Abstract
The human blood-brain barrier (BBB) transporter P-glycoprotein (P-gp) can efflux amyloid-β out of the central nervous system. Amyloid-β is thought to be the causative agent for Alzheimer’s Disease (AD). Using PET imaging, we have shown that BBB P-gp activity is reduced in AD, as quantified by the in vivo brain distribution of the P-gp probe, [11C]-verapamil. Therefore, the aim of this study was to determine whether this reduced BBB P-gp activity in AD was due to decreased P-gp abundance at the BBB. Using targeted proteomics, we quantified the abundance of P-gp and other drug transporters in gray matter brain microvessels isolated from 43 AD subjects and 38 age-matched controls (AMC) from regions affected by AD (hippocampus and the parietal lobe of the brain cortex) and not affected by AD (cerebellum). First, P-gp abundance was decreased in the BBB of the hippocampus versus cerebellum in both AD subjects and AMC, and therefore was not AD-related. In addition, gray matter BBB abundance of P-gp (and of other transporters) in the hippocampus and the parietal lobe was not different between AD and AMC. The gray matter BBB abundance of all drug transporters decreased with age, likely due to age-dependent decrease in the density of brain microvessels. Collectively, the observed reduced in vivo cerebral BBB P-gp activity in AD cannot be explained by reduced abundance of P-gp at the BBB. Nevertheless, the drug transporter abundance at the human gray matter BBB data provided here can be used to predict brain distribution of drugs targeted to treat CNS diseases, including AD.
Keywords: Alzheimer’s Disease, blood-brain barrier, transporter abundance, P-glycoprotein, targeted proteomics
INTRODUCTION
Dementia is a global health burden that is increasing worldwide. In 2019, more than 40 million people were living with dementia. With aging of the population, by 2050, this number is expected to increase to 120 million. Alzheimer’s Disease (AD) is the leading cause of dementia. Indeed, 60–70% of all cases of dementia in subjects >65 years are related to AD 1,2.
Physiologically, AD is characterized by the deposition of amyloid beta (Aβ) plaques in the extracellular matrix of the human brain cortex and the formation of neurofibrillary tangles in the neurons 3. According to the Aβ cascade hypothesis, the accumulation of Aβ in the extracellular matrix of the human cortex is thought to be the triggering factor for the pathogenesis of AD 3. In an elegant study where stable labeled amino acids were administered to AD subjects followed by cerebrospinal fluid (CSF) sampling, Mawuenyega et al. conclusively demonstrated that Aβ accumulation in the brain is due to reduced Aβ brain clearance and not to increase Aβ production4,5. However, other causative factors may also play a role, such as inflammation 6–8.
Both P-glycoprotein (P-gp) and Lipoprotein Receptor-related Protein 1 (LRP1), located respectively at the abluminal and luminal membrane of the endothelial cells forming the blood-brain-barrier (BBB), are involved in Aβ efflux from the brain interstitial fluid 9–11. The in vivo role of P-gp in Aβ clearance from the brain was first shown in a Mdr1a/1b-knockout (KO) mice model, where KO mice had a significantly reduced (~50%) Aβ clearance from the brain 12. This role was confirmed by our human positron emission tomography (PET) imaging study where we measured the brain distribution of [11C]verapamil, a selective P-gp substrate in various brain substructures of subjects with AD and age-matched controls 13. We showed that BBB P-gp-mediated clearance of [11C]verapamil was significantly reduced in AD in the parietotemporal lobe, hippocampus and amygdalae, areas typically affected in mild to moderate AD, and not in brain regions unaffected by AD such as the cerebellum.
This region-specific decrease in P-gp activity could be due to reduced BBB P-gp abundance (E) or its catalytic activity (kcat) (resulting in reduced maximal velocity of transport Vmax = kcat·E), a change in the affinity (Km) of Aβ for P-gp, or all three. Using immunochemistry, several groups have found reduced abundance of P-gp protein at the BBB in AD 14–16. Moreover, using immunostaining of a large brain sample set, an inverse correlation was observed between Aβ plaques and P-gp expression in non-demented human subjects 17. However, all those studies are limited by a small sample size (n<10) and/or challenges related to quantification by immunohistochemistry. Using quantitative proteomics, others found no difference in BBB P-gp abundance between AD subjects (n=5) and healthy controls (n=12) 18, possibly due to the small sample size and therefore lack of power. In addition, the latter study did not correct for the inter-subject variability in brain microvascular endothelial cells (BMEC) isolation.
