Abstract
Background:
Mid-life obesity is associated with cognitive impairment, though the relationship for late-life obesity is equivocal, and may depend on the anthropometric measure.
Objective:
We examined the relationship between adiposity and cognition across age categories, cognitive domains, and by measures of obesity in a multi-ethnic population-based cohort.
Methods:
The study included 1179 Northern Manhattan Study participants with obesity measures at baseline (44% overweight, 30% obese), an initial neuropsychological assessment conducted within 7y (mean age=70), and a second cognitive assessment conducted on average 6y later. Z-scores were derived for cognitive domains (episodic and semantic memory, executive function, processing speed) and averaged to calculate global cognition. BMI and waist:hip ratio (WHR) were examined in relation to cognitive performance and change over time, stratified by age, using linear regression models adjusting for vascular risk factors.
Results:
Among those age <65y at baseline, greater WHR was associated with worse global cognitive performance at initial assessment and directly associated with decline in performance between assessments. The association with initial performance was strongest for non-Hispanic Whites (beta=−0.155/standard deviation, p=0.04), followed by non-Hispanic Black/African Americans (beta=−0.079/standard deviation, p=0.07), and Hispanics (beta=−0.055/standard deviation, p=0.03). The associations were most apparent for the domains of processing speed and executive function. There was no association for BMI among those <65y. Among those age ≥65, there was no association for BMI or WHR with cognitive performance at initial assessment nor decline over time.
Conclusions:
Our results support the detrimental effect of mid-life rather than later life obesity, particularly abdominal adiposity, on cognitive impairment and decline.
Keywords: Cognition, Obesity, Adiposity, Cognitive Dysfunction, Epidemiology
Introduction
The public health burden of cognitive impairment in older populations is substantial and growing [1], underscoring the importance of identifying and targeting modifiable risk factors. Mid-life adiposity has been associated with an increased risk of cognitive impairment and dementia [2,3]. However, inconsistencies exist in the literature relating measures of adiposity to cognitive health. The relationship between adiposity and cognition may depend on the anthropometric measure of obesity. Although BMI may perform well as an adiposity measure in mid-life, it may not be an ideal measure in the elderly due to the concurrent loss of lean body mass and increase in adipose tissue in the absence of weight change that comes with aging. Waist circumference and waist:hip circumference ratio have been suggested as more reliable measures of adiposity in the elderly [4–8], and abdominal adiposity may be most detrimental for cognition [9–12]. The effect of adiposity on cognition may be age-dependent and change over time, with some studies suggesting that late-life overweight/obesity may in fact be associated with better cognition [6, 13].
This study examines the relationship between obesity and cognitive performance and decline in a racially/ethnically diverse prospective population-based cohort, and whether these associations vary by age, across cognitive domains, and by measure of adiposity. We hypothesized that adiposity would be associated with worse cognitive performance and greater cognitive decline, and that this association would be attenuated, and possibly even reversed, at older ages. Further, we hypothesized that waist:hip ratio, a measure of abdominal obesity, would be a greater risk factor for cognitive impairment and decline compared to BMI.
Methods
Data Sharing Statement
The data that support our findings are available upon reasonable request.
Study Population
This study was conducted using the MRI subcohort of the Northern Manhattan Study (NOMAS). NOMAS is an ongoing prospective population-based cohort study initiated in 1993 to examine stroke epidemiology in a multi-ethnic urban population. Adults over age 40 who had resided in Northern Manhattan for >3 months and had never been diagnosed with a stroke were sampled using random-digit dialing. In 2003 the MRI subcohort began recruitment during annual telephone follow-up, comprised of participants who were clinically stroke-free, age >50 years, with no contraindications to MRI (N=1290). For these analyses we excluded participants who self-classified as “other” race/ethnicity, those who were underweight (BMI<18.5), and those with cognitive impairment at baseline (MMSE<17 for those with less than eight years of education, and a MMSE<24 among those with ≥8 years of education (N=93 excluded)) [14]. The study was approved by the IRBs of Columbia University and the University of Miami, and all subjects provided written informed consent.
