Abstract
Background
Esophagogastric junction outflow obstruction (EGJOO) as defined by Chicago Classification of esophageal motility disorders (CCv3.0) encompasses a broad range of diagnoses thus posing clinical challenges. Our aims were to evaluate Multiple Rapid Swallow (MRS) and Rapid Drink Challenge (RDC) during high-resolution manometry (HRM) to aid identifying clinically relevant EGJOO.
Methods
Patients with a HRM diagnosis of EGJOO based on CCv3.0 that also completed MRS and RDC during HRM and barium esophagram were retrospectively identified. Radiographic EGJOO (RAD-EGJOO) was defined by either liquid barium retention or delayed passage of a barium tablet on barium esophagram. 30 healthy asymptomatic controls that completed HRM were also included. MRS involved drinking 2 mL for 5 successive swallows. RDC involved rapid drinking of 200 mL liquid. Integrated relaxation pressure (IRP) and presence of pan-esophageal pressurization (PEP) during MRS and RDC were assessed.
Key Results
101 patients, mean (SD) age 56 (16) years, were included; 32% had RAD-EGJOO, 68% did not. RAD-EGJOO patients more frequently had elevated (>12mmHg) upright-IRP (100%), MRS-IRP (56%), RDC-IRP (53%), and PEP during RDC (66%) than both controls [17%; 0%; 7%; 3%] and patients without RAD-EGJOO [83%; 35%; 39%; 41%] Having IRP>12mmHg during both MRS and RDC was twice as likely to be associated with RAD-EGJOO (19%) than those without RAD-EGJOO (9%) among patients with upright IRP >12mmHg.
Conclusions & Inferences
Adjunctive HRM maneuvers MRS and RDC appear to help identify clinically significant EGJOO. While future outcome studies are needed, comprehensive multimodal evaluation helps clarify relevance of EGJOO on HRM.
Keywords: Dysphagia, Endoscopy, Motility, Diagnostic Tests, Achalasia
Introduction
Evaluation for esophageal motility disorders utilizing high-resolution manometry (HRM) is pursued during the clinical evaluation in patients presenting with dysphagia, non-cardiac chest pain, and other esophageal symptoms when upper gastrointestinal endoscopy does not reveal an objective diagnosis.1 The Chicago Classification of esophageal motility disorders utilizes parameters generated from ten 5-ml liquid test swallows performed in the supine position to categorize HRM findings into motility diagnoses.2,3 Achalasia, perhaps the most important of esophageal motility disorders, is characterized by abnormal deglutitive lower esophageal sphincter (LES) relaxation, which is quantified on HRM by an elevated integrated relaxation pressure (IRP). However, when IRP is elevated on HRM, but contractility or pressurization parameters do not meet criteria for an achalasia subtype, the manometric classification of esophagogastric junction outflow obstruction (EGJOO) is reached.
Although EGJOO is classified as a major esophageal motor disorder by the Chicago Classification, this manometric classification is clinically heterogeneous: This HRM pattern can be observed in patients with a variant of achalasia, but also among those without achalasia that instead have hiatal hernia, subtle or extrinsic mechanical obstruction, and even elevated IRP resulting from pressure or contact artifact.4–8 In this last scenario, the clinical-HRM diagnosis may be normal esophageal motility. Thus, this clinical differential diagnosis generated by this HRM classification spans the spectrum from achalasia to normal motility and this creates significant clinical management challenges, i.e. pursuit of appropriate versus inappropriate LES myotomy. While supplementary testing to manometry such as barium esophagram may ultimately be needed to clinically characterize patients, additional methods to aid clarification of the significance of IRP elevation on HRM remain essential.
Incorporation of upright swallows into the HRM protocol can aid excluding patients without clinically significant EGJOO (defined solely by a supine swallow protocol) when the IRP normalizes in the upright position.8,9 However, a recent study demonstrated that normalization of upright IRP was primarily an exclusionary finding as the upright IRP remained elevated in 75–85% of patients with supine swallow IRP elevation of unclear clinical relevance. Thus, additional maneuvers with HRM that may assist in identifying clinically relevant EGJOO include Multiple Rapid Swallow (MRS) and Rapid Drink Challenge (RDC).10–13 Therefore, the aim of this study was to evaluate the role of MRS and RDC in identifying clinically relevant EGJOO, defined by radiographic evidence of EGJOO on barium esophagram.
Material and Methods
Subjects
Patients with a diagnosis of EGJOO based on CCv3.0 were identified retrospectively from the Esophageal Center motility laboratory registry of HRM studies completed between January 2015 and July 2017. This registry includes English-speaking patients 18–85 years of age. Patients with previous foregut surgery and mechanical esophageal obstruction (including esophageal stricture, Los Angeles C or D erosive esophagitis, and hiatal hernia > 3cm) were excluded from this study, as were patients without available esophagram, HRM-MRS, or HRM-RDC. Northwestern University Institutional Review Board approved the study protocol and a waiver of informed consent was obtained for this retrospective analysis. This cohort was previously described.9
Additionally, a cohort of healthy, asymptomatic (i.e. free of esophageal symptoms including dysphagia, heartburn, and chest pain), adult volunteers were enrolled: “controls”. Potential subjects were excluded for any previous diagnosis of esophageal, autoimmune, or eating disorders. Subjects were also excluded for use of antacids or proton pump inhibitors, body mass index > 30 kg/m2, or a history of tobacco use or alcohol abuse. The study protocol was approved by the Northwestern University Institutional Review Board. Informed consent was obtained from all controls, who were paid for their participation. This cohort was previously described.14,15
High Resolution Manometry
Patients fasted for a minimum of 6-hours prior to completing HRM. The HRM studies used a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals (Medtronic, Shoreview, MN). The HRM device was placed transnasally to record from the hypopharynx to the stomach with approximately 3 intragastric pressure sensors.
The HRM protocol included 5 minutes of baseline recording, ten 5ml liquid supine swallows and five 5ml upright swallows. HRM also included MRS, which involved drinking 2-mL of water for 5 successive swallows, separated by 2–3 second intervals in an upright, seated position.16,17 The RDC involved drinking 200-mL of water, with patients instructed to drink as fast as possible, in an upright, seated position.10,11,18,19 The standard HRM protocol at the Esophageal Center of Northwestern that is attempted on every patient includes supine and upright 5-ml swallows, the MRS, and RDC. However, not all patients were able to tolerate or complete all portions and those without MRS or RDC were excluded.
