Abstract
Objectives
To establish the evidence for rehabilitation interventions tested in populations of patients admitted to ICU and critical care with severe respiratory illness, and consider whether the evidence is generalizable to patients with COVID-19.
Methods
The authors undertook a rapid systematic review. Medline (via OvidSP), CINAHL Complete (via EBSCOhost), Cochrane Library, Cochrane Database of Systematic Reviews and CENTRAL (via Wiley), Epistemonikos (via Epistemonikos.org), PEDro (via pedro.org.au) and OTseeker (via otseeker.com) searched to 7 May 2020. The authors included systematic reviews, RCTs and qualitative studies involving adults with respiratory illness requiring intensive care who received rehabilitation to enhance or restore resulting physical impairments or function. Data were extracted by one author and checked by a second. TIDier was used to guide intervention descriptions. Study quality was assessed using Critical Skills Appraisal Programme (CASP) tools.
Results
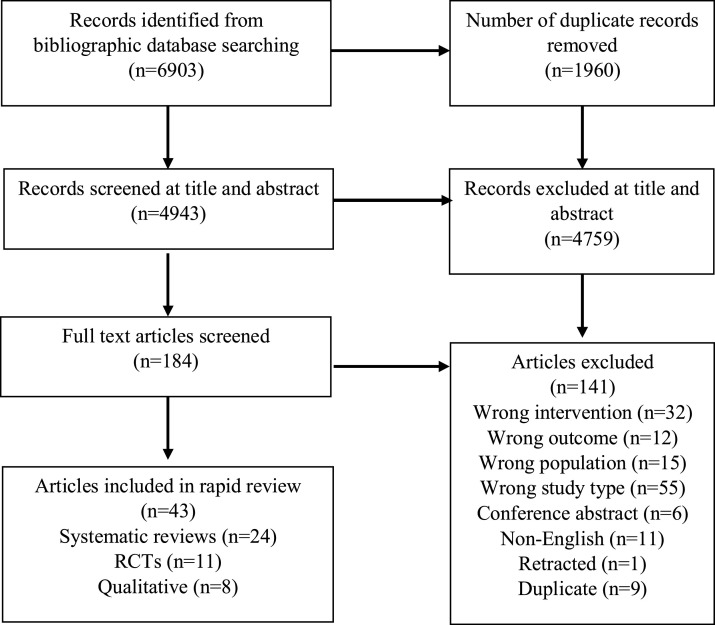
Six thousand nine hundred and three titles and abstracts were screened; 24 systematic reviews, 11 RCTs and eight qualitative studies were included. Progressive exercise programmes, early mobilisation and multicomponent interventions delivered in ICU can improve functional independence. Nutritional supplementation in addition to rehabilitation in post-ICU hospital settings may improve performance of activities of daily living. The evidence for rehabilitation after discharge from hospital following an ICU admission is inconclusive. Those receiving rehabilitation valued it, engendering hope and confidence.
Conclusions
Exercise, early mobilisation and multicomponent programmes may improve recovery following ICU admission for severe respiratory illness that could be generalizable to those with COVID-19. Rehabilitation interventions can bring hope and confidence to individuals but there is a need for an individualised approach and the use of behaviour change strategies. Further research is needed in post-ICU settings and with those who have COVID-19.
Registration: Open Science Framework https://osf.io/prc2y
Keywords: COVID-19, Rehabilitation, Systematic review
Background
On March 11th 2020 the World Health Organisation (WHO) classified COVID-19 as a pandemic. In December 2020 in the UK, there were over 1.75 million confirmed cases, with 62,033 deaths [1]. The main focus of all health services has been to maximise survival in those infected with a strong emphasis on sufficient critical care facilities and pharmacological treatments, along with the development of a vaccine. Observational studies describe acute shortness of breath, myalgia and fatigue as presenting symptoms [2], similar to other severe respiratory illnesses such as influenza [3]. However, it has also been recognised that ongoing symptoms may last several months after being infected including pain, fatigue, difficulty thinking, vertigo and insomnia [4]. Critical care can also result in muscle atrophy, weakness and functional impairment [5]. In addition, many who present with COVID-19 are older and some have pre-existing frailty, which is exacerbated during acute clinical care [6]. Some of these short and long terms symptoms may be responsive to rehabilitation to aid recovery, such as pain and fatigue which will impact on functional ability, participation and quality of life. However, the field of rehabilitation has been neglected [7] and experienced significant disruption during this pandemic [8].
Pragmatic recommendations were quickly developed including acute physiotherapy management of those with COVID-19 [9] focussing on respiratory care and COVID-19 specific precautions. However, when dealing with a new disease, the authors also need to utilise relevant and best available research from studies of similar, severe respiratory illnesses. Thus, the authors aimed to establish the evidence base for rehabilitation interventions tested in populations of patients admitted to an intensive care unit (ICU) and critical care with severe respiratory illness, and to consider whether this evidence is generalizable to patients with COVID-19. Our primary objective was:
-
•
To establish whether rehabilitation interventions could improve functional ability and quality of life for adults recovering from severe COVID-19.
Secondary objectives were:
-
•
To establish whether rehabilitation interventions could improve functional ability and quality of life in older people (aged 65+) and people with pre-existing conditions or frailty recovering from severe COVID-19?
-
•
To explore the views and experiences of those undergoing such rehabilitation.
Methods
The authors undertook a systematic review of rehabilitation interventions for those with severe respiratory illness requiring intensive or critical care such as Severe Adult Respiratory Syndrome (SARS) where there are symptom parallels [3], [10]. The authors followed Cochrane rapid review methods guidance [11] and reported according to PRISMA guidelines [12]. The protocol was registered (https://osf.io/prc2y).
Data sources and search strategy
Seven bibliographic databases (Medline (via OvidSP), CINAHL Complete, Cochrane Library, CDSR and CENTRAL, Epistemonikos, PEDro and OTseeker) were searched from inception to May 2020 using a search strategy (Supplementary data).
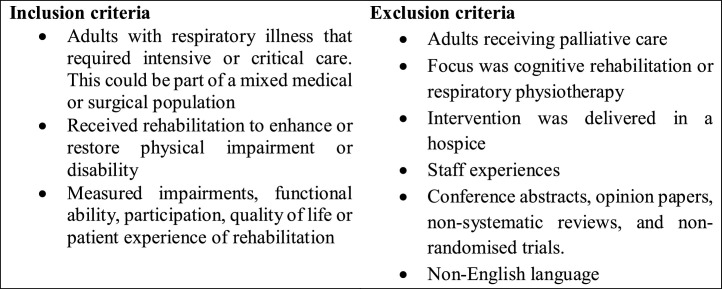
Inclusion and exclusion criteria (Fig. 1)
Fig. 1.
Inclusion and exclusion criteria.
Study selection and data extraction
The authors used a stepwise approach starting with systematic reviews. Twenty five percent of titles and abstracts were dual-screened with one reviewer screening remaining abstracts and a second screening all excluded abstracts. One reviewer screened all, and a second screened excluded full text papers. A third reviewer was involved where necessary. Any RCTs and primary qualitative studies included in the systematic reviews were listed to avoid inclusion in the subsequent study selection. The same screening process was then followed for RCTs and qualitative studies.
Data were extracted by one reviewer, checked by a second. Data included population, intervention, setting, outcomes, participants and results. Study quality was assessed using the relevant Critical Appraisal Skills (CASP) tools [13].
Analysis
In order to categorise interventions, the authors modified an existing intervention taxonomy [14] and added additional categories for mobility training, early mobilisation and neuromuscular electrical stimulation (NMES). ‘Other’ was used where there was no description of an intervention other than, e.g. the term physical rehabilitation. The authors explored the impact of primary studies appearing in multiple included reviews. A narrative synthesis was undertaken, structured by intervention type and setting.
