Abstract
Introduction:
This study explores how human papillomavirus vaccination initiation and completion among men and women aged 18–34 years varies by geographic region.
Methods:
Data from the 2015–2017 Behavioral Risk Factor Surveillance System were analyzed. Geographic regions for the selected states were defined as South, Northeast, and Midwest/West. Human papillomavirus vaccination initiation was defined as receipt of ≥1 dose and completion as receipt of ≥3 doses. Weighted, multivariable logistic regression models estimated the association between geographic region and vaccine uptake, adjusting for sociodemographic, health, and healthcare factors. Analyses were performed in November 2019.
Results:
A total of 18,078 adults were included in the study, 80% of whom resided in the South. The overall vaccination initiation rate was 23.4% and the completion rate was 11.0%. Initiation was higher among those who resided in the Northeast (38.6%), followed by Midwest/West (23.8%), and lowest for those in the South (21.8%) (p<0.0001). Completion rates followed the same trend as initiation. In the adjusted models, compared with adults residing in the Northeast, those living in the South were less likely to initiate (AOR=0.47, 95% CI=0.40, 0.55) and complete (AOR=0.56, 95% CI=0.46, 0.68) human papillomavirus vaccination.
Conclusions:
Human papillomavirus vaccine uptake was low for all regions, but vaccine uptake was significantly lower in the South region. This demonstrates the need to identify barriers specifically associated with the Southern population, which may include differing levels of education and insurance. Such work is especially pertinent as many Southern states face increased risk of human papillomavirus–associated cancers, such as cervix and oral cavity and pharynx cancers.
The abstract of this study was presented at the 12th American Association for Cancer Research Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Under-served, San Francisco, CA.
INTRODUCTION
Human papillomavirus (HPV) is the most common sexually transmitted infection in the U.S. and it is associated with virtually all cases of cervical and anal (90% of each), vaginal (69%), oropharyngeal (60%), vulvar (51%), and penile (40%) cancer.1 HPV infection is not only associated with primary cancers but also contributes to second primary cancers.2–4 Since 2006, bivalent vaccine and later quadrivalent and nonavalent vaccines have been developed to prevent HPV-associated cancers and genital warts. The vaccine was first recommended exclusively for adolescent girls in 2006 and then in 2011 for adolescent boys. The Advisory Committee on Immunization Practices now recommends routine HPV vaccination for adolescents between age 11 and 12 years.5,6 Catch-up vaccination is also recommended for both male and female individuals aged 13–26 years and shared clinical decision making through age 45 years.5,6 In 2017, a total 65.5% of adolescents aged 13–17 years received ≥1 dose of HPV vaccine (68.6% for girls and 62.6% for boys) and 48.6% completed the series (53.1% for girls and 44.3 % for boys).7 For young adults aged 18–26 years, this uptake is even lower, with national initiation rates of 40% for women and only 8% for men.8 Many young adults can benefit from the vaccine; therefore, better understanding of vaccination rates among young adults is warranted.
Previous research on geographic variation in HPV vaccination showed that female individuals age 9–26 years living in the Northeast were significantly more likely to initiate/complete vaccination than those in the South.9–15 Rahman et al.10 found on further analysis in 2012 that male and female residents of the South were less likely to initiate or complete the HPV vaccine series compared with those living in the Northeast. Only the study by Rahman and colleagues10 examined geographic variations in HPV vaccination among male individuals. However, the study used 2012 data, when the vaccine had been recommended for male patients for 1 year. This study includes male individuals and used 2015–2017 data when the vaccine has been recommended for male patients for 4–6 years. Including men is very important because they have a higher burden of HPV-associated oropharyngeal cancer than women,16,17 and are at risk of anal and penile cancers.18 In addition, using current data is important as they can capture current geographic differences in male HPV vaccination rates. Furthermore, since 2012, adolescent HPV vaccination rates have increased in the West and Northeast whereas rates remain low in the South.19 The geographic variation may continue to exist owing to differences in state immunization requirements and insurance mandates from the Affordable Care Act. It is therefore warranted to further examine current geographic variations in HPV vaccination in young adults. This study examines the association between geographic regions and HPV vaccination initiation and completion (vaccine uptake) among men and women aged 18–34 years.
METHODS
Study Sample
Data were extracted from the 2015–2017 Behavioral Risk Factor Surveillance System (BRFSS). The BRFSS is an annual, nationally representative telephone survey that collects data from non-institutionalized adults from the U.S. and U.S. territories (Puerto Rico, Guam, and the U.S. Virgin Islands) regarding their health-related risk behaviors, chronic health conditions, and use of preventive services. It is the largest continuously conducted health survey in the world, is administered by state health departments with oversight from the Centers for Disease Control and Prevention (CDC), and reaches >500,000 respondents. Details of the survey methods used have been published in the literature.20 The BRFSS has 3 overall components: core modules (sets of questions consistently administered to all states and territories to establish national estimates), optional modules (CDC–developed questions that states can include in their BRFSS survey depending on their priorities), and state-added questions (state-customized items).