ManoView version 3.0 analysis software (Medtronic) was used for analysis. IRP was applied to the supine and upright swallows per the Chicago Classification, and the median value across the 10 supine 5-ml liquid swallow and five 5-ml upright swallows, respectively. Esophageal motility diagnosis of EGJOO was defined by CCv3.0 as an elevated median supine (≥15 mmHg) but not meeting criteria for an achalasia subtype on HRM.3 Contractility patterns were based on the ten supine swallows and considered hypercontractile if ≥ 20% swallows with distal contractile integral >8000 mmHg•cm•s, ineffective if ≥50% ineffective (i.e. failed, weak, or fragmented) swallows, or normal peristalsis if neither of the former.3
The IRP was measured for both MRS and RDC; Figure 1.11,20 For RDC, several iterations of IRP measurement were first assessed, and then subjected to receiver operating characteristic (ROC) analysis. RDC-IRP was measured during the first 10 seconds, 20 seconds, and 30 seconds of the RDC (RDC-IRP-10s, -20s, -30s, respectively). RDC was measured over the entire RDC-duration (RDC-IRP-total) and also the RDC-IRP-AVG was the averaged value of 10-second increments over the entire duration (‘RDC-time’). (Figure S1). The RDC-IRP-30s was ultimately chosen based on test performance and ease of measurement and included for subsequent analysis as “RDC-IRP”.
Figure 1: Multiple rapid swallow (MRS) and rapid drink challenge (RDC) Interpretation.

MRS (A) and RDC (B) from a control and MRS (C) and RDC (D) from a patient are displayed. Integrated relaxation pressure (IRP) values were 5 mmHg in A, 1 mmHg in B, 31 mmHg in C, and 20 mmHg in D. Panesophageal pressurization (PEP) was observed in the patient (C and D), but not the control. Figure used with permission from the Esophageal Center at Northwestern.
Presence of PEP, defined as continuous pressurization from the upper esophageal sphincter to the EGJ at an isobaric contour of 30 mmHg, during MRS and during RDC was dichotomized as present or absent. Esophageal-gastric (EG) gradient during RDC was measured as the difference between the mean esophageal pressure and mean gastric pressure 2 cm above and below the LES, respectively.20 RDC shortening was defined by an elevation of the LES to ≥ 3 cm that occurs during or within the following 60 seconds of the RDC.10,12
Esophagram
Barium esophagrams were performed in the upright position. Timed barium esophagrams (TBE) were performed following consumption of 200-mL of low density barium sulfate and images were obtained at 1, 2, and 5 minutes. The height of the barium column was measured vertically from the EGJ. Patients without liquid barium retention or patients who did not have timed images recorded, underwent ingestion of a 12.5-mm barium tablet. As the primary outcome, patients were categorized as having radiographic EGJOO (“RAD-EGJOO”) if they had either liquid barium retention on TBE at 1 minute or delayed passage (based on radiologist impression) of a 12.5 mm barium tablet on barium esophagram.23 Those with complete clearance of barium at 1 min on TBE and when applicable, normal passage of a barium tablet, were categorized as being without RAD-EGJOO. The controls did not complete barium esophagram.
Patient reported symptom scores
Subjects completed validated self-reported symptom scores, Brief Esophageal Dysphagia Questionnaire (BEDQ) and GERDQ on the day of HRM.21,22 The BEDQ is a self-report measure of dysphagia severity that consists of eight 6-point Likert scale questions (scored 0–5) assessing the frequency and severity of dysphagia and odynophagia over the preceding 14-days. The 8 Likert-scaled items were summed to yield scores ranging from 0 (asymptomatic) – 40, with greater scores indicating greater dysphagia severity. A BEDQ score ≥10 was determined as the ideal threshold.21 The GERDQ is a 6-item self-report measure used to support GERD diagnosis. The items assess the frequency of symptoms and medication use over the preceding 7 days. The GERDQ is score in generated by summing four graded Likert scale items of four positive predictors (scored 0–3) and two reverse Likert scale items of negative predictors (scored 3–0). GERDQ scores ≤8 are considered normal, while scores > 8 are considered positive for a GERD diagnosis.22
Statistical Analyses
Data were analyzed using SPSS v 26 (IBM Corporation, Armonk, NY). Comparisons between cohorts were assessed using two-tailed t-test or Mann-Whitney U tests, depending on data distribution for continuous variables. Χ2-tests were applied for group comparison of categorical variables. Intra-subject comparisons were made using Wilcoxon Rank Sum test. Receiver operating characteristic (ROC) curves were also generated for prediction of RAD-EGJOO. Binary logistic regression to predict RAD-EGJOO was performed on the EGJOO patient sample via incorporation of variables with significant associations to RAD-EGJOO. Independent variables with significant univariable associations were applied to multivariable binary logistic regression; multicollinearity was evaluated using standard cutoff scores for variance inflation factor (< 5.0) and tolerance (> 0.2) with no violations indicated. All analyses assumed a 5% level of statistical significance.
Results
Study Subjects
101 patients with a CCv3.0 diagnosis of EGJOO that completed MRS and RDC during HRM and also barium esophagram were included in this study: mean (SD) age 56 (16) years, 60% female. The 30 asymptomatic controls had a mean (SD) age of 30 (6) years and 77% were female.
Clinical characteristics by cohort are included in Table 1. Esophagram was completed at median (IQR) 32 (14–88) days from HRM. Radiographic EGJ outflow obstruction (RAD-EGJOO) was observed in 32 (32%), thus 69 (68%) were without RAD-EGJOO. Among the 32 patients with RAD-EGJOO, 22 (69%) had liquid barium retention (4 of whom also had delayed barium tablet passage; 14 did not have a tablet applied) and 10 (31%) had delayed passage of barium tablet without retention of liquid barium. Of those with liquid barium retention, 9/32 had a TBE with column present at 5-minutes (median; range column height: 4.7: 1.2 – 22 cm); 4 of which had a 5-min TBE column height >5cm. Upper endoscopies within one year of HRM were available in 84 patients (83%; Table 1); the controls all had normal endoscopy. The BEDQ was completed by 89 (88%) patients and the GERDQ by 76 (75%). Patients were older, had greater body mass index (BMI), and greater symptom scores than controls. Patients with RAD-EGJOO had greater BEDQ scores than patients without RAD-EGJOO; otherwise demographics, endoscopic features, and GERDQ scores were similar between patients with and without RAD-EGJOO.
Table 1.