Baseline Risk Factor Assessment
At enrollment extensive interviews were conducted with trained bilingual research assistants, and physical/neurological examinations were conducted by study physicians. Race/ethnicity was based upon self-identification through questions modeled after the US census and conforming to standard definitions outlined by Directive 15 [15]. Based on these questions, participants were categorized as Hispanic, non-Hispanic Black or African American (NHB), non-Hispanic White (NHW), and non-Hispanic other race.
Standardized questions were adapted from the Behavioral Risk Factor Surveillance System by the CDC regarding vascular risk factors, including hypertension, diabetes mellitus, smoking, and cardiac conditions [16]. Covariate data collection and definitions, including former/current smoking, alcohol consumption, physical activity, hypertension, diabetes, and hypercholesterolemia, have been described previously [17,18]. The Hamilton Rating Scale for Depression was included in the baseline questionnaire, and participants who scored ≥8 or self-reported taking anti-depressant medications were classified as depressed [19].
Anthropometric variables included height, weight, waist circumference (cm), and hip circumference (cm). The two exposures of interest were body mass index (BMI), calculated as kg/m2 as a measure of overall obesity, and the ratio of waist:hip circumference (WHR) as a measure of abdominal obesity [20]. BMI, measured at study baseline, was examined continuously and categorically (reference: normal 18.5–24.9, overweight 25–29.9, obese 30+). WHR, measured at baseline, was examined continuously.
Neuropsychological Testing
Participants underwent a neuropsychological (NP) battery, in English or Spanish, by trained research assistants. The mean time from baseline NOMAS enrollment to the first NP assessment was 7.2 (+/−2.4) years. Domain-specific Z scores were calculated for cognitive domains (episodic memory, processing speed, semantic memory, and executive function) by averaging construct-relevant Z-transformed NP test scores. The tests included in each domain were determined based on inter-relationships observed among the tests in an exploratory factor analysis and findings presented in previous studies [21, 22]. Episodic memory was assessed with a 12-word five trial list-learning task: total score, delayed recall score, and delayed recognition score. Executive function was assessed by the difference in time to complete the Color Trails test Form 1 and Form 2, and the sum of the Odd-Man-Out subtests 2 and 4. Processing speed was assessed with the Grooved Pegboard task in the non-dominant hand, the Color Trails test Form 1, and the Visual-Motor Integration test [23]. Regarding the Color Trails test Form 1 and Form 2 and Grooved Pegboard task, for participants who did not complete the task within the maximum time, we estimated their expected completion time by adding penalty time to the maximum time allowed using Kaplan-Meier. Semantic memory was assessed with the picture naming (modified Boston Naming) test, category fluency (Animal Naming) test and phonemic fluency (C, F, L in English speakers and F, A, S in Spanish speakers). The mean of the four domain-specific z-scores was calculated as a global cognition score.
A second NP was conducted at a mean of 6 +/− 2 years later. The Z-score for performance at the initial assessment was subtracted from that at the second assessment to calculate change in performance. Change in global cognitive performance was defined as the mean of the difference of the four domain-specific z-scores over time.
Statistical Analysis
We examined the distribution of the covariates stratified by age at baseline (<65 years vs ≥65) and BMI. Multivariable-adjusted linear regression models were constructed to examine associations between the anthropometric measures of adiposity (BMI, WHR, per standard deviation) and Z-scores for global cognitive performance and cognition change from the first to follow-up assessment. When associations between the measures of adiposity and global cognition were suggested at P<0.10, secondary analyses examining the four cognitive domains were conducted to determine which specific domains were driving the associations. Model 1 included age at neuropsychological testing, sex, race/ethnicity, years of education, medical insurance (Medicaid or no insurance versus Medicare without Medicaid or private insurance), and the time from baseline data collection to NP assessment. Model 2 additionally adjusted for smoking, moderate alcohol consumption, moderate-heavy physical activity, and depression. Model 3 additionally included hypertension, diabetes, and hypercholesterolemia. For the analyses of the Z-scores representing the change in performance from first assessment to follow-up, we additionally included the time between the two neuropsychological assessments and the Z-score for performance at the initial assessment. All analyses were stratified by baseline age < 65 vs ≥65 (approximate median age at baseline). A secondary analysis was conducted by adding HOMA insulin resistance [24] to model 3 and restricting to those without diabetes at baseline.