To overcome the limitations of the aforementioned studies, the primary objective of this study was to quantify P-gp and LRP1 cerebral abundance in a larger number of AD and age-matched non-demented controls (AMC) (n=82) in regions affected (e.g. the parietal lobe and hippocampus) and unaffected (e.g. cerebellum) by mild to moderate AD 19,20. To do so, we used a quantitative targeted proteomics approach which accounts for the variability in BMEC isolation 21. In addition, since there is considerable interest in developing drugs to treat AD and other central nervous system (CNS) diseases, we quantified the abundance of a number of additional BBB transporters, namely Breast Cancer Resistance Protein (BCRP), Organic Anion Transporting Protein 2B1 (OATP2B1) and Equilibrative Nucleoside Transporter 1 (ENT1), that could be important in the brain distribution of drugs developed to treat CNS diseases. Moreover, the abundance of BMEC transporters may be affected by age. Therefore, we also compared the abundance of the above mentioned transporters in BMEC isolated from the elderly (72–83 years) versus younger controls (YC; 24–48 years) previously reported by us 21
MATERIALS AND METHOD
Human brain samples
Frozen brain tissues were provided by the Sepulveda Research Corporation, the Harvard Brain Tissue Resource Center and the University of Washington Alzheimer’s Disease Research Center (UW ADRC). Control subjects were defined as those with no known psychiatric or neurological diagnosis. Other diagnoses not primarily affecting the brain were not excluded. The severity of AD was assessed by the biobanks based on clinical and/or neuropathological observations as per the Braak stage classification 22, the criteria of the National Institute of Ageing (NIA) 23 or the criteria of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) 24. Upon reception, the frozen brain samples were stored at −80°C until analysis.
Quantification of drug transporters and LRP1 abundance in human gray brain matter by quantitative targeted proteomics
The abundance of P-gp, BCRP, OATP2B1, ENT1 and LRP1 was quantified in BMEC isolated from gray brain matter by targeted LC-MS/MS as previously described 21 (except that no bovine serum album was included in the isolation of BMEC and cell lysis was achieved using extraction buffer II with protease inhibitors from the Calbiochem ProteoExtract Native Membrane Protein Extraction kit + 0.2% SDS). Additional information on targeted proteomic quantification of LRP1, which was not quantified in that previous study, is reported in Supplementary Materials. The ratio of Glucose Transporter 1 (GLUT1, a marker of endothelial cells) peak area ratio normalized with the injected protein amount in isolated BMEC versus gray matter homogenates was used as an enrichment factor correcting for the variability in the isolation of BMEC. Except for LRP1, transporter protein abundance in gray matter was then calculated as follows:
LRP1 was quantifiable in brain homogenates and because LRP1 is localized in neurons as well as at the BBB 25,26, its abundance was estimated as follows:
As in our previous study 21, both analytical (at low, medium and high levels of the calibration curve) and biological controls were used as quality controls. The biological controls were a pool of human liver total membrane proteins that was trypsin digested and analyzed by LC-MS/MS to ensure lack of bias when comparing data from different studies. The biological and quality control samples were within ± 30% of the expected values.
Statistics
In our PET imaging study, we observed a 50% lower BBB P-gp activity in AD subjects versus controls, as determined by [11C]verapamil brain extraction ratio 13. Therefore, the study reported here was powered to observe a 50% difference in P-gp abundance in a region affected by AD (hippocampus) versus one not affected by AD (cerebellum) in paired brain samples obtained from AD subjects (primary endpoint). Analysis of our preliminary data from samples obtained from the National Institute of Health (NIH) Neurobiobank (17 AD + 28 AMC subjects), the number of samples needed to observe a significant regional difference of 50% in P-gp abundance in paired hippocampus versus cerebellum samples with 80% power and α value of 5% was 51 (30 AD + 21 AMC). Therefore, additional samples from AD and AMC subjects were obtained from UW ADRC. A secondary endpoint of our analyses was the difference in transporter abundance in brain samples available to us from AD versus AMC and AMC versus YC.
Statistically significant difference in age, postmortem interval (PMI), protein abundance and protein yields in AD subjects versus AMC were determined using the nonparametric Mann-Whitney U Test. A Chi-square test was performed to determine difference in sex distribution between AD subjects and AMC. To determine regional differences in protein abundance between paired samples, the paired Wilcoxon signed rank test was performed. The Spearman test was used to determine the correlation between P-gp and LRP1 cerebral abundance. A Jonckheere-Terpstra test was performed to determine if the Braak score affected P-gp abundance (IBM SPSS Statistics version 25, Chicago, IL, USA). All otherstatistical tests were performed using Graphpad Prism version 8.2 (GraphPad Software, San Diego, CA, USA). P-values ≤ 0.05 were considered statistically significant. Unless otherwise specified, results are presented as arithmetic mean ± standard deviation (SD). When transporter abundance was not quantifiable in a sample, that sample was not included in the analysis of that transporter.