Patient characteristics.
| Controls | EGJOO-all | Without RAD-EGJOO | RAD-EGJOO | |
|---|---|---|---|---|
| n | 30 | 101 | 69 | 32 |
| Age, mean (SD) years | 30 (6) | 56 (16)* | 56 (16)* | 58 (15)* |
| Female, n (%) | 23 (77) | 61 (60) | 42 (61) | 19 (59) |
| BMI, kg/m2, mean (SD) | 23 (3) | 28 (7)* | 28 (7)* | 28 (7)* |
| Indication, n (%) | na | |||
| Dysphagia | 84 (83) | 55 (80) | 29 (91) | |
| Reflux-symptoms | 9 (9) | 8 (12) | 1 (3) | |
| Chest pain | 5 (5) | 4 (6) | 1 (3) | |
| Other | 3 (3) | 2 (3) | 1 (3) | |
| Endoscopy findings† | ||||
| Hiatal hernia | 0 | 10 (12) | 8 (14) | 2 (8) |
| Erosive esophagitis‡ | 0 | 3 (4) | 3 (5) | 0 |
| Diverticula | 0 | 7 (8) | 6 (10) | 1 (4) |
| BEDQ score, median (IQR)§ | 0 (0–0) | 10 (4 – 19)* | 7 (3 – 15)*, ** | 18 (12 – 23)*, ** |
| BEDQ score≥10, n (%) | 0 | 47 (53)* | 25 (41)* | 22 (78)*, ** |
| GERDQ score, mean (SD)¶ | 6 (0) | 9 (3)* | 9 (3)* | 9 (3)* |
| GERDQ score >8, n (%) | 0 | 39 (51)* | 27 (51)* | 12 (52)* |
P<0.05 compared with control.
P<0.05 for comparison of patients without radiographic EGJ outflow obstruction (RAD-EGJOO) vs patients with RAD-EGJOO.
Endoscopy available for 84 patients, 58/69 (84%) without RAD-EGJOO and 26/32 (81%) with RAD-EGJOO.
All 3 patients had Los Angeles grade A esophagitis.
BEDQ was completed by 89 patients, 61/69 (88%) without RAD-EGJOO and 28/32 (88%) with RAD-EGJOO.
GERDQ was completed by 76 patients, 53/69 (77%) without RAD-EGJOO and 23/32 (72%) with RAD-EGJOO.
High-resolution manometry findings among controls
Among the controls, median (5–95th) supine IRP 11 (4 – 17) mmHg the median (5–95%) upright IRP was 9 (0 – 17) mmHg, MRS-IRP was 3 (0 – 12) mmHg, RDC-IRP was 5 (−3 – 12) mmHg. The median (5–95th) RDC-EG-gradient was 2 (−6 – 10) mmHg. HRM classification from the supine swallows among the controls were of normal motility in 26 (87%) and ineffective esophageal motility in two (7%). Two (7%) controls had supine IRP > 15 mmHg (16 and 17 mmHg): both had normal peristalsis and had upright IRPs of 10 and 14 mmHg, MRS-IRPs of 6 and 12 mmHg, RDC-IRP of 8 and 10 mmHg, and RDC-EG-gradients of 2 and 5 mmHg, respectively.
High-resolution manometry findings in patients with EGJ outflow obstruction.
IRP values from supine swallows, upright swallows, MRS, and RDC, RDC-EG-gradient, frequency of PEP at MRS and RDC, and esophageal shortening events with RDC were greater or more frequent in patients than controls (Table 2). By selection, all EGJOO patients had a median supine IRP > 15 mmHg which was associated with contractility patterns during supine swallows meeting classification for normal peristalsis in 58 (57%), ineffective in 23 (23%), and hypercontractile in 20 (20%). There was a trend toward a greater proportion of hypercontractile and less normal peristalsis in patients with RAD-EGJOO than without (P=0.050): contractility patterns in RAD-EGJOO included normal peristalsis in 13 (41%), ineffective in 9 (28%), and hypercontractile in 10 (31%) and in patients without RAD-EGJOO, normal peristalsis in 45 (65%), ineffective in 14 (20%), and hypercontractile in 10 (15%).
Table 2.
High-resolution manometry (HRM) findings between controls and patients with and without radiographic EGJ outflow obstruction (RAD-EGJOO).
| Controls | EGJOO-all | Without RAD-EGJOO | RAD-EGJOO | |
|---|---|---|---|---|
| n | 30 | 101 | 69 | 32 |
| Values reflect median (IQR) | ||||
| Basal EGJ-pressure, mmHg | 15 (8 – 19) | 22 (11 – 39)* | 23 (11 – 42)* | 20 (10–31)* |
| Supine IRP, mmHg | 11 (8 – 14) | 21 (18 – 26)* | 21 (17 – 25)* | 22 (18 – 28)* |
| Upright IRP, mmHg | 9 (4 – 10) | 19 (15 – 25)* | 18 (14 – 22)*,† | 22 (16 – 32)*,† |
| MRS-IRP, mmHg | 3 (0 – 6) | 11 (8 – 16)* | 10 (7 – 13)*,† | 13 (9 – 17)*,† |
| RDC-IRP, mmHg | 5 (1 – 7) | 11 (6 – 16)* | 11 (6 – 16)* | 13 (7 – 18)* |
| RDC-time, seconds | 39 (22 – 61) | 36 (22 – 61) | 36 (24 – 52) | 37 (20 – 78) |
| RDC-EG-gradient, mmHg | 2 (4 – 7) | 5 (1 – 14)* | 4 (1 – 13)* | 9 (2 – 15)* |
| Values reflect n (%) | ||||
| MRS-PEP present | 0 | 17 (17)* | 10 (15)* | 7 (22)* |
| RDC-PEP present | 1 (3) | 49 (49)* | 28 (41)*,† | 21 (66)*, † |
| RDC-shortening present | 0 | 27 (27)* | 17 (25)* | 10 (31)* |
| Upright-IRP >12mmHg | 5 (17) | 89 (88)* | 57 (83)*, † | 32 (100)*, † |
| MRS-IRP > 8mmHg | 2 (7) | 77 (76)* | 47 (68)*, † | 30 (94),*,† |
| MRS-IRP >12mmHg | 0 | 42 (42)* | 24 (35)*,† | 18 (56)*,† |
| RDC-IRP > 8mmHg | 6 (20) | 66 (65)* | 44 (64)*,† | 22 (69)*,† |
| RDC-IRP >12mmHg | 2 (7) | 44 (44)* | 27 (39)* | 17 (53)* |
| MRS-IRP >8mmHg AND MRS-PEP | 0 | 15 (15)* | 9 (13)* | 6 (19)* |
| RDC-IRP >8mmHg AND RDC-PEP | 0 | 36 (36)* | 21 (30)* | 15 (47)* |
| MRS-IRP>8mmHg AND RDC-IRP>8mmHg | 1 (3) | 59 (58)* | 37 (54)* | 22 (69)* |
| MRS-IRP >12mmHg AND MRS-PEP | 0 | 11 (11) | 5 (7) | 6 (19)* |
| RDC-IRP >12mmHg AND RDC-PEP | 0 | 25 (25)* | 13 (19)*,† | 12 (38)*,† |
| MRS-IRP>12mmHg AND RDC-IRP>12mmHg | 0 | 20 (20)* | 8 (12)† | 12 (38)*,† |
Continuous variables displayed as median (IQR) while categorical variables displayed as n (% cohort). Thresholds for categorization of continuous variables were determined based on 95th percentile of controls and from ROC curves (Figure 2).
