Abstract
INTRODUCTION:
The effect of bilingualism on age at onset has yet to be examined within different clinical variants of Alzheimer’s disease.
METHODS:
We reviewed the research charts of 287 well-characterized participants with either amnestic Alzheimer’s dementia (AD) or logopenic variant primary progressive aphasia (lvPPA) and identified bilingual speakers based on regular use of two or more languages and/or ability to communicate with native speakers in two or more languages. We evaluated whether bilingual speakers demonstrated a delay in age of symptom onset relative to monolingual speakers while controlling for other variables known to influence cognitive reserve.
RESULTS:
A five-year delay in age at symptom onset was observed for bilingual relative to monolingual speakers with lvPPA. This delay in onset was not observed in the amnestic AD cohort.
DISCUSSION:
Bilingualism may serve as a unique cognitive reserve variable in lvPPA, but not in amnestic AD.
Keywords: Primary progressive aphasia, Alzheimer’s disease, bilingualism, cognitive reserve, dementia, multilingualism
1. Background
It has been postulated that individuals with high cognitive reserve can sustain a greater degree of pathological burden before displaying clinical symptoms1,2. A number of activities and experiences that regularly engage cognition in a sustained and taxing fashion have been found to be contributors to cognitive reserve3,4. Bilingualism may contribute to cognitive reserve and serve as a protective factor against the onset of Alzheimer’s dementia (AD)5–7, but results have been mixed8–11. In frontotemporal dementia (FTD), results suggest that the effects of bilingualism may differ by clinical variant12.
Like FTD, AD comprises distinct clinical variants, including amnestic AD, characterized by episodic memory deficits and bilateral hippocampal and mesial temporal lobe atrophy13, and logopenic variant primary progressive aphasia (lvPPA)14, characterized by impaired phonological processing and left temporo-parietal degeneration15. Although these two variants often share AD pathology and biomarkers16–20, they differ in the underlying neural networks (episodic memory versus phonological)13–15 that are implicated.
The aim of this retrospective study was to examine the effect of bilingualism on age of symptom onset in a large, historical cohort of monolingual and bilingual patients presenting with lvPPA or amnestic AD based on established clinical and imaging criteria13,15. We predicted that bilingual speakers in each clinical variant would show a delay in age of symptom onset relative to monolingual speakers, and that effects would persist when accounting for other variables known to influence cognitive reserve. This prediction was based upon previous literature indicating that bilingualism may confer a delay in symptom onset in individuals with AD5–7,21–23.
2. Methods
2.1. Participants
Individuals seen at the UCSF Memory and Aging Center (MAC) between August 2005 and September 2017 were considered for inclusion in this retrospective study. All participants were previously recruited for a longitudinal research study through an NIH Program Project Grant (PPG) or the Alzheimer’s Disease Research Center (ADRC) at the University of California, San Francisco. Participants were evaluated by a team consisting of a behavioral neurologist, a neuropsychologist and a nurse practitioner. At the research visit, each participant gave a complete clinical history, underwent neuropsychological testing and received a neurological examination, and their study partners participated in a nursing interview to discuss the participant’s functional status. Diagnosis was reached by a multidisciplinary team applying current diagnostic criteria13,15.
All patients or their decision-making surrogate gave written consent for the ongoing, longitudinal study from which the data reported in this study were derived. The study was approved by the UCSF institutional review board for human research.
2.2. Chart Review
In order to determine speaker status (monolingual or bilingual), we conducted a comprehensive chart review following a four-step procedure (Figure 1). These steps were taken not only to discern speaker status, but also to restrict our extensive review of the MAC database to diagnoses of interest (AD variants, per our a priori hypotheses), and to extract other variables of interest (when available; e.g., languages spoken). The four-step procedure was conducted as follows:
Step 1) A filtering process using a comprehensive list of search terms related to a potential positive history of bilingualism (see Appendix Table A.1 for full list) was conducted on all research visit summaries (N = 1,628) in the MAC database. Charts that were flagged by this filtering process (due to the presence of a search term), were reviewed in detail. Patients were classified as bilingual if their chart indicated that they regularly used two or more languages and/or they had the ability to communicate with native speakers in two or more languages12. For the purposes of this study, patients who spoke more than two languages were classified as bilingual24. Conversely, individuals were classified as monolingual if their charts did not state information regarding exposure to or experience with a second language. Participants were excluded if it was not clear whether they were monolingual or bilingual according to the above criteria. More specifically, participants were excluded if their chart revealed that a) they had enrolled in classes to learn a second language for only a few years and did not report any other exposure or ongoing experience with the language outside of the classroom; b) they immigrated to a country that had a different majority language from the country of their previous residence, but it was unclear whether they engaged in formal schooling or were employed in their adopted country in an environment that required usage of the primary language spoken in that country; or c) they reported minimal usage of a second language such that it was unclear if they had attained high proficiency or regularly used two or more languages.
Figure 1.