RESULTS
Demographics
Tissues were obtained from three different regions: hippocampus, BA39 (parietal lobe) and cerebellum (Table 1 and Figure 1). Information on age, gender, PMI and disease stage of all cases used in this study are listed in Supplementary Materials. A significant difference in both age (3 years difference in averages) and PMI (4 hours difference in averages) was found between AD and AMC groups (Table 1). These differences do not affect the primary endpoint of the study since it is based on paired comparison of P-gp abundance in the hippocampus versus cerebellum in AD subjects. In addition, the age difference is biologically irrelevant and we have previously reported that PMI (range 2–32 h) does not affect transporter abundance 21.
Table 1.
Demographics of brain tissue samples obtained from both Alzheimer’s Disease (AD) subjects and age-matched controls (AMC). Data presented as arithmetic mean ± standard deviation.
| AD | AMC | |
|---|---|---|
| Number of donors | 43 | 38 |
| Age (years)* | 84 ± 7 | 81 ± 7 |
| PMI1 (hours)* | 8 ± 6 | 12 ± 8 |
| % Female | 50 | 44 |
| Regions | Cerebellum (n=43) | BA39 (parietal lobe) (n=17) |
| BA39 (parietal lobe) (n=11) | BA39 (parietal lobe) (n=17) | |
| Hippocampus (n=32) | Hippocampus (n=24) | |
| Initial tissue weight (g) | ||
| Cerebellum | 1.39 ± 0.80 | 1.72 ± 0.81 |
| BA39 (parietal lobe) | 0.89 ± 0.29 | 1.22 ± 0.41 |
| Hippocampus | 0.68 ± 0.25 | 0.99 ± 0.36 |
PMI, post-mortem interval
Statistically significant differences were found in age (P= 0.01) and post-mortem interval (PMI) (P= 0.006), but not sex (P= 0.69) between the two groups.
Initial tissue weight represents the initial weight of the sample upon reception and thawing. From this, white matter and vascular tissue were discarded by dissection and contaminating white matter was further removed using dextran gradient, as described previously (Billington et al., 2019).
Figure 1.
Flowchart of Alzheimer’s Disease (AD) and age-matched control (AMC) brain samples available and analyzed in this study.
Abundance of cerebral drug transporters, but not LRP1, is reduced in hippocampus versus cerebellum in both Alzheimer’s Disease subjects and age-matched controls
P-gp abundance in the hippocampus of AD subjects was significantly lower (P=0.003) than that in the cerebellum of the same subjects (4.4 ± 2.4 versus 6.67 ± 2.97 pmol/g gray matter). The same was true in AMC (4.6 ± 3.3 versus 6.6 ± 2.89 pmol/g gray matter, P=0.002). A similar observation was made for other drug transporters BCRP, OATP2B1 and ENT1 (although statistical significance was not reached for ENT1 in AD subjects) (Figure 2 and Table 2). In contrast, for either AD (P=0.73) or AMC (P=0.54), LRP1 abundance in the hippocampus was not significantly different than in cerebellum. (Figure 2). Notably, GLUT1 abundance was also lower in hippocampus compared to cerebellum in both AD subjects (79 ± 23 versus 189 ± 34, P=0.0002, n=14) and AMC subjects (101 ± 25.6 versus 190 ± 35, P=0.0129, n=10). BMEC protein abundance (in mg BMEC protein/ g gray matter), although more variable, followed the same trend: 0.71 ± 1.02 in hippocampus versus 1.26 ± 0.84 in cerebellum in AD subjects, P= 0.0017) and AMC subjects (0.30 ± 0.39 in hippocampus versus 1.21 ± 1.32 in cerebellum in AMC, P<0.0001). We found no significant effect of Braak score on P-gp abundance (Figure S1).
Figure 2.
The abundance of P-gp, BCRP, OATP2B1 and ENT1, but not LRP1, was reduced in the gray matter of hippocampus versus cerebellum in Alzheimer’s Disease (AD) subjects (not significant for ENT1) and age-matched controls (AMC). Mild to moderate AD affects the hippocampus but not the cerebellum. Data were analyzed using a nonparametric Wilcoxon paired signed rank test. Horizontal purple lines represent arithmetic mean. The golden lines connect paired samples.
Table 2.