P<0.05 compared with control.
P<0.05 for comparison between patients without RAD-EGJOO and patients with RAD-EGJOO.
Among patients, paired comparisons demonstrated higher supine IRP than upright IRP, MRS-IRP, and RDC-IRP (P-values ≤0.002) and higher upright IRP values than MRS-IRP and RDC-IRP (P-values <0.001); Table 2. Supine IRP, upright IRP, MRS-IRP, and RDC-IRP were also significantly correlated among patients (rho values 0.363 – 0.716; P-values <0.001; Table S1).
Prediction of radiographic EGJ outflow obstruction
Patients with RAD-EGJOO had greater upright IRP values, greater MRS-IRP more frequent PEP during RDC than patients without RAD-EGJOO, but similar RDC IRP values than patients without RAD-EGJOO (Table 2). Patients with RAD-EGJOO had similar RDC-EG-gradients with both controls and patients without RAD-EGJOO.
ROC curves for prediction of RAD-EGJOO versus control and RAD-EGJOO versus HRM-EGJOO without RAD-EGJOO demonstrated greatest area under the curves (AUCs) for upright IRP and MRS-IRP (Figure 2), as compared with RDC-IRP and EG-gradient. Based on the ROC curves, ideal thresholds were estimated as 12 mmHg for upright IRP, 8 mmHg for MRS-IRP, 8 mmHg for RDC-IRP, which were applied for comparisons displayed in Table 2. Applying these thresholds to differentiate HRM-EGJOO with RAD-EGJOO from HRM-EGJOO without RAD-EGJOO yielded sensitivity and specificity of 100% and 17% for upright-IRP 12mmHg, 94% and 32% for MRS-IRP 8mmHg, 56% and 65% for MRS-IRP 12mmHg, 69% and 36% for RDC-IRP 8mmHg, and 53% and 61% for RDC-IRP 12mmHg. Variables with significant difference between RAD-EGJOO and no RAD-EGJOO were also assessed via logistic regression (Table 3). Multivariable analysis including upright IRP, PEP during RDC, and MRS-IRP and RDC-IRP > 12mmHg demonstrated that upright IRP was the only significant predictor for RAD-EGJOO.
Figure 2: Receiver operating characteristic (ROC) curves to predict radiographic esophagogastric junction outflow obstruction (RAD-EGJOO).

A. Prediction of RAD-EGJOO on comparison with controls. B. Prediction of RAD-EGJOO on comparison with patients without RAD-EGJOO. MRS – multiple rapid swallows. RDC – rapid drink challenge. IRP – integrated relaxation pressure. AUC – area under the curve 1Median value of supine or upright swallows, respectively.
Table 3.
Binary logistic regression for predictors of radiographic EGJ-outflow obstruction. A) Among the entire patient cohort, N=101. B) Among the patient cohort with upright integrated relaxation pressure (IRP) > 12 mmHg. MRS – multiple rapid swallows. RDC – rapid drink challenge. PEP – panesophageal pressurization.
| A | ||||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| B (SE) | OR (5–95 CI) | P | B (SE) | OR (5–95 CI) | P | |
| Upright IRP | 0.09 (0.03) | 1.1 (1.0–1.1) | 0.002 | 0.06 (0.03) | 1.1 (1.0–1.1) | 0.045 |
| MRS-IRP | 0.09 (0.04) | 1.1 (1.0– 1.2) | 0.013 | |||
| RDC-PEP present | 1.03 (0.45) | 2.8 (1.2–6.7) | 0.021 | 0.37 (0.54) | 1.5 (0.5–4.2) | 0.49 |
| MRS-IRP >12mmHg | 0.88 (0.44) | 2.4 (1.0–5.7) | 0.044 | |||
| RDC-IRP > 8mmHg | 0.2 (0.46) | 1.3 (0.5–3.1) | 0.625 | |||
| RDC-IRP >12mmHg AND RDC-PEP | 0.95 (0.48) | 2.6 (1.0–6.6) | 0.047 | |||
| MRS-IRP>12mmHg AND RDC-IRP>12mmHg | 1.5 (0.52) | 4.6 (1.6–12.8) | 0.004 | 0.79 (0.65) | 0.23 (0.6–7.9) | 0.23 |
| B | |||
|---|---|---|---|
| Univariable | |||
| B (SE) | OR (5–95 CI) | P | |
| RDC-PEP present | 0.83 (0.46) | 2.3 (0.93–5.6) | 0.072 |
| MRS-IRP >8mmHg | 1.4 (0.8) | 4.0 (0.8–19.1) | 0.083 |
| MRS-IRP>12mmHg AND RDC-IRP>12mmHg | 1.3 (0.53) | 3.7 (1.3–10.3) | 0.014 |
Focused examination of the 4 patients with a 5-minute column height >5cm on TBE (Table S2) demonstrated that 3 of 4 patients had IRP values >12mmHg on upright, MRS, and RDC; all three of whom also had PEP on MRS or RDC. The fourth patient (with MRS and RDC IRP < 12mmHg) had esophageal shortening after RDC. All four patients with a 5-minute column height >5cm on TBE had a BEDQ score > 10.
As reduction of IRP in the upright position to <12mmHg was previous demonstrated to carry a high negative predictive value for RAD-EGJOO (all 12 such patients included in this cohort were without RAD-EGJOO), the patients with IRP > 12mmHg in the upright position were analyzed separately with regard to radiographic EGJOO (Table 4).9 In doing so, BEDQ score was found to be greater and more frequently ≥10 in those with than those without RAD-EGJOO. Among HRM parameters, a greater proportion of patients with both MRS and RDC IRP values >12mmHg were observed in the RAD-EGJOO group. Logistic regression demonstrated that MRS-IRP and RDC-IRP > 12mmHg had an OR of 3.7 (5–95th CI 1.3 – 10.3) for prediction of RAD-EGJOO (Table 3). There were also trends toward more frequent RDC-PEP (P = 0.081) and MRS-IRP > 8 mmHg (P = 0.077) in the RAD-EGJOO patients compared to those without RAD-EGJOO. Compared HRM parameters were otherwise similar between patients with and without RAD-EGJOO. Application of a stepwise decision process to apply first upright IRP and then MRS-IRP and RDC-IRP are displayed in Figure 3. Patient examples are displayed in Figure 4.