From 2015 to 2017, an optional module focused on adult HPV vaccination was administered by 16 states (Alabama, Arkansas, Connecticut, Georgia, Hawaii, Massachusetts, Mississippi, Missouri, New Hampshire, Nebraska, North Carolina, South Carolina, South Dakota, Tennessee, Texas, and West Virginia) (Appendix Figure 1), representing 28.7% of the U.S. population. The overall response rate for all 3 years ranged from 45% to 48%, and totaled 18,876 individuals aged 18–34 years. Respondents who did not know or refused to answer if they had been vaccinated (n=798) were removed. This resulted in a final sample of 18,078 individuals. The censored upper age was selected because these adults would have been eligible to have received the HPV vaccine at some point after it was licensed in the U.S. in 2006. As all data used in this study were de-identified and publicly available, downloaded from BRFSS website, IRB approval was not warranted.
Measures
The primary outcomes were self-reported HPV vaccination initiation and completion. Initiation was assessed with the question: A vaccine to prevent the human papillomavirus or HPV infection is available and is called the cervical cancer or genital warts vaccine, HPV shot, (GARDASIL or CERVARIX). Have you ever had the HPV vaccination? Respondents with a yes response were deemed to have initiated the vaccination. Respondents who had initiated vaccination were subsequently asked: How many HPV shots did you receive? Respondents indicating receipt of ≥3 vaccine doses were deemed to have completed the vaccination. It is worth noting that although Advisory Committee on Immunization Practices guidelines for those aged 11–14 years specify only 2 doses are required for completion, this age range is not included in the BRFSS data.21
As the HPV vaccination module was optional and only included by a minority of states, the states could not be grouped according to commonly defined regions (such as the 10 used by HHS).22 Thus, the study used grouping previously employed by Rahman et al.,9 which divided into 3 regions: South (Alabama, Arkansas, Georgia, Mississippi, North Carolina, South Carolina, Tennessee, Texas, West Virginia), Northeast (Connecticut, Massachusetts, New Hampshire), and Midwest/West (Hawaii, Missouri, Nebraska, South Dakota). The Midwest and West were combined owing to the small sample size for each region.
Covariates identified from other studies8,9,23 included sociodemographic variables: age category (18–24, 25–29, 30–34 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic other), marital status (married, divorced/separated/widowed, never married), educational attainment (college graduate or higher, some college or associate degree, high school diploma, less than a high school diploma), income level (≥$50,000, $25,000–<$50,000, <$25,000, refused/missing), health insurance coverage (yes or no). Other covariates concerned health and health care: regular provider (yes or no), time since last medical checkup (within the past year, >1 year), and general health (excellent/very good, good, poor/fair).
Statistical Analysis
To account for the BRFSS complex survey design, survey weights were used throughout all analyses to reduce bias owing to non-response and non-coverage using SAS procedures SURVEYFREQ and SURVEYLOGISTIC. Descriptive statistics for the overall sample, stratified by geographic region, were estimated across covariates (age, sex, race/ethnicity, marital status, educational attainment, income level, health insurance coverage, regular provider, time since last medical checkup, and general health). Wald’s design-based chi-square tests of independence analyses were performed to test for differences in geographic region by HPV vaccine uptake and sociodemographic, health, and healthcare characteristics. Weighted, multivariable binary logistic regression models were used to assess the association between geographic region and HPV vaccine uptake, adjusting for sociodemographic and health and healthcare factors. The Advisory Committee on Immunization Practices recommends “catch-up” vaccination for those aged 13–26 years; therefore, sensitivity analysis was conducted where respondents were restricted to age 18–26 years. Also, owing to the vaccination being recommended at different years for female compared with male individuals, a sensitivity analysis was conducted stratified by sex. Analyses were performed in November 2019. All statistical analyses were performed using SAS, version 9.4.
RESULTS
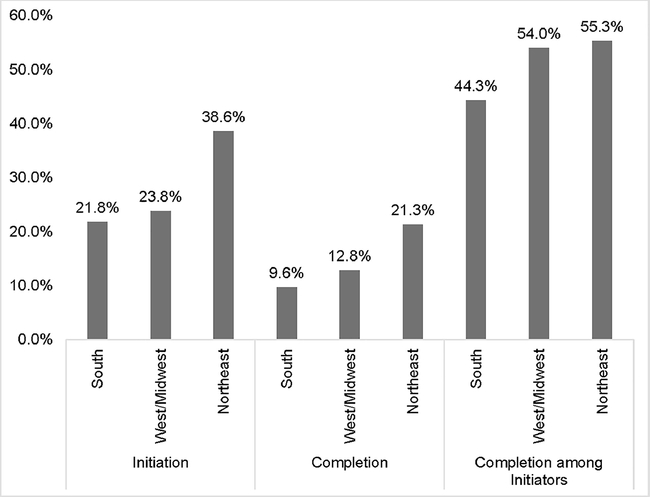
Descriptive statistics for this study sample are shown in Table 1. A total of 18,078 adults were included in the study, 80% of whom resided in the South. Chi-square analyses comparing geographic region with age, race/ethnicity, marital status, educational attainment, level of income, insurance coverage, regularity of healthcare provider, time since last checkup, and general health showed statistically significant differences by geographic region. Overall, 4,338 (23.4%) adults initiated the HPV vaccine, while 2,287 (11.0%) completed the vaccine. Figure 1 shows that the highest proportion of vaccine initiators (38.6%) and vaccine completers (21.3%) resided in the Northeast whereas the lowest proportion of initiators (21.8%) and vaccine completers (9.6%) resided in the South (all with p<0.0001).
