Abstract
Objective
Approximately 10% of stillbirths (SB) are attributed to fetal anomalies, but anomalies are also common in live births (LB). We aimed to assess the relationship between anomalies, by system, and stillbirth.
Design
Secondary analysis of a prospective, case-control study
Setting
Multi-center-59 hospitals in five regional catchment areas in the United States (U.S.).
Population or Sample
All stillbirths and representative live birth controls.
Methods
Standardized postmortem examinations performed in stillbirths, medical record abstraction for stillbirths and live births.
Main Outcome Measures
Incidence of major anomalies, by type, compared between stillbirths and live births with univariable and multivariable analyses utilizing weighted analysis to account for study design and differential consent.
Results
Of 465 singleton stillbirths included, 23.4% had one or more major anomalies compared to 4.3% of 1871 live births. Having an anomaly increased the odds of stillbirth; an increasing number of anomalies was more highly associated with stillbirth. Regardless of organ system affected, the presence of an anomaly increased the odds of stillbirth. These relationships remained significant if stillbirths with known genetic abnormalities are excluded. After multivariable analyses, the odds of stillbirth for any anomaly was aOR 4.33, 95% CI 2.80–6.70 and the systems most strongly associated with stillbirth were cystic hygroma (aOR 29.97, 95% CI 5.85–153.57), thoracic (aOR16.18, 95% CI 4.30–60.94), and craniofacial (aOR 35.25, 95% CI 9.22–134.68).
Conclusions
In pregnancies affected by anomalies, the odds of stillbirth are higher with increasing numbers of anomalies. Anomalies of nearly any organ system increased the odds of stillbirth even when adjusting for gestational age and maternal race.
Keywords: Stillbirth, anomaly, nomalies, congenital anomaly, congenital anomalies, fetal anomaly, fetal anomalies
Tweetable abstract
Stillbirth risk increases with anomalies of nearly any organ system and with number of anomalies seen.
Introduction
Stillbirth, defined by the WHO as fetal death at 28 weeks estimated gestational age (EGA) or more, affects 18.4 per 1000 births worldwide (1). In the United States (U.S.), fetal death at or beyond 20 weeks EGA is routinely classified as stillbirth and affects one in 168 pregnancies. The 2017 U.S. National Vital Statistics Report shows that a stillbirth rate of 5.786 stillbirths per 1000 livebirths, nearly identical to that of infant deaths, at 5.79 per 1000 livebirths (2,3).
The cause of stillbirth varies and is often multifactorial with maternal, fetal, and obstetric contributions. National Vital Statistics Reports investigating the causes of fetal death report a rate of anomalies of 10.8% among stillbirths (3). Similar results were noted in a large U.S. study wherein stillbirth was attributed to fetal genetic and / or structural abnormalities in 13.7% of cases (4). Anomalies are also relatively common in live births, affecting 3.0% (1/33) when all anomalies are included (5,6). In the absence of data delineating stillbirth risk by anomaly system, clinicians are limited in providing accurate counseling regarding this risk. While it is logical to speculate that complex and/or multisystem anomalies are at higher risk of stillbirth than isolated or mild anomalies, this has not been systematically assessed.
While “fetal genetic and/or structural abnormalities” have been described as a cause of stillbirth, further detailed description of this group is lacking. Additionally, the relative contribution of various anomalies to stillbirth by anatomic system is uncertain. Thus, we aimed to characterize pregnancies affected by anomalies (stillbirths and live births) and assess the associations of stillbirth with specific anomalies and groups of anomalies.
Methods
We conducted a secondary analysis of data from the SCRN prospective case-control study (March 2006 through September 2008). This is cross-sectional data from patients enrolled at the time of delivery. Accordingly, women may have had adequate, inadequate, or no prenatal care. The study design, methodology, and primary findings have been described (6). Stillbirths and live births were enrolled at the time of delivery in five regional catchment areas in Rhode Island, Massachusetts, Georgia, Texas, and Utah. Stillbirth was defined as a fetus with Apgar scores of 0 at 1 and 5 minutes without signs of life by direct observation. Deliveries performed as a result of termination of pregnancy were excluded from the study.