We explored potential effect modification by race/ethnicity, sex, education, depression, and physical activity. Interaction terms between these variables with WHR were added individually to model 2, and when these interactions reached statistical significance (P<0.05), stratified analyses were conducted. Lastly, we examined the potential interaction between WHR and BMI in model 2 for both cognitive performance and decline.
Analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Results
The study population included 1179 NOMAS participants with data on BMI and WHR and NP testing. The mean age at baseline was 64 (SD=8) years and at neuropsychological assessment was 70 (SD=9) years, with 40% men, 67% Hispanic, 16% NHW, and 18% NHB. At study baseline, 44% were overweight, and 30% were obese. At baseline the mean BMI was 28.2 (SD=4.7) and the mean WHR was 0.90 (SD=0.09). Table 1 shows the characteristics of the study population in relation to the covariates stratified by both baseline age and BMI.
Table 1.
Description of the study sample stratified by age and BMI categories at baseline.
| Age <65 | Age 65+ | |||||
|---|---|---|---|---|---|---|
| BMI<25 | BMI 25–29.9 | BMI 30+ | BMI<25 | BMI 25–29.9 | BMI 30+ | |
| N=145 | N=304 | N=214 | N=161 | N=218 | N=137 | |
| Male sex, N(%) | 65(45) | 145 (48) | 57(27) | 75(47) | 86(39) | 39(28) |
| Medicaid/uninsured, N(%) | 76(52) | 168(55) | 120(56) | 50(31) | 80(37) | 60(44) |
| Race/ethnicity, N(%) | ||||||
| NHB | 28(19) | 36(12) | 33(15) | 31(19) | 41(19) | 42(31) |
| NHW | 16(11) | 25(8) | 20(9) | 59(37) | 48(22) | 15(11) |
| Hispanic | 101(70) | 243(80) | 161(75) | 71(44) | 129(49) | 80(58) |
| Depressed, N(%) | 24 (16) | 37 (12) | 39 (18) | 19 (12) | 25 (11) | 19 (14) |
| Smoking, N(%) | ||||||
| Never | 57(39) | 147(48) | 108(50) | 76(47) | 94(43) | 77(56) |
| Former | 48(33) | 102(34) | 74(35) | 64(40) | 98(45) | 48(35) |
| Current | 40(28) | 55(18) | 32(15) | 21(13) | 26(12) | 12(9) |
| Moderate alcohol use, N(%) | 67(46) | 151(50) | 71(33) | 80(50) | 87(40) | 41(30) |
| Moderate-heavy physical activity, N(%) | 17(12) | 36(12) | 20(9) | 20(13) | 19(9) | 7(5) |
| Hypertension, N(%) | 69(48) | 191(63) | 159(74) | 98(61) | 162(74) | 124(91) |
| Diabetes, N(%) | 16(11) | 49(16) | 65(30) | 14(9) | 45(21) | 37(27) |
| Hypercholesterolemia, N(%) | 86(59) | 190(63) | 146(68) | 112(70) | 143(66) | 93 (68) |
| Subclinical infarcts, N(%) | 5 (3) | 18 (6) | 15 (7) | 15 (9) | 30 (13) | 16 (12) |
| Age, Mean (SD) | 64(6) | 65(5) | 64(6) | 79(6) | 78(6) | 77(5) |
| Years of education, Mean (SD) | 11(5) | 9(5) | 10(5) | 11(5) | 10(5) | 9(5) |
| WMHV (% total cranial volume), Mean (SD) | 0.49 (0.62) | 0.45 (0.59) | 0.42 (0.46) | 0.89 (1.00) | 0.91 (0.93) | 0.93 (1.05) |
| Cerebral volume (% total cranial volume), Mean (SD) | 75 (4) | 74 (3) | 74 (4) | 70 (4) | 70 (5) | 71 (3) |