Table 1.
Characteristics of Young Adults Aged 18–34 Years in the U.S. Overall and Stratified by Region of Residence, BRFSS (2015–2017)
| n (w%) | Weighted percent | p-value | |||
|---|---|---|---|---|---|
| Characteristics | Total n=18,078 | Southern n=10,354 (80.0%) | West/Midwest n=5,295 (11.8%) | Northeast n=2,429 (8.2%) | |
| HPV vaccine initiation: yes | 4,338 (23.4) | 21.8 | 23.8 | 38.6 | <0.0001 |
| HPV vaccine completion overall: yes | 2,287 (11.0) | 9.6 | 12.8 | 21.3 | <0.0001 |
| HPV vaccine completion among initiators: yes | 2,287 (46.9) | 44.3 | 54.0 | 55.3 | <0.0001 |
| Age, years | 0.0010 | ||||
| 18–24 | 6,303 (42.4) | 42.3 | 42.8 | 44.0 | |
| 25–29 | 5,414 (25.8) | 26.0 | 22.6 | 28.4 | |
| 30–34 | 6,361 (31.8) | 31.8 | 34.6 | 27.6 | |
| Sex | 0.7498 | ||||
| Female | 9,676 (50.9) | 50.8 | 51.1 | 52.1 | |
| Male | 8,397 (49.1) | 49.2 | 48.9 | 47.9 | |
| Race/Ethnicity | <0.0001 | ||||
| Non-Hispanic White | 11,090 (48.9) | 43.8 | 71.0 | 66.6 | |
| Non-Hispanic Black | 2,526 (16.3) | 18.2 | 9.6 | 7.0 | |
| Hispanic | 2,399 (26.3) | 30.1 | 8.1 | 15.6 | |
| Non-Hispanic other | 2,063 (8.5) | 7.9 | 11.3 | 10.9 | |
| Marital status | 0.0015 | ||||
| Married | 7,360 (38.9) | 39.2 | 40.3 | 33.7 | |
| Not married | 10,718 (61.1) | 60.8 | 59.7 | 66.3 | |
| Educational attainment | <0.0001 | ||||
| College graduate or higher | 5,609 (19.0) | 17.7 | 20.8 | 29.4 | |
| Some college or associate degree | 5,868 (35.2) | 35.1 | 38.5 | 31.6 | |
| High school diploma | 5,205 (31.5) | 32.4 | 28.8 | 26.3 | |
| Less than high school diploma | 1,396 (14.3) | 14.8 | 11.9 | 12.7 | |
| Income level | <0.0001 | ||||
| ≥$50,000 | 6,166 (30.8) | 28.8 | 35.6 | 43.0 | |
| $25,000 to $49,999 | 4,301 (22.0) | 22.4 | 24.7 | 15.2 | |
| <$25,000 | 4,723 (29.2) | 32.2 | 22.8 | 18.8 | |
| Refused/missing | 2,888 (18.0) | 17.6 | 16.8 | 23.0 | |
| Has insurance coverage | <0.0001 | ||||
| Yes | 14,591 (72.8) | 70.0 | 80.3 | 88.7 | |
| No | 3,487 (27.2) | 30.0 | 19.7 | 11.3 | |
| Has regular provider | <0.0001 | ||||
| Yes | 11,423 (56.1) | 53.1 | 62.6 | 75.6 | |
| No | 6,655 (43.9) | 46.9 | 37.4 | 24.4 | |
| Time since last medical checkup | <0.0001 | ||||
| Within past year | 10,061 (53.9) | 53.2 | 50.9 | 65.3 | |
| More than a year | 8,017 (46.1) | 46.8 | 49.1 | 34.7 | |
| General health | <0.0001 | ||||
| Excellent/Very good | 10,841 (56.8) | 55.6 | 60.2 | 63.3 | |
| Good | 5,335 (31.4) | 32.1 | 30.7 | 25.9 | |
| Poor/Fair | 1,902 (11.8) | 12.3 | 9.1 | 10.8 | |
Note: Boldface indicates statistical significance (p<0.05). p-value based on chi-square tests.
n, frequency; w%, weighted percentage; BRFSS, Behavioral Risk Factor Surveillance System; HPV, human papillomavirus.
Figure 1.
