenovirus expressing IL-12 significantly potentiated phagocytosis by macrophages and antitumor CD8+ cytotoxic T cell response.85
Immunotherapy that targets liver CSCs is currently investigated. Kiatomab is a specific monoclonal antibody against KIAA1114, which is a recently identified cell surface marker of liver CSCs with unknown function.86 The inhibitory effect of kiatomab on HCC tumor growth and metastasis was demonstrated in a murine syngeneic tumor transplantation model and was suggested to involve antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity.87
DCs are potent immune adjuvant by presenting antigens to naïve T cells and releasing cytokines. DCs fused with CD90+ CSCs of HepG2 hepatoma cell line showed the increased expression of co-stimulatory molecules, such as CD80, CD83, and CD86, and MHC class I and II molecules (human leukocyte antigen-A, -B, -C, and -DR).88 Moreover, the fusion with CSCs induced more potent activation of DCs, including higher expression of inflammatory cytokines, than that with bulk HepG2 cells.88 Interestingly, DCs fused with CSCs activated cytotoxic T lymphocytes (CTL) against both HepG2 CSCs and bulk cells.88 However, DCs fused with bulk cells induced less activation of CTL against bulk cells than did CSCs-fused DC and failed to induce CSCs-specific CTL response.88
Cytokine-induced killer cells (CIKs) are a population of cytotoxic effector cells generated by treating peripheral blood mononuclear cells with specific cytokines, such as IL-2 and interferon-γ, and contain CD3-/CD56+ natural killer (NK) cells, CD3+/CD56- T cells, and CD3+/CD56+ CIKs.89 These “bona fide” CIKs are also positive for NK-activating receptor NKG2D and exhibit NK-like MHC-unrestricted cytotoxicity against a wide variety of malignant cells possibly through NKG2D but not toward normal cells.89 Since antigen-pulsed DCs directly or indirectly activate the killer activity of CIKs, it was demonstrated that autologous CIKs co-cultured with autologous DCs from patients with HCC efficiently suppressed liver CSCs-derived tumor growth in vivo.90 Notably, when DCs were pulsed with liver CSCs, the suppression effect was more prominent than when DCs were pulsed with bulk HCC cells.90 These results indicate that liver CSCs are promising therapeutic antigens for the establishment of immunotherapy for HCC.
CONCLUSIONS
A large part of the regulatory mechanisms of CSCs remains unclear although enormous attempts have been made to understand CSCs, and the existence of CSC itself remains debated. The CSC markers and functions depicted in the present review are just a part of those identified so far. Some of these CSC markers solely regulate CSC-like properties whereas others cooperate with each other to promote CSC-like properties. By contrast, some CSC markers are mutually exclusively expressed in cancer cells even those of the same HCC tissue. To develop innovative medicines to combat HCC, the complex functions of these CSC-related molecules in the maintenance of CSC-like properties should be urgently elucidated.
Clinical needs for HCC treatment are being met owing to the recent approval of several molecular-targeted drugs.7 Moreover, with the advancement of surgical treatments, the prognosis of HCC patients is slowly but steadily being improved. However, in line with the prolonged prognosis by those recent advances, recurrence risk appears to be slightly increased. Because CSCs are involved in recurrence as well as de novo tumor occurrence, CSC-targeted therapy will also be a good option for the prevention of cancer recurrence in the future. We believe that deepening our understanding of CSCs will provide a great advance toward achieving the complete eradication of HCC.
Acknowledgments
Acknowledgments: We would like to thank Enago (www.enago.jp) for the English language review. This work was supported in part by JSPS KAKENHI Grant Number JP19K08469 (HT).
Footnotes
Conflicts of interest: GS holds more than 5% of the total shares of KanonCure Inc. and receives compensation as a member of KanonCure Inc. The other author has no competing interests.