| Waist:Hip Ratio, Mean (SD) | 0.86 (0.08) | 0.90 (0.08) | 0.90 (0.08) | 0.90 (0.10) | 0.91 (0.08) | 0.92 (0.09) |
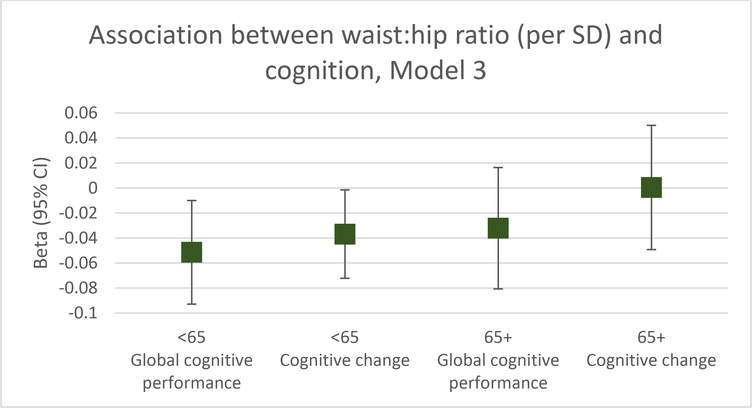
The associations between BMI and WHR with cognitive performance and change, stratified by baseline age, are shown in Table 2. Among participants <65 years, there was no association between BMI and cognitive performance at initial assessment or change over time. However, greater WHR was associated with worse global cognitive performance at initial assessment (model 3 beta=−0.051 per SD, p=0.02, Figure 1) and with decline in performance between the two assessments (model 3 beta=−0.037/SD, p=0.04, Figure 1). For perspective, the effect estimate for a one year increase in age in model 3 in relation to initial cognitive performance was beta=−0.014, and the effect estimate in relation to change in performance was also beta=−0.014. Therefore, among those <65 the effect estimate for a one SD (0.09) increase in WHR was similar to that for a 3.6 year increase in age in relation to initial global cognitive performance in model 3, and the effect estimate for a one SD increase in WHR was similar to that for a 2.6 year increase in age in relation to change in global cognitive performance.
Table 2.
Association between measures of adiposity and cognitive performance and change, stratified by age.
| Baseline age <65 | Baseline age 65+ | |||
|---|---|---|---|---|
| Global cognition | Cognitive change | Global cognition | Cognitive change | |
| Beta, P-value | Beta, P-value | Beta, P-value | Beta, P-value | |
| BMI (per SD) | ||||
| Model 1 | −0.030, 0.09 | −0.009, 0.55 | −0.011, 0.68 | 0.041, 0.11 |
| Model 2 | −0.025, 0.16 | −0.012, 0.45 | −0.009, 0.73 | 0.045, 0.08 |
| Model 3 | −0.011, 0.54 | −0.008, 0.61 | −0.011, 0.67 | 0.048, 0.07 |
| Overweight | ||||
| Model 1 | −0.025, 0.59 | −0.013, 0.75 | −0.065, 0.23 | −0.009, 0.87 |
| Model 2 | −0.033, 0.48 | −0.025, 0.55 | −0.061, 0.27 | −0.014, 0.80 |
| Model 3 | −0.019, 0.68 | −0.021, 0.61 | −0.061, 0.27 | −0.015, 0.79 |
| Obese | ||||
| Model 1 | −0.092, 0.06 | −0.037, 0.39 | −0.059, 0.36 | 0.067, 0.30 |
| Model 2 | −0.077, 0.12 | −0.044, 0.32 | −0.049, 0.45 | 0.072, 0.27 |
| Model 3 | −0.043, 0.40 | −0.034, 0.45 | −0.053, 0.43 | 0.091, 0.17 |
| Waist:hip ratio (per SD) | ||||
| Model 1 | −0.064, 0.002 | −0.040, 0.02 | −0.038, 0.12 | −0.001, 0.97 |
| Model 2 | –0.062, 0.003 | −0.039, 0.03 | −0.035, 0.15 | −0.004, 0.87 |
| Model 3 | −0.051, 0.02 | −0.037, 0.04 | −0.032, 0.19 | 0.0004, 0.99 |
Model 1: sex, insurance, race/ethnicity, age, education, time from baseline to MRI
Model 2: variables in model 1 + smoking, alcohol consumption, physical activity, depression
Model 3: variables in model 2 + hypertension, diabetes, hypercholesterolemia
Figure 1.