TABLE 4.
Patients with EGJ outflow obstruction on HRM by abnormal integrated relaxation in both supine and upright positions.
| HRM-EGJOO with upright IRP >12mmHg | |||
|---|---|---|---|
| ALL | Without RAD-EGJOO | RAD-EGJOO | |
| n | 89 | 57 | 32 |
| Values reflect median (IQR), unless otherwise specific | |||
| Basal EGJ-pressure, mmHg | 21 (11 – 37) | 22 (11 – 31) | 20 (10 – 39) |
| Supine IRP, mmHg | 22 (18 – 27) | 21 (18 – 26) | 22 (18 – 28) |
| Upright IRP, mmHg | 20 (17 – 26) | 20 (17 – 23) | 22 (16 – 32) |
| MRS-IRP, mmHg | 12 (9 – 16) | 11 (9 – 15) | 13 (9 – 17) |
| RDC-IRP, mmHg | 12 (7 – 17) | 11 (7 – 16) | 13 (7 – 18) |
| RDC-time, seconds | 38 (24 – 63) | 38 (25 – 61) | 37 (20 – 78) |
| RDC-EG-gradient, mmHg | 6 (2 – 15) | 5 (2 – 13) | 9 (2 – 15) |
| BEDQ score, mean (SD) | 12 (10) [n =79] |
9 (8) [n =51] |
17 (10) [n =28] |
| Values reflect n (%) | |||
| BEDQ score ≥10 | 43 (54) [n =79] |
21 (41)* [n =51] |
22 (77)* [n =28] |
| MRS-PEP present | 17 (19) | 10 (18) | 7 (22) |
| RDC-PEP present | 47 (53) | 26 (46) | 21 (66) |
| RDC-shortening present | 26 (29) | 16 (28) | 10 (31) |
| MRS-IRP > 8mmHg | 75 (84) | 45 (79) | 30 (94) |
| MRS-IRP >12mmHg | 42 (47) | 24 (42) | 18 (56) |
| RDC-IRP > 8mmHg | 61 (69) | 39 (68) | 22 (69) |
| RDC-IRP >12mmHg | 42 (47) | 25 (44) | 17 (53) |
| MRS-IRP >8mmHg AND MRS-PEP | 15 (17) | 9 (16) | 6 (19) |
| RDC-IRP >8mmHg AND RDC-PEP | 34 (38) | 19 (33) | 15 (47) |
| MRS-IRP>8mmHg AND RDC-IRP>8mmHg | 57 (64) | 35 (61) | 22 (69) |
| MRS-IRP >12mmHg AND MRS-PEP | 11 (12) | 5 (9) | 6 (19) |
| RDC-IRP >12mmHg AND RDC-PEP | 25 (28) | 13 (23) | 12 (38) |
| MRS-IRP>12mmHg AND RDC-IRP>12mmHg | 20 (23) | 8 (14)* | 12 (38)* |
Continuous variables displayed as median (IQR) while categorical variables displayed as n (% cohort), unless otherwise specified.
P <0.05 on comparison between with and without RAD-EGJOO.
Figure 3: Application of decision tree to assess for radiographic esophagogastric junction outflow obstruction (RAD-EGJOO).

Application of a the stepwise approach to apply 1) Upright IRP, then 2) MRS-IRP and RDC-IRP, demonstrated the greatest proportion of esophagram/HRM positive/positive (red-shading) were identified with upright, MRS, and RDC IRPs > 12mmHg, and greatest proportion of negative/negative (blue shading) had upright, MRS, RDC IRP <12mmHg. Persistent overlap of outcome groups is still notable however. MRS – multiple rapid swallows. RDC – rapid drink challenge. IRP – integrated relaxation pressure. Figure used with permission from the Esophageal Center at Northwestern.
Figure 4. Examples of patients with EGJ outflow obstruction on high-resolution manometry.
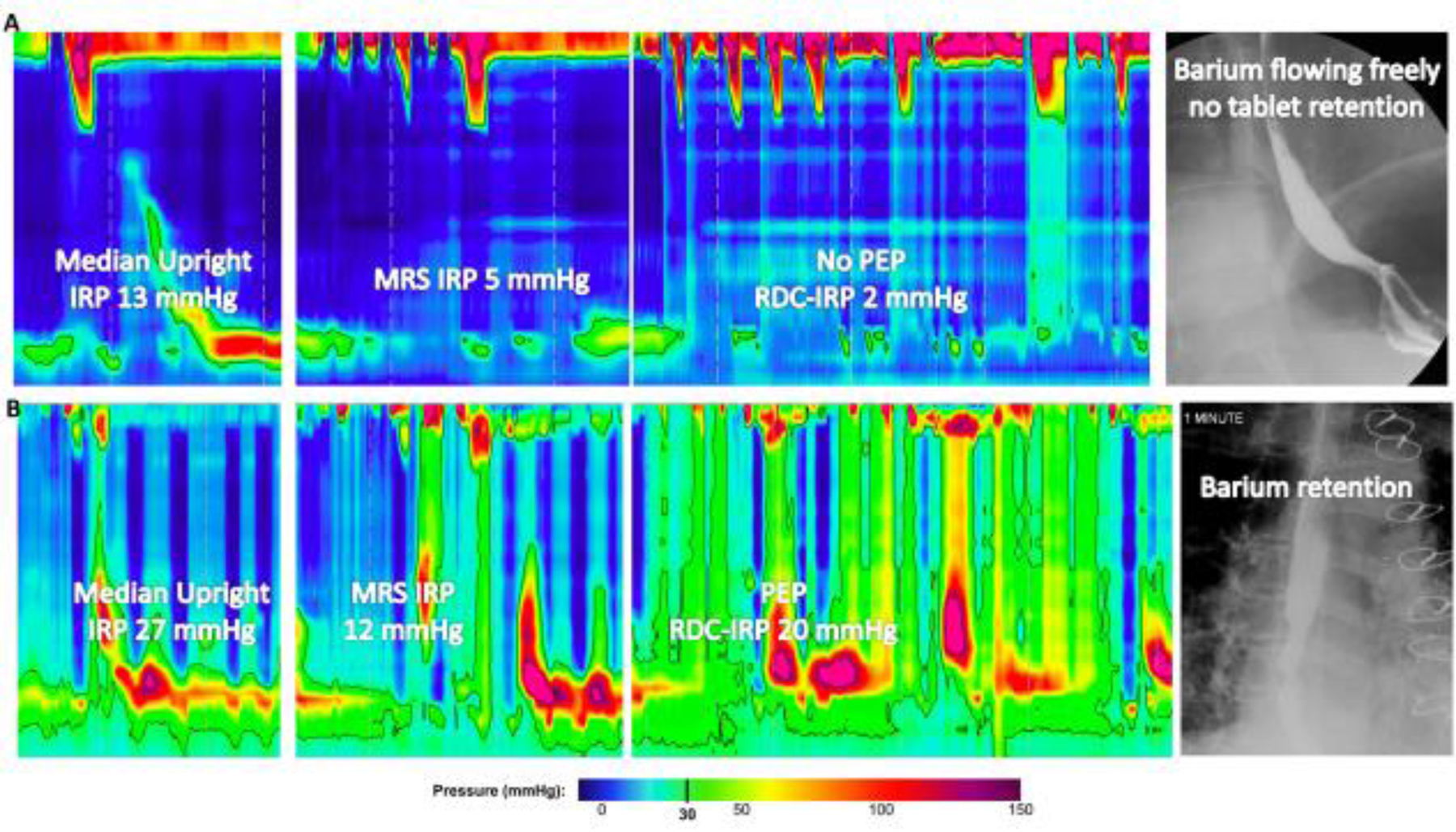
A. After presenting with chest pain and having a normal endoscopy, HRM demonstrated EGJOO with median supine integrated relaxation pressure (IRP) of 21 mmHg. IRP remained >12mmHg in the upright position, but normalized with both multiple rapid swallows (MRS) and rapid drink challenge (RDC). Barium esophagram was normal. B. After presenting with dysphagia and having a normal endoscopy, HRM demonstrated EGJOO with median supine IRP 18 mmHg. IRP elevation persisted with upright swallows, MRS, and RDC. Panesophageal pressurization (PEP) was also observed with both MRS and RDC. Timed-barium esophagram demonstrated barium retention. Figure used with permission from the Esophageal Center at Northwestern.
Discussion
We evaluated HRM parameters associated with MRS and RDC among asymptomatic controls and patients with EGJOO classified per CCv3.0 to identify factors associated with clinically relevant EGJOO, which was defined based on barium esophagram. In doing so, we demonstrated that both MRS and RDC can supplement identification of an abnormal esophagram with elevation in IRP or PEP occurring during these maneuvers. While neither of these parameters appeared to have negative predictive values that performed as well as normalization of IRP in the upright swallows,9 elevation of IRP (>12 mmHg) on both MRS and RDC provided adjuvant characterization among people in whom IRP does not normalize in the upright position. Thus, MRS and RDC improved identification of clinically relevant EGJOO, as opposed to clinically misleading IRP elevation related to pressure artifact on supine test swallows. Therefore, incorporation of MRS and RDC should be considered for inclusion in the HRM test protocol to provide complementary information to both supine and upright liquid single test swallows.