REFERENCES
- 1.Asrani SK,Devarbhavi H,Eaton J,Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-71. 10.1016/j.jhep.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-31. 10.1056/NEJMra011775 [DOI] [PubMed] [Google Scholar]
- 3.Roth GA,Abate D,Abate KH,Abay SM,Abbafati C,Abbasi N,et al. ; GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z,Jiang Y,Yuan H,Fang Q,Cai N,Suo C,et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70:674-83. 10.1016/j.jhep.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Estes C,Razavi H,Loomba R,Younossi Z,Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-33. 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ocker M. Challenges and opportunities in drug development for nonalcoholic steatohepatitis. Eur J Pharmacol. 2020;870:172913. 10.1016/j.ejphar.2020.172913 [DOI] [PubMed]
- 7.Kudo M. Systemic Therapy for Hepatocellular Carcinoma: latest Advances. Cancers (Basel). 2018;10:412. 10.3390/cancers10110412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapidot T,Sirard C,Vormoor J,Murdoch B,Hoang T,Caceres-Cortes J,et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645-8. 10.1038/367645a0 [DOI] [PubMed] [Google Scholar]
- 9.Al-Hajj M,Wicha MS,Benito-Hernandez A,Morrison SJ,Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-8. 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke MF,Dick JE,Dirks PB,Eaves CJ,Jamieson CHM,Jones DL,et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339-44. 10.1158/0008-5472.CAN-06-3126 [DOI] [PubMed] [Google Scholar]
- 11.Wang JCY,Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494-501. 10.1016/j.tcb.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 12.Bruce WR,Van Der Gaag H. A QUANTITATIVE ASSAY FOR THE NUMBER OF MURINE LYMPHOMA CELLS CAPABLE OF PROLIFERATION IN VIVO. Nature. 1963;199:79-80. 10.1038/199079a0 [DOI] [PubMed] [Google Scholar]
- 13.Hamburger A,Salmon S. Primary bioassay of human tumor stem cells. Science. 1977;197:461-3. 10.1126/science.560061 [DOI] [PubMed] [Google Scholar]
- 14.Zheng H,Pomyen Y,Hernandez MO,Li C,Livak F,Tang W,et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology. 2018;68:127-40. 10.1002/hep.29778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W,Quan Y,Fu Q,Liu Y,Liang Y,Wu J,et al. Dynamics between cancer cell subpopulations reveals a model coordinating with both hierarchical and stochastic concepts. PLoS One. 2014;9:e84654. 10.1371/journal.pone.0084654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiba T,Kita K,Zheng YW,Yokosuka O,Saisho H,Iwama A,et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240-51. 10.1002/hep.21227 [DOI] [PubMed] [Google Scholar]
- 17.Goodell MA,Brose K,Paradis G,Conner AS,Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797-806. 10.1084/jem.183.4.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimano K,Satake M,Okaya A,Kitanaka J,Kitanaka N,Takemura M,et al. Hepatic oval cells have the side population phenotype defined by expression of ATP-binding cassette transporter ABCG2/BCRP1. Am J Pathol. 2003;163:3-9. 10.1016/S0002-9440(10)63624-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiba T,Miyagi S,Saraya A,Aoki R,Seki A,Morita Y,et al. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008;68:7742-9. 10.1158/0008-5472.CAN-07-5882 [DOI] [PubMed] [Google Scholar]
- 20.Liu H,Wang Y,Xing X,Sun Y,Wei D,Chen G,et al. Comparative proteomics of side population cells derived from human hepatocellular carcinoma cell lines with varying metastatic potentials. Oncol Lett. 2018;16:335-45. 10.3892/ol.2018.8666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suetsugu A,Nagaki M,Aoki H,Motohashi T,Kunisada T,Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820-4. 10.1016/j.bbrc.2006.10.128 [DOI] [PubMed] [Google Scholar]
- 22.Mima K,Okabe H,Ishimoto T,Hayashi H,Nakagawa S,Kuroki H,et al. CD44s regulates the TGF-β-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. 2012;72:3414-23. 10.1158/0008-5472.CAN-12-0299 [DOI] [PubMed] [Google Scholar]