Gestational age was determined by the best clinical estimate. Multiple sources were used including information from assisted reproductive technology, last menstrual period, and/or obstetric ultrasound (8). Fetal deaths occurring at 18 through 19 weeks 6 days estimated gestational age with poor gestational dating criteria were also included so as to avoid inadvertent exclusion of fetuses that may have been ≥20 weeks’ gestation (7). All participants provided written informed consent and the study was approved by the institutional review boards at each of the participating sites as well as the Data Coordinating and Analysis Center.
Women were enrolled at the time of diagnosis of stillbirth or at delivery in the case of live births. Women underwent a standardized maternal interview, medical record abstraction, biospecimen collection, postmortem examinations of the fetus and placenta, as well as attempted karyotype. Fetal autopsy and placental examinations were performed by perinatal pathologists using study specific standardized protocols, that included centralized training (9,10). Biospecimens collected included maternal blood, fetal blood (umbilical cord) when available, placental tissue, and fetal tissue (in the case of stillbirths). In stillbirth cases, additional laboratory evaluation was recommended (based on clinically recommended evaluation of stillbirth) and included karyotype, testing for fetal-maternal hemorrhage, antibody screen, infectious serologies (syphilis and parvovirus), antiphospholipid antibodies, and toxicology screen. When possible, biospecimens were stored to complete additional testing not completed at time of delivery including chromosomal microarray (7,11,12).
For this analysis we included stillbirths and live births with and without major fetal anomalies. Multifetal gestations and stillbirths without perinatal postmortem examination data were excluded. Major anomalies were grouped by anatomic system and specific anomaly type including cystic hygroma, central nervous system (open neural tube defect, anencephaly, hydranencephaly, hydrocephalus, holoprosencephaly, and other), thoracic (congenital diaphragmatic hernia, cystic adenomatoid malformation (CAM), pulmonary sequestration, other), cardiac (atrioseptal defect, ventricular septal defect, atrioventricular canal defect, transposition of the great vessels, tetralogy of fallot, other), gastrointestinal (gastroschisis, omphalocele, duodenal atresia, other), genitourinary (hydronephrosis/ureteropelvic junction obstruction, autosomal recessive polycystic kidney disease, multicystic/dysplastic kidney, posterior urethral valves, renal agenesis, other), skeletal (skeletal dysplasia, club feet, other), umbilical cord, craniofacial (cleft palate, other), hydrops fetalis, and other. A “write in” option was available to further describe abnormalities, particularly those listed as “other”. These “write in” sections were individually reviewed to ensure they were appropriately categorized. A “craniofacial anomalies” category was added after data inspection revealed that these were not consistently categorized. Additionally, a category of “hydrops” was added. True “knots” were not considered as anomalies and were not considered causes of death in the SCRN study. When multiple abnormalities were noted and consistent with hydrops (i.e., ascites, pleural effusions, skin edema), they were categorized as a single diagnosis of hydrops. When multiple anomalies were noted, they were coded separately to provide an accurate total anomaly count.
Statistical Analysis
Baseline demographics and clinical characteristics were reported for stillbirths with and without anomalies, and live births with and without anomalies. Demographics were compared between stillbirths with and without anomalies, and among anomalous deliveries between stillbirths and live births. The incidence of anomalies, overall (any) and by type, was compared between stillbirths and live births. Anomaly types (by system and subcategory) were then compared between stillbirths and live births using univariable and multivariable analyses.
The outcome of stillbirth delivery was modeled in a logistic regression model, to estimate the association of any anomaly, and each system with stillbirth. In adjusted modeling, the four covariates considered for inclusion were gestational age at delivery (preterm, early term, full/late term), race/ethnicity (white, black, Hispanic, other), maternal age (<35 vs 35+), and maternal education (college or more vs less than college). Variable selection occurred in the model of the association of any anomaly with stillbirth occurred by removing variables for which the overall effect was non-significant with p≥0.05. The same set of covariates that remained in the model of any anomalies and stillbirth were included in adjusted analyses for models of the association of each system with stillbirth.