Abundance of drug transporters in grey matter of Alzheimer’s Disease patients (AD) and age-matched controls (AMC). Data shown are arithmetic mean ± standard deviation (sample size).
| Transporter/receptor abundance in grey matter (pmol/ g grey matter) | ||
|---|---|---|
| AD | AMC | |
| Cerebellum | ||
| P-gp | 6.8 ± 2.8 (41) | 6.6 ± 2.9 (38) |
| BCRP | 16.0.8 ± 5.7 (41) | 16.3 ± 7.5 (38) |
| OATP2B1 | 1.16 ± 0.87 (34) | 1.01 ± 0.86 (36) |
| LRP1 | 14.5 ± 5.2 (27) | 19.6 ± 17.7 (23) |
| ENT1 | 3.37 ± 5.91 (28) | 2.05 ± 1.56 (18) |
| Hippocampus | ||
| P-gp | 4.4 ± 2.4 (30) | 4.6 ± 3.3 (21) |
| BCRP | 8.5 ± 3.6 (28) | 8.7 ± 5.0 (21) |
| OATP2B1 | 0.85 ± 0.68 (25) | 0.82 ± 0.61 (18) |
| LRP1 | 16.7 ± 6.6 (28) | 15.4 ± 5.5 (24) |
| ENT1 | 1.75 ± 1.16 (26) | 1.38 ± 1.17 (17) |
| BA39 (parietal lobe) | ||
| P-gp | 5.1 ± 0.8 (6) | 4.7 ± 2.0 (17) |
| BCRP | 8.5 ± 1.4 (6) | 10.8 ± 5.4 (17) |
| OATP2B1 | 0.38 ± 0.06 (6) | 0.64 ± 0.43 (17) |
| LRP1 | 22.1 ± 8.0 (5) | 17.5 ± 5.9 (4) |
| ENT1 | NA | NA |
NA, data not available
The abundance of drug transporters and LRP1 was not different between AD and AMC in regions affected by AD
Consistent with the above findings, no difference was found in the abundance of the measured proteins between AD and AMC in either the hippocampus or parietal lobe (Figure 3 and Table 2). Furthermore, after excluding the AMC specimens with any presence of neuritic plaques (i.e. CERAD score other than C0 or those with no CERAD score), the abundance of hippocampus P-gp was not significantly different between the two groups (P=0.32, n=7). Except for ENT1 (P=0.0495), the same was true for the other proteins (P>0.05).
Figure 3.
Total cerebral abundance of drug transporters P-gp, BCRP, OATP2B1 and ENT1 as well as LRP1 was not different between Alzheimer’s Disease (AD) subjects and age-matched controls (AMC) for both the hippocampus (left panels) and BA39 (parietal lobe, right panels). Data (also shown in Table 2) were analyzed using a non-parametric Mann-Whitney U Tests. Lines indicate arithmetic mean ± SD.
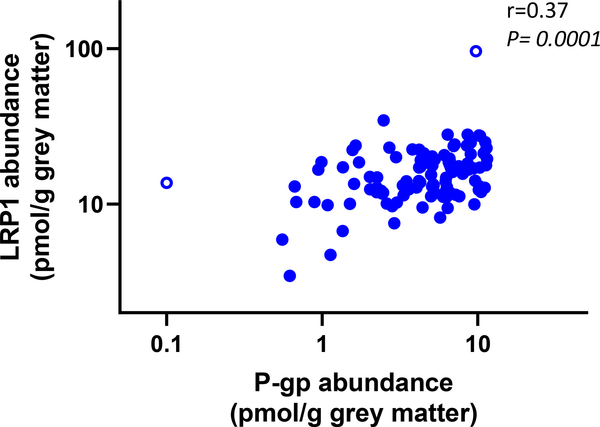
The difference in hippocampus GLUT1 abundance in AD subjects (82 ± 25, n=14) compared to AMC (101 ± 26, n=10), was not significant (P=0.13). A significant correlation was found between cerebral P-gp and LRP1 abundance in the gray matter when all samples were combined (P = 0.0001, r=0.37) (Figure 4).
Figure 4.
A positive correlation (r=0.37, P= 0.0001) was observed between gray matter LRP1 and P-gp abundance. Pooled data from the hippocampus, parietal lobe and cerebellum from both Alzheimer’s Disease subjects and age-matched controls were used for analysis using a nonparametric Spearman test. The correlation remained significant even when potential outliers (empty circles) were removed.