Association between WHR (per SD) and cognitive performance and change, stratified by baseline age (controlling for time from baseline to MRI, sex, insurance, race/ethnicity, age, education, smoking, alcohol consumption, physical activity, depression, hypertension, diabetes, and hypercholesterolemia).
When the individual cognitive domains were examined separately among participants <65, controlling for the variables in model 2, WHR was inversely associated with initial performance on the domains of processing speed (beta=−0.087/SD, p=0.002) and executive function (beta=−0.066/SD, p=0.03), but not with episodic memory (beta=−0.043/SD, p=0.16) or semantic memory (beta=−0.041/SD, p=0.14). Similarly, WHR was associated with decline over time on the domains of processing speed (beta=−0.068/SD, p=0.02) and executive function (beta=−0.090/SD, p=0.002), but not with episodic memory (beta=−0.017/SD, p=0.54) or semantic memory (beta=−0.008/SD, p=0.73).
Among those age ≥65, there was no association for BMI or WHR with cognitive performance at initial assessment nor with change in cognitive performance between the two assessments (Table 2, Figure 1).
In analyses that included HOMA insulin resistance in model 3 and excluded those with diabetes at baseline, the results were mostly consistent: the association between high WHR and greater cognitive decline among those age <65 was attenuated and no longer reached statistical significance (beta=−0.015 per SD, p=0.52) though the association with initial cognitive performance remained strong (beta=−0.102 per SD, p=0.0002).
Interactions between WHR and race/ethnicity, sex, education, physical activity, and depression were examined for cognitive performance and change, again stratified by age. No effect modification was suggested for sex, physical activity or depression (P>0.05). An interaction with years of education was suggested for change in cognitive performance among those age ≥65, with non-significant effect estimates in opposite directions between strata (completed highschool: beta=0.050/SD, p=0.16; did not complete highschool: beta=−0.038/SD, p=0.28). Effect modification by race/ethnicity was suggested for the association between WHR and cognitive performance at initial assessment among those age <65 (p<0.05). The effect estimate for WHR was strongest for NHW (beta=−0.155/SD, p=0.04), followed by NHB (beta=−0.079/SD, p=0.07), and then Hispanics (beta=−0.055/SD, p=0.03).
An interaction was also suggested between WHR and BMI in relation to cognitive decline among those < 65 years (P<0.10), such that the association for WHR was stronger for those who were obese (BMI=30+) at baseline (beta=−0.077/SD, p=0.04) than those who were not obese (beta=−0.024/SD, p=0.22).
Discussion
This study elucidates the relationship between abdominal adiposity and cognitive performance and cognitive decline in a diverse population-based cohort. Greater abdominal obesity, as measured by WHR, was associated with worse cognitive performance and more decline among those age <65 at the time of anthropometric measurement, though these associations were not apparent among those who were older. Processing speed and executive function were the domains most associated with abdominal obesity. Although abdominal obesity was a risk factor for poor cognitive health, overall adiposity as measured by BMI was not a risk factor.