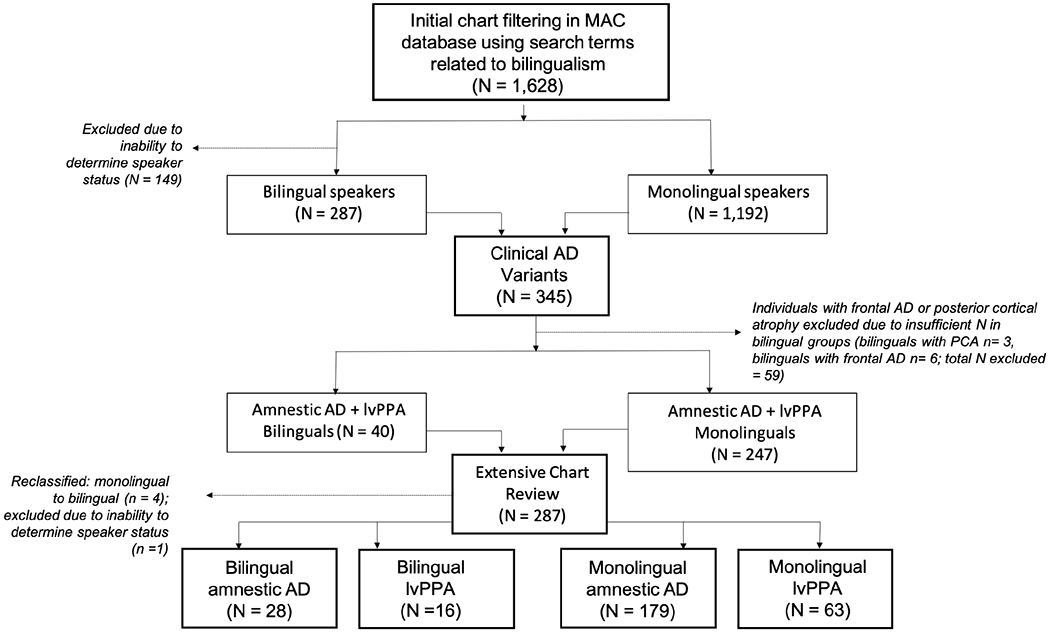
Flowchart describing selection and classification of participants
The raters (JD or SG) independently determined monolingual or bilingual status for each patient. In cases of disagreement, the raters discussed the case to reach consensus. A total of 895 charts were flagged for review through this filtering process. Of these, 287 individuals were classified as bilingual, 459 individuals were classified as monolingual, and 149 were excluded due to inability to determine speaker status. The remaining 733 charts did not contain a search term related to a potential positive history of bilingualism and were therefore considered to be monolingual. In sum, the initial step in our chart review process yielded a total of 1,479 charts to be considered in the next step of our chart review procedure (n = 287 bilinguals; n = 1192 monolinguals).
Step 2) Given that the focus of this study pertained to the effect of bilingualism on age of onset in AD variants, our second step was to identify which of the 1,479 monolingual and bilingual participants met diagnostic criteria for amnestic AD13, lvPPA15, posterior cortical atrophy (PCA)13 or frontal/executive AD13. A total of 345 participants met diagnostic criteria for one of these four AD variants. However, due to an insufficient number of bilingual patients diagnosed with PCA (N = 3) or frontal/executive AD (N = 6), all participants with these diagnoses (both monolingual and bilingual) were not considered for further analyses. Therefore, a total of 287 individuals with amnestic AD or lvPPA were considered for further inclusion in this study.
Step 3) Our third step was to perform a more in-depth chart review of the 287 remaining charts. This chart review identified four bilingual participants who were initially and incorrectly classified as monolingual by the filtering process in Step 1 and one participant for whom speaker status was unable to be determined. This resulted in our final cohort of 286 monolingual and bilingual participants with diagnoses of either amnestic AD or lvPPA.
Step 4) Our fourth step was to collect available information regarding patients’ first language (L1), second language (L2), additional languages, country of birth, occupation and any history of learning disability from the reviewed charts. Demographic information, including sex, education, handedness, age at first symptom and clinical diagnosis was collected through an established research database. Age at first symptom was defined as the age that the participant or family member first observed a clinical symptom indicative of a dementia syndrome.
2.3. Statistical analysis
All statistical analyses were performed using Stata 14.1 (StataCorp). 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
2.3.1. Demographic variables and neuropsychological assessment scores
The monolingual and bilingual speakers from each diagnostic group were compared to each other for the following variables using unequal samples t-tests: years of education, age at disease onset, age at diagnosis, CDR, MMSE and additional measures from a standard, comprehensive neuropsychological battery25. Tasks from the battery were grouped by cognitive domains (i.e., episodic memory, speech and language, visuospatial and executive/frontal) and a Bonferroni correction was applied to analyses conducted within each domain. Fisher’s exact test was used to test for between-group differences (monolingual versus bilingual) on categorical variables including sex, handedness, history of learning disability26, immigrant status, occupational level and diagnostic phenotype.
2.3.2. Effect of bilingualism and clinical phenotype on age of onset
Analyses of covariance (ANCOVA) were used to assess the effects of bilingualism and clinical diagnosis on age of symptom onset while controlling for other variables known to influence cognitive reserve. Data used in our analyses met assumptions for ANCOVA. Our omnibus test comprised a two-way ANCOVA with the independent variables of clinical variant (amnestic AD and lvPPA) and speaker status (monolingual and bilingual), the dependent variable of age at first symptom onset, and the covariates of sex, immigrant status, and years of education, with alpha set at p < 0.05. Post-hoc analyses were conducted, as appropriate.
3. Results
3.1. Demographic and neuropsychological assessment results of the entire cohort
The current study included a total of 286 participants. The cohort was 48% male. The mean level of education was 15.9 years (SD = 3.0). Mean age at symptom onset was 60.6 years (range 40-89 years) with a mean age at diagnosis of 64.6 years (range 44-92 years).
The entire cohort consisted of 242 monolingual speakers and 44 bilingual speakers. With regard to demographic variables, monolingual and bilingual speakers did not differ significantly from each other on the basis of sex, education, handedness, history of learning disability, or occupation (Table 1). Consistent with the demography of the general population of the United States of America27, bilingual relative to monolingual speakers were more likely to have immigrated from another country (2% of the monolingual group versus 55% of the bilingual group; p < .0001; Table 1). Within the bilingual cohort, a variety of languages were spoken (Appendix Table A.2). Performance on neuropsychological assessment did not differ between monolingual and bilingual speakers (Table 2).
Table 1.