Results
Searches identified 6903 articles: twenty-four systematic reviews, with eleven RCTs and eight qualitative studies (that were not included in any of the reviews) were included (Fig. 2 ). Table 1, Table 2 summarise the included studies with intervention components reported as Supplementary data. Fig. 3 provides an overall summary of findings.
Fig. 2.
PRISMA flow diagram.
Table 1.
Description of study characteristics and findings by Intervention type.
| Source | Study design | Setting | Participants | Outcome domains | Primary Outcome Measure (time point) | Intervention details | Study characteristics | Key findings |
|---|---|---|---|---|---|---|---|---|
| Exercise only | ||||||||
| Battle et al 2019 (UK) [18] | RCT | Outpatient | Adults, who had been patients on ICU | Impairment | 6MWT (7 weeks, 6 months, 12 months) | Six-week, twice weekly individualised exercise programme including cardiopulmonary, balance and strengthening exercises. Control – usual care | 62 mixed medical/surgical patients; Median age (IQR) 62 (49 to 72) years; 31/60 (52%) male | Mean difference in 6MWT at 7 weeks was −70.5 (95% CI −179.1 to 38.0). No difference in any outcome at any time point |
| Ferguson et al 2019 (UK) [21] | Qualitative using semi-structured interviews | Outpatient/home | ICU survivors taking part in an RCT | Perceptions | Perceptions of exercise programme | ICU survivors taking part in RCT of a six-week programme including aerobic and strength exercises | 21 mixed medical patients; mean age (SD) 53 (13) years; 10/21 (48%) male | Benefit of exercise more likely if pre-existing and new issues optimally managed. Personalisation of programme is a key facilitator |
| Schujmann et al 2019 (Brazil) [19] | RCT | ICU | Adults on ICU with 100 points on Barthel Index 2 weeks prior to admission | Impairment; activity limitation | Barthel Index (discharge from ICU) | Individualized progressive programme of strength and aerobic exercises and gait training during ICU stay. Control – usual care. | 135 mixed medical/surgical patients; mean (SD) age controls 55 (12) and intervention 48 (15); 85/135 (63%) male | Differences in Barthel (76 ± 20 vs 97 ± 5; 95% CI −26.3 to −14.5). Improvements in functional independence, mobility and light/moderate physical activity; inconsistent findings in muscle and respiratory function |
| Wright et al 2018 (UK) [20] | RCT | ICU | Adults requiring MV | Impairment; activity limitation; HRQoL; Service outcomes | Physical component summary (PCS) of the SF-36 (6 months) | Individualised and progressive strength training 90 minutes a day, 5 days a week whilst on ICU. Control – usual care | 308 mixed medical/surgical patients; Mean (SD) age controls 64 (16) and intervention 60 (16) years; 180/308 male (58%) | Mean difference in PCS at 6 months was −1.1 (95% CI −7.1 to 5.0). No difference in outcomes at any time point except in functional independence measure at 3 months. |
| Lau et al 2005 (China) [17] | RCT | Outpatient | People referred for physiotherapy from the SARS review clinic | Impairment; HRQoL | 6MWT (6 weeks) | Six-week group aerobic and strength training programme (4 to 5 sessions per week). Control – usual care | 133 patients with SARS; mean age (SD) intervention 35.9 (9.3) and controls 38.3 (11.2) years; 45/133 male (34%) | 6MWT–56.7 (95% CI −86.7 to −26.8). Inconsistent findings in muscle strength; no difference in SF36 domains |
| Exercise and mobility training | ||||||||
| Amundadottir et al 2019 (Iceland) [24] | RCT | ICU | Adults requiring MV for >48 hours | Impairment; activity limitation; HRQoL; service outcomes; adverse events | Duration of mechanical ventilation, ICU and hospital LoS | Twice daily, progressive strength, balance and mobility training. Control – daily passive and active exercises and transfers | 50 mixed medical/surgical patients; median (IQR) age intervention participants = 62 (50 to 70) and controls = 64 (58 to 74) years; 33/50 (66%) male | Median difference in ventilation duration −0.8 days (95% CI −4.3 to 3.0). No difference in any outcome at any time point |
| Parry et al 2017 [23] | Mixed methods SR | ICU and post-ICU | Adults admitted to ICU | Perceptions | Barriers and enablers to physical activity | Any physical activity | 89 studies of mixed medical/surgical patients (4 RCTs; 4 qualitative studies of patient experiences. No summary data regarding participants | Barriers and enablers are multidimensional and include: Physical and psychological capacity; safety; culture and team; motivation and beliefs; environment |
| Tipping et al 2017 [22] | Meta-analysis | ICU | Adults admitted to ICU > 24 hours | Impairment; activity limitation; adverse events | Mortality (hospital discharge) | Active mobilisation and rehabilitation | 14 RCTs/CCTs; 1753 mixed medical/surgical patients. No summary data regarding participants. | Risk difference in mortality 0.02 (95% CI −0.01 to 0.05). Improved muscle weakness, activity limitation and participation. |
| Vitacca et al 2016 (Italy) [25] | RCT | Patients’ home | Adults discharged from rehabilitation unit following critical care | Impairment; activity limitation; HRQoL; Service outcomes; | Maximum inspiratory pressure (6 months) | 6-month, daily home based programme supervised by carer including flexibility, mobility training, strengthening and aerobic exercises. Control – usual care | 48 mixed medical/surgical patients; mean age (range) controls 63 (43 to 81) and intervention 68.3 (49 to 83) years; 22/48 (46%) male | Effect sizes not reported. Inconsistent findings in respiratory outcomes; no difference in strength, quality of life or survival. No adverse events |
| Connolly et al 2015 & 2016 [15], [16] | Narrative SR | Post ICU (hospital and community) | Adults admitted to ICU > 24 hours | Impairment; activity limitation; HRQoL; adverse events | Not specified | Exercise interventions | 6 RCTs; 483 mixed medical/surgical patients. No summary data regarding participants. | No difference in outcomes |
| Early mobilisation | ||||||||
| Ding et al 2019 [27] | Network meta-analysis | ICU | Adults undergoing MV | Impairments; Service outcomes | ICU-AW (not specified) | Early mobilization versus usual nursing care | 15 RCTs; 1726 mixed medical/surgical patients. No summary data regarding participants. | Improved ICU-AW when started within 72 to 96 hours of MV compared with 24 to 48 hours (Mean difference 0.11, 95% CI 0.02 to 0.58); no difference in LoS |
| Okada et al 2019 [33] | Meta-analysis | ICU | Adults admitted to ICU | HRQoL; Service outcomes; Adverse events | In hospital mortality, length of ITY/hospital stay and SF-36 (not specified) | Early mobilisation started within 1 week of ICU admission | 12 RCTs; 1322 mixed medical/surgical patients. No summary data regarding participants. | Difference in mortality 1.12 (95% CI 0.80 to 1.58). No difference in outcomes |
| Zhang et al 2019 [28] | Meta-analysis | ICU | Adults admitted to ICU | Impairment; activity limitation; service outcomes; adverse events | Muscle strength, ICU-AW, functional mobility, duration of MV, ventilator free days, mortality rates, adverse events (not specified) | Early mobilisation versus routine care | 23 RCTs; 2308 mixed medical/surgical patients. Mean ages ranged from 44.9 to 65.5 years. 1352/2308 (59%) male | Improved mobility, incidence of ICU-AW (relative risk 0.60, 95% CI 0.40 to 0.90) and discharge home rate. Reduced duration of MV. No different in mortality or adverse events |
| Laerkner et al 2019 (Denmark) [36] | Qualitative (ethnography) with semi-structured interviews and observation | ICU | Adult undergoing MV | Interactions | Nurse-patient interactions in relation to mobilisation | Mobilisation | 13 interviews with mixed medical/surgical patients; age range 30 to 86 years; 8/13 (62%) male N = 25 observations; age range 37 to 80 years; 17/25 (68%) male | Mobilisation is more than physical activity and involves negotiation and behaviour change techniques |
| Doiron et al 2018 [30] | Narrative SR | ICU | Adults admitted to ICU | Impairment; activity limitation; service outcomes; adverse events | ADLs | Early mobilisation or active exercise of criticality ill participants either during or after MV | 4 RCTs and quasi-RCTs; 690 mixed medical/surgical patients. Mean/median age range from 56 to 62 years. No summary data on sex. | No difference in outcomes |
| Fuke et al 2018 [32] | Meta-analysis | ICU | Adults admitted to ICU | Impairments; HRQoL | ICU-AW, delirium free days, HADS (during hospitalisation) | Early rehabilitation | 6 RCTs; 709 mixed medical/surgical patients. No summary data on participants. | Difference in incidence of ICU-AW (Odds ratio 0.42 (95% CI 0.22 to 0.82); no difference in other outcomes |