HPV vaccine initiation and completion rates of young adults aged 18‒34 years by geographic region, BRFSS (2015‒2017).
Notes: There were significant differences between geographic region and vaccine initiation, completion and completion among initiators (all with p<0.0001) based on Chi-square tests.
BRFSS, Behavioral Risk Factor Surveillance System.
In the multivariable models in Table 2, respondents residing in the South were less likely to initiate (AOR=0.47, 95% CI=0.40, 0.55) and complete (AOR=0.56, 95% CI=0.46, 0.68) the HPV vaccination than those in the Northeast. Midwestern/Western residents were also less likely to initiate (AOR=0.55, 95% CI=0.45, 0.68) and complete (AOR=0.70, 95% CI=0.56, 0.89) the HPV vaccination than those in the Northeast. Younger age groups (18–24 years and 25–29 years) were more likely to initiate vaccination compared with those aged 30–34 years. Similarly, respondents aged 18–24 years were more likely to complete the vaccine series than those aged 30–34 years. Male participants were less likely than female participants to initiate and complete the vaccine. Compared with married young adults, unmarried young adults were more likely to initiate and complete the vaccine; adults with health insurance were more likely to initiate and complete the vaccine compared with those without health insurance. The study also showed a linear relationship between level of education and vaccine uptake, with the highest uptake among participants who had a college education or higher.
Table 2.
Association Between Region of Residence and HPV Vaccination Uptake Among Young Adults 18–34 Years, BRFSS (2015–2017)
| AOR (95% CI) | |||
|---|---|---|---|
| Variable | HPV vaccine initiation (n=18,078) | HPV vaccine completion – overall (n=18,078) | HPV vaccine completion among initiators (n=4,336) |
| Geographic region | |||
| Northeast | ref | ref | ref |
| West/Midwest | 0.55 (0.45, 0.68) | 0.70 (0.56, 0.89) | 1.14 (0.82, 1.57) |
| Southern | 0.47 (0.40, 0.55) | 0.56 (0.46, 0.68) | 1.02 (0.78, 1.33) |
| Age, years | |||
| 18–24 | 6.32 (5.12, 7.81) | 5.63 (4.26, 7.44) | 1.46 (1.03, 2.07) |
| 25–29 | 2.41 (1.97, 2.95) | 2.77 (2.14, 3.58) | 1.34 (0.94, 1.90) |
| 30–34 | ref | ref | ref |
| Sex | |||
| Female | ref | ref | ref |
| Male | 0.19 (0.16, 0.22) | 0.12 (0.10, 0.15) | 0.33 (0.25, 0.44) |
| Race/Ethnicity | |||
| Non-Hispanic White | ref | ref | ref |
| Non-Hispanic Black | 1.00 (0.82, 1.22) | 0.46 (0.35, 0.60) | 0.33 (0.24, 0.46) |
| Hispanic | 0.92 (0.72, 1.17) | 0.62 (0.46, 0.84) | 0.53 (0.37, 0.76) |
| Non-Hispanic other | 0.80 (0.60, 1.08) | 0.65 (0.42, 1.01) | 0.68 (0.39, 1.18) |
| Marital status | |||
| Married | ref | ref | ref |
| Not married | 1.35 (1.13, 1.61) | 1.45 (1.16, 1.81) | 1.23 (0.93, 1.64) |
| Educational attainment | |||
| College graduate or higher | ref | ref | ref |
| Some college or associate degree | 0.82 (0.68, 0.98) | 0.57 (0.46, 0.70) | 0.52 (0.39, 0.69) |
| High school diploma | 0.69 (0.56, 0.84) | 0.47 (0.36, 0.62) | 0.46 (0.33, 0.64) |
| Less than high school diploma | 0.48 (0.34, 0.66) | 0.35 (0.20, 0.58) | 0.41 (0.22, 0.76) |
| Income level | |||
| ≥$50,000 | ref | ref | ref |
| $25,000 to $49,999 | 0.96 (0.78, 1.18) | 0.88 (0.68, 1.15) | 0.89 (0.62, 1.27) |
| <$25,000 | 0.89 (0.72, 1.10) | 0.89 (0.69, 1.15) | 1.01 (0.73, 1.41) |
| Refused/missing | 0.63 (0.50, 0.80) | 0.59 (0.45, 0.77) | 0.78 (0.54, 1.13) |
| Has insurance coverage | |||
| Yes | ref | ref | ref |
| No | 0.69 (0.56, 0.85) | 0.69 (0.51, 0.92) | 0.83 (0.57, 1.19) |
| Has regular provider | |||
| Yes | ref | ref | ref |
| No | 0.88 (0.74, 1.04) | 0.76 (0.61, 0.94) | 0.76 (0.58, 1.00) |
| Time since last medical checkup | |||
| Within past year | ref | ref | ref |
| More than a year | 0.79 (0.68, 0.92) | 0.87 (0.71, 1.06) | 0.92 (0.70, 1.21) |
| General health | |||
| Excellent/Very good | ref | ref | ref |
| Good | 0.84 (0.71, 1.00) | 0.83 (0.67, 1.03) | 0.85 (0.65, 1.13) |
| Poor/Fair | 0.94 (0.73, 1.21) | 0.89 (0.66, 1.19) | 0.83 (0.58, 1.21) |
| Survey year | |||
| 2017 | ref | ref | ref |
| 2016 | 0.72 (0.59, 0.86) | 0.77 (0.60, 0.97) | 1.08 (0.80, 1.45) |
| 2015 | 1.00 (0.83, 1.22) | 1.20 (0.94, 1.53) | 1.45 (1.05, 1.99) |
Note: Boldface indicates statistical significance (p<0.05).