To address whether additional anomalies are associated with stillbirth, we compared number of anomalies, categorized as 1, 2, 3+ versus zero anomalies, in a logistic regression model where the outcome was stillbirth.
Mean and SD reported for continuous characteristics with comparisons tested with a two-sample t-test. P-values are reported from a Wald chi square for categorical characteristics. Unadjusted and adjusted odds ratios (aOR) with 95% confidence intervals are reported for logistic regressions. In a sensitivity analysis, the impact of removing stillbirth deliveries at gestational ages 18 weeks to 19 weeks 6 days are evaluated. Statistical significance was defined as p <0.05. All analyses were completed using the SCRN analytical weights (7) in SAS 9.4 using survey-analysis procedures (surveymeans, surveyfreq, and surveylogistic).
Results
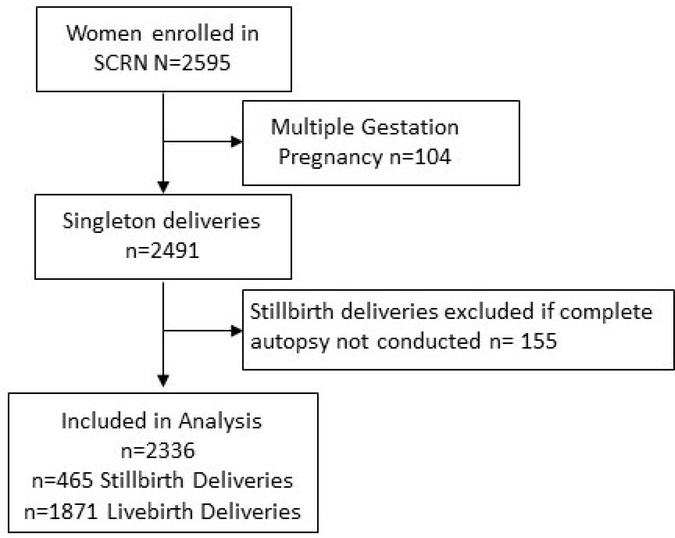
The SCRN database included 2595 women. After excluding 104 multigestation pregnancies and 155 cases of stillbirth without perinatal postmortem examination data available, a total of 465 stillbirth and 1871 live birth cases were included in our analysis (Figure 1). Among stillbirths, 23.4% had one or more major anomalies compared to 4.3% of live births. In pregnancies ending in stillbirth, those that were affected by anomalies were more commononly born to Hispanic women, had lower maternal education, were born preterm, and had genetic abnormalities (Table S1a). Among all pregnancies affected by anomalies, gestational age at delivery was the only baseline demographic or clinical characteristic associated with stillbirth (Table S1b).
Figure 1.
Study Enrollment.