The HRM classification of EGJOO encompasses a spectrum of clinical diagnoses that range from the clinically-relevant, such as variant-achalasia and un-recognized mechanical obstruction, to clinically insignificant: elevated IRP values related to pressure artifact. Applying a threshold of a 95th percentile of normal, by definition, could identify findings that overlap with the extreme of healthy controls (as observed in 2/30 controls with a supine IRP >15mmHg in this study). Thus, an effective diagnostic approach would ideally both differentiate health from pathology (e.g. controls from patients), but also aid identification of clinically-relevant pathologic findings. Similar to previous studies comparing patients with controls, this study also demonstrated differences associated with various parameters during RDC. However, the current study also applied an objective assessment for esophageal obstruction that was independent of the HRM study by applying barium esophagram, a marker of clinically relevant esophageal obstruction.23 When doing so, the IRP during MRS, as well as PEP and IRP with RDC differed between HRM-EGJOO patients with RAD-EGJOO and those without. Similarly, another previous study found that IRP during RDC was predictive of esophageal retention on TBE.13
The recent focus of MRS has primarily been utilization to assess for contractile reserve as it associates with gastroesophageal reflux.17,18,24 However, this study demonstrates the utility of MRS to also assess LES relaxation and esophageal outflow obstruction. Similar to RDC, the rapid swallows of MRS generates increased inhibitory signaling with expected greater relaxation of LES – if inhibitory innervation to the LES is intact. Impaired LES relaxation even with increased stimuli would still be expected in the setting of inhibitory neuronal loss with achalasia representing the prime example. The degree of inhibitory ganglion loss among the sub-set of HRM-EGJOO that entails ‘early’ or ‘evolving’ achalasia is under explored, though inhibitory ganglion loss was reported among one of three EGJOO patients studied.25
We previously reported findings from a greater portion of this patient cohort that an upright IRP of >12 mmHg carried a 98% sensitivity, but only 16% specificity for identifying patients with radiographic evidence of EGJOO.9 While this supports the importance of consistent bi-positional IRP elevation for clinical significant EGJ outflow obstruction, relying solely on the two IRP values is still subject to clinically irrelevant classification of ‘EGJOO’. Thus, we specifically assessed patients in which IRP elevation persisted in the upright position, and found that combined application of elevated MRS and RDC-IRPs (thus IRP elevation across supine, upright, MRS, and RDC) was associated with an increased probability for an abnormal esophagram, i.e. clinically relevant outflow obstruction. However, even consistent IRP elevation across multiple HRM tests did not identify, nor exclude, all cases (Figure 3). In some cases, position change or provocative maneuvers may not correct or overcome IRP elevation related to pressure, contact, or impingement artifact.26 However, other features such as presence of PEP, a marker of outflow obstruction, appeared to carry value and has the added benefit of relative immunity from pressure artifact. Additionally, esophageal shortening, which was an ‘abnormal’ finding in our study (as in not observed in controls), may reflect abnormality other than obstruction.27 This finding was associated with ‘incomplete’ achalasia in another study applying RDC to HRM-EGJOO.12 Finally, greater BEDQ scores remained associated with RAD-EGJOO, even among the study cohort composed primarily of patients evaluated with an indication of dysphagia. Thus, although abnormal symptoms scores are not unique between esophageal motility disorders,28 their complementary role in the clinical evaluation is supported by this study.