| Ringdal et al 2018 (Sweden) [37] | Qualitative involving semi-structured interviews | ICU | Adults in ICU | Experiences | Experiences of early mobilisation and in bed cycling | In bed cycling, 20 minutes a day for 5 consecutive days | 11 mixed medical/surgical patients; age range 31 to 77 years; 5 (45%) male | Activity enables feelings of engagement, control and normalisation |
| Doroy 2016 (USA) [35] | Qualitative (phenomenology) using semi-structured interviews | ICU | Adults on ICU employing a care bundle including early mobilisation | Experiences | Experiences of receiving care using an early mobility care bundle | Care bundle included pain management, breathing/awakening trials, sedation choice, delirium monitoring, early mobility/exercise and family involvement | 12 ICU patients; age range 25 to 65 years; 6 (50%) male | The care bundle not sufficient to improve patient experience of ICU. The role of follow up care needs to be considered |
| Da Silva Azevedo et al 2015 [26] | Narrative SR | ICU | Adults admitted to ICU | Activity limitation | Not specified | Early mobilisation | 4 RCTs and 2 cohort studies; 806 mixed medical/surgical patients. No summary data on participants | Improved function |
| Castro- Avila et al 2015 [29] | Meta-analysis | ICU | Adults admitted to ICU > 48 hours | Functional status; Walking ability; muscle strength; HRQoL; Duration of MV, LoS; Time in rehab | Functional status (not specified) | Early rehabilitation | 7 RCTs/CCTs; 774 mixed medical/surgical patients. 481/774 (62%) were male. | Walking without assistance improved (Risk ratio 1.42; 95% CI 1.17 to 1.72); no difference in other outcomes |
| Laurent et al 2015 [31] | Narrative SR | ICU | Adults admitted to ICU under MV | Adverse events | Unclear | Early exercise | 22 studies (19 RCTs; 2 case series; 1 retrospective study); 2307 mixed medical/surgical patients. No summary data on participants | Safe and feasible. |
| Silva et al 2014 [34] | Narrative SR | ICU | Adults admitted to ICU | Impairment; activity limitation; HRQoL; service outcomes; adverse events | Function; duration of MV and ICU (not specified) | Early mobilisation | 8 RCTs; 731 mixed medical/surgical patients. No summary data on participants | Improvement across all outcomes |
| NMES | ||||||||
| Zayed et al 2020 [40] | Meta-analysis | ICU | Adults admitted to ICU | Impairment; service outcomes; adverse events | Muscle strength (not specified) | NMES applied to different muscle groups | 6 RCTs; 718 mixed medical/surgical patients. Mean age (SD) 60 ± 15.3 years; 435.718 (60.6%) male | Mean difference in muscle strength 0.45 (95% CI −2.89 to 3.80). No difference any outcomes |
| Chen et al 2019 (Taiwan) [38] | RCT | Sub-acute care | Adults requiring MV > 21 days | Impairment; activity limitation; service outcomes | Pulmonary function, muscle function and physical function (10 days) | Daily electrical stimulation for two 30 minute sessions per day 5 days a week for 2 weeks on vastus lateralis and rectus femoris bilaterally. Control group had similar electrode placement but stimulator power was turned off | 33 mixed medical/surgical patients; mean (SD) age controls 73.8(17.8) and intervention 77.7 (14.3) years; 17/33 (52%) male | Inconsistent findings in muscle function. No difference in other outcomes |
| Koutsioumpa et al 2018 (Greece) [39] | RCT | ICU | Adults requiring >96 hours MV | Impairment; service outcomes | Histologically diagnosed myopathy on 14th day of ICU admission | Transcutaneous electrical neuromuscular stimulation on bilateral quadriceps for 60 minutes for 10 days. Control – usual care | 80 mixed medical/surgical patients; mean (SD) age controls 66 (13.1) and intervention 64 (12.4) years; 60/80 (75%) male | Effect sizes not reported. No difference in any outcomes |
| Maffiuletti et al 2013 [41] | Narrative SR | ICU | Adults with critical illness | Impairment | Muscle strength and mass (not specified) | NMES using a defined protocol | 8 RCTs; 172 mixed medical/surgical patients. 126/172 (73%) were male. No summary data on age of participants. | Improved muscle weakness; no difference in muscle mass |
| Parry et al 2013 [42] | Narrative SR | ICU | Adults admitted to ICU | Impairment | Not specified | Electrical muscle stimulation applied to peripheral muscles | 9 RCTs/CCTs; 136 mixed medical/surgical patients. No summary data on participants. | Improvements in strength for those with long-term admissions |
| Mixed interventions (combination of exercise/mobility training/early mobilisation/NMES | ||||||||
| Anekewe et al 2020 [43] | Meta-analysis | ICU | Adults with critical illness | Impairment; service outcomes | ICU-AW | Early mobilisation and/or NMES compared to usual care. | 9 RCTs; 841 mixed medical/surgical patients. No summary data on participants. | Improved ICU-AW with early rehabilitation (Odds ratio 0.71, 95% CI 0.53 to 0.95); more likely to return home |
| Taito et al 2019 [45] | Meta-analysis | Post ICU (hospital and community) | Adults discharged from ICU | Activity limitation; HRQoL; adverse events | SF36 physical and mental component scores, ADL function and mortality (1 month and 6 months) | Protocolised rehabilitation following ICU discharge earlier than/more intensive than usual care | 10 RCTs; 1110 mixed medical/surgical patients. Mean/median age raged from 40.5 to 68.5 years. No summary data on sex | SMD for PCS 0.06 (−0.12 to 0.24). No difference in other outcomes |
| Akar et al 2017 (Turkey) [49] | RCT | ICU | Adults with COPD requiring MV for >24 hours | Impairments; activity limitation; | Muscle strength; mobility (not specified) | Group 1 – active extremity exercise training plus NMES bilaterally on deltoid, quadriceps 5 days per week for 20 sessions; Group 2 – NMES only. Group 3 – active extremity training only | 30 people with COPD; Mean (SD) age Group 1 70 (12.3), Group 2 62.3 (6.8), Group 3 68 (17.8) years; 15/30 (50%) male | No effect sizes presented. No difference in any outcome |
| Gruther et al 2017 (Austria) [50] | RCT | General hospital ward | Aged >16 with >5 days ICU stay | Impairments; service outcomes; adverse events | Number of days from discharge to general ward until hospital discharge | Early rehabilitation (aerobic and resistance exercises programme and NMES) 2 hours a day, 5 days a week versus usual care | 50 mixed medical/surgical patients; median (IQR) age controls 59 (48 to 70) and intervention 64 (46 to 70) years; 14/50 (28%) male | No effect sizes presented. No difference in outcomes. Hospital costs were lower in the intervention group. No adverse events |
| Connolly et al 2016 [47] | Narrative SR of reviews | ICU; post ICU | Adults with critical illness | Impairments; HRQoL; service outcomes; adverse events | Not specified | Physical rehabilitation that addressed exercise and/or mobility programme, use of cycle ergometry or NMES | 5 systematic reviews; 2479 mixed medical/surgical patients. No summary data on participants. | Improvements (short-term) in strength, LoS and duration of MV; Inconclusive outcomes post discharge; few adverse events reported |
| Kayambu et al 2013 [44] | Meta-analysis | ICU | Adults with critical illness | Impairments; activity limitation; HRQoL; service outcomes; adverse events | Mortality, length of hospital and ICU stay, physical function, quality of life, muscle strength, ventilator free days (not specified) | EMS, exercise, mobility training | 10 RCTs; 790 mixed medical/surgical patients. Mean age was 59.3 years (control) and 63.6 (intervention). Amongst controls 69% were male and 73% of intervention participants. | Improvements across a range of outcomes including physical function (pooled effect size 0.46, 95% CI 0.13 to 0.78); no difference in mortality |
| Stiller 2013 [46] | Narrative SR | ICU | Adults admitted to ICU | Impairments; activity limitation; service outcomes; adverse events | Not specified | Any physiotherapy interventions | 85 studies of mixed medical/surgical patients (12 systematic reviews; 20 RCTs; 8 CCTs; 22 observational studies; 15 surveys; 3 opinion papers). No summary data on participants | Improvement in function and LoS; Inconsistent evidence for NMES |
| Pinheiro et al 2012 [48] | Narrative SR | ICU | Adults admitted to ICU | Impairments; activity limitation; service outcomes; adverse events | Not specified | Physiotherapy, mobility and mobilisation in the ICU. Included electrostimulation, cycle ergometry and kinesiotherapy | 8 studies (7 RCTs); 332 mixed medical/surgical patients. Unclear data on participant characteristics | Improvements in strength and function |
Table 2.