BRFSS, Behavioral Risk Factor Surveillance System; HPV, human papillomavirus.
The sensitivity analyses, which limited data to adults aged 18–26 years, provided similar results to the main analyses. Appendix Table 1 shows that the highest proportion of young adults who initiated (55.9%) and completed (30.9%) the HPV vaccine resided in the Northeast, whereas the lowest proportion of those who initiated (33.4%) and completed (14.5%) resided in the South (all with p<0.0001). Appendix Table 2 shows the association between HPV vaccination and geographic region among participants aged 18–26 years. In the adjusted models, respondents residing in the South were less likely to initiate (AOR=0.44, 95% CI=0.34, 0.57) and complete (AOR=0.53, 95% CI=0.40, 0.71) the vaccination compared with those in the Northeast. The same was true for respondents in the Midwest/West compared with those in the Northeast. In the second sensitivity analyses, when the analyses were stratified by sex, the results observed were similar to the original results. Both female and male residents of the South were less likely to have initiated and completed the vaccination compared with those in the Northeast (Appendix Table 3).
DISCUSSION
Based on the states included in the study, the study noted regional variations in HPV vaccine uptake among adults aged 18–34 years, with vaccination rates being low across all regions. However, vaccination uptake was significantly lower in the South region compared with the Northeast even after adjusting for covariates. These results mirror previous published geographical HPV vaccination disparities observed in 2008–2012 among young adults.9,10 It should be noted that Rahman and colleagues9 in 2013 focused on only women and Rahman et al.10 in 2015 observed only 2012 data, whereas this study included both men and women with data from 2015–2017. Compared with the BRFSS data between 2008 and 2010, the weighted vaccine uptake increased over time among young adult women in the South, from 14% of vaccine initiation to 21.8% and from 6.6% of vaccine completion to 9.6%. Similar regional variation in HPV initiation and completion was reported among adolescents in Texas.24 The study’s finding on the impact of region, similar to the Texas finding by Conrey et al.,24 exemplifies the significance of considering region-tailored interventions when developing programs aimed at increasing HPV vaccine uptake. Additionally, these findings also underscore the importance of improving awareness and knowledge about the benefits of HPV vaccination especially in Southern states as this will enhance shared decision making between clients and healthcare providers as postulated by Thomas and colleagues.25
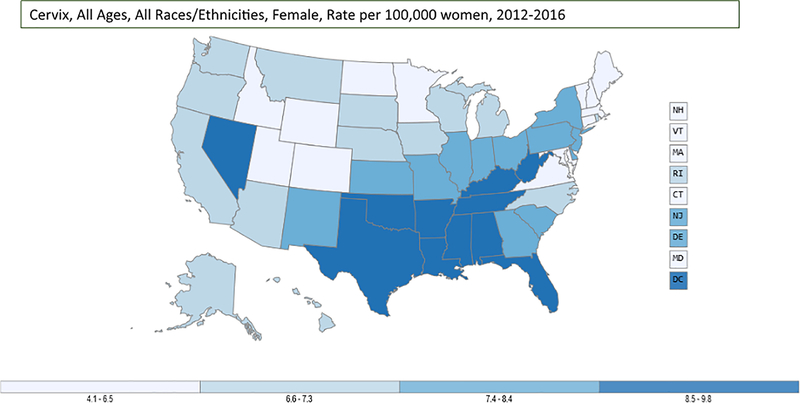
Low vaccination rates in the South are concerning given the high HPV infection prevalence in the region. Hirth et al.26 found that respondents in the Midwest and South had higher HPV infection prevalence compared with Northeastern and Western regions. A consistent lower rate of HPV vaccine uptake in the South may contribute to the higher burden of HPV-associated diseases and cancers. Whitney and colleagues27 indicated that there is a higher burden of HPV-associated cancers in the Southern regions of the U.S. Cervical cancer, which is primarily caused by HPV infection, is more prevalent in the South. CDC data from 2012–2016 shows higher rates of cervical cancer in the South than the other regions (Figure 2A).28 Studies have also shown that cervical cancer disproportionately affects Black women in the South.29,30 In this study, a higher proportion of Black individuals (18.2%) resided in the South than the Northeast (7.0%), underscoring the need to improve vaccination rates in the South.
Figure 2A.
Rate of new cervical cancers in the U.S., 2012‒2016.