Demographics for the full cohort of patients (monolingual and bilingual speakers) diagnosed with a clinical variant of AD
| All AD (amnestic AD + lvPPA) | |||||
|---|---|---|---|---|---|
| All Patients | Monolingual | Bilingual | p (mono vs. bi) | N (mono/bi) | |
| (N = 286) | (N = 242) | (N = 44) | |||
| Sex, Female, n (%) | 148 (52) | 128 (53) | 20 (45) | 0.414 | - |
| Education, mean (SD), y | 15.9 (3.0) | 15.8 (3.3) | 16.2 (2.7) | 0.341 | - |
| History of learning disability, n (%) | 26 (14) | 20 (8) | 6 (14) | 0.227 | 158/27 |
| Right-handed, n (%) | 255 (89) | 215 (89) | 40 (91) | 0.910 | - |
| Occupation | 217/57 | ||||
| Professionals, n (%) | 155 (60) | 128 (59) | 42 (74) | 0.607 | |
| Associate professionals, n (%) | 33 (13) | 28 (13) | 5 (9) | 1.000 | |
| Skilled workers, n (%) | 67 (26) | 57 (26) | 10 (18) | 0.848 | |
| Elementary, n (%) | 4 (2) | 4 (2) | 0 (0) | 1.000 | |
| Immigrant, n (%) | 29 (10) | 5 (2) | 24 (55) | 0.000 | - |
| Age at onset, mean (SD), y | 60.6 (9.8) | 60.3 (9.8) | 61.9 (9.8) | 0.338 | - |
| Age at diagnosis, mean (SD), y | 64.6 (9.7) | 64.5 (9.7) | 65.5 (9.9) | 0.546 | - |
| MMSE (30), mean (SD) | 20.8 (6.3) | 21.0 (6.9) | 19.6 (6.8) | 0.216 | 225/33 |
| BNT (15), mean (SD) | 10.92 (3.8) | 11.07 (3.7) | 9.9 (4.0) | 0.108 | 214/31 |
| CDR Total (3), mean (SD) | 0.84 (0.5) | 0.85 (0.5) | 0.83 (0.6) | 0.855 | 232/41 |
Note: BNT = Boston Naming Test, CDR = Clinical Dementia Rating scale, MMSE = Mini-Mental State Examination. A dash (-) in the N column indicates that the full dataset was available. Occupational skill level was determined using the International Standard Classification of Occupations (ISCO-08).
Table 2.
Comparison of performance on neuropsychological measures for monolingual and bilingual patients across the full cohort
| Full Cohort | ||||
|---|---|---|---|---|
| Monolingual | Bilingual | p | N | |
| (N = 242) | (N = 44) | (mono/bi) | ||
| Age at Testing, mean (SD), y | 64.24 (9.6) | 65.41 (9.82) | 0.494 | 229/37 |
| MMSE (30) | 21.00 (6.91) | 19.55 (6.83) | 0.216 | 225/33 |
| GDS (30) | 7.17 (5.46) | 9.12 (6.15) | 0.101 | 185/25 |
| Episodic Memory | ||||
| CVLT Trials 1-4 (36) | 15.69 (7.03) | 15.63 (4.50) | 0.964 | 192/30 |
| CVLT 10 min (9) | 1.86 (2.32) | 1.53 (2.08) | 0.468 | 192/30 |
| Rey recall (17) | 3.68 (3.91) | 3.88 (3.41) | 0.783 | 216/33 |
| Speech and Language | ||||
| Sentence repetition (5) | 3.32 (1.58) | 2.85 (1.42) | 0.104 | 194/33 |
| Animal fluency | 10.96 (5.71) | 9.66 (5.92) | 0.251 | 211/29 |
| BNT (15) | 11.07 (3.73) | 9.9 (4.00) | 0.108 | 214/31 |
| Sentence comprehension (5) | 3.57 (1.56) | 3.31 (1.42) | 0.365 | 183/29 |
| PPVT (16) | 13.79 (2.48) | 13.23 (2.92) | 0.249 | 190/31 |
| Visuospatial | ||||
| VOSP (10) | 7.14 (2.59) | 7.71 (2.12) | 0.266 | 184/28 |
| Rey copy (17) | 12.13 (4.7) | 12.5 (4.32) | 0.666 | 217/34 |
| Calculations (5) | 3.32 (1.35) | 3.09 (1.10) | 0.344 | 219/33 |
| Frontal/Executive | ||||
| Digits Forward | 5.28 (1.29) | 5.07 (1.64) | 0.474 | 148/27 |
| Digits Backward | 3.28 (1.31) | 3.52 (1.46) | 0.356 | 211/31 |
| D words | 9.40 (5.50) | 9.45 (4.82) | 0.962 | 219/31 |
| Trails (lines) | 85.23 (37.73) | 100.04 (31.49) | 0.063 | 175/25 |
| Trails (seconds) | 9.49 (5.52) | 8.56 (5.80) | 0.436 | 175/25 |
| Design Fluency | 5.62 (2.63) | 4.93 (2.89) | 0.339 | 188/28 |
| Stroop color naming | 54.39 (20.99) | 49.21 (20.97) | 0.262 | 155/24 |
| Stroop inhibition | 22.27 (14.56) | 18.45 (15.93) | 0.254 | 169/22 |
Abbreviations: BNT = Boston Naming Test, CVLT = California Verbal Learning Test, GDS = Geriatric Depression Scale, MMSE = Mini-Mental State Examination, PPVT = Peabody Picture Vocabulary Test, VOSP = Visual Object and Space Perception battery, mono = monolingual, bi = bilingual.
Note. Asterisk denotes significance with Bonferroni correction applied within each cognitive domain. These measures are derived from a neuropsychological battery described further in Kramer et al., 200325.
A comparison of the lvPPA group to the amnestic AD group revealed the expected pattern of differences on neuropsychological assessment per diagnostic criteria13–15 (Table 3). More specifically, the lvPPA cohort performed significantly worse than the amnestic AD cohort on tasks assessing language and phonological short-term memory. In addition, individuals in the amnestic AD cohort performed significantly worse than individuals with lvPPA on tasks assessing episodic memory.
Table 3.