Description of study characteristics and findings for other interventions.
| Source | Study design | Setting | Participants | Outcome domains | Primary Outcome Measure (time point) | Intervention details | Study characteristics | Key findings |
|---|---|---|---|---|---|---|---|---|
| Other interventions | ||||||||
| Van Willigen et al 2020 (UK) [52] | Qualitative using semi-structured interviews | ICU | ICU survivors | Perspectives | Patient and family perspectives on physical rehabilitation | Physical rehabilitation | N = 5; age range 23 to 68 years; 4 (80%) male N = 5 family members | Rehab should focus on building relationships and good communication, be consistent and start as soon as possible. |
| Kou et al 2019 [54] | Meta-analysis | Hospital | Adults with an acute and critical illness undergoing rehabilitation | Impairments; Activity limitation; HRQoL; Adverse events | ADLs (not specified) | Nutritional interventions (lectures, counselling, fortified foods, oral nutritional supplements or parenteral/enteral nutrition) plus rehabilitation (defined as comprehensive or individualised expert programme) | 2 RCTs; 293 mixed medical patients. No summary data on paticipants | Improvements in muscle mass; Short term improvements in Barthel Index at 6 months (SMD 0.30, 95% CI 0.02 to 0.58). No effect on HRQoL. Adverse events not reported |
| Corner et al 2018 (UK) [53] | Qualitative (grounded theory) using semi-structured interviews | ICU and post discharge | ICU survivors and family members | Experiences | Experience of rehabilitation and recovery | Physical rehabilitation | N = 15 mixed medical/surgical patients; age range 30 to 89 years; 11/15 (73%) male 4 family members (dyads) | Need to recalibrate past, present and future self and differences between expectation and reality; recovering autonomy needs motivation and support |
| Suwardianto et al 2018 (Indonesia) [51] | RCT | ICU | Adults admitted to ICU > 24 hours | Impairments; activity limitation. MMSE, PFIT | Not specified | Physical and cognitive therapy. Control no intervention | N = 64 mixed medical patients; mean (SD) age controls 48 (11.4) and intervention 59.9 (11) years; 35/64 (55%) male | Effect sizes not clearly reported. Improved bed transfers and cognitive function |
| Felten-Barentz et al 2018 (Netherlands) [71] | Qualitative (phenomenology) using semi-structured interviews | ICU and post discharge | Ventilated adults receiving hydrotherapy | Experiences | Meaning and experience of hydrotherapy | Hydrotherapy | N = 8 mixed medical/surgical patients; age range 33 to 73 years; 4/8 (50%) male | Feelings of safety and ability to move that can involve families. A turning point in the recovery journey |
| Ramsay et al 2016 (UK) [56] | Mixed methods process evaluation using a questionnaire and focus groups | Hospital (post-ICU) | Participants from an RCT (intervention and control) RECOVER trial | Experiences | Experiences of rehabilitation and quality of care | Physical (MDT) rehabilitation (enhanced physiotherapy, nutritional care and information provision, case management. Usual care comparator | N = 14 focus group participants (+8 family members) 182 experience questionnaires. Median age (IQR for intervention participants 55 years (36, 69) and controls 70 years (63, 78). 50% of intervention participants were male and 66% controls | Individualised care and information highly valued. Enabled greater access to physiotherapy and nutritional care |
| Mehlhorn et al 2014 [55] | Narrative SR | Post ICU (hospital and community) | Adults post ICU admission | Impairments; activity limitations; HRQoL; Service outcomes; Adverse events | Not specified | Rehabilitation | 19 studies (9 RCTs); 2510 mixed medical/surgical patients. No summary data of participants | PTSD may be reduced; no effect on other outcomes |
Fig. 3.
Summary of findings.
Study and participant characteristics
Systematic reviews
Sixty-one unique RCTs and three unique qualitative studies were included in the 24 systematic reviews. Thirty studies were included in more than one review. Two different papers that were included reported the same Cochrane review [15], [16]. Where reported, the total sample sizes ranged from 136 to 2510 participants.
RCTs
Eleven additional RCTs that were not included in any of the reviews, were undertaken in ten countries with 993 participants. The majority reported a mean or median age between 60 and 69 years. The mean proportion of men was 53% (490/993). Where described, interventions tended to be delivered by physiotherapists. Outcomes varied between studies in term of follow up time points (longest was a year) and the measures used.
Qualitative studies
Of the eight qualitative studies not included in any of the reviews, four were undertaken in the UK. Sample sizes ranged from eight to 25 with three studies interviewing both patients and family. Studies included a broad range of ages up to 89 years, with men accounting for 45% to 80% of participants overall.
Quality of included studies
Methodological quality of the included studies is reported in Supplementary files. The quality of the systematic reviews was generally good with all but one assessing the quality of the included studies. However, 8/24 (33%) were deemed to not have considered all important outcomes and only ten (24%) considered potential harms. The quality of RCTs and qualitative studies was more variable. Four of the eleven RCTs (36%) did not describe the method of randomisation, only six accounted for those recruited at follow-up and only three reported harms. As is common in trials of rehabilitation, it is not always possible to blind participants and those delivering the interventions to allocation, but assessors were blinded in eight RCTs (73%) although this was unclear in two RCTs. Most trials (8/11; 73%) did not clearly report adverse outcomes. For the qualitative studies, the recruitment strategy was not clear in half of the studies and the relationship between the researcher and participants was only considered in four of the eight studies. However, analysis appeared sufficiently rigorous in six of the studies.
Summary of evidence by intervention and setting
Exercise
Four high quality RCTs and one qualitative study [17], [18], [19], [20], [21] involving 659 participants evaluated exercise interventions. Four included participants who were aged 65 and over [18], [19], [20], [21]. No adverse events were recorded in two trials [18], [19] and one reported a tracheostomy issue.