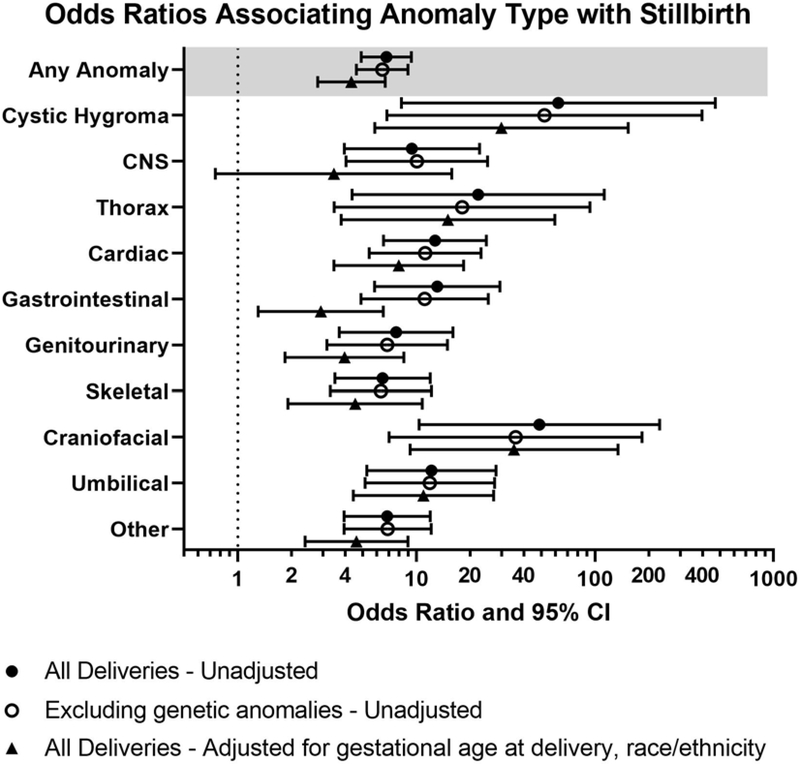
Anomalies (overall) and affecting each organ system were significantly associated with stillbirth (Table 1); however not all specific anomaly types (subcategories) were associated with stillbirth (Table S2). The odds of stillbirth varied by organ system with increased odds of stillbirth for anomalies in all systems in unadjusted analysis (Figure 2). When excluding cases with known genetic abnormalities, these trends were unchanged. (Figure 2, Table S3). Additionally, in a subgroup analysis of those cases diagnosed in the antenatal period, trends were unchanged (Table S4). Of note, because there were no cases of hydrops fetalis in the live birth group, an odds ratio could not be calculated for this finding.
Table 1.
Presence of anomalies by system in stillbirths versus live births
| Value | Stillbirth | Live birth | p |
|---|---|---|---|
| Unweighted | 465 | 1871 | |
| Weighted | 461 | 1412 | |
| n (%) | n (%) | ||
| Any Anomaly | 108 (23.4) | 61 (4.3) | <.001 |
| Number of anomalies | |||
| 1 | 42 (9.1) | 47 (3.3) | <.001 |
| 2 | 17 (3.6) | 9 (0.7) | |
| 3+ | 49 (10.6) | 5 (0.3) | |
| System | |||
| Cystic Hygroma | 16 (3.5) | 1 (0.1) | <.001 |
| CNS | 17 (3.6) | 6 (0.4) | <.001 |
| Thorax Defects | 12 (2.6) | 2 (0.1) | 0.001 |
| Cardiac Defects | 35 (7.7) | 9 (0.6) | <.001 |
| Gastrointestinal defects | 26 (5.6) | 6 (0.4) | <.001 |
| Genitourinary defects | 22 (4.8) | 9 (0.6) | <.001 |
| Skeletal defects | 30 (6.5) | 15 (1.1) | <.001 |
| Umbilical cord abnormalities | 26 (5.6) | 7 (0.5) | <.001 |
| Craniofacial | 26 (5.6) | 2 (0.1) | <.001 |
| Hydrops | 30 (6.4) | 0 (0) | * |
| Other anomaly | 37 (8.1) | 18 (1.3) | <.001 |
p-value not available when zero events observed in one group.
Statistics shown in the table are based on weighted data
Figure 2.
Odds ratio of stillbirth vs live birth by anomaly type.
In the model of the association of any anomaly with stillbirth, variable selection first excluded maternal education, then maternal age. After adjustment for race/ethnicity and gestational age at delivery, any anomaly remained significantly associated with stillbirth, but with an odds ratio estimate 37% smaller in magnitude than that in unadjusted analyses. Similarly, for each system the association with stillbirth was smaller but still significant, with the exception of CNS anomaly which was no longer significant when adjusted for maternal race and gestational age at delivery (Figure 2). The odds of stillbirth for any anomaly was aOR 4.33, 95% CI 2.80–6.70 and the odds of stillbirth were highest for cystic hygroma (aOR 29.97, 95% CI 5.85–153.57), thoracic (aOR16.18, 95% CI 4.30–60.94), and craniofacial anomalies (aOR 35.25, 95% CI 9.22–134.68) (Figure 2, Table S2).