Comparison of performance on neuropsychological measures for AD and lvPPA patients across the full cohort
| Full Cohort | ||||
|---|---|---|---|---|
| AD | lvPPA | p | N | |
| (N = 207) | (N = 79) | (AD/lvPPA) | ||
| Age at Testing, mean (SD), y | 64.72 (10.06) | 63.59 (8.43) | 0.388 | 190/76 |
| MMSE (30) | 21.51 (5.68) | 19.07 (7.32) | 0.005* | 184/74 |
| GDS (30) | 7.30 (5.81) | 7.67 (4.93) | 0.667 | 150/60 |
| Episodic Memory | ||||
| CVLT Trials 1-4 (36) | 16.25 (6.27) | 14.30 (7.64) | 0.050 | 158/64 |
| CVLT 10 min (9) | 1.50 (2.03) | 2.59 (2.68) | 0.001* | 158/64 |
| Rey recall (17) | 3.03 (3.46) | 5.38 (4.28) | <.001* | 177/72 |
| Speech and Language | ||||
| Sentence repetition (5) | 3.80 (1.35) | 1.88 (1.15) | <.001* | 163/64 |
| Animal fluency | 11.56 (5.51) | 8.97 (5.90) | 0.001* | 170/70 |
| BNT (15) | 11.81 (3.17) | 8.75 (4.24) | <.001* | 174/71 |
| Sentence comprehension (5) | 3.73 (1.42) | 3.03 (1.43) | 0.002* | 154/58 |
| PPVT (16) | 14.01 (2.29) | 13.02 (2.97) | 0.007* | 155/66 |
| Visuospatial | ||||
| VOSP (10) | 7.09 (2.60) | 7.58 (2.32) | 0.223 | 159/53 |
| Rey copy (17) | 11.94 (4.70) | 12.75 (4.48) | 0.210 | 178/73 |
| Calculations (5) | 3.34 (1.33) | 3.17 (1.28) | 0.348 | 181/71 |
| Frontal/Executive | ||||
| Digits Forward | 5.62 (1.24) | 4.45 (1.22) | <.001* | 119/56 |
| Digits Backward | 3.52 (1.28) | 2.80 (1.33) | 0.0001* | 171/71 |
| D words | 10.22 (5.33) | 7.35 (5.06) | 0.0001* | 179/71 |
| Trails (lines) | 85.86 (38.37) | 90.37 (34.21) | 0.449 | 146/54 |
| Trails (seconds) | 9.24 (5.58) | 9.72 (5.48) | 0.586 | 146/54 |
| Design Fluency | 5.43 (3.68) | 5.78 (3.15) | 0.523 | 157/59 |
| Stroop color naming | 55.31 (21.45) | 49.02 (19.08) | 0.080 | 133/46 |
| Stroop inhibition | 21.92 (15.28) | 21.55 (12.88) | 0.883 | 147/44 |
Abbreviations: BNT = Boston Naming Test, CVLT = California Verbal Learning Test, GDS = Geriatric Depression Scale, MMSE = Mini-Mental State Examination, PPVT = Peabody Picture Vocabulary Test, VOSP = Visual Object and Space Perception battery, mono = monolingual, bi = bilingual.
Note. Asterisk denotes significance with Bonferroni correction applied within each cognitive domain. These measures are derived from a neuropsychological battery described further in Kramer et al., 200325.
3.2. Demographic and neuropsychological assessment results of monolingual/bilingual speakers within each clinical variant
The monolingual and bilingual speakers in each AD variant did not differ significantly from each other with regard to sex, education, handedness, history of learning disability, occupation, diagnostic category or CDR (Table 4). In the lvPPA cohort, bilingual speakers were significantly older than monolinguals at time of diagnosis (M = 68.2 ± 8.6 years for the bilingual lvPPA group versus M = 62.8 ± 8.1 years for the monolingual lvPPA group; p = 0.021; Table 4). The monolingual and bilingual speakers within the AD and lvPPA cohorts did not differ in their performance on neuropsychological measures (Appendix Tables A.3 and A.4).
Table 4.
Demographic variables and performance on measures indicative of severity for monolingual and bilingual speakers by clinical phenotype
| Amnestic AD | lvPPA | |||||||
|---|---|---|---|---|---|---|---|---|
| Monolingual | Bilingual | p | N | Monolingual | Bilingual | p | N | |
| (N = 179) | (N = 28) | (mono/bi) | (N = 63) | (N = 16) | (mono/bi) | |||
| Sex, Female, n (%) | 94 (53) | 15 (54) | 1.000 | - | 34 (54) | 5 (31) | 0.161 | - |
| Education, mean (SD), y | 15.7 (3.2) | 16.3 (3.6) | 0.435 | - | 16.2 (2.6) | 16.3 (3.3) | 0.842 | - |
| History of learning disability, n (%) | 14 (8) | 5 (18) | 0.182 | 118/22 | 6 (10) | 1 (6) | 1.000 | 40/5 |
| Right-handed, n (%) | 162 (91) | 27 (96) | 0.806 | - | 53 (84) | 13 (81) | 0.838 | - |
| Occupation | 159/26 | 58/16 | ||||||
| Professionals, n (%) | 93 (58) | 17 (61) | 0.667 | 35 (56) | 10 (63) | 1.000 | ||
| Associate professionals, n (%) | 20 (13) | 2 (7) | 0.744 | 8 (13) | 3 (19) | 0.694 | ||
| Skilled workers, n (%) | 44 (28) | 7 (25) | 1.000 | 13 (21) | 3 (19) | 1.000 | ||
| Elementary, n (%) | 2 (1) | 0 (0) | 1.000 | 2 (3) | 0 (0) | 1.000 | ||
| Immigrant, n (%) | 3 (2) | 19 (68) | 0.000 | - | 2 (3) | 5 (31) | 0.003 | - |
| Age at diagnosis, mean (SD), y | 65.1 (10.1) | 63.9 (10.4) | 0.560 | - | 62.8 (8.1) | 68.2 (8.6) | 0.021 | - |
| MMSE (30), mean (SD) | 21.6 (5.6) | 20.8 (6.6) | 0.560 | 165/19 | 19.4 (7.4) | 17.9 (7.0) | 0.487 | 60/14 |
| BNT (15), mean (SD) | 11.8 (3.2) | 11.7 (2.9) | 0.824 | 157/17 | 8.9 (4.3) | 7.8 (4.3) | 0.356 | 57/14 |
| CDR Total (3), mean (SD) | 0.91 (0.5) | 0.98 (0.7) | 0.506 | 170/26 | 0.68 (0.39) | 0.57 (0.18) | 0.283 | 62/15 |
Note: BNT = Boston Naming Test, CDR = Clinical Dementia Rating scale, MMSE = Mini-Mental State Examination, mono = monolingual, bi = bilingual. A dash (-) in the N column indicates that the full dataset was available. Occupational skill level was determined using the International Standard Classification of Occupations (ISCO-08).