- 23.Asai R,Tsuchiya H,Amisaki M,Makimoto K,Takenaga A,Sakabe T,et al. CD44 standard isoform is involved in maintenance of cancer stem cells of a hepatocellular carcinoma cell line. Cancer Med. 2019;8:773-82. 10.1002/cam4.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee TKW,Castilho A,Cheung VCH,Tang KH,Ma S,Ng IOL. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50-63. 10.1016/j.stem.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 25.Yamashita T,Forgues M,Wang W,Kim JW,Ye Q,Jia H,et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451-61. 10.1158/0008-5472.CAN-07-6013 [DOI] [PubMed] [Google Scholar]
- 26.Yang ZF,Ho DW,Ng MN,Lau CK,Yu WC,Ngai P,et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153-66. 10.1016/j.ccr.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 27.Yang ZF,Ngai P,Ho DW,Yu WC,Ng MNP,Lau CK,et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919-28. 10.1002/hep.22082 [DOI] [PubMed] [Google Scholar]
- 28.Haraguchi N,Ishii H,Mimori K,Tanaka F,Ohkuma M,Kim HM,et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326-39. 10.1172/JCI42550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W,Yan HX,Chen L,Liu Q,He YQ,Yu LX,et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287-95. 10.1158/0008-5472.CAN-07-6691 [DOI] [PubMed] [Google Scholar]
- 30.Ma S,Chan KW,Lee TKW,Tang KH,Wo JYH,Zheng BJ,et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146-53. 10.1158/1541-7786.MCR-08-0035 [DOI] [PubMed] [Google Scholar]
- 31.Koyama S,Tsuchiya H,Amisaki M,Sakaguchi H,Honjo S,Fujiwara Y,et al. NEAT1 is a long noncoding RNA required for the expression of the liver cancer stem cell marker CD44. Int J Mol Sci. 2020;21:1927. 10.3390/ijms21061927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jászai J,Fargeas CA,Florek M,Huttner WB,Corbeil D. Focus on Molecules: Prominin-1 (CD133). Exp Eye Res. 2007;85:585-6. 10.1016/j.exer.2006.03.022 [DOI] [PubMed] [Google Scholar]
- 33.Ho JWY,Pang RWC,Lau C,Sun CK,Yu WC,Fan ST,et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44:836-43. 10.1002/hep.21353 [DOI] [PubMed] [Google Scholar]
- 34.Tang KH,Ma S,Lee TK,Chan YP,Kwan PS,Tong CM,et al. CD133+ liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55:807-20. 10.1002/hep.24739 [DOI] [PubMed] [Google Scholar]
- 35.Jang JW,Song Y,Kim SH,Kim J,Kim K,Choi EK,et al. CD133 confers cancer stem-like cell properties by stabilizing EGFR-AKT signaling in hepatocellular carcinoma. Cancer Lett. 2017;389:1-10. 10.1016/j.canlet.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 36.Liu YM,Li XF,Liu H,Wu XL. Ultrasound-targeted microbubble destruction-mediated downregulation of CD133 inhibits epithelial-mesenchymal transition, stemness and migratory ability of liver cancer stem cells. Oncol Rep. 2015;34:2977-86. 10.3892/or.2015.4270 [DOI] [PubMed] [Google Scholar]
- 37.Yan Y,Zuo X,Wei D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl Med. 2015;4:1033-43. 10.5966/sctm.2015-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishimoto T,Nagano O,Yae T,Tamada M,Motohara T,Oshima H,et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387-400. 10.1016/j.ccr.2011.01.038 [DOI] [PubMed] [Google Scholar]
- 39.Wan S,Zhao E,Kryczek I,Vatan L,Sadovskaya A,Ludema G,et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393-404. 10.1053/j.gastro.2014.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu AY,Cai Y,Mao Y,Lin Y,Zheng H,Wu T,et al. Twist2 promotes self-renewal of liver cancer stem-like cells by regulating CD24. Carcinogenesis. 2014;35:537-45. 10.1093/carcin/bgt364 [DOI] [PubMed] [Google Scholar]
- 41.Yamashita T,Budhu A,Forgues M,Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831-9. 10.1158/0008-5472.CAN-07-0908 [DOI] [PubMed] [Google Scholar]