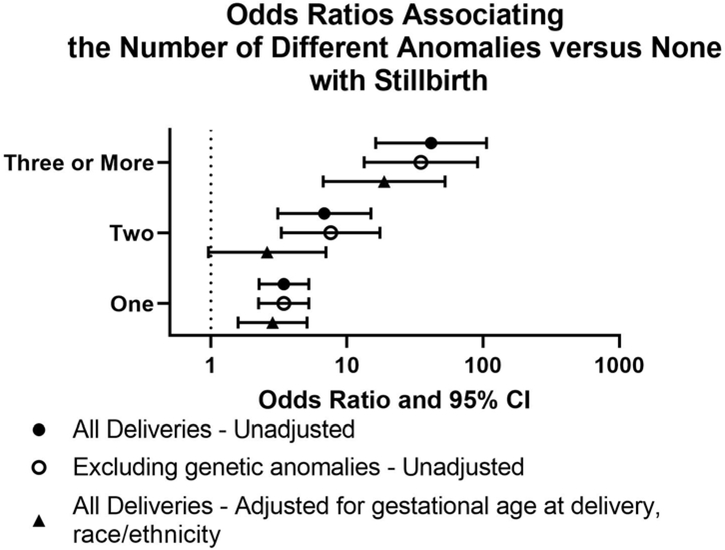
The number of anomalies was significantly associated with stillbirth (p<0.001) (Table 1). The distribution of anomaly count in the stillbirth group is wider than those in the live birth group and remained the case when excluding genetic abnormalities (Figure S1). The odds of stillbirth increased monotonically with additional anomalies (as compared to zero anomalies) (Figure 3). This remained true when excluding cases with genetic abnormalities, and for one and three anomalies adjustment for maternal race/ethnicity and gestational age at delivery (Table S5).
Figure 3.
Impact of additional anomalies.
Ten stillbirth deliveries occurred at gestational ages 18 and 19 weeks. None of these were affected by anomalies. Excluding these deliveries impacts results by a non-substantial amount; increasing differences between stillbirth and livebirth deliveries very slightly (data not shown).
Discussion
Main Findings
In this case-control study of pregnancies affected by anomalies, with the exception of CNS anomalies, the odds of stillbirth increase in the presence of an anomaly in any system and this effect is additive when multiple anomalies are present. This analysis may provide a framework for counseling families with pregnancies affected by anomalies.
Strengths and Limitations
Our study has some limitations. First, we ascertained malformations in live births by medical record abstraction and patient interview. Although these approaches are likely to identify all major abnormalities, they are not as robust as perinatal postmortem examination. Also, we did not enroll 100% of cases of stillbirth or live birth, providing a potential source of bias. Another limitation of our study was the inability to evaluate the decision to pursue a termination, additional antenatal surveillance (serial ultrasounds for example), or iatrogenic preterm birth in the context of known anomalies. Accordingly, our observations of an increased proportion of stillbirths with anomalies in Hispanic women or preterm births may be due to unmeasured confounders. Since cases of termination of pregnancy were excluded, those women with poor access to abortion care, or religious or cultural objections to pregnancy termination may be more likely to end up in the stillbirth group, inflating the calculated stillbirth risk in this population. Given small numbers of events for anomalies of specific organ systems, the confidence intervals were wide, potentially limiting the interpretation of the magnitude of the effect size. Finally, the number of stillbirths and live births was too small for meaningful analysis of individual abnormalities.