Notes: Rate per 100,000 women.
Source: U.S. Cancer Statistics Working Group, U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999‒2016). HHS, Centers for Disease Control and Prevention, National Cancer Institute. https://www.cdc.gov/cancer/dataviz. June 2019.
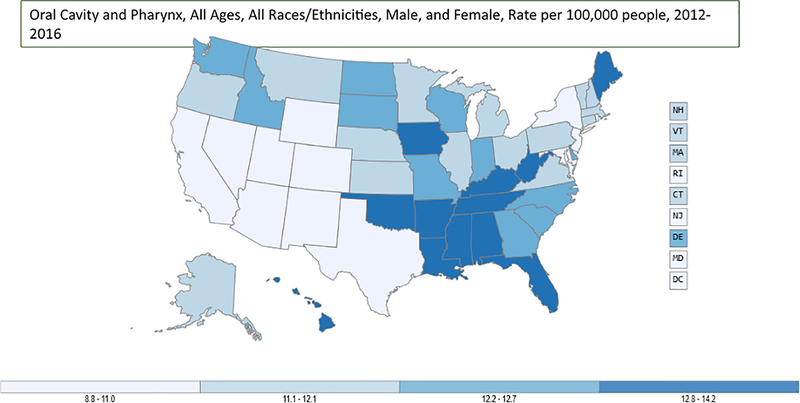
Similarly, for head and neck cancer, CDC data from 2012–2016 show higher rates of oral cavity and pharynx cancer in the South than other regions (Figure 2B).28 Head and neck cancer affects men more than women with a 3:1 ratio; however; after controlling for covariates, the study uncovered a significant gap in vaccine uptake between male and female participants, where male participants were 81% and 88% less likely to initiate and complete the vaccine, respectively, compared with female participants. It should be noted oral cavity and pharynx cancers are caused not only by HPV, but also tobacco use; thus, the effect of HPV should not be underestimated. There is a need to design strategies to improve the HPV vaccine uptake in the South to lower the burden of HPV-associated diseases and cancers in the future.
Figure 2B.
Rate of new oral cavity and pharynx cancers in the U.S., 2012‒2016.
Notes: Rate per 100,000 women.
Source: U.S. Cancer Statistics Working Group, U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999‒2016). HHS, Centers for Disease Control and Prevention, National Cancer Institute. https://www.cdc.gov/cancer/dataviz. June 2019.
People affected by HIV are disproportionately affected by HPV-associated cancers even without profound immunosuppression.31,32 In 2017, Southern states had a greater proportion of new HIV diagnoses (an estimate of 52%) than all other regions combined annually.33 In addition, diagnosis rates for people in the South are higher than for Americans overall: 8 of the 10 states with the highest rates of new HIV diagnoses are in the South.33 The intersectional effect of socioeconomic disparity, HIV prevalence, and low vaccination rates discovered in this study highlights how profoundly the South is at risk for HPV-associated sequelae. There is a need to develop targeted education and public health programs dedicated to addressing HPV vaccination disparities in the U.S.
Reasons that could explain the low vaccination in the South may include the lower SES of individuals in the South. For instance, in this study, a higher proportion of participants in the South indicated not having health insurance (30%) compared with the Northeast (11%). Lack of health insurance in Southern states may have been complicated by contextual factors such as lack of state Medicaid expansion and history of Jim Crow laws.34,35 For example, respondents have shown statistically increased vaccination report for influenza in states where the cost of vaccination was covered by Medicaid.34 Also, because of the history of Jim Crow law in the south, racial disparities continued to exist in the receipt of primary care.35 The HPV vaccine is covered by most private insurances, public programs and the Vaccines for Children36 for boys and girls aged ≤18 years. Merck offers a Vaccine Patience Assistance Program for uninsured young adults aged 19–26.37 However, currently there is no federal funding stream that supports young adults who do not get assistance. There should therefore be programs that provide funding for young adults especially now that the vaccine is recommended for adults up to age 45 years. Another potential reason that could explain low HPV uptake in the south is rurality: Most Southern states have been noted to consist of large proportion of rural counties.38,39 Some of the reasons that were identified as likely associated with decreased vaccination uptake among rural dwellers are lower income, less educational attainment, higher rates of being uninsured, and less knowledge and awareness of HPV vaccination and its benefits.38,39
Limitations
This study has limitations. First, the BRFSS survey is a telephone survey and relies on self-report; thus, the data may have been subject to recall bias and social desirability bias. Second, not all states of different regions of the U.S. conducted the HPV vaccination module. Similarly, different states participated in different years during 2015–2017. Third, this was a cross-sectional study and therefore this study cannot infer causality. Because this is a cross-sectional study, mediation analysis was unable to be performed.40 Fourth, the sample size consisted largely of people who lived in the Southern U.S. (80%); this may affect generalizability of the findings and the ability to detect associations between other regions and HPV vaccination. In addition to Southern states being 80% of the sample size, most southern states have been noted to consist of large proportion of rural counties; however, rurality was unable to be controlled within the study. Finally, the effects of other variables such as provider recommendation, sexual behaviors, rural–urban status, and age of vaccination (BRFSS do not assess these variables) were unable to be assessed, which could have explained some of the variations in vaccination uptake.