- 42.Yamashita T,Ji J,Budhu A,Forgues M,Yang W,Wang HY,et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012-1024.e4. 10.1053/j.gastro.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armeanu-Ebinger S,Hoh A,Wenz J,Fuchs J. Targeting EpCAM (CD326) for immunotherapy in hepatoblastoma. OncoImmunology. 2013;2:e22620. 10.4161/onci.22620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y,Wang B,Xiao S,Li Y,Chen Q. miR‐125a/b inhibits tumor‐associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J Cell Biochem. 2019;120:3046-55. 10.1002/jcb.27436 [DOI] [PubMed] [Google Scholar]
- 45.Yamashita T,Honda M,Nakamoto Y,Baba M,Nio K,Hara Y,et al. Discrete nature of EpCAM+ and CD90 + cancer stem cells in human hepatocellular carcinoma. Hepatology. 2013;57:1484-97. 10.1002/hep.26168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HM,Haraguchi N,Ishii H,Ohkuma M,Okano M,Mimori K,et al. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol. 2012;19(suppl 3):539-48. 10.1245/s10434-011-2040-5 [DOI] [PubMed] [Google Scholar]
- 47.Dunsford HA,Sell S. Production of monoclonal antibodies to preneoplastic liver cell populations induced by chemical carcinogens in rats and to transplantable Morris hepatomas. Cancer Res. 1989;49:4887-93. [PubMed] [Google Scholar]
- 48.Yang W,Wang C,Lin Y,Liu Q,Yu L,Tang L,et al. OV6+ tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J Hepatol. 2012;57:613-20. 10.1016/j.jhep.2012.04.024 [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Torres M,Allan AL. Aldehyde dehydrogenase as a marker and functional mediator of metastasis in solid tumors. Clin Exp Metastasis. 2016;33:97-113. 10.1007/s10585-015-9755-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreb JS,Gabr A,Vartikar GR,Gowda S,Zucali JR,Mohuczy D. Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroperoxycyclophosphamide and acetaldehyde. J Pharmacol Exp Ther. 2005;312:339-45. 10.1124/jpet.104.072496 [DOI] [PubMed] [Google Scholar]
- 51.Moreb JS,Ucar-Bilyeu DA,Khan A. Use of retinoic acid/aldehyde dehydrogenase pathway as potential targeted therapy against cancer stem cells. Cancer Chemother Pharmacol. 2017;79:295-301. 10.1007/s00280-016-3213-5 [DOI] [PubMed] [Google Scholar]
- 52.Adriaens C,Standaert L,Barra J,Latil M,Verfaillie A,Kalev P,et al. p53 induces formation of NEAT1lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med. 2016;22:861-8. 10.1038/nm.4135 [DOI] [PubMed] [Google Scholar]
- 53.Yang X,Qu S,Wang L,Zhang H,Yang Z,Wang J,et al. PTBP3 splicing factor promotes hepatocellular carcinoma by destroying the splicing balance of NEAT1 and pre-miR-612. Oncogene. 2018;37:6399-413. 10.1038/s41388-018-0416-8 [DOI] [PubMed] [Google Scholar]
- 54.Wang C,Jiang X,Li X,Song S,Meng Q,Wang L,et al. Long noncoding RNA HULC accelerates the growth of human liver cancer stem cells by upregulating CyclinD1 through miR675-PKM2 pathway via autophagy. Stem Cell Res Ther. 2020;11:8. 10.1186/s13287-019-1528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang HL,Bamodu OA,Ong JR,Lee WH,Yeh CT,Tsai JT. Targeting the Epigenetic Non-Coding RNA MALAT1/Wnt Signaling Axis as a Therapeutic Approach to Suppress Stemness and Metastasis in Hepatocellular Carcinoma. Cells. 2020;9:1020. 10.3390/cells9041020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao J,Dai C,Yu X,Yin XB,Zhou F. Long noncoding RNA LINC00324 exerts protumorigenic effects on liver cancer stem cells by upregulating fas ligand via PU box binding protein. FASEB J. 2020;34:5800-17. 10.1096/fj.201902705RR [DOI] [PubMed] [Google Scholar]
- 57.Gui X,Li H,Li T,Pu H,Lu D. Long Noncoding RNA CUDR Regulates HULC and β-Catenin to Govern Human Liver Stem Cell Malignant Differentiation. Mol Ther. 2015;23:1843-53. 10.1038/mt.2015.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen HA,Kuo TC,Tseng CF,Ma JT,Yang ST,Yen CJ,et al. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology. 2016;64:1637-51. 10.1002/hep.28773 [DOI] [PubMed] [Google Scholar]