Cerebral drug transporters abundance in the parietal lobe is decreased with age in non-demented subjects
The gray matter abundance of drug transporters P-gp, BCRP and OATP2B1 in the parietal lobe was reduced by 38% (P=0.0002), 24% (P=0.032) and 31% (P=0.007), respectively, in the 72–83 years old group compared to the younger 24–48 years old group (YC; Figure 5; data for ENT1 & GLUT1 were not available). The BMEC (Figure S2, see ref. 21 for YC data) and homogenate (Figure S3, see ref. 21 for YC data) protein yields were significantly lower in AMC vs. YC: 0.37 ± 0.19 mg versus 0.72 ± 0.48 mg protein/g gray matter for BMEC (P=0.008) and 60.2 ± 17.9 versus 82.0 ± 12.8 mg protein/g gray matter for homogenates (P<0.0001).
Figure 5.
The gray matter BBB abundance of drug transporters P-gp, BCRP and OATP2B1, in BA39 (parietal lobe) was reduced with increasing age. Data in young controls (age range 24–48 years) were taken from our previously published proteomic study (Billington et al. 2019) using the same methodology as in the present study. The data on abundance of drug transporters in older controls (age range 72–83 years) are from the present study. Data were analyzed using a non-parametric Mann-Whitney U Test. Lines indicates arithmetic mean ± SD.
DISCUSSION
In this study, we quantified the BBB abundance of P-gp and other proteins in human gray matter in both AD subjects and AMC in brain regions affected (hippocampus, parietal lobe) or unaffected (cerebellum) by AD. Our study incorporated several unique study designs that were not incorporated in previous studies. First, we used targeted proteomics (using LC-MS/MS), a highly quantitative method with respect to selectivity, sensitivity and reproducibility 27. Second, unlike previous proteomic studies 18,we corrected for the variability in BMEC enrichment by quantifying BMEC enrichment using the ratio of GLUT1 abundance in isolated BMEC versus homogenates (Figure S3). GLUT1 serves as an excellent enrichment marker because it is expressed only in BMEC, it is highly abundant and quantifiable in both brain homogenates and isolated BMEC. Although GLUT1 abundance is reduced in AD 28, this does not in any way detract from its use as an enrichment factor because it will be similarly reduced in both the homogenate and BMEC isolated from AD brain samples. To note, other BMEC markers, cluster of differentiation protein 31 (CD31) and von Willebrand Factor (vWF), were previously found not to be quantifiable in the brain homogenate 21. Third, most of the previous studies had a limited sample size (<10). Here we powered our sample size to detect a difference in paired analysis of the abundance of P-gp between brain areas affected by AD versus those not affected by AD.
As mentioned above, the primary endpoint of this study was the difference in transporter abundance between regions affected by AD (hippocampus, parietal lobe) and regions not affected (cerebellum) in both AD and AMC, rather than the difference in transporter abundance in AD versus AMC. Gray matter BBB P-gp abundance in hippocampus was reduced compared to that in cerebellum in both AD subjects and AMC (Figure 2). These findings show that the reduction in P-gp abundance is not due to AD. This is supported by the fact that we did not observe a difference in P-gp abundance between AD and AMC in regions affected by AD (Figure 3). The reason(s) for the regional difference in P-gp abundance between the hippocampus and the cerebellum are not clear (but see below for a possible reason). In our previous study on younger brain tissue samples, minor brain regional differences in transporter abundance were reported between the parietotemporal and the frontal lobes 21. Nevertheless, these results indicate that total P-gp abundance at the human gray matter BBB cannot explain the observed decreased BBB P-gp activity in regions affected by AD in our PET imaging study 13.
The above in vitro in vivo discrepancy could be due to AD affecting the abundance of P-gp at the plasma membrane of BMEC, but not its total BMEC abundance. This hypothesis can be tested by quantifying the plasma membrane abundance of P-gp in BMEC of AD subjects 29. Recently, Hartz et al. found higher levels of ubiquitinated P-gp in human BMEC isolated from AD subjects compared to AMC 30. Consequently, they showed that P-gp ubiquitination and internalization was triggered by Aβ, thereby enhancing degradation of the transporter 10. In our study, we did not observe a decrease in P-gp abundance (the consequence of enhanced degradation). However, it is worth mentioning that ubiquitination has multiple consequences other than enhanced cellular internalization and protein degradation such as regulation of protein activity by modulating the substrate affinity of the transporter31. To our knowledge, no study on the modulation of P-gp activity by ubiquitination has been performed to date. Alternatively, an endogenous factor present in AD brain could have altered in vivo P-gp activity in our PET imaging study by reducing P-gp’s affinity for verapamil (Km) or its catalytic activity (kcat) to transport verapamil. This hypothesis is difficult to test without identifying the perpetrator.