While the current study assessed a large cohort of patients that were comprehensively evaluated with HRM including provocative maneuvers, esophagram, and validated symptom scores, this study does carry limitations. As is similar in most of esophageal motor disorders, a perfect ‘gold standard’ to assess for specific esophageal motility disorders is lacking. In this study, we placed the primary assessment for ‘clinically-significant’ EGJOO based on esophagram results as it does represent an objective outcome that is independent of the HRM protocol.23 However, esophagram is also not a perfect diagnostic tool and carries its own inherent limitations. Further, we applied a fairly liberal definition for abnormal esophagram using barium retention at 1 minute during a TBE protocol or tablet retention (as opposed to an achalasia-outcome type assessment requiring significant retention on TBE at 5 minutes), as this would only have considered 4/101 (4%) of patients ‘abnormal’. Thus it was not our intent to suggest that all patients with barium retention or delayed passage of barium tablet represent the variant-achalasia spectrum of HRM-EGJOO, nor that all such patients should be offered achalasia-type intervention (e.g. myotomy). Instead, retention on esophagram represents an objective abnormality in esophageal function and may reflect clinically-relevant abnormalities other than achalasia (e.g. subtle stricture or extrinsic compression). Further, even subtle barium retention may represent a clinically-relevant abnormality when occurring in the setting of an ‘abnormal’ HRM per our inclusion criteria. Overall, generation of the clinical diagnosis and related management decisions requires comprehensive review of the clinical data, to which HRM with MRS and RDC appear to provide a useful addition.
Other study limitations include our inclusion requirement for completion of esophagram that likely generated a selection-bias among this cohort as suspicion for clinically relevant obstruction (based on symptom-severity, endoscopic, or HRM features) were likely factors related to referral for and completion of esophagram. Additionally, there are other parameters or promising HRM maneuvers that may further enhance identification of clinically significant obstruction, such as those involved with high-resolution impedance manometry and the solid test meal, however these were not included in this study.29–32 Finally, the study’s retrospective, cross-sectional design carries limitations in lack of longitudinal follow-up after consistently implemented management strategies. Future prospective studies are needed to assess predictors related to clinical outcomes.
In conclusion, EGJOO on HRM can create diagnostic challenges related to the heterogeneity of clinical etiologies it encompasses that carry widely varying management strategies. This study demonstrated that application of additional HRM maneuvers of MRS and RDC improves identification of clinically significant EGJOO, but that neither are capable to do so in isolation. Future studies to involve more sophisticated analytic approaches, such as machine-learning techniques, could aid optimization of parameter thresholds and clinical application of the multiple parameters generated by a comprehensive HRM testing protocol.33 Overall, identification of clinical-pathology among patients meeting the current classification of HRM-EGJOO likely requires a multifactorial approach that incorporates symptomatic presentation, careful endoscopic examination, HRM with a comprehensive testing protocol, as well as complementary testing such as esophagram or functional luminal imaging probe.5,34 Ultimately, comprehensive clinical evaluation can hopefully facilitate identification of specific clinical entities toward which targeted management approaches can be directed.
Supplementary Material
Key points:
Esophagogastric junction outflow obstruction (EGJOO) on high-resolution manometry (HRM) is a manometric classification that is associated with a broad spectrum of clinical diagnoses ranging from variant-achalasia to normal esophageal motility (the latter associated with pressure artifact), thus methods to improve assessment of this HRM pattern are needed.
On evaluation of 101 patients with EGJOO classified on supine HRM test swallows, elevated integrated relaxation pressure during MRS and RDC were associated with esophageal retention on barium esophagram.
Both the multiple rapid swallows and rapid drink challenge demonstrated utility to predict clinical relevance of EGJOO classified on HRM and thus appear to be useful additions to the HRM test protocol.
Esophagogastric junction outflow obstruction (EGJOO) on high-resolution manometry (HRM) is a manometric classification that is associated with a broad spectrum of clinical diagnoses ranging from variant-achalasia to normal esophageal motility (the latter associated with pressure artifact), thus methods to improve assessment of this HRM pattern are needed.
The study aimed to evaluate the utility of the manometric provocative maneuvers of multiple rapid swallows and rapid drink challenge to predict esophageal retention on barium esophagram among 101 patients with EGJOO classified on HRM.
Both the multiple rapid swallows and rapid drink challenge demonstrated utility to predict retention on barium esophagram among patients and appear to be useful additions to the HRM test protocol.
Key message:
Provocative manometry maneuvers of demonstrated utility to predict retention on barium esophagram among patients with esophagogastric junction outflow obstruction (EGJOO) classified on high-resolution manometry (HRM).
Esophagogastric junction outflow obstruction (EGJOO) on high-resolution manometry (HRM) is a manometric classification that is associated with a broad spectrum of clinical diagnoses ranging from variant-achalasia to normal esophageal motility (the latter associated with pressure artifact), thus methods to improve assessment of this HRM pattern are needed.
The study evaluated HRM findings from multiple rapid swallows (MRS) and rapid drink challenge (RDC) to predict esophageal retention on barium esopagram among 101 patients with EGJOO classified on HRM. Having integrated relaxation pressure >12mmHg during both MRS and RDC was twice as likely to be associated with retention on barium esophagram than a normal esophagram.
Acknowledgments
Funding: This study was funded in part by the Public Health service and American College of Gastroenterology Junior Faculty Development Award, grant number P01 DK117824 (JEP).
Footnotes
Disclosures
Dustin A. Carlson: Medtronic (Speaking. Consulting)
John E. Pandolfino: Crospon, Inc (stock options), Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking), Medtronic (Speaking. Consulting), Torax (Speaking, Consulting), Ironwood (Consulting), Impleo (Grant).