3.3. Effects of bilingualism and clinical diagnosis on age of symptom onset
The omnibus ANCOVA revealed a marginally significant crossover interaction of clinical variant and speaker status on age of symptom onset (F(1, 279) = 3.78, ηp2 = .013, p = .05). To investigate this interaction, we conducted post-hoc analyses comparing monolinguals and bilinguals within each clinical variant. An ANCOVA (covariates = sex, immigrant status and years of education) revealed no significant difference in age of symptom onset between monolingual and bilingual patients with amnestic AD (F(1, 202) = 3.64, ηp2 = .018, p = 0.06; Bilinguals M = 60.9 years; Monolinguals M = 60.9 years; Table 4, Figure 2). Conversely, an ANCOVA (covariates = sex, immigrant status and years of education) revealed that bilingual lvPPA patients developed symptoms significantly later than monolingual lvPPA patients (F(1, 74) = 4.40, ηp2 = 0.056, p = 0.04; Bilinguals M = 63.6 years; Monolinguals M = 58.7 years; Table 4, Figure 2). On average, bilingual speakers with lvPPA manifested clinical symptoms 5 years later than monolingual speakers.
Figure 2.
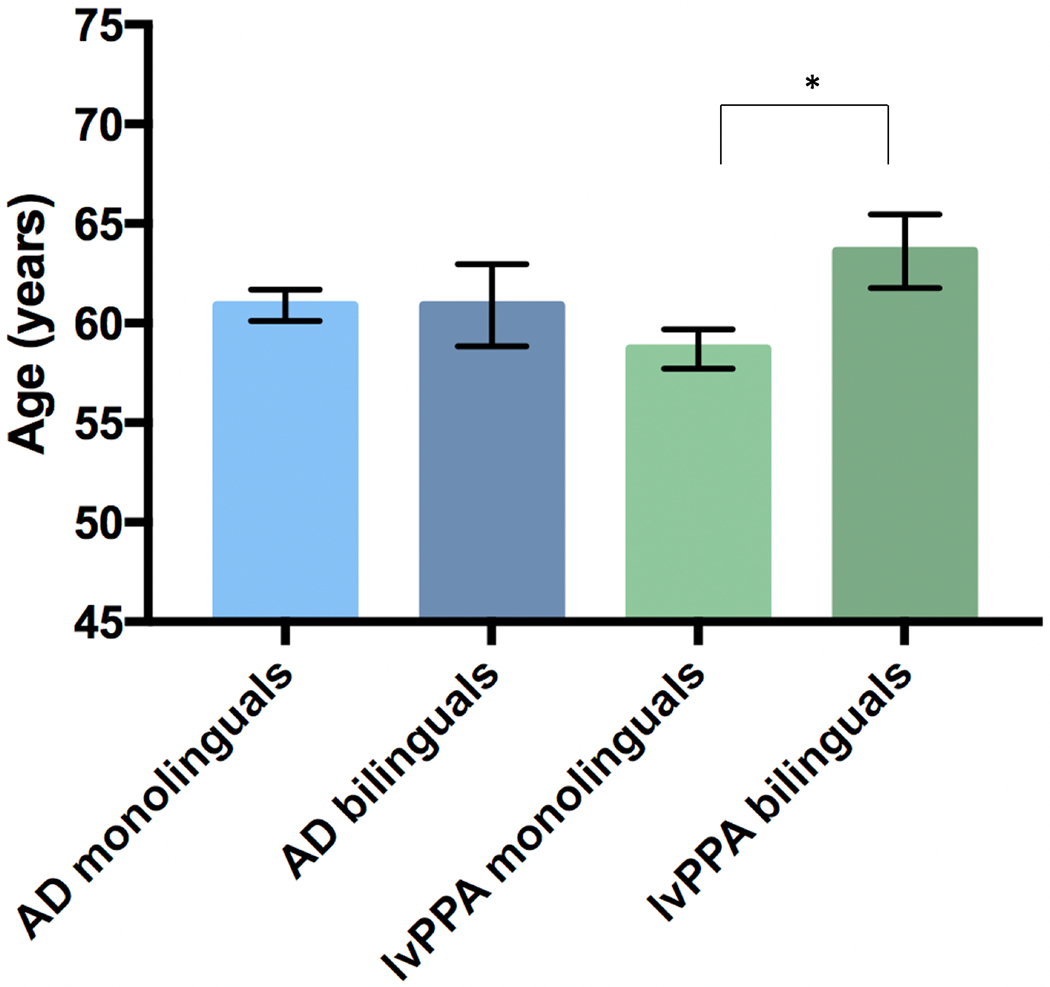
Age at symptom onset by clinical diagnosis and speaker group.
In order to further investigate whether speaker status interacted with other variables known to influence cognitive reserve in the lvPPA cohort, we conducted additional post-hoc ANOVAs. There were no significant interaction effects of speaker status and sex (F(1,75) = 0.05; ηp2 = 0.0006; p = 0.83), speaker status and education (F(1,75) = 0.45; ηp2 = 0.006; p = 0.50), or speaker status and immigrant status (F(1,75) = 0.41; ηp2 = 0.005; p = 0.52) on age of symptom onset.
4. Discussion
To our knowledge, this is the first study to address the potential effects of bilingualism on age of symptom onset in two distinct clinical variants of AD. Our data indicate that, within this cohort, bilingualism confers a significant, 5-year delay in symptom onset in bilingual relative to monolingual speakers with lvPPA, but not in those with amnestic AD. Whereas some studies have shown up to a 5-year delay in AD symptom onset in bilinguals compared to monolinguals5–7,21–23, others have not replicated this effect8–11. Although the different findings may be attributed to a number of factors7,22,28,29, our results indicate that clinical variant is a delineating variable that warrants consideration. In other words, inconsistent findings may be due, in part, to the fact that previous studies did not examine patterns within clinical AD variants or were published before the development of diagnostic criteria for AD variants. Results from this study may help to elucidate mixed findings with regard to bilingualism as a contributor to cognitive reserve in AD, given that we not only controlled for known confounding variables (i.e., sex, years of education and immigrant status), but also accounted for different behavioral phenotypes that implicate different underlying brain networks.
4.1. Effects of bilingualism on cognitive reserve in primary progressive aphasia
Previous evidence from Alladi and colleagues has demonstrated the importance of examining the effects of bilingualism across different clinical variants12. Results from their study indicated that bilingualism was protective against symptom onset in bvFTD, but not in nfvPPA or svPPA (the language variants of FTD), suggesting the potential for network-specific effects of bilingualism. Each of the three PPA variants is characterized by unique speech and language deficits15, and distinct patterns of network-based neurodegeneration30. Results from our study suggest that bilingualism confers a delay in age of onset for individuals with lvPPA, which is characterized by impairments in word-retrieval and repetition due to an underlying phonological deficit. Therefore, it may be the case that bilingualism confers a benefit to those with primary involvement of the phonological network (lvPPA), but not for those with primary involvement of the semantic (svPPA) or syntax and motor speech (nfvPPA) networks. Indeed, previous studies have shown differing effects of bilingualism on the core speech and language domains that are implicated in the three variants of PPA. With regard to phonology, studies have investigated phonological processing and phonological short-term memory in healthy bilingual relative to monolingual speakers. Although these studies have yielded mixed findings31,32, results are suggestive of bilingual advantages in phonological awareness33 and on tasks of phonological working memory31. Therefore, the phonological network may be enhanced in bilinguals and therefore more robust in the face of pathological processes. On the other hand, advantages related to bilingualism may not be evident on tasks of semantic processing34, sentence production35 and verbal fluency36,37, which rely upon processes that are affected in svPPA and nfvPPA. Taken together, these studies show that bilingualism may differentially influence the specific linguistic domains that are implicated in each PPA variant.
4.2. Effects of bilingualism on cognitive reserve in AD variants
Our finding that bilingualism conferred a delay for individuals with lvPPA but not for those with amnestic AD may be explained by the pattern of neuroanatomical enhancements associated with bilingualism. Bilingualism is associated with greater grey matter volume and white matter connectivity in phonological processing networks. Results from voxel-based morphometry (VBM) studies of neurotypical adults have found increased grey matter in the left inferior parietal cortex, Heschl’s gyrus, the superior temporal gyrus, and inferior frontal regions for bilingual relative to monolingual speakers (see Wong et al.,38 and García-Pentón et al.,39 for reviews). In a diffusion tensor imaging (DTI) study, bilinguals who performed well on a sound-to-word learning paradigm were shown to have enhanced fractional anisotropy (FA) in the left parietal-temporal region40. Converging evidence was reported in a study of early bilinguals and monolinguals using diffusion-weighted MRI (DWI) analyzed via network-based statistics, wherein bilinguals were found to have greater connection density in two networks: 1) a left frontal and temporo-parietal network and 2) a left occipital, temporo-parietal and right superior frontal network41. Together, these studies indicate that bilinguals have enhanced gray matter volume and white matter integrity in regions that have a high degree of overlap with the affected pathological network in lvPPA30,42. Therefore, it is plausible that these neuroanatomical enhancements may counteract symptom onset in the early stages of lvPPA, but not necessarily in amnestic AD, which is associated with degeneration of memory-predominant networks, particularly medial temporal and anterior default mode networks16.
Our results should also be considered in the context of previous studies on amnestic AD and amnestic mild cognitive impairment (MCI), a clinical syndrome that has a high probability of progression to Alzheimer’s dementia43–45. Compared to monolingual speakers with amnestic MCI, bilingual speakers presented with a later age of onset46. At first, the finding of a later age of symptom onset in bilingual speakers with amnestic MCI may seem to imply that bilingual speakers should also have a later age of symptom onset of amnestic AD, which we did not find in our patient cohort. As such, the rate of decline in bilingual patients who progress from amnestic MCI to amnestic AD may be faster than that of their monolingual counterparts, resulting in the two groups reaching an amnestic AD stage at a similar age. This notion is supported by a recent paper by Berkes et al.47, which reports a faster rate of conversion from MCI to AD for bilingual relative to monolingual speakers, and this pattern is consistent with the theory of cognitive reserve 1,48.
4.3. Strengths and Limitations
This study included a large sample of patients with lvPPA and AD with cohorts of monolingual and bilingual speakers matched for years of education, level of occupation and neuropsychological profile. Whereas some previous studies have interpreted results in the context of potentially confounding variables (i.e. those known to influence cognitive reserve), our sample was relatively homogeneous with regard to several of these cognitive reserve variables (sex, education, highest classification of occupation). Additionally, and importantly, because these patients were seen in a tertiary care center, they were all seen by behavioral neurologists with high levels of expertise in diagnosing and differentiating between AD variants.
Our study does have several limitations. This study was retrospective in nature, and therefore, data were limited with regard to characterization of patients’ history of language exposure and use. We acknowledge the importance of these factors in determining the nuances of bilingualism as a contributor to cognitive reserve. Additionally, there is no available objective data on L2 level of proficiency; nonetheless, we note that previous studies have found that subjective report of proficiency correlates well with objective proficiency measures49. Future prospective studies should document detailed language history and should treat bilingualism as a continuous rather than a categorical variable.
Another potential weakness is that bilingual relative to monolingual speakers in our study were more likely to have immigrated to the U.S. Past studies have shown varying results of the protective effect of bilingualism depending on immigrant status6,28, and this has been attributed to differences in baseline characteristics between non-immigrant and immigrant bilinguals, such as manner of L2 acquisition, socioeconomic status and other cognitive reserve variables (see Calvo et al.29 for a review). However, we note that our bilingual cohort is representative of the broader population of bilingual speakers in the U.S.27, and that results from our lvPPA cohort were significant even after controlling for this potentially confounding variable. In addition, no significant interaction effect was observed between immigrant status and speaker status on age of symptom onset in our lvPPA cohort. Nevertheless, future large-scale prospective studies should account for all potentially confounding variables and should continue to examine the differential effects of bilingualism on distinct clinical phenotypes. In addition, future research should continue to analyze data by clinical phenotype with samples that are more closely matched for number of cases in each group, as greater statistical power can be achieved with large, equal samples.
5. Conclusion
In summary, we have documented a 5-year delay in symptom onset for bilingual speakers who developed lvPPA, and no such effect for bilingual speakers with amnestic AD. These findings suggest that bilingualism may be an important mediator of cognitive reserve for language networks that are susceptible to Alzheimer’s disease. One potential explanation for this finding may be that the neural enhancements associated with bilingualism lend a protective benefit against negative effects of pathology in lvPPA in the early stages of disease progression. This study may help to uncover the basis for inconsistent findings with regard to the protective effect of bilingualism in AD by showing that bilingualism delays symptom onset in the language variant but not the amnestic variant of AD.
Supplementary Material
Acknowledgements
The authors thank the research participants and their study partners for their time and dedication to this work.
This work is supported by the National Institutes of Health [(MLGT,NINDS R01 NS050915), (MLGT, NIDCD K24 DC015544), (MH, NIDCD R01 DC016291), (BM, NIA P50 AG023501), (BM, NIA P01 AG019724), (SG, NIDCD F31DC016229), and (JD, NIDCD K23 DC018021)].
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Stern Y What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society : JINS 8, 448–460 (2002). [PubMed] [Google Scholar]
- 2.Jones RN et al. Conceptual and measurement challenges in research on cognitive reserve. Journal of the International Neuropsychological Society : JINS 17, 593–601, doi: 10.1017/s1355617710001748 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarmeas N, Levy G, Tang MX, Manly J & Stern Y Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 57, 2236–2242, doi: 10.1212/wnl.57.12.2236 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CB et al. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 73, 356–361, doi: 10.1212/WNL.0b013e3181b04ae3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bialystok E, Craik FI & Freedman M Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia 45, 459–464, doi: 10.1016/j.neuropsychologia.2006.10.009 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Craik FI, Bialystok E & Freedman M Delaying the onset of Alzheimer disease: bilingualism as a form of cognitive reserve. Neurology 75, 1726–1729, doi: 10.1212/WNL.0b013e3181fc2a1c (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman-Velez E & Tranel D Does bilingualism contribute to cognitive reserve? Cognitive and neural perspectives. Neuropsychology 29, 139–150, doi: 10.1037/neu0000105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crane PK et al. Midlife use of written Japanese and protection from late life dementia. Epidemiology (Cambridge, Mass.) 20, 766–774, doi: 10.1097/EDE.0b013e3181b09332 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders AE, Hall CB, Katz MJ & Lipton RB Non-native language use and risk of incident dementia in the elderly. J Alzheimers Dis 29, 99–108, doi: 10.3233/jad-2011-111631 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahodne LB, Schofield PW, Farrell MT, Stern Y & Manly JJ Bilingualism does not alter cognitive decline or dementia risk among Spanish-speaking immigrants. Neuropsychology 28, 238–246, doi: 10.1037/neu0000014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukadam N, Sommerlad A & Livingston G The Relationship of Bilingualism Compared to Monolingualism to the Risk of Cognitive Decline or Dementia: A Systematic Review and Meta-Analysis. J Alzheimers Dis 58, 45–54, doi: 10.3233/jad-170131 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Alladi S et al. Bilingualism delays the onset of behavioral but not aphasic forms of frontotemporal dementia. Neuropsychologia 99, 207–212, doi: 10.1016/j.neuropsychologia.2017.03.021 (2017). [DOI] [PubMed] [Google Scholar]
- 13.McKhann GM et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 7, 263–269, doi: 10.1016/j.jalz.2011.03.005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55, 335–346, doi: 10.1002/ana.10825 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorno-Tempini ML et al. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014, doi: 10.1212/WNL.0b013e31821103e6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ossenkoppele R et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Human brain mapping 36, 4421–4437, doi: 10.1002/hbm.22927 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snowden J, Neary D & Mann D Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol 114, 31–38, doi: 10.1007/s00401-007-0236-3 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Mesulam M et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 63, 709–719, doi: 10.1002/ana.21388 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos-Santos MA et al. Rates of Amyloid Imaging Positivity in Patients With Primary Progressive Aphasia. JAMA Neurol 75, 342–352, doi: 10.1001/jamaneurol.2017.4309 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinelli EG et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 81, 430–443, doi: 10.1002/ana.24885 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gollan TH, Salmon DP, Montoya RI & Galasko DR Degree of bilingualism predicts age of diagnosis of Alzheimer’s disease in low-education but not in highly educated Hispanics. Neuropsychologia 49, 3826–3830, doi: 10.1016/j.neuropsychologia.2011.09.041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alladi S et al. Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology 81, 1938–1944, doi: 10.1212/01.wnl.0000436620.33155.a4 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Woumans E & Duyck W The bilingual advantage debate: Moving toward different methods for verifying its existence. Cortex; a journal devoted to the study of the nervous system and behavior 73, 356–357, doi: 10.1016/j.cortex.2015.07.012 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Grosjean F Bilingual: Life and reality. (Harvard University Press, 2010). [Google Scholar]
- 25.Kramer JH et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology 16, 211–218, doi: 10.1097/00146965-200312000-00002 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Miller ZA et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain 136, 3461–3473, doi: 10.1093/brain/awt242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumbaut RG & Massey DS Immigration and Language Diversity in the United States. Daedalus 142, 141–154, doi: 10.1162/daed_a_00224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chertkow H et al. Multilingualism (but not always bilingualism) delays the onset of Alzheimer disease: evidence from a bilingual community. Alzheimer Dis Assoc Disord 24, 118–125, doi: 10.1097/WAD.0b013e3181ca1221 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Calvo N, Garcia AM, Manoiloff L & Ibanez A Bilingualism and Cognitive Reserve: A Critical Overview and a Plea for Methodological Innovations. Frontiers in aging neuroscience 7, 249, doi: 10.3389/fnagi.2015.00249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galantucci S et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain 134, 3011–3029, doi: 10.1093/brain/awr099 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo J & Kaushanskaya M Phonological memory in bilinguals and monolinguals. Memory & cognition 40, 1314–1330, doi: 10.3758/s13421-012-0237-x (2012). [DOI] [PubMed] [Google Scholar]
- 32.Kaushanskaya M, Blumenfeld HK & Marian V The relationship between vocabulary and short-term memory measures in monolingual and bilingual speakers. The international journal of bilingualism : cross-disciplinary, cross-linguistic studies of language behavior 15, 408–425, doi: 10.1177/1367006911403201 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo LJ & Anderson RC Effects of early bilingualism on learning phonological regularities in a new language. Journal of experimental child psychology 111, 455–467, doi: 10.1016/j.jecp.2011.08.013 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Gollan TH, Montoya RI, Fennema-Notestine C & Morris SK Bilingualism affects picture naming but not picture classification. Memory & cognition 33, 1220–1234, doi: 10.3758/bf03193224 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Runnqvist E, Gollan TH, Costa A & Ferreira VS A disadvantage in bilingual sentence production modulated by syntactic frequency and similarity across languages. Cognition 129, 256–263, doi: 10.1016/j.cognition.2013.07.008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandoval TC, Gollan TH, Ferreira VS & Salmon DP What causes the bilingual disadvantage in verbal fluency? The dual-task analogy. Bilingualism: Language and Cognition 13, 231–252, doi: 10.1017/S1366728909990514 (2010). [DOI] [Google Scholar]
- 37.Luo L, Luk G & Bialystok E Effect of language proficiency and executive control on verbal fluency performance in bilinguals. Cognition 114, 29–41, doi: 10.1016/j.cognition.2009.08.014 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Wong B, Yin B & O’Brien B Neurolinguistics: Structure, Function, and Connectivity in the Bilingual Brain. BioMed research international 2016, 7069274, doi: 10.1155/2016/7069274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Pentón L, Fernández García Y, Costello B, Duñabeitia JA & Carreiras M The neuroanatomy of bilingualism: how to turn a hazy view into the full picture. Language, Cognition and Neuroscience 31, 303–327, doi: 10.1080/23273798.2015.1068944 (2016). [DOI] [Google Scholar]
- 40.Wong FC, Chandrasekaran B, Garibaldi K & Wong PC White matter anisotropy in the ventral language pathway predicts sound-to-word learning success. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 8780–8785, doi: 10.1523/jneurosci.0999-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Penton L, Perez Fernandez A, Iturria-Medina Y, Gillon-Dowens M & Carreiras M Anatomical connectivity changes in the bilingual brain. NeuroImage 84, 495–504, doi: 10.1016/j.neuroimage.2013.08.064 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Lehmann M et al. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America 110, 11606–11611, doi: 10.1073/pnas.1221536110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell AJ & Shiri-Feshki M Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta psychiatrica Scandinavica 119, 252–265, doi: 10.1111/j.1600-0447.2008.01326.x (2009). [DOI] [PubMed] [Google Scholar]
- 44.Lopez OL et al. Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol 64, 416–420, doi: 10.1001/archneur.64.3.416 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Larrieu S et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 59, 1594–1599, doi: 10.1212/01.wnl.0000034176.07159.f8 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Ossher L, Bialystok E, Craik FI, Murphy KJ & Troyer AK The effect of bilingualism on amnestic mild cognitive impairment. The journals of gerontology. Series B, Psychological sciences and social sciences 68, 8–12, doi: 10.1093/geronb/gbs038 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berkes M, Bialystok E, Craik FIM, Troyer A & Freedman M Conversion of Mild Cognitive Impairment to Alzheimer Disease in Monolingual and Bilingual Patients. Alzheimer Dis Assoc Disord, doi: 10.1097/wad.0000000000000373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. The Lancet. Neurology 11, 1006–1012, doi: 10.1016/s1474-4422(12)70191-6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vega-Mendoza M, West H, Sorace A & Bak TH The impact of late, non-balanced bilingualism on cognitive performance. Cognition 137, 40–46, doi: 10.1016/j.cognition.2014.12.008 (2015). [DOI] [PubMed] [Google Scholar]
- 50.U.S. Census Bureau; American Community Survey, 2016 American Community Survey 1-Year Estimates, Table B05006; generated by Jessica DeLeon; using American FactFinder; <http://factfinder.census.gov>; (1 August 2017).
- 51.Parkvall M “Världens 100 största språk 2007” (The World’s 100 Largest Languages in 2007) in Nationalencyklopedin. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