As mentioned in the introduction, LRP1 has also been reported to be involved in the clearance of Aβ, by mediating the abluminal uptake of Aβ into the BMEC followed by its efflux from the BMEC into blood by P-gp 9,10. In this study, we found that the gray matter abundance of P-gp and LRP1 was correlated (Figure 4), which supports their dual expression in the BMEC allowing vectorial transport of Aβ from the brain interstitial fluid to the blood. However, contrary to P-gp, we did not observe any difference in LRP1 abundance in hippocampus versus cerebellum, in AD nor in AMC (Figure 2). In addition, we did not see any difference in the abundance of this receptor in the parietal lobe and the hippocampus between AD subjects and AMC (Figure 3). We speculate that this is because LRP1 is present in the neurons and in BMECs. For this reason, we quantified LRP1 in homogenates rather than in isolated BMECs.
Interestingly, P-gp is not the only transporter that has been implicated in the pathophysiology of AD. In a study that investigated the role of Bcrp and Mrp1 in Aβ clearance in a transporter KO mouse model 32, Mrp1 was shown to play a major role in the clearance of this peptide. Unfortunately, the significance of these findings for the human pathophysiology of the disease has not been investigated as we currently lack specific PET MRP1 probe substrates to quantify the cerebral activity of this transporter in humans. In addition, the presence of MRP1 at the human BBB remains controversial 33. Consistent with our study, MRP1 abundance in BMEC is lower than the lower limit of quantification (LLOQ) in all of the brain proteomic studies published so far 18,21,34,35. ENT1 has also been associated with AD in a mouse AD model, where inhibition of adenosine transport (mediated by ENT1) improved AD-related loss of memory and impaired neuronal plasticity and decreased the size of Aβ plaques 36. However, in our study, we could not identify a difference in ENT1 gray matter BBB abundance in AD versus AMC and observed a (non-significant) tendency towards decreased ENT1 abundance in hippocampus versus cerebellum in our paired analysis of samples obtained from the same AD subjects.
Because quantification of drug transporter abundance at the human BBB as well as their modulation in AD is critical to predict the CNS penetration of AD-targeting drug candidates and support dose selection and individualization, we also quantified other drug transporters in gray matter of both AD subjects and AMC. This allowed us to determine and distinguish between the effect of AD (AD versus AMC) and age (YC versus AMC, using individual data from our previously published study 21) on drug transporter abundance in gray matter. With respect to the effect of AD on gray matter transporter abundance, similar to P-gp, we found lower gray matter BBB abundance of BCRP, OATP2B1 and ENT1 in the hippocampus compared to cerebellum in both AD subjects and AMC, (Figure 2). In addition, similar to P-gp, we could not find any difference in the abundance of these transporters in AD subjects in regions affected by AD (hippocampus and the parietal lobe) versus AMC (Figure 3).
Regarding the effect of age, the parietal lobe was the only region that was analyzed in both YC (in our previous study 21) and AMC. Cerebellum (the “control” region, not affected by AD) was not analyzed in our previous study in YC. Therefore, interpretation of the effect of age on transporter abundance needs to be reproduced by analysis of other regions of the brain. Nevertheless, we found that the gray matter BBB abundance of all quantifiable drug transporters (P-gp, BCRP, OATP2B1) in the parietal lobe was significantly reduced by age, as determined by comparison of YC versus elderly samples (Figure 5). Of note, other drug transporters were under the LLOQ in our previous report 21 and therefore were not quantified in this study. PET imaging studies with [11C]verapamil as a selective P-gp substrate also show reduced whole brain P-gp activity with age 37.
Taken together, all drug transporters at the human gray matter BBB, including GLUT1, seem to follow a same pattern, that is decreased abundance in hippocampus versus cerebellum (in both AD subjects and AMC). Interestingly, we found that the BMEC protein yield, although variable, followed the same trend, that is significantly lower BMEC protein yield from the hippocampus versus cerebellum in both AD subjects and AMC (Figure S2). While caution should be exercised when interpreting BMEC protein yields data (BMEC protein yields are more a measure of the efficiency of BMEC isolation, which might be region-specific, rather than a measure of actual BMEC density), this could suggest a potential difference in the density of brain vessels between these two regions. In contrast, this was not observed for LRP1, which was quantified in brain homogenates and is expressed in multiple brain cell types. Therefore, the observed difference in P-gp activity from our PET imaging study 13 cannot be explained by a difference in total P-gp abundance at the human gray matter BBB, nor by a difference in total cerebral abundance of LRP1. In addition, the gray matter BBB abundance of the quantified transporters in the parietal lobe decreased with age as did the yield of BMEC. These parallel trends suggest that the effect of age, as well as the regional difference in transporter abundance, is not transporter-specific and seems to be explained by a both regional (hippocampus versus cerebellum) and age-related difference in BMEC density. This hypothesis is supported by increasing body of evidence pointing to BBB disruption in ageing and in AD: increased brain permeability of gadolinium, capillary leakage of blood-derived proteins, extravasation of red blood cells and cellular infiltration of macrophages and neutrophils, reduced expression of tight junction proteins, increased concentration of albumin in cerebrospinal fluid 38. In addition, our observations suggest that aging will affect not only the CNS distribution of drugs that are actively effluxed from the brain but also those that passively permeate across the BBB.