The results are consistent with a growing body of literature demonstrating a role of mid-life vascular risk factors, and adiposity specifically, in cognitive impairment and decline. Though it is now well-established that obesity is a risk factor for Alzheimer disease and age-related cognitive decline [2.3], some of the nuances of these relationships remain unclear. Inconsistencies exist in the literature relating adiposity to cognitive health [6,13]. These inconsistencies may be partly explained by differences in the anthropometric measures used across studies, which motivated our examination of both BMI and WHR as related, but distinct, obesity measures. Inconsistencies may also be explained by age, which motivated our decision to stratify the cohort a priori by age [25]. A 2016 meta-analysis that defined obesity based on BMI showed that mid-life obesity (age <65 years) was associated with a significant 41% increased risk of incident dementia, whereas late-life obesity (age ≥65 years) was associated with a significant 17% decreased risk of incident dementia [13].
The problem with using BMI as a measure of adiposity in the elderly is multi-fold. First, if the hypothesis that central, and not peripheral, adiposity is most clinically relevant for brain health is correct, then BMI is not a specific marker of central adiposity and cannot distinguish between varying patterns of fat distribution. For example, among those age 65 and older with a BMI of 25 or higher, 47% had a WHR less than 0.90 in our study. Data supports an inverse association between visceral fat and cognitive performance, with greater deleterious effects than subcutaneous fat [10–12], and suggests that while abdominal fat in late-life may increase risk for dementia and cognitive impairment, overall obesity may be protective [9]. Second, BMI does not consider body composition or shape, and therefore is unable to distinguish between lean body mass and fat mass and their distribution. As muscle mass diminishes and adipose tissue grows in the elderly the validity of BMI as a measure of obesity decreases. In this way, a stable BMI with age fails to adequately reflect the changes in body composition and shape that characterize the aging process, whereas measures of abdominal adiposity may better reflect obesity in the elderly [4–8].
The results also support the possibility of synergistic effects between abdominal and overall obesity, consistent with previous reports [26]. The association of WHR with cognitive decline was stronger among those who were obese according to the BMI cutpoint of ≥30. This is consistent with previous data that have similarly shown abdominal obesity (e.g., WHR) to be a risk factor for cognitive impairment specifically among those with high BMI, underscoring the importance of efforts to reduce abdominal adiposity particularly among those with high BMI [27].
Combined data from the Minority Aging Research Study and the Rush Memory and Aging Project showed that lower BMI in late-life was associated with faster decline in semantic and episodic memory in both White and Black participants [28], and the Cardiovascular Health Study showed that late-life obesity was associated with a decreased risk of dementia vs normal weight, and those who were underweight in late-life had an increased risk of dementia [25]. The impact of late-life obesity remains equivocal, and doubt persists regarding the causal nature of observed associations between late-life overweight/obesity with a reduced risk of dementia and improved cognitive performance [6]. A positive association between BMI and cognition can be explained by the fact that low BMI in the elderly can be a marker of frailty, and a low BMI may be the result of cognitive impairment, suggesting reverse causality. To reduce the possibility of this type of bias we excluded those who were underweight with a BMI less than 18.5 from our study, and there were too few in this category to reliably examine them as a separate group. However, we were unable to capture significant weight loss prior to baseline which may indicate frailty even in the normal BMI range. In the current study, we found WHR was a stronger correlate of worse cognitive performance at the initial assessment compared to BMI, supporting our hypothesis that abdominal obesity is a better marker of cognitive health than general obesity. We also found that the inverse association between WHR and cognitive performance at the baseline neuropsychological assessment was stronger among those age <65, supporting our hypothesis that adiposity in mid-life may be more related to cognitive health than that in later life.
We previously found that individuals with more ideal cardiovascular health, as defined by the American Heart Association’s Life Simple 7 metric, had better performance on the initial assessment of processing speed and less decline, and that processing speed was more sensitive to these cardiovascular health metrics than the other cognitive domains, with a healthy BMI an important contributor [29]. In the current study, which delves more deeply into the relationship between adiposity and cognitive health, processing speed remains a specific cognitive domain associated with WHR.