CONCLUSIONS
Although HPV vaccination recommendations have been developed at the federal level, specific HPV vaccination implementation is needed at state level considering the regional differences in HPV vaccine uptake observed in this study. Currently, Rhode Island, Virginia, the District of Columbia, and Puerto Rico require HPV vaccination for school entry.41 Mandating school entry administration of the HPV vaccine could reduce regional disparities in HPV vaccine uptake. Moreover, this study demonstrates the critical need to develop and implement interventional programs that promote HPV vaccination for young adults in the catch-up group, especially residents of the South. As provider recommendation is the primary predictor of vaccine uptake,42 providers in all regions—especially the South—should take every doctor visit as an opportunity to recommend the vaccine to eligible patients. Each year, approximately 44,000 new cases of cancer in the U.S. are attributable to HPV infection, and the HPV vaccine could potentially prevent up to 92% of those cancers.43 Raising HPV vaccination rates is therefore warranted.
This study noted geographic disparities in HPV vaccination among young adults. Although HPV vaccination initiation and completion rates were low for all regions, they were significantly lower in the South region. The results of this study provide important evidence to highlight the need for public health interventions to reduce geographical disparities in HPV vaccination and ultimately increase HPV vaccination rates.
Supplementary Material
ACKNOWLEDGMENTS
Oluwole A. Babatunde was supported by a National Cancer Institute’s F99/K00 Fellowship grant (1F99CA22272201).
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(S10):3036–3046. 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjei Boakye E, Buchanan P, Hinyard L, Osazuwa-Peters N, Schootman M, Piccirillo JF. Incidence and risk of second primary malignant neoplasm after a first head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2018;144(8):727–737. 10.1001/jamaoto.2018.0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suk R, Mahale P, Sonawane K, et al. Trends in risks for second primary cancers associated with index human papillomavirus–associated cancers. JAMA Netw Open. 2018;1(5):e181999 10.1001/jamanetworkopen.2018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Sharma A, Osazuwa-Peters N, et al. Risk of subsequent malignant neoplasms after an index potentially-human papillomavirus (HPV)-associated cancers. Cancer Epidemiol. 2020;64:101649 10.1016/j.canep.2019.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrosky EY, Liu G, Hariri S, Markowitz LE. Human papillomavirus vaccination and age at first sexual activity, National Health and Nutrition Examination Survey. Clin Pediatr (Phila). 2017;56(4):363–370. 10.1177/0009922816660541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13‒17 years - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(33):909–917. 10.15585/mmwr.mm6733a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adjei Boakye E, Lew D, Muthukrishnan M, et al. Correlates of human papillomavirus (HPV) vaccination initiation and completion among 18‒26 year olds in the United States. Hum Vaccin Immunother. 2018;14(8):2016–2024. 10.1080/21645515.2018.1467203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman M, Laz TH, Berenson AB. Geographic variation in human papillomavirus vaccination uptake among young adult women in the United States during 2008‒2010. Vaccine. 2013;31(47):5495–5499. 10.1016/j.vaccine.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman M, Islam M, Berenson AB. Differences in HPV immunization levels among young adults in various regions of the United States. J Community Health. 2015;40(3):404–408. 10.1007/s10900-015-9995-2. [DOI] [PubMed] [Google Scholar]
- 11.Hirth JM, Rahman M, Smith JS, Berenson AB. Regional variations in HPV vaccination among 9–17 year old adolescent females from the BRFSS, 2008–2010. Hum Vaccin Immunother. 2014;10(12):3475–3483. https://doi.org/10.416½1645515.2014.980202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laz TH, Rahman M, Berenson AB. An update on human papillomavirus vaccine uptake among 11–17 year old girls in the United States: National Health Interview Survey, 2010. Vaccine. 2012;30(24):3534–3540. 10.1016/j.vaccine.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei F, Moore PC, Green AL. Geographic variability in human papillomavirus vaccination among U.S. young women. Am J Prev Med. 2013;44(2):154–157. 10.1016/j.amepre.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong CA, Berkowitz Z, Dorell CG, Anhang Price R, Lee J, Saraiya M. Human papillomavirus vaccine uptake among 9- to 17-year-old girls: National Health Interview Survey, 2008. Cancer. 2011;117(24):5612–5620. 10.1002/cncr.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Kong L, Du P. HPV vaccine completion and dose adherence among commercially insured females aged 9 through 26 years in the US. Papillomavirus Res. 2016;2:1–8. 10.1016/j.pvr.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. 10.1200/jco.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15(22):6758–6762. 10.1158/1078-0432.ccr-09-0784. [DOI] [PubMed] [Google Scholar]
- 18.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers - United States, 2008‒2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661–666. 10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- 19.Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13‒17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882. 10.15585/mmwr.mm6633a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokdad AH. The Behavioral Risk Factors Surveillance System: past, present, and future. Annu Rev Public Health. 2009;30:43–54. 10.1146/annurev.publhealth.031308.100226. [DOI] [PubMed] [Google Scholar]
- 21.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination-updated recommendations of the Advisory Committee on Immunization Practices. Am J Transplant. 2017;17(3):834–837. 10.1111/ajt.14206. [DOI] [PubMed] [Google Scholar]
- 22.HHS. Office of Intergovernmental and External Affairs: Regional Offices. https://www.hhs.gov/about/agencies/iea/regional-offices/index.html Published 2014. Accessed April 15, 2014.