ICU
One exercise programme (90 minutes, five days/week) was compared with usual care (30 minutes rehabilitation per day during ICU stay) [20]. No differences in any outcomes at any point during the six-month follow-up were found, with the exception of one secondary outcome measure. The Functional Independence Measure at three months showed an effect in favour of the exercise group (adjusted mean difference 9.7, 95% CI 0.9 to 18.5). Those undergoing a progressive exercise programme for 40 minutes daily, five days/week, compared with daily mobilisation, were more likely to be independent at discharge (Relative risk 0.07, 95% CI 0.02 to 0.23).
Post-hospital discharge
Lau et al. [17] and Battle et al. [18] evaluated six-week outpatient exercise programmes. Lau reported improvements in the six-minute walk test (6MWT). However, this was not supported by Battle who found no differences in any outcome (including 6MWT) at discharge, six or twelve months. These inconclusive findings may be due to the high loss to follow-up (42%; 26/62) resulting in an underpowered study. In addition, Lau et al. [17] included a younger population (in their 30 second) with SARS whereas Battle [18] included an older sample (median 62 years; IQR 49 to 72) with medical/surgical conditions.
Ferguson et al. found that undertaking an exercise programme brought feelings of hope for both physical and mental health recovery [21]. However, lower levels of physical ability and mental health created barriers to engagement whereas individually tailored programmes provided confidence and motivation.
Exercise and mobility training
Four systematic reviews [15], [16], [22], [23] and two RCTs [24], [25] examined exercise plus mobility training. The mean age range was 60–69 years. Few adverse events were reported.
ICU
Tipping et al. [22] evaluated exercise and mobility programmes in a high quality systematic review and found a reduction in muscle weakness using the Medical Research Council Sum Score (pooled mean difference 8.62, 95% CI 1.39 to 15.86) and increased probability of walking independently at hospital discharge (odds ratio 2.13, 95% CI 1.19 to 3.83) in favour of the intervention. There was no difference in mortality at six months (risk difference 0.01, 95% CI −0.06 to 0.08).
Post-ICU
Connolly et al. [15], [16] included six RCTs in a Cochrane review examining interventions delivered post-ICU. Narrative analysis concluded that inconsistent findings, issues with methodological rigour and heterogeneity prevented conclusions being reached. One low quality trial [25] conducted a carer delivered, home-based rehabilitation programme with cardiorespiratory/neurological participants recovering following acute respiratory failure. Benefits were reported for cardiorespiratory participants in some respiratory function measures in the intervention group, but there was no of benefit in ADL, muscle strength or quality of life. No serious adverse events were reported although there were more deaths in the control group (6/24) compared with the rehabilitation group (2/24).
In a mixed-methods systematic review of barriers and facilitators to physical activity and mobilisation in ICU and post-ICU settings [23] there were only three included qualitative studies relating to patient experiences (out of 89 included studies). Physical and psychological factors, and, motivations and beliefs about physical activity were key considerations when promoting recovery following critical illness.
Early mobilisation
Nine systematic reviews [26], [27], [28], [29], [30], [31], [32], [33], [34] and three qualitative studies of mixed quality focussed on early mobilisation in ICU. Sixteen trials were reported in more than one review. One review [28] included 23 RCTs and over 2300 participants and found a reduced incidence of ICU–Acquired weakness (ICU-AW) (relative risk 0.6; 95% confidence intervals 0.4, 0.9) following early mobilisation programmes comprising flexibility, strength and mobility training. However, no benefit was identified in muscle strength at ICU discharge (Weighted Mean Difference WMD 0.95 [95% CI −1.72, 3.61]). The network meta-analysis undertaken by Ding et al. found that optimum time to commence early mobilisation to reduce ICU-AW was during the first 72 to 96 hours of mechanical ventilation (Mean 0.11, 95% CI 0.02 to 0.58) [27].
Functional ability, in particular walking, was consistently found to improve following early mobilisation [26], [28], [29], [30], [34]. One study of 7 RCTs and 774 participants [29] reported improved walking independently (RR 1.42, 95% CI 1.17 to 1.72). Two systematic reviews [28], [33] found no difference in ICU length of stay or HRQoL. Reporting adverse events was not consistent across studies but, where reported, there were few [28], [30], [31], [34]. No detrimental effect on mortality due to early mobilisation was found in two studies [28], [33].
One qualitative study highlighted conflicting feelings of participants regarding fear and safety concerns versus moving around [35]. Laerkner et al.’ ethnographic study explored nurse-patient interactions of mobilisation on intensive care [36] and demonstrated different perspectives of nurses and patients where patients found the idea of mobilisation engendered feelings of fear and harm whereas nurses viewed this positively. In contrast, participants using in-bed cycling in ICU described positive feelings of recovery, control and normalisation [37].
Neuromuscular electrical stimulation
Five studies, comprising two low quality RCTs [38], [39] and three mixed quality systematic reviews [40], [41], [42] evaluated NMES.
ICU
Although early systematic reviews [41], [42] suggested possible benefits from NMES in reducing muscle weakness, a meta-analysis [40], which included six RCTs with 718 participants, was inconclusive for impairment, service or adverse event outcomes. The most recent RCT finding also supports this [39].
Post-ICU
A small RCT [38] in a sub-acute hospital setting with older participants (mean age [SD] 75.8 [16] years) reported an improvement in muscle strength in favour of the intervention.
Multicomponent interventions
Eight studies, including six systematic reviews of generally good quality [43], [44], [45], [46], [47], [48], and two RCTs [49], [50] evaluated interventions that comprised multiple components including exercise, mobilisation or NMES. The studies varied in their combined components (Supplementary data).
ICU
Anekwe et al. [43] evaluated early mobilisation and/or NMES in nine RCTs involving 841 participants. They reported a reduced likelihood of developing ICU-AW (Odds Ratio 0.71; 95% CI 0.53 to 0.95) in favour of the intervention group. One small, low quality RCT [49], with 30 participants evaluating NMES vs NMES plus strength training vs strength training alone, found no differences in muscle strength between the different arms.
ICU and post-ICU
An overview of reviews examining multicomponent rehabilitation programmes across the care continuum concluded that exercise and mobilisation programmes based in ICU may improve muscle strength and are safe but interventions targeting those discharged from ICU are inconclusive [47].
Post ICU
One high quality RCT [50] involving patients on a general hospital ward after transfer from ICU found no difference in outcomes although reported overall hospital costs were lower for those who received the intervention. Taito and colleagues [45] found no difference in the SF-36 physical and mental components scales, respectively (SMD 0.06, 95% CI −0.12 to 0.24; −0.04, −0.20 to 0.11).
Other interventions
Two systematic reviews and one RCT evaluated the effectiveness of other interventions. Four qualitative studies explored experiences of those undergoing rehabilitation.
ICU
Suwardianto et al. [51] reported improvements in bed transfers and cognition following a physical and cognitive rehabilitation programme compared with no rehabilitation, however both the intervention content and study details were poorly described.
Qualitative studies illustrate how setting rehabilitation goals, early in ICU, may not be a priority for patients or families who could only focus on survival. They described initially needing a paternalistic approach to goal setting [52], [53]. Critical care survivors described a lost sense of self which rehabilitation began to rebuild: therapy staff were perceived as trusted advocates who could provide motivation and person-centred approaches to help reconstruct a new future [53].
Post-ICU
One well conducted systematic review evaluating nutritional interventions in addition to rehabilitation in hospital suggested short-term benefits on the Barthel Index (SMD 0.30, 95% CI 0.02 to 0.58) in favour of the intervention group but no effect on quality of life [54]. A mixed quality narrative systematic review of post-ICU rehabilitation both in hospital and after discharge included a broad range of rehabilitation interventions and models of care, such as follow-up programmes. This concluded that post-traumatic stress disorder may be reduced but found no effect on other outcomes [55].
In a mixed-methods process evaluation examining the effectiveness of hospital-based multidisciplinary rehabilitation following ICU discharge, the importance of individualised rehabilitation was raised by participants. They described physiotherapy and nutritional care as particularly important in recovery [56].