Effect modification by sex for the relationship between adiposity and cognitive health has been suggested, but the direction is inconsistent [3,25]. We did not observe effect modification by sex in our age-stratified models, though power was limited. We did observe potential effect modification by race/ethnicity. Increased WHR was significantly associated with poorer cognitive performance in the younger subset in all race/ethnic groups, though the association was strongest for whites and weakest for Hispanics, an observation that deserves further exploration in other multi-ethnic cohorts. Nonetheless, our study is one of the first to explore the role of adiposity in a Hispanic population, in which the burden of obesity is higher than other US populations.
Strengths of the study include the large diverse population-based sample, two neuropsychological testing sessions an average of 6 years apart, and comprehensive data on associated vascular risk factors. However, there are a few important limitations. We are unable to estimate the association of underweight with cognitive health as only a small proportion of our study population was underweight. Second, we lack data on fat distribution. Ideally we would have measured visceral and subcutaneous fat, as data support an inverse relationship between visceral fat with cognitive performance that may be more relevant than subcutaneous fat [10–12]. We cannot infer causality, nor rule out the possibility of residual confounding by measured and unmeasured brain health risk factors. Additionally, this study was conducted within a subsample that was healthier than the overall NOMAS population because they had not suffered strokes and were able to visit the center and undergo MRI, and had lower BMIs. Thus, we may be underestimating the adiposity effects and cannot assume generalizability to other populations.
In conclusion, data from this diverse population-based prospective cohort support the deleterious role of adiposity in cognitive health. Achieving and maintaining a normal BMI and specifically a healthy waist circumference by mid-life may be important for preserving cognitive health for many decades. Further research is needed to elucidate whether weight loss in late life has the potential to be neuroprotective, how fat distribution impacts cognitive trajectories, and how adiposity changes over the life course impact brain morphology and cognition.
Acknowledgements:
Funding: National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01 NS 29993) and the Evelyn F. McKnight Brain Institute.
Footnotes
Conflicts of interest: None
References
- [1].Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9, 63–75. [DOI] [PubMed] [Google Scholar]
- [2].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11, 718–726. [DOI] [PubMed] [Google Scholar]
- [3].Anstey KJ, Cherbuin N, Budge M, Young J (2011) Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev 12, e426–e437. [DOI] [PubMed] [Google Scholar]
- [4].Stevens J, Cai J Juhaeri, Thun MJ, Williamson DF, Wood JL (1999) Consequences of the use of different measures of effect to determine the impact of age on the association between obesity and mortality. Am J Epidemiol 150, 399–407. [DOI] [PubMed] [Google Scholar]
- [5].Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC (2001) A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord 25, 1730–1735. [DOI] [PubMed] [Google Scholar]
- [6].Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R (2007) Measures of adiposity and dementia risk in elderly persons. Arch Neurol 64, 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Janssen I, Katzmarzyk PT, Ross R (2004) Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 79, 379–384. [DOI] [PubMed] [Google Scholar]
- [8].Allison DB, Faith MS, Heo M, Kotler DP (1997) Hypothesis concerning the U-shaped relation between body mass index and mortality. Am J Epidemiol 146, 339–349. [DOI] [PubMed] [Google Scholar]
- [9].West NA, Haan MN. Body adiposity in late life and risk of dementia or cognitive impairment in a longitudinal community-based study (2009) J Gerontol A Biol Sci Med Sci 64, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mazzoccoli G, Dagostino MP, Vinciguerra M, Ciccone F, Paroni G, Seripa D, Addante F, Montella RC, De Cosmo S, Sera F, Greco A (2014) An association study between epicardial fat thickness and cognitive impairment in the elderly. Am J Physiol Heart Circ Physiol 307, H1269–H1276. [DOI] [PubMed] [Google Scholar]