In conclusion, our data do not support the hypothesis of reduced BMEC abundance of P-gp in AD to explain the observed decreased P-gp in vivo activity in AD. Other factors, such as altered plasma membrane abundance and/or modification of transport activity (substrate affinity or transport capacity), may explain the clinical observation of decreased in vivo P-gp activity in AD and should be further investigated. In addition, we provide an extensive dataset on transporter abundance in brain regions of AD, AMC and YC. These data can inform age- and disease-related interindividual variability in brain penetration of drugs and therefore a better understanding of the extent of drug penetration in these elderly populations. These data can help build Physiologically Based Pharmacokinetic (PBPK) models to support dose selection and individualization as a function of ageing and drug interactions. If the hypothesis of reduced BMEC density and integrity in ageing can be verified using appropriate markers, drug penetration in elderly subjects will likely be greater than that in younger healthy subjects. Consequently, the contribution of active versus passive permeability (i.e. the fraction transported) will be lower in elderly subjects, thereby reducing the vulnerability of this population to transporter-related drug-drug interactions and pharmacogenetic variations.
Supplementary Material
STUDY HIGHLIGHTS.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Using PET imaging and [11C]verapamil, we have shown that BBB P-glycoprotein (P-gp) activity is reduced in AD. However, whether this reduced activity was due to decreased BBB P-gp abundance has never been investigated in detail.
WHAT QUESTION DID THIS STUDY ADDRESS?
We quantified the gray matter BBB abundance of P-gp in AD subjects and age-matched controls, in regions affected and not affected by the disease, to determine whether this reduced activity in vivo can be explained by a reduced abundance of the transporter. In addition, the abundance of BCRP, OATP2B1 & ENT1 at the human gray matter BBB was also quantified in order to predict the penetration of CNS drugs.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This targeted proteomic study provides the largest set of transporters’ abundance data at the human BBB in AD and elderly subjects.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The impact of AD and age on abundance of BBB transporters will help development of drugs targeted to treat AD and other CNS diseases.
ACKNOWLEDGEMENTS
Human tissues were obtained from the Harvard Brain Tissue Resource Center and Sepulveda Research Corporation, which are Brain and Tissue Repositories of the National Institute of Health NeuroBioBank, and from the University of Washington Neuropathology Core, which is supported by the Alzheimer’s Disease Research Center (AG05136) and the Adult Changes in Thought Study (AG006781).
This work was funded by the University of Washington Research Affiliate Program on Transporters (UWRAPT) through funding from Gilead, Amgen, Merck, Genentech, Biogen and Takeda.
ABBREVIATIONS
- Aβ
amyloid-β
- ABC
ATP-Binding Cassette
- AMC
age-matched control
- AD
Alzheimer’s Disease
- BBB
blood-brain barrier
- BCRP
Breast Cancer Resistance Protein
- BMEC
brain microvascular endothelial cells
- CD31
cluster of differentiation protein 31
- CNS
central nervous system
- CV
coefficient of variation
- DMET
drug metabolizing enzymes and transporters
- ENT1
Equilibrative Nucleoside Transporter
- GLUT1
glucose transporter 1
- kcat
catalytic constant of a protein
- Km
affinity constant of a protein for a substrate
- KO
knockout
- LC-MS/MS
liquid chromatography coupled to tandem mass spectrometry
- LLOQ
lower limit of quantification
- LRP1
Lipoprotein Receptor-related Protein 1
- MRP1
Multidrug Resistance Protein 1
- OATP2B1
Organic Anion Transporter 2B1
- PBPK model
physiologically-based pharmacokinetic model
- PET
positron emission tomography
- PMI
postmortem interval
- P-gp
P-glycoprotein
- REF
relative expression factor
- SD
standard deviation
- Vmax
maximal velocity of a reaction
- vWF
von Willebrand Factor
- YC
younger controls
Footnotes
All authors declared no competing interests for this work.
Supplemental Information
Supplemental Material
REFERENCES
- 1.Alzheimer’s Association 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 15, 321–387 (2019). [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization & Alzheimer’s Disease International Dementia: a public health priority. (2012).