Discussion
This systematic review aimed to synthesise current evidence for physical rehabilitation interventions performed in adults who were admitted to ICU or critical care that may be generalizable to adults with or recovering from severe COVID-19. The authors found evidence in those with severe respiratory illness and in mixed respiratory and surgical populations that interventions could improve muscle strength, walking and functional ability. These findings in relation to the 6MWT and Barthel index suggest effect sizes were both statistically and clinically significant [57], [58]. However findings regarding quality of life were inconclusive. The quality of included studies varied. No studies were identified for those with COVID-19. Almost all studies included some older people who are often excluded from research studies [59]. It has been recommended that during the current pandemic, if capacity becomes limited, then critical care should be prioritised for those most likely to survive, which would likely exclude those living with pre-existing frailty [60]. As none of the included studies reported exclusion criteria related to frailty and none reported pre-admission frailty status, the authors cannot be sure our findings apply to this population, although the overarching rehabilitation principles are unlikely to be very different. Most interventions were delivered in intensive care with a paucity of research conducted after hospital discharge. Outcomes reported were varied and often short term. Where reported, adverse events were few in number while there is good evidence that individually tailored exercise programmes can reduce the deleterious effects of inactivity without harm in ICU and acute settings [61].
Qualitative research showed that rehabilitation can bring hope and build confidence on the recovery journey, however an individualised approach is needed. These are key issues for those surviving COVID-19 [62]. Behaviour change strategies, such as goal setting, were perceived to be key components of motivation and recovery in the qualitative literature but these were not component parts of the interventions evaluated in our review. When developing and delivering rehabilitation programmes to support recovery from COVID-19 the inclusion of behaviour change should be integral and must be explicit and well described to facilitate implementation in healthcare settings [63].
The strength of this systematic review is the comprehensive search developed by a multidisciplinary team and adherence to best practice methodological guidance [11]. Where necessary, the authors prioritised findings from the most recent and highest quality systematic reviews to minimise the impact on our findings from individual primary studies that were cited in multiple reviews. This approach also reduced the contribution to our findings from earlier reviews that were generally narrative syntheses and included observational studies as well as RCTs. Nonetheless, there are some limitations. Firstly, by the rapid nature of this review, the authors could have omitted relevant studies by not e.g. undertaking forwards/backwards citation chasing. However, the broad range of databases searched would minimise missing key published studies [11]. In addition, our screening process identified eleven potentially relevant papers that were not available in English as full-text, which is a limitation. Secondly, by limiting inclusion criteria to those with severe respiratory illness requiring intensive care, these findings may not address the emerging rehabilitation needs of all those recovering from COVID-19, such as those who required hospital care but were not deemed critical or those who were not admitted to hospital. The inclusion of studies with mixed respiratory and surgical populations could be seen as non-generalizable to a COVID-19 population. However, in these studies, all included participants with severe respiratory illness and rehabilitation interventions predominantly focussed on the cardiorespiratory and musculoskeletal impairments experienced by both these groups of patients, which have also been observed in those with COVID-19, such as muscle weakness. The mechanistic reasoning underpinning how the interventions may work [64], e.g. strength training to improve neuromuscular function could apply to those from both COVID-19 and non-COVID-19 medical and surgical populations requiring critical care, and to those who may have less severe symptoms. The participants in our review tended to be slightly younger than those admitted to hospital with COVID-19 in the UK [2]. Since the authors conducted our review, there have been increasing reports of additional wide ranging manifestations of COVID-19, such as delirium, peripheral neuropathy, dizziness and mood disorders. In the absence of COVID-19 specific evidence for managing these symptoms, NICE recommend individually tailored self-management, multidisciplinary rehabilitation and social care interventions [4]. Finally, our use of the CASP tools to assess study quality was for pragmatic reasons as it enabled multiple study designs to be assessed within the same framework.
A number of other reports are emerging providing recommendations for the rehabilitation of those recovering from COVID-19. Some suggest broad approaches in relation to service delivery rather than recommendations for specific interventions [65], [66], [67]. Others have combined a literature review with consensus statements [68], [69]. Our review now provides a rigorous evidence base to support the consensus statements, that had been developed using less robust methods, regarding the benefits of mobilisation and exercise in the acute setting [9], goal setting and individualised rehabilitation [66]. Uniquely, our review also included programmes targeting post-hospital rehabilitation which is important not just for those who are discharged from hospital, but also to those with COVID-19 not admitted to hospital. This said, there was a paucity of evidence in this setting with limited benefit of interventions, and no studies based in residential/nursing care homes. There is also a lack of consensus on which outcome measures should be used but these should reflect what is important to those affected by COVID-19 [70].
Where reported, interventions were delivered mainly by physiotherapists. There were no studies reporting programmes delivered by a multidisciplinary team. This may be as a result of our search strategy as the authors excluded cognitive rehabilitation, which is more likely to be delivered by occupational therapists or psychologists. However, it is equally plausible that no research has been published including these professionals. This also limits application of existing evidence as these professionals are clearly supporting the rehabilitation of patients with COVID-19.
There remain unanswered questions about recovery and rehabilitation from COVID-19. The authors do not yet fully understand the short and long term rehabilitation needs of survivors, and importantly but this is starting to change through recent research funding. The PHOSP-COVID study is investigating the longer term recovery from COVID-19 following hospital admission (https://www.leicesterbrc.nihr.ac.uk/themes/respiratory/research/phosp-covid/). The Research and Innovation for Post-COVID-19 Rehabilitation Unit (RICOVR) has also been established to understand what interventions may work to aid physical, psychological, social and economic recovery (https://www.shu.ac.uk/research/specialisms/advanced-wellbeing-research-centre/ricovr). Commonly cited issues for survivors of COVID-19 include frailty, sarcopenia and fatigue, all of which may be amenable to rehabilitation interventions – but there are currently no RCTs underway to establish the effectiveness of programmes. Such trials should include outcomes that are important to those with the disease and consider cost-effectiveness as well as clinical effectiveness. Moving forwards, clinicians and academics need to agree on core outcomes for documenting recovery from COVID-19 to examine progress accurately. Any future rehabilitation research also needs to take into account practical considerations, such as personal protective equipment, as well as considering the use of technology to deliver and monitor programmes and the location of care.
Conclusion
Based on the best available evidence, our rapid systematic review found that those with severe respiratory illness and mixed respiratory and surgical populations admitted for critical care may benefit from progressive exercise, early mobilisation and multicomponent programmes to improve functional independence and walking. Qualitative evidence from those participating in these rehabilitation programmes valued an individualised approach and the bringing of hope and confidence to their recovery. This evidence could be generalised to those with, or recovering from, COVID-19. This said, there is room for improvement in the quality of research in this field and there is a paucity of evidence for effective interventions after discharge from ICU. There is a lack of evidence specifically relating to older people and those with frailty and a lack of consensus regarding outcome measures. Future research needs to better understand the trajectory and rehabilitation needs of those with COVID-19 across the care continuum in order to develop and evaluate relevant interventions.
Key messages
-
•
Due to the novel nature of COVID-19, there is currently no evidence specifically evaluating the benefits of rehabilitation for those in recovery.
-
•
The authors found evidence that some rehabilitation programmes for adults requiring ICU for severe respiratory illness could be beneficial for people recovering from COVID-19
-
•
There is limited evidence for programmes that could aid longer term recovery after discharge from hospital following severe respiratory illness that required an ICU admission
Ethical approval: Not applicable. Conflict of interest: SL was on the Health Technology Assessment (HTA) Additional Capacity Funding Board, HTA End of Life Care and Add-on Studies Board, HTA Prioritisation Group Board and the HTA Trauma Board. The other authors declare no competing interests.
VG is an Associate Editor and was not involved in the peer review of this article.