- [11].Isaac V, Sim S, Zheng H, Zagorodnov V, Tai ES, Chee M (2011) Adverse Associations between Visceral Adiposity, Brain Structure, and Cognitive Performance in Healthy Elderly. Front Aging Neurosci 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yoon DH, Choi SH, Yu JH, Ha JH, Ryu SH, Park DH (2012) The relationship between visceral adiposity and cognitive performance in older adults. Age Ageing 41, 456–461. [DOI] [PubMed] [Google Scholar]
- [13].Pedditzi E, Peters R, Beckett N (2016) The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies [published correction appears in Age Ageing. 2016 Sep;45(5):740]. Age Ageing 45, 14–21. [DOI] [PubMed] [Google Scholar]
- [14].Gambhir IS, Khurana V, Kishore D, Sinha AK, Mohapatra SC (2014) A clinico-epidemiological study of cognitive function status of community-dwelling elderly. Indian J Psychiatry 56, 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wallman KK, Hodgdon J (1977) Race and ethnic standards for Federal statistics and administrative reporting. Stat Report 450–4. [PubMed] [Google Scholar]
- [16].Kargman DE, Sacco RL, Boden-Albala B, Paik MC, Hauser WA, Shea S (1999) Validity of telephone interview data for vascular disease risk factors in a racially mixed urban community: the Northern Manhattan Stroke Study. Neuroepidemiology 18, 174–184. [DOI] [PubMed] [Google Scholar]
- [17].Willey JZ, Moon YP, Paik MC, Boden-Albala B, Sacco RL, Elkind MS (2009) Physical activity and risk of ischemic stroke in the Northern Manhattan Study. Neurology 73, 1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Willey J, Gardener H, Cespedes S, Cheung YK, Sacco RL, Elkind MSV (2017) Dietary Sodium to Potassium Ratio and Risk of Stroke in a Multiethnic Urban Population: The Northern Manhattan Study. Stroke 48, 2979–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Suk SH, Sacco RL, Boden-Albala B, Cheun JF, Pittman JG, Elkind MSV, Paik MC, Northern Manhattan Study (2003) Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study. Stroke 34, 1586–1592. [DOI] [PubMed] [Google Scholar]
- [21].Marquine MJ, Attix DK, Goldstein LB, Samsa GP, Payne ME, Chelune GJ, Steffens DC (2010) Differential patterns of cognitive decline in anterior and posterior white matter hyperintensity progression. Stroke 41, 1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Siedlecki KL, Rundek T, Elkind MS, Sacco RL, Stern Y, Wright CB (2012) Using contextual analyses to examine the meaning of neuropsychological variables across samples of english-speaking and spanish-speaking older adults. J Int Neuropsychol Soc 18, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Berry DC, Banbury S, Henry L (1997) Transfer across form and modality in implicit and explicit memory. Q J Exp Psychol A 50, 1–24. [DOI] [PubMed] [Google Scholar]
- [24].Rundek T, Gardener H, Xu Q, Goldberg RB, Wright CB, Boden-Albala B, Disla N, Paik MC, Elkind MSV, Sacco RL (2010) Insulin resistance and risk of ischemic stroke among nondiabetic individuals from the northern Manhattan study. Arch Neurol 67, 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Luchsinger JA (2009) Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol 66, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu Z, Yang H, Chen S, Cai J, Huang Z (2019) The association between body mass index, waist circumference, waist-hip ratio and cognitive disorder in older adults. J Public Health (Oxf) 41, 305–312. [DOI] [PubMed] [Google Scholar]
- [27].Zhang T, Yan R, Chen Q, Ying X, Zhai Y, Li F, Wang X, He F, Ye C, Lin J (2018) Body mass index, waist-to-hip ratio and cognitive function among Chinese elderly: a cross-sectional study BMJ Open 8, e022055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Arvanitakis Z, Capuano AW, Bennett DA, Barnes LL (2018) Body Mass Index and Decline in Cognitive Function in Older Black and White Persons. J Gerontol A Biol Sci Med Sci 73, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gardener H, Wright CB, Dong C, Cheung K, DeRosa J, Nannery M, Stern Y, Elkind MSV, Sacco RL (2016) Ideal Cardiovascular Health and Cognitive Aging in the Northern Manhattan Study. J Am Heart Assoc 5, e002731. [DOI] [PMC free article] [PubMed] [Google Scholar]