There also were several strengths of the study. Most notably, as a large multicenter case-control study with five catchment areas including 59 hospitals, the population is geographically, ethnically, and racially diverse, optimizing generalizability of our findings. Stillbirths underwent standardized perinatal postmortem examination, ensuring accurate ascertainment of all abnormalities. Although this was not performed on live births, abnormalities in controls were assessed via medical record abstraction and patient interview, ensuring identification of major problems. In addition, numbers were large for a study of stillbirths, and granular data were collected relative to studies using vital statistics.
Interpretation (in light of other evidence)
The incidence of stillbirth in the context of anomalies was described in a European population based study. However, this study did not report the odds of stillbirth associated with anomalies as our study has (14). In a multicenter prospective cohort study of 1025 stillbirths, congenital anomalies were the cited cause of stillbirth in 4.8% of cases (14). This was lower than the rate of stillbirths reported due to anomalies using U.S. vital statistics (10.8%) (2) as well as by the SCRN study (13.7%) (4). Notably, our reported 23.4% incidence of anomalies among stillbirths only indicates the presence of one or more anomalies that were not necessarily considered a cause of death. We previously reported that anomalies were a cause of death in 13.7% of our study population (4). The higher rate of reported anomalies in the two U.S. studies is likely due in part to the inclusion of all anomalies, rather than only those considered a cause of death. Other reasons for the differences noted include variation in rates of pregnancy termination in the setting of prenatally diagnosed anomalies and differences in the classification systems used to assign a cause of death.
Conclusion
Historically, counseling surrounding some anomalies has generically included an increased risk of stillbirth. However, these data go further to support this claim as well as to delineate the magnitude by which that risk may change based on the type and number of anomalies present. Since the odds of stillbirth were higher with nearly all anomaly types, our findings support consideration of antenatal testing in the setting of major anomalies when the goal is to optimize fetal outcome. Of course, our data do not allow assessment of the cost effectiveness or clinical efficacy of antenatal testing in pregnancies with anomalies, but raise the question as a potential focus for future studies. Additionally, these data can facilitate the refinement of stillbirth classification systems when considering fetal anomalies as a cause of death.
Supplementary Material
Acknowledgements
Additional Contributions: We acknowledge the members of the NICHD Scientific Advisory and Safety Monitoring Board, including Rev Phillip Cato, PhD; James W. Collins Jr, MD, MPH; Terry Dwyer, MD, MPH; William P. Fifer, PhD; John Ilekis, PhD; Marc Incerpi, MD; George Macones, MD, MSCE; Richard M. Pauli, MD, PhD; Raymond W. Redline, MD; Elizabeth Thom, PhD (chair); as well as all of the other physicians, study coordinators, research nurses, and patients who participated in the Stillbirth Collaborative Research Network. We also acknowledge Elizabeth Gates, MBA, University of Utah Health Sciences Center, for her editorial assistance. No compensation was received by any of these individuals in association with their contributions to this article.
Stillbirth Collaborative Research Network: University of Texas Health Science Center at San Antonio: Donald J. Dudley, Deborah Conway, Karen Aufdemorte, Angela Rodriguez, Monica Pina; University of Utah School of Medicine: Robert M. Silver, Michael W. Varner, Kristi Nelson; Emory University School of Medicine and the Rollins School of Public Health: Carol J. Rowland Hogue, Barbara J.Stoll, Janice Daniels Tinsley, Bahig Shehata, Carlos Abramowsky; Brown University: Donald Coustan, Halit Pinar, Marshall Carpenter, Susan Kubaska; University of Texas Medical Branch at Galveston: George R. Saade, Radek Bukowski, Jennifer Lee Rollins, Hal Hawkins, Elena Sbrana; RTI International: Corette B. Parker, Matthew A. Koch, Vanessa R. Thorsten, Holly Franklin, Pinliang Chen; Pregnancy and Perinatalogy Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health: Marian Willinger, Uma M. Reddy; Drexel University School of Medicine: Robert L. Goldenberg.
Disclosure of interest: NICHD grants were received by the instutitions listed. No other conflicts of interest reported by authors. Completed disclosure of interest forms are available to view online as supporting information.