Acknowledgements
This research was supported by the National Institute for Health Research (NIHR) Applied Research Collaboration South West Peninsula. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.physio.2021.01.007.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Public Health England . 2020. Coronavirus (COVID-19) in the UK.https://coronavirus.data.gov.uk/ [Google Scholar]
- 2.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Piu R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . 2020. Similarities and Differences between Flu and COVID-19.https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm [updated 02/07/20]. Available from: [Google Scholar]
- 4.National Institute for Health and Care Excellence . NICE; 2020. COVID-19 rapid guideline: managing the long-term effects of COVID-19.https://www.nice.org.uk/guidance/ng188/chapter/common-symptoms-of-ongoing-symptomatic-covid-19-and-post-covid-19-syndrome [PubMed] [Google Scholar]
- 5.Jolley S.E., Bunnell A.E., Hough C.L. ICU-acquired weakness. Chest. 2016;150(5):1129–1140. doi: 10.1016/j.chest.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lithander F.E., Neumann S., Tenison E., et al. COVID-19 in older people: a rapid clinical review. Age Ageing. 2020 doi: 10.1093/ageing/afaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinitz P., Mills K., Connolly B., Skelton P., Smith G., Clift Z. Pan American Health Organization and World Health Organization; 2020. Rehabilitation considerations during the COVID-19 outbreak. [Google Scholar]
- 8.COVID-19 significantly impacts health services for noncommunicable diseases [press release]. 1/6/20 2020.
- 9.Thomas P., Baldwin C., Bissett B., et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73–82. doi: 10.1016/j.jphys.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garritty C., Gartlehner G., Kamel C., et al. 2020. Interim Guidance from the Cochrane Rapid Reviews Methods Group. [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;338:b2535. [PMC free article] [PubMed] [Google Scholar]
- 13.Critical Skills Appraisal Programme . 2020. CASP Checklists.https://casp-uk.net/casp-tools-checklists/ Available from: [Google Scholar]
- 14.Lamb S., Becker C., Gillespie L., et al. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall-prevention interventions. Trials. 2011;12(1):125. doi: 10.1186/1745-6215-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connolly B., Salisbury L., O’Neill B., et al. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev. 2015;(6) doi: 10.1002/14651858.CD008632.pub2. N.PAG–N.PAG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly B., Salisbury L., O’Neill B., et al. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle. 2016;7(5):520–526. doi: 10.1002/jcsm.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau H.M., Ng G.Y., Jones A.Y., Lee E.W., Siu E.H., Hui D.S. A randomised controlled trial of the effectiveness of an exercise training program in patients recovering from severe acute respiratory syndrome. Aust J Physiother. 2005;51(4):213–219. doi: 10.1016/S0004-9514(05)70002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battle C., James K., Temblett P., Hutchings H. Supervised exercise rehabilitation in survivors of critical illness: a randomised controlled trial. J Intensive Care Soc. 2019;20(1):18–26. doi: 10.1177/1751143718767061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schujmann D.S., Teixeira Gomes T., Lunardi A.C., et al. Impact of a progressive mobility program on the functional status, respiratory and muscular systems of ICU patients: a randomized and controlled trial. Crit Care Med. 2019;19:19. doi: 10.1097/CCM.0000000000004181. [DOI] [PubMed] [Google Scholar]
- 20.Wright S.E., Thomas K., Watson G., et al. Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax. 2018;73(3):213–221. doi: 10.1136/thoraxjnl-2016-209858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson K., Bradley J.M., McAuley D.F., Blackwood B., O’Neill B. Patients’ perceptions of an exercise program delivered following discharge from hospital after critical illness (the revive trial) J Intensive Care Med (Sage Publications Inc) 2019;34(11–12):978–984. doi: 10.1177/0885066617724738. [DOI] [PubMed] [Google Scholar]
- 22.Tipping C., Harrold M., Holland A., et al. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–183. doi: 10.1007/s00134-016-4612-0. [DOI] [PubMed] [Google Scholar]
- 23.Parry S., Knight L., Connolly B., et al. Factors influencing physical activity and rehabilitation in survivors of critical illness: a systematic review of quantitative and qualitative studies. Intensive Care Med. 2017;43(4):531–542. doi: 10.1007/s00134-017-4685-4. [DOI] [PubMed] [Google Scholar]
- 24.Amundadottir O.R., Jonasdottir R.J., Sigvaldason K., et al. Effects of intensive upright mobilisation on outcomes of mechanically ventilated patients in the intensive care unit: a randomised controlled trial with 12-months follow-up. Eur J Physiother. 2019 [Google Scholar]
- 25.Vitacca M., Barbano L., Vanoglio F., et al. Does 6-month home caregiver-supervised physiotherapy improve post-critical care outcomes?: A randomized controlled trial. Am J Phys Med Rehabil. 2016;95(8):571–579. doi: 10.1097/PHM.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 26.da Silva Azevedo P.M.D., Pereira Gomes B. Effects of early mobilisation in the functional rehabilitation of critically ill patients: a systematic review. Rev Enferm Ref. 2015;(5):129–138. [Google Scholar]
- 27.Ding N., Zhang Z., Zhang C., et al. What is the optimum time for initiation of early mobilization in mechanically ventilated patients? A network meta-analysis. PLoS One [Electronic Resource] 2019;14(10) doi: 10.1371/journal.pone.0223151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Hu W., Cai Z., et al. Early mobilization of critically ill patients in the intensive care unit: a systematic review and meta-analysis. PLoS One [Electronic Resource] 2019;14(10) doi: 10.1371/journal.pone.0223185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro-Avila A.C., Seron P., Fan E., Gaete M., Mickan S. Effect of early rehabilitation during intensive care unit stay on functional status: systematic review and meta-analysis. PLoS One [Electronic Resource] 2015;10(7) doi: 10.1371/journal.pone.0130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doiron K.A., Hoffmann T.C., Beller E.M. Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database Syst Rev. 2018;3 doi: 10.1002/14651858.CD010754.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurent H., Aubreton S., Richard R., et al. Systematic review of early exercise in intensive care: a qualitative approach. Anaesth Crit Care Pain Med. 2016;35(2):133–149. doi: 10.1016/j.accpm.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Fuke R., Hifumi T., Kondo Y., et al. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: a systematic review and meta-analysis. BMJ Open. 2018;8(5) doi: 10.1136/bmjopen-2017-019998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada Y., Unoki T., Matsuishi Y., Egawa Y., Hayashida K., Inoue S. Early versus delayed mobilization for in-hospital mortality and health-related quality of life among critically ill patients: a systematic review and meta-analysis. J Intensive Care. 2019;7:57. doi: 10.1186/s40560-019-0413-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva V.S., Pinto J.G., Martinez B.P., Camelier F.W.R. Mobilization in the intensive care unit: systematic review. Fisioter Pesq. 2014;21(4):398–404. [Google Scholar]
- 35.Doroy A. 2016. Exploring the Lived Experiences of Patients who have Participated in an Early Mobility Program; p. 1. [Google Scholar]
- 36.Laerkner E., Egerod I., Olesen F., Toft P., Hansen Hp. Negotiated mobilisation: an ethnographic exploration of nurse-patient interactions in an intensive care unit. J Clin Nurs. 2019;28(11–12):2329–2339. doi: 10.1111/jocn.14828. [DOI] [PubMed] [Google Scholar]