- 3.Masters CL et al. Alzheimer’s disease. Nat Rev Dis Primers 1, 15056 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Mawuenyega KG et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330, 1774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman RJ et al. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat. Med. 12, 856–861 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominy SS et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5, eaau3333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrup K The case for rejecting the amyloid cascade hypothesis. Nat Neurosci 18, 794–799 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Ising C et al. NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nazer B, Hong S & Selkoe DJ LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-β peptide in a blood-brain barrier in vitro model. Neurobiol Dis 30, 94–102 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storck SE et al. The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM. Brain, Behavior, and Immunity 73, 21–33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai AB, Leung GKF, Callaghan R & Gelissen IC P‐glycoprotein: A role in the export of amyloid‐β in Alzheimer’s disease? FEBS J febs 15148 (2019).doi: 10.1111/febs.15148 [DOI] [PubMed] [Google Scholar]
- 12.Cirrito JR et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J Clin Invest 115, 3285–3290 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deo AK et al. Activity of P-Glycoprotein, a -Amyloid Transporter at the Blood-Brain Barrier, Is Compromised in Patients with Mild Alzheimer Disease. Journal of Nuclear Medicine 55, 1106–1111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijesuriya HC, Bullock JY, Faull RLM, Hladky SB & Barrand MA ABC efflux transporters in brain vasculature of Alzheimer’s subjects. Brain Res. 1358, 228–238 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Chiu C et al. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: preliminary observations. Neurobiol. Aging 36, 2475–2482 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Carrano A et al. ATP-binding cassette transporters P-glycoprotein and breast cancer related protein are reduced in capillary cerebral amyloid angiopathy. Neurobiol. Aging 35, 565–575 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Vogelgesang S et al. Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 12, 535–541 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Al-Majdoub ZM et al. Proteomic Quantification of Human Blood–Brain Barrier SLC and ABC Transporters in Healthy Individuals and Dementia Patients. Mol. Pharmaceutics 16, 1220–1233 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Scahill RI, Schott JM, Stevens JM, Rossor MN & Fox NC Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proceedings of the National Academy of Sciences 99, 4703–4707 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J et al. Regional protein expression in human Alzheimer’s brain correlates with disease severity. Commun Biol 2, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billington S et al. Interindividual and Regional Variability in Drug Transporter Abundance at the Human Blood-Brain Barrier Measured by Quantitative Targeted Proteomics. Clin. Pharmacol. Ther. 106, 228–237 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Braak H & Braak E Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- 23.Sperling RA et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7, 280–292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fillenbaum GG et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): The first twenty years. Alzheimer’s & Dementia 4, ALZJJALZ200708005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf BB, Lopes MB, VandenBerg SR & Gonias SL Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am. J. Pathol. 141, 37–42 (1992). [PMC free article] [PubMed] [Google Scholar]
- 26.Bu G, Maksymovitch EA, Nerbonne JM & Schwartz AL Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J. Biol. Chem. 269, 18521–18528 (1994). [PubMed] [Google Scholar]
- 27.Prasad B & Unadkat JD Optimized approaches for quantification of drug transporters in tissues and cells by MRM proteomics. AAPS J 16, 634–648 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szablewski L Glucose Transporters in Brain: In Health and in Alzheimer’s Disease. JAD 55, 1307–1320 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Kumar V, Nguyen TB, Tóth B, Juhasz V & Unadkat JD Optimization and Application of a Biotinylation Method for Quantification of Plasma Membrane Expression of Transporters in Cells. AAPS J 19, 1377–1386 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Hartz AMS et al. Aβ40 Reduces P-Glycoprotein at the Blood-Brain Barrier through the Ubiquitin-Proteasome Pathway. J. Neurosci. 36, 1930–1941 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ZJ & Sun LJ Nonproteolytic Functions of Ubiquitin in Cell Signaling. Molecular Cell 33, 275–286 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Krohn M et al. Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice. J. Clin. Invest. 121, 3924–3931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamura T, Kikuchi T & Irie T PET Imaging of MRP1 Function in the Living Brain: Method Development and Future Perspectives. CTMC 10, 1810–1819 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Shawahna R et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol. Pharm. 8, 1332–1341 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Uchida Y et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 117, 333–345 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Lee C-C et al. Adenosine Augmentation Evoked by an ENT1 Inhibitor Improves Memory Impairment and Neuronal Plasticity in the APP/PS1 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 55, 8936–8952 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Bartels AL et al. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol. Aging 30, 1818–1824 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Sweeney MD, Sagare AP & Zlokovic BV Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14, 133–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.