- 59.Feng Y,Guo X,Huang X,Wu M,Li X,Wu S,et al. Metformin reverses stem cell‑like HepG2 sphere formation and resistance to sorafenib by attenuating epithelial‑mesenchymal transformation. Mol Med Rep. 2018;18:3866-72. 10.3892/mmr.2018.9348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang T,Qin Z,Wen L,Guo Y,Liu Q,Lei Z,et al. Epigenetic restriction of Hippo signaling by MORC2 underlies stemness of hepatocellular carcinoma cells. Cell Death Differ. 2018;25:2086-100. 10.1038/s41418-018-0095-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jing L,Ruan Z,Sun H,Li Q,Han L,Huang L,et al. Epithelial–mesenchymal transition induced cancer‐stem‐cell‐like characteristics in hepatocellular carcinoma. J Cell Physiol. 2019;234:18448-58. 10.1002/jcp.28480 [DOI] [PubMed] [Google Scholar]
- 62.Li XY,Jiang LJ,Chen L,Ding ML,Guo HZ,Zhang W,et al. RIG-I modulates Src-mediated AKT activation to restrain leukemic stemness. Mol Cell. 2014;53:407-19. 10.1016/j.molcel.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 63.Zhong M,Zhong C,Cui W,Wang G,Zheng G,Li L,et al. Induction of tolerogenic dendritic cells by activated TGF-β/Akt/Smad2 signaling in RIG-I-deficient stemness-high human liver cancer cells. BMC Cancer. 2019;19:439. 10.1186/s12885-019-5670-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malfettone A,Soukupova J,Bertran E,Crosas-Molist E,Lastra R,Fernando J,et al. Transforming growth factor-β-induced plasticity causes a migratory stemness phenotype in hepatocellular carcinoma. Cancer Lett. 2017;392:39-50. 10.1016/j.canlet.2017.01.037 [DOI] [PubMed] [Google Scholar]
- 65.Liu T,Zhou L,Li D,Andl T,Zhang Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front Cell Dev Biol. 2019;7:60. 10.3389/fcell.2019.00060 [DOI] [PMC free article] [PubMed]
- 66.Neophytou CM,Pierides C,Christodoulou MI,Costeas P,Kyriakou TC,Papageorgis P. The Role of Tumor-Associated Myeloid Cells in Modulating Cancer Therapy. Front Oncol. 2020;10:899. 10.3389/fonc.2020.00899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chantrain CF,Henriet P,Jodele S,Emonard H,Feron O,Courtoy PJ,et al. Mechanisms of pericyte recruitment in tumour angiogenesis: A new role for metalloproteinases. Eur J Cancer. 2006;42:310-8. 10.1016/j.ejca.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 68.Paolillo M,Schinelli S. Extracellular Matrix Alterations in Metastatic Processes. Int J Mol Sci. 2019;20:4947. 10.3390/ijms20194947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan QM,Jing YY,Yu GF,Kou XR,Ye F,Gao L,et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial–mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160-8. 10.1016/j.canlet.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 70.Xu M,Zhao Z,Song J,Lan X,Lu S,Chen M,et al. Interactions between interleukin-6 and myeloid-derived suppressor cells drive the chemoresistant phenotype of hepatocellular cancer. Exp Cell Res. 2017;351:142-9. 10.1016/j.yexcr.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 71.Mazzoldi EL,Pavan S,Pilotto G,Leone K,Pagotto A,Frezzini S,et al. A juxtacrine/paracrine loop between C-Kit and stem cell factor promotes cancer stem cell survival in epithelial ovarian cancer. Cell Death Dis. 2019;10:412. 10.1038/s41419-019-1656-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuribayashi W,Takizawa K,Sugata K,Kuramitsu M,Momose H,Sasaki E,et al. Impact of the SCF signaling pathway on leukemia stem cell-mediated ATL initiation and progression in an HBZ transgenic mouse model. Oncotarget. 2016;7:51027-43. 10.18632/oncotarget.10210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L,Wang J,Li Z,Liu Y,Jiang M,Li Y,et al. Silencing stem cell factor attenuates stemness and inhibits migration of cancer stem cells derived from Lewis lung carcinoma cells. Tumour Biol. 2016;37:7213-27. 10.1007/s13277-015-4577-6 [DOI] [PubMed] [Google Scholar]