- 23.Adjei Boakye E, Zeng W, Governor S, et al. Differences in human papillomavirus (HPV) vaccine uptake by nativity status among men aged 18‒34 years. Prev Med Rep. 2019;16:101010 10.1016/j.pmedr.2019.101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conrey R, Valencia V, Cioletti A, Williams-Brown MY. Regional variation in human papillomavirus vaccination uptake and completion among adolescents 13‒17 in the state of Texas. Vaccine. 2020;38(25):4119–4124. 10.1016/j.vaccine.2020.03.059. [DOI] [PubMed] [Google Scholar]
- 25.Thompson EL, Wheldon CW, Rosen BL, Maness SB, Kasting ML, Massey PM. Awareness and knowledge of HPV and HPV vaccination among adults ages 27‒45 years. Vaccine. 2020;38(15):3143–3148. 10.1016/j.vaccine.2020.01.053. [DOI] [PubMed] [Google Scholar]
- 26.Hirth JM, Kuo YF, Starkey JM, et al. Regional variations in human papillomavirus prevalence across time in NHANES (2003‒2014). Vaccine. 2019;37(30):4040–4046. 10.1016/j.vaccine.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zahnd WE, James AS, Jenkins WD, et al. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1265–1274. 10.1158/1055-9965.epi-17-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999–2016). HHS, Centers for Disease Control and Prevention and National Cancer Institute; 2016. [Google Scholar]
- 29.Yoo W, Kim S, Huh WK, et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PloS One. 2017;12(2):e0172548 10.1371/journal.pone.0172548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benard VB, Thomas CC, King J, et al. Vital signs: cervical cancer incidence, mortality, and screening - United States, 2007‒2012. MMWR Morb Mortal Wkly Rep. 2014;63(44):1004–1009. [PMC free article] [PubMed] [Google Scholar]
- 31.Cubie HA, Seagar AL, Beattie GJ, Monaghan S, Williams ARW. A longitudinal study of HPV detection and cervical pathology in HIV infected women. Sex Transm Infect. 2000;76(4):257–261. 10.1136/sti.76.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21(1):122 10.1158/1055-9965.epi-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. HIV Surveillance Report. Atlanta, GA: HHS; 2018. [Google Scholar]
- 34.Stoecker C, Stewart AM, Lindley MC. The cost of cost-sharing: the impact of Medicaid benefit design on influenza vaccination uptake. Vaccines (Basel). 2017;5(1):8 10.3390/vaccines5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith DB. Racial and ethnic health disparities and the unfinished civil rights agenda. Health Aff (Millwood). 2005;24(2):317–324. 10.1377/hlthaff.24.2.317. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Vaccines for Children Program (VFC). https://www.cdc.gov/vaccines/programs/vfc/index.html Published 2014. Accessed June 14, 2017.
- 37.Helps Merck. Merck Patient Assistance Programs. https://www.merckhelps.com/Home.aspx Accessed January 28, 2020.
- 38.Swiecki-Sikora AL, Henry KA, Kepka D. HPV vaccination coverage among US teens across the rural-urban continuum. J Rural Health. 2019;35(4):506–517. 10.1111/jrh.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammed KA, Subramaniam DS, Geneus CJ, et al. Rural-urban differences in human papillomavirus knowledge and awareness among US adults. Prev Med. 2018;109:39–43. 10.1016/j.ypmed.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen AB, Robinson J, O’Brien EK, Zhao X. Racial and ethnic differences in tobacco information seeking and information sources: findings from the 2015 Health Information National Trends Survey. J Health Commun. 2017;22(9):743–752. 10.1080/10810730.2017.1347216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Conference of State Legislatures. HPV Vaccine: State Legislation and Statutes. https://www.ncsl.org/research/health/hpv-vaccine-state-legislation-and-statutes.aspx Published 2018. Accessed January 31, 2020.
- 42.Mohammed KA, Geneus CJ, Osazuwa-Peters N, Adjei Boakye E, Tobo BB, Burroughs TE. Disparities in provider recommendation of human papillomavirus vaccination for U.S. adolescents. J Adolesc Health. 2016;59(5):592–598. 10.1016/j.jadohealth.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus–attributable cancers—United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2019;68(33):724 10.15585/mmwr.mm6833a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.