- 37.Ringdal M., Warren Stomberg M., Egnell K., Wennberg E., Zatterman R., Rylander C. In-bed cycling in the ICU; patient safety and recollections with motivational effects. Acta Anaesthesiol Scand. 2018;62(5):658–665. doi: 10.1111/aas.13070. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y.H., Hsiao H.F., Li L.F., Chen N.H., Huang C.C. Effects of electrical muscle stimulation in subjects undergoing prolonged mechanical ventilation. Respir Care. 2019;64(3):262–271. doi: 10.4187/respcare.05921. [DOI] [PubMed] [Google Scholar]
- 39.Koutsioumpa E., Makris D., Theochari A., et al. Effect of transcutaneous electrical neuromuscular stimulation on myopathy in intensive care patients. Am J Crit Care. 2018;27(6):495–503. doi: 10.4037/ajcc2018311. [DOI] [PubMed] [Google Scholar]
- 40.Zayed Y., Kheiri B., Barbarawi M., et al. Effects of neuromuscular electrical stimulation in critically ill patients: a systematic review and meta-analysis of randomised controlled trials. Aust Crit Care. 2020;33(2):203–210. doi: 10.1016/j.aucc.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Maffiuletti N.A., Roig M., Karatzanos E., Nanas S. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: a systematic review. BMC Med. 2013;11:137. doi: 10.1186/1741-7015-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parry S.M., Berney S., Granger C.L., Koopman R., El-Ansary D., Denehy L. Electrical muscle stimulation in the intensive care setting: a systematic review. Crit Care Med. 2013;41(10):2406–2418. doi: 10.1097/CCM.0b013e3182923642. [DOI] [PubMed] [Google Scholar]
- 43.Anekwe D.E., Biswas S., Bussieres A., Spahija J. Early rehabilitation reduces the likelihood of developing intensive care unit-acquired weakness: a systematic review and meta-analysis. Physiotherapy. 2020;107:1–10. doi: 10.1016/j.physio.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Kayambu G., Boots R., Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41(6):1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 45.Taito S., Yamauchi K., Tsujimoto Y., Banno M., Tsujimoto H., Kataoka Y. Does enhanced physical rehabilitation following intensive care unit discharge improve outcomes in patients who received mechanical ventilation? A systematic review and meta-analysis. BMJ Open. 2019;9(6) doi: 10.1136/bmjopen-2018-026075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stiller K. Physiotherapy in intensive care: an updated systematic review. Chest. 2013;144(3):825–847. doi: 10.1378/chest.12-2930. [DOI] [PubMed] [Google Scholar]
- 47.Connolly B., O’Neill B., Salisbury L., Blackwood B., Enhanced Recovery After Critical Illness Programme G Physical rehabilitation interventions for adult patients during critical illness: an overview of systematic reviews. Thorax. 2016;71(10):881–890. doi: 10.1136/thoraxjnl-2015-208273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinheiro A.R., Christofoletti G. Motor physical therapy in hospitalized patients in an intensive care unit: a systematic review. Rev Bras Ter Intensiva. 2012;24(2):188–196. [PubMed] [Google Scholar]
- 49.Akar O., Gunay E., Sarinc Ulasli S., et al. Efficacy of neuromuscular electrical stimulation in patients with COPD followed in intensive care unit. Clin Respir J. 2017;11(6):743–750. doi: 10.1111/crj.12411. [DOI] [PubMed] [Google Scholar]
- 50.Gruther W., Pieber K., Steiner I., Hein C., Hiesmayr J.M., Paternostro-Sluga T. Can early rehabilitation on the general ward after an intensive care unit stay reduce hospital length of stay in survivors of critical illness?: A randomized controlled trial. Am J Phys Med Rehabil. 2017;96(9):607–615. doi: 10.1097/PHM.0000000000000718. [DOI] [PubMed] [Google Scholar]
- 51.Suwardianto H., Prasetyo A., Utami R. Effects of physical-cognitive therapy (PCT) on criticaly ill patients in intensive care unit. Hiroshima J Med Sci. 2018;67:63–69. [Google Scholar]
- 52.van Willigen Z., Ostler C., Thackray D., Cusack R. Patient and family experience of physical rehabilitation on the intensive care unit: a qualitative exploration. Physiotherapy. 2020;04:04. doi: 10.1016/j.physio.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Corner E.J., Murray E.J., Brett S.J. Qualitative, grounded theory exploration of patients’ experience of early mobilisation, rehabilitation and recovery after critical illness. BMJ Open. 2019;9(2) doi: 10.1136/bmjopen-2018-026348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kou K., Momosaki R., Miyazaki S., Wakabayashi H., Shamoto H. Impact of nutrition therapy and rehabilitation on acute and critical illness: a systematic review. J UOEH. 2019;41(3):303–315. doi: 10.7888/juoeh.41.303. [DOI] [PubMed] [Google Scholar]
- 55.Mehlhorn J., Freytag A., Schmidt K., et al. Rehabilitation interventions for postintensive care syndrome: a systematic review. Crit Care Med. 2014;42(5):1263–1271. doi: 10.1097/CCM.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 56.Ramsay P., Huby G., Merriweather J., et al. Patient and carer experience of hospital-based rehabilitation from intensive care to hospital discharge: mixed methods process evaluation of the RECOVER randomised clinical trial. BMJ Open. 2016;6(8) doi: 10.1136/bmjopen-2016-012041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bohannon R.W., Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377–381. doi: 10.1111/jep.12629. [DOI] [PubMed] [Google Scholar]
- 58.Bouwstra H., Smit E.B., Wattel E.M., et al. Measurement properties of the barthel index in geriatric rehabilitation. J Am Med Directors Assoc. 2019;20(4) doi: 10.1016/j.jamda.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 59.McMurdo M.E.T., Roberts H., Parker S., et al. Improving recruitment of older people to research through good practice. Age Ageing. 2011;40(6):659–665. doi: 10.1093/ageing/afr115. [DOI] [PubMed] [Google Scholar]
- 60.British Medical Association . British Medical Association; London: 2020. Guidance for doctors on ethical issues likely to arise when providing care and treatment during the COVID-19 outbreak.https://www.bma.org.uk/media/2226/bma-covid-19-ethics-guidance.pdf Available from: [Google Scholar]
- 61.Parry S.M., Puthucheary Z.A. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Biase S., Cook L., Skelton D.A., Witham M., ten Hove R. The COVID-19 rehabilitation pandemic. Age Ageing. 2020 doi: 10.1093/ageing/afaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michie S., Ashford S., Sniehotta F.F., Dombrowski S.U., Bishop A., French D.P. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- 64.Howick J., Glasziou P., Aronson J.K. Evidence-based mechanistic reasoning. J R Soc Med. 2010;103(11):433–441. doi: 10.1258/jrsm.2010.100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phillips M., Turner-Stokes L., Wade D., Walton K. British Society of Rehabilitation Medicine (BSRM); 2020. Rehabilitation in the wake of Covid-19—a phoenix from the ashes.www.bsrm.org.uk 27.4.2020. [Google Scholar]
- 66.Chartered Society of Physiotherapy . 2020. CSP COVID-19: Rehabilitation Standards. London: Chartered Society of Physiotherapy. 13/5/20. [Google Scholar]
- 67.Chartered Society of Physiotherapy . Chartered Society of Physiotherapy; London: 2020. Rehabilitation and COVID-19: CSP policy statement. May 2020. [Google Scholar]
- 68.Barker-Davies R.M., O’Sullivan O., Senaratne K.P.P., et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020 doi: 10.1136/bjsports-2020-102596. bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vitacca M., Carone M., Clini E.M., et al. Joint statement on the role of respiratory rehabilitation in the COVID-19 crisis: the Italian position paper. Respiration. 2020:1–7. doi: 10.1159/000508399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.COMET Initiative . 2014. COMET. Initiative Public Involvement Strategy.https://www.comet-initiative.COMET.rg/assets/downloads/COMET%20Public%20Involvement%20strategy_website.pdf [Google Scholar]
- 71.Felten-Barentsz Km, van Oorsouw R., Haans Ajc, Staal Jb, van der Hoeven Jg, Nijhuis-van der Sanden M.G.W. Patient views regarding the impact of hydrotherapy on critically ill ventilated patients: a qualitative exploration study. J Crit Care. 2018;48:321–327. doi: 10.1016/j.jcrc.2018.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.