- 74.Huang B,Lei Z,Zhang GM,Li D,Song C,Li B,et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269-79. 10.1182/blood-2008-03-147033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Z,Zhang B,Li D,Lv M,Huang C,Shen GX,et al. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS One. 2010;5:e8922. 10.1371/journal.pone.0008922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee SH,Charmoy M,Romano A,Paun A,Chaves MM,Cope FO,et al. Mannose receptor high, M2 dermal macrophages mediate nonhealing Leishmania major infection in a Th1 immune environment. J Exp Med. 2018;215:357-75. 10.1084/jem.20171389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Staines K,Hunt LG,Young JR,Butter C. Evolution of an expanded mannose receptor gene family. PLoS One. 2014;9:e110330. 10.1371/journal.pone.0110330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan W,Yang X,Huang F,Tong X,Zhu L,Wang S. Identification of CD206 as a potential biomarker of cancer stem-like cells and therapeutic agent in liver cancer. Oncol Lett. 2019;18:3218-26. 10.3892/ol.2019.10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo Z,Jiang JH,Zhang J,Yang HJ,Yang FQ,Qi YP,et al. COX-2 Promotes Migration and Invasion by the Side Population of Cancer Stem Cell-Like Hepatocellular Carcinoma Cells. Medicine (Baltimore). 2015;94:e1806. 10.1097/MD.0000000000001806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang D,Fu L,Sun H,Guo L,DuBois RN. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology. 2015;149:1884-1895.e4. 10.1053/j.gastro.2015.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kundu N,Ma X,Kochel T,Goloubeva O,Staats P,Thompson K,et al. Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties. Breast Cancer Res Treat. 2014;143:19-31. 10.1007/s10549-013-2779-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chu TH,Chan HH,Kuo HM,Liu LF,Hu TH,Sun CK,et al. Celecoxib suppresses hepatoma stemness and progression by up-regulating PTEN. Oncotarget. 2014;5:1475-90. 10.18632/oncotarget.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chu TH,Chan HH,Hu TH,Wang EM,Ma YL,Huang SC,et al. Celecoxib enhances the therapeutic efficacy of epirubicin for Novikoff hepatoma in rats. Cancer Med. 2018;7:2567-80. 10.1002/cam4.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okuda H,Kobayashi A,Xia B,Watabe M,Pai SK,Hirota S,et al. Hyaluronan synthase HAS2 promotes tumor progression in bone by stimulating the interaction of breast cancer stem-like cells with macrophages and stromal cells. Cancer Res. 2012;72:537-47. 10.1158/0008-5472.CAN-11-1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodríguez MM,Fiore E,Bayo J,Atorrasagasti C,García M,Onorato A,et al. 4Mu Decreases CD47 Expression on Hepatic Cancer Stem Cells and Primes a Potent Antitumor T Cell Response Induced by Interleukin-12. Mol Ther. 2018;26:2738-50. 10.1016/j.ymthe.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim SW,Yang HG,Kang MC,Lee S,Namkoong H,Lee SW,et al. KIAA1114, a full-length protein encoded by the trophinin gene, is a novel surface marker for isolating tumor-initiating cells of multiple hepatocellular carcinoma subtypes. Oncotarget. 2014;5:1226-40. 10.18632/oncotarget.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SW,Park HW,Kim H,Lee S,Choi SY,Park Y,et al. Evaluating Antitumor Activity of Kiatomab by Targeting Cancer Stem Cell-Specific KIAA1114 Antigen in Mice. Immune Netw. 2019;19:e43. 10.4110/in.2019.19.e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pang YB,He J,Cui BY,Xu S,Li XL,Wu MY,et al. A Potential Antitumor Effect of Dendritic Cells Fused with Cancer Stem Cells in Hepatocellular Carcinoma. Stem Cells Int. 2019;2019:1-10. 10.1155/2019/5680327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Introna M. CIK as therapeutic agents against tumors. J Autoimmun. 2017;85:32-44. 10.1016/j.jaut.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 90.Yang T,Zhang W,Wang L,Xiao C,Wang L,Gong Y,et al. Co-culture of dendritic cells and cytokine-induced killer cells effectively suppresses liver cancer stem cell growth by inhibiting pathways in the immune system. BMC Cancer. 2018;18:984. 10.1186/s12885-018-4871-y [DOI] [PMC free article] [PubMed] [Google Scholar]