Funding/Support: This research was supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): U10-HD045953 Brown University; U10-HD045925 Emory University; U10-HD045952 University of Texas Medical Branch at Galveston; U10- HDO45955 University of Texas Health Sciences Center at San Antonio; U10-HD045944 University of Utah Health Sciences Center; and U01-HD045954 RTI International, RTP. The Stillbirth Collaborative Research Network is solely responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript
Footnotes
The University of Utah Institutional Review Board: This secondary analysis was found to qualify for IRB exemption 5/9/2019 IRB#122488 due to the deidentified nature of the data.
References:
- 1.Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. The Lancet Global Health. 2016;4(2):e98–e108. doi: 10.1016/S2214-109X(15)00275-2 [DOI] [PubMed] [Google Scholar]
- 2.Ely DM, Driscoll AK. Infant Mortality in the United States, 2017: Data From the Period Linked Birth/Infant Death File. Natl Vital Stat Rep. 2019;68(10):1–19. [PubMed] [Google Scholar]
- 3.Hoyert DL, Gregory ECW. Cause of Fetal Death: Data From the Fetal Death Report, 2015–2017. Natl Vital Stat Rep. 2020;69(4):1–19. [PubMed] [Google Scholar]
- 4.Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA. 2011;306(22):2459–2468. doi: 10.1001/jama.2011.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Update on Overall Prevalence of Major Birth Defects --- Atlanta, Georgia, 1978–-2005. Accessed November 19, 2019 https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.html [PubMed]
- 6.Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019;111(18):1420–1435. doi: 10.1002/bdr2.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker CB, Hogue CJR, Koch MA, Willinger M, Reddy UM, Thorsten VR, et al. Stillbirth Collaborative Research Network: Design, Methods and Recruitment Experience. Paediatr Perinat Epidemiol. 2011;25(5):425–435. doi: 10.1111/j.1365-3016.2011.01218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, et al. Metronidazole to Prevent Preterm Delivery in Pregnant Women with Asymptomatic Bacterial Vaginosis. 10.1056/NEJM200002243420802. doi: 10.1056/NEJM200002243420802 [DOI] [PubMed] [Google Scholar]
- 9.Pinar H, Koch MA, Hawkins H, Jeim-Hall J, Shehata B, Thorsten VR, et al. The Stillbirth Collaborative Research Network (SCRN) Placental and Umbilical Cord Examination Protocol. Am J Perinatol. 2011;28(10):781–792. doi: 10.1055/s-0031-1281509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Abramowsky CR, Thorsten VR, et al. The Stillbirth Collaborative Research Network Postmortem Examination Protocol. Am J Perinatol. 2012;29(3):187–202. doi: 10.1055/s-0031-1284228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy UM, Page GP, Saade GR, Silver RM, Thorsten VR, Parker CB, et al. Karyotype versus Microarray Testing for Genetic Abnormalities after Stillbirth. N Engl J Med. 2012;367(23):2185–2193. doi: 10.1056/NEJMoa1201569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy UM, Page GP, Saade GR. The role of DNA microarrays in the evaluation of fetal death. - PubMed - NCBI. Accessed May 17, 2020 http://www.ncbi.nlm.nih.gov/pubmed/22467168 [DOI] [PubMed]
- 13.Groen H, Bouman K, Pierini A, Rankin J, Rissmann A, Haeusler M, et al. Stillbirth and neonatal mortality in pregnancies complicated by major congenital anomalies: Findings from a large European cohort. Prenatal Diagnosis. 2017;37(11):1100–1111. doi: 10.1002/pd.5148 [DOI] [PubMed] [Google Scholar]
- 14.Korteweg FJ, Erwich JJHM, Timmer A, van der Meer J, Ravise JM, Veeger NJGM, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol. 2012;206(1):53.e1–53.e12. doi: 10.1016/j.ajog.2011.10.02 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.