None: Amanda J. Krause, Hui Su, Joseph R. Triggs, Claire Beveridge, Alexandra J. Baumann, Erica Donnan
References
- 1.Pandolfino JE, Kahrilas PJ. American Gastroenterological Association medical position statement: Clinical use of esophageal manometry. Gastroenterology. 2005;128(1):207–208. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103(1):27–37. [DOI] [PubMed] [Google Scholar]
- 3.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13(12):2219–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton SB, Patel R, Richter JE. Functional and Anatomic Esophagogastic Junction Outflow Obstruction: Manometry, Timed Barium Esophagram Findings, and Treatment Outcomes. Clin Gastroenterol Hepatol. 2016;14(6):907–911. [DOI] [PubMed] [Google Scholar]
- 6.Schupack D, Katzka DA, Geno DM, Ravi K. The clinical significance of esophagogastric junction outflow obstruction and hypercontractile esophagus in high resolution esophageal manometry. Neurogastroenterol Motil. 2017;29(10):1–9. [DOI] [PubMed] [Google Scholar]
- 7.van Hoeij FB, Smout AJ, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil. 2015;27(9):1310–1316. [DOI] [PubMed] [Google Scholar]
- 8.Babaei A, Shad S, Szabo A, Massey BT. Pharmacologic interrogation of patients with esophagogastric junction outflow obstruction using amyl nitrite. Neurogastroenterol Motil. 2019;31(9):e13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triggs JR, Carlson DA, Beveridge C, et al. Upright Integrated Relaxation Pressure Facilitates Characterization of Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol. 2019. [DOI] [PMC free article] [PubMed]
- 10.Marin I, Serra J. Patterns of esophageal pressure responses to a rapid drink challenge test in patients with esophageal motility disorders. Neurogastroenterol Motil. 2016;28(4):543–553. [DOI] [PubMed] [Google Scholar]
- 11.Ang D, Hollenstein M, Misselwitz B, et al. Rapid Drink Challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil. 2017;29(1). [DOI] [PubMed] [Google Scholar]
- 12.Biasutto D, Mion F, Garros A, Roman S. Rapid drink challenge test during esophageal high resolution manometry in patients with esophago-gastric junction outflow obstruction. Neurogastroenterol Motil. 2018;30(6):e13293. [DOI] [PubMed] [Google Scholar]
- 13.Woodland P, Gabieta-Sonmez S, Arguero J, et al. 200 mL Rapid Drink Challenge During High-resolution Manometry Best Predicts Objective Esophagogastric Junction Obstruction and Correlates With Symptom Severity. J Neurogastroenterol Motil. 2018;24(3):410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson DA, Kou W, Lin Z, et al. Normal Values of Esophageal Distensibility and Distension-Induced Contractility Measured by Functional Luminal Imaging Probe Panometry. Clin Gastroenterol Hepatol. 2019;17(4):674–681 e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su H, Krause A, Masihi M, et al. Normal values of high-resolution manometry parameters with provocative maneuvers. UNDER REVIEW. 2020. [DOI] [PMC free article] [PubMed]
- 16.Fornari F, Bravi I, Penagini R, Tack J, Sifrim D. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil. 2009;21(7):718–e741. [DOI] [PubMed] [Google Scholar]
- 17.Shaker A, Stoikes N, Drapekin J, Kushnir V, Brunt LM, Gyawali CP. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol. 2013;108(11):1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daum C, Sweis R, Kaufman E, et al. Failure to respond to physiologic challenge characterizes esophageal motility in erosive gastro-esophageal reflux disease. Neurogastroenterol Motil. 2011;23(6):517–e200. [DOI] [PubMed] [Google Scholar]
- 19.Elvevi A, Mauro A, Pugliese D, et al. Usefulness of low- and high-volume multiple rapid swallowing during high-resolution manometry. Dig Liver Dis. 2015;47(2):103–107. [DOI] [PubMed] [Google Scholar]
- 20.Marin I, Cisternas D, Abrao L, et al. Normal values of esophageal pressure responses to a rapid drink challenge test in healthy subjects: results of a multicenter study. Neurogastroenterol Motil. 2017;29(6). [DOI] [PubMed] [Google Scholar]
- 21.Taft TH, Riehl M, Sodikoff JB, et al. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol Motil. 2016;28(12):1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonasson C, Wernersson B, Hoff DA, Hatlebakk JG. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37(5):564–572. [DOI] [PubMed] [Google Scholar]
- 23.Blonski W, Kumar A, Feldman J, Richter JE. Timed Barium Swallow: Diagnostic Role and Predictive Value in Untreated Achalasia, Esophagogastric Junction Outflow Obstruction, and Non-Achalasia Dysphagia. Am J Gastroenterol. 2018;113(2):196–203. [DOI] [PubMed] [Google Scholar]
- 24.Martinucci I, Savarino EV, Pandolfino JE, et al. Vigor of peristalsis during multiple rapid swallows is inversely correlated with acid exposure time in patients with NERD. Neurogastroenterol Motil. 2016;28(2):243–250. [DOI] [PubMed] [Google Scholar]
- 25.Sodikoff JB, Lo AA, Shetuni BB, Kahrilas PJ, Yang GY, Pandolfino JE. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil. 2016;28(1):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babaei A, Szabo A, Yorio SD, Massey BT. Pressure exposure and catheter impingement affect the recorded pressure in the Manoscan 360 system. Neurogastroenterol Motil. 2018. [DOI] [PMC free article] [PubMed]
- 27.Kwiatek MA, Post J, Pandolfino JE, Kahrilas PJ. Transient lower oesophageal sphincter relaxation in achalasia: everything but LOS relaxation. Neurogastroenterol Motil. 2009;21(12):1294–e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson D, Gyawali CP, Roman S, et al. Esophageal hypervigilance and visceral anxiety are major contributors to symptom severity among patients evaluated with high-resolution esophageal manometry. Gastroenterol. 2019;156(6):s999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ang D, Misselwitz B, Hollenstein M, et al. Diagnostic yield of high-resolution manometry with a solid test meal for clinically relevant, symptomatic oesophageal motility disorders: serial diagnostic study. Lancet Gastroenterol Hepatol. 2017;2(9):654–661. [DOI] [PubMed] [Google Scholar]
- 30.Misselwitz B, Hollenstein M, Butikofer S, Ang D, Heinrich H, Fox M. Prospective serial diagnostic study: the effects of position and provocative tests on the diagnosis of oesophageal motility disorders by high-resolution manometry. Aliment Pharmacol Ther. 2020;51(7):706–718. [DOI] [PubMed] [Google Scholar]
- 31.Singendonk MJ, Lin Z, Scheerens C, et al. High-resolution impedance manometry parameters in the evaluation of esophageal function of non-obstructive dysphagia patients. Neurogastroenterol Motil. 2018:e13505. [DOI] [PMC free article] [PubMed]
- 32.Lin Z, Carlson DA, Dykstra K, et al. High-resolution impedance manometry measurement of bolus flow time in achalasia and its correlation with dysphagia. Neurogastroenterol Motil. 2015;27(9):1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Z, Kahrilas PJ, Roman S, Boris L, Carlson D, Pandolfino JE. Refining the criterion for an abnormal Integrated Relaxation Pressure in esophageal pressure topography based on the pattern of esophageal contractility using a classification and regression tree model. Neurogastroenterol Motil. 2012;24(8):e356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triggs JR, Carlson DA, Beveridge C, Kou W, Kahrilas PJ, Pandolfino JE. Functional Luminal Imaging Probe Panometry Identifies Achalasia-Type Